High Temperature Alters Secondary Metabolites and Photosynthetic Efficiency in Heracleum sosnowskyi
Abstract
:1. Introduction
2. Results and Discussion
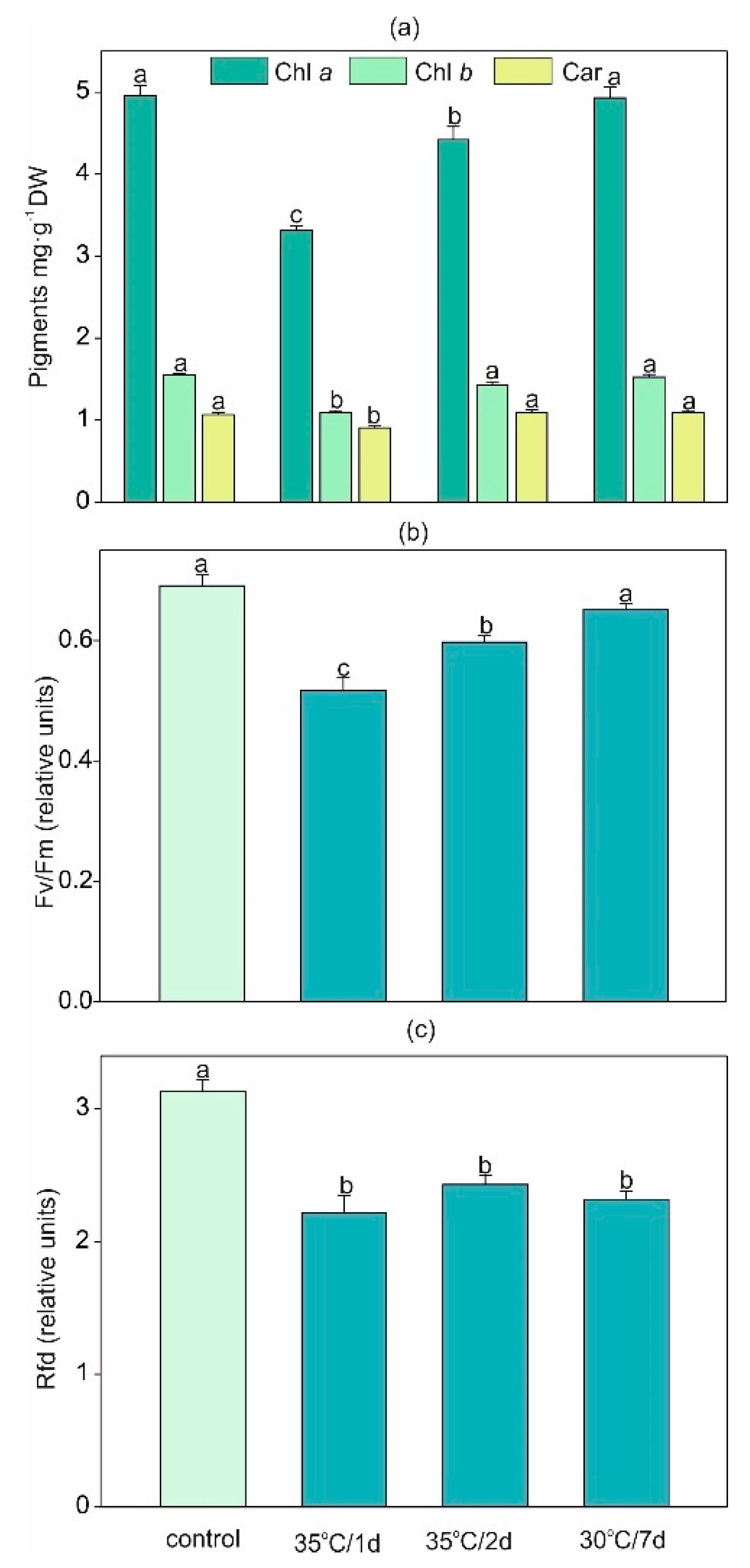
2.1. Impact of Heat Stress on Pigments Content and Parameters of Chlorophyll a Fluorescence
2.2. High-Temperature-Induced Changes in Lipid Peroxidation and the H2O2 and Proline Content
2.3. Impact of High Temperature on SMs and Antioxidant Capacity
2.4. Principle Component Analysis
3. Materials and Methods
3.1. Plant Materials and Experimental Design
3.2. Photosynthetic Pigment Content and Chlorophyll Fluorescence Measurement
3.3. Detection of H2O2 and Determination of Lipid Peroxidation and Free Proline Accumulation
3.4. Determination of Secondary Metabolites and Antioxidant Capacity
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity Scenarios for the Year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Byers, J.E. Impact of Non-Indigenous Species on Natives Enhanced by Anthropogenic Alteration of Selection Regimes. Oikos 2002, 97, 449–458. [Google Scholar] [CrossRef]
- Crowl, T.A.; Crist, T.O.; Parmenter, R.R.; Belovsky, G.; Lugo, A.E. The Spread of Invasive Species and Infectious Disease as Drivers of Ecosystem Change. Front. Ecol. Environ. 2008, 6, 238–246. [Google Scholar] [CrossRef]
- Somero, G.N. The Physiology of Climate Change: How Potentials for Acclimatization and Genetic Adaptation Will Determine ‘Winners’ and ‘Losers’. J. Exp. Biol. 2010, 213, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Somero, G.N. The Physiology of Global Change: Linking Patterns to Mechanisms. Ann. Rev. Mar. Sci. 2012, 4, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Karmalkar, A.V.; Bradley, R.S. Consequences of Global Warming of 1.5 °C and 2 °C for Regional Temperature and Precipitation Changes in the Contiguous United States. PLoS ONE 2017, 12, e0168697. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Temperature Stress Can Alter the Photosynthetic Efficiency and Secondary Metabolite Concentrations in St. John’s Wort. Plant Physiol. Biochem. 2005, 43, 977–984. [Google Scholar] [CrossRef]
- Liu, Y.; Lin-Wang, K.; Espley, R.V.; Wang, L.; Li, Y.; Liu, Z.; Zhou, P.; Zeng, L.; Zhang, X.; Zhang, J.; et al. StMYB44 Negatively Regulates Anthocyanin Biosynthesis at High Temperatures in Tuber Flesh of Potato. J. Exp. Bot. 2019, 70, 3809–3824. [Google Scholar] [CrossRef] [Green Version]
- Weryszko-Chmielewska, E.; Chwil, M. Structures of Heracleum Sosnovskii Manden. Stem and Leaves Releasing Photodermatosis-Causing Substances. Acta Agrobot. 2012, 67, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Balal, R.M.; Shahid, M.A.; Javaid, M.M.; Iqbal, Z.; Anjum, M.A.; Garcia-Sanchez, F.; Mattson, N.S. The Role of Selenium in Amelioration of Heat-Induced Oxidative Damage in Cucumber under High Temperature Stress. Acta Physiol. Plant 2016, 38, 158. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.S. Impact of Combined Heat and Drought Stress on the Potential Growth Responses of the Desert Grass Artemisia Sieberi Alba: Relation to Biochemical and Molecular Adaptation. Plants 2019, 8, 416. [Google Scholar] [CrossRef] [Green Version]
- Hawrylak-Nowak, B.; Dresler, S.; Rubinowska, K.; Matraszek-Gawron, R.; Woch, W.; Hasanuzzaman, M. Selenium Biofortification Enhances the Growth and Alters the Physiological Response of Lamb’s Lettuce Grown under High Temperature Stress. Plant Physiol. Bioch. 2018, 127, 446–456. [Google Scholar] [CrossRef]
- Efeoglu, B.; Terzioglu, S. Photosynthetic Responses of Two Wheat Varieties to High Temperature. Eurasian J. Biosci. 2009, 3, 97–106. [Google Scholar] [CrossRef]
- Yamane, Y.; Shikanai, T.; Kashino, Y.; Koike, H.; Satoh, K. Reduction of QA in the Dark: Another Cause of Fluorescence Fo Increases by High Temperatures in Higher Plants. Photosynth. Res. 2000, 63, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Haldimann, P.; Feller, U. Inhibition of Photosynthesis by High Temperature in Oak (Quercus Pubescens L.) Leaves Grown under Natural Conditions Closely Correlates with a Reversible Heat-Dependent Reduction of the Activation State of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase. Plant Cell Environ. 2004, 27, 1169–1183. [Google Scholar] [CrossRef]
- Allakhverdiev, S.; Kreslavskii, V.; Klimov, V.; Los, D.; Carpentier, R.; Mohanty, P. Heat Stress: An Overview of Molecular Responses in Photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Srivastava, A.; Guissé, B.; Greppin, H.; Strasser, R.J. Regulation of Antenna Structure and Electron Transport in Photosystem II of Pisum Sativum under Elevated Temperature Probed by the Fast Polyphasic Chlorophyll a Fluorescence Transient: OKJIP. Biochim. Biophys. Acta Bioenerg. 1997, 1320, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Berry, J.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher Plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Reszczyńska, E.; Hanaka, A. Lipids Composition in Plant Membranes. Cell Biochem. Biophys. 2020, 78, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Pastenes, C.; Horton, P. Effect of High Temperature on Photosynthesis in Beans (I. Oxygen Evolution and Chlorophyll Fluorescence). Plant Physiol. 1996, 112, 1245–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to High Temperature Stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Yang, X.; Wen, X.; Gong, H.; Lu, Q.; Yang, Z.; Tang, Y.; Liang, Z.; Lu, C. Genetic Engineering of the Biosynthesis of Glycinebetaine Enhances Thermotolerance of Photosystem II in Tobacco Plants. Planta 2007, 225, 719–733. [Google Scholar] [CrossRef]
- Kreslavskii, V.; Zorina, A.; Los, D.; Fomina, I.; Allakhverdiev, S. Molecular Mechanisms of Stress Resistance of Photosynthetic Machinery. In Molecular Stress Physiology of Plants; Springer: New Delhi, India, 2013; pp. 21–51. ISBN 978-81-322-0806-8. [Google Scholar]
- Aminaka, R.; Taira, Y.; Kashino, Y.; Koike, H.; Satoh, K. Acclimation to the Growth Temperature and Thermosensitivity of Photosystem II in a Mesophilic Cyanobacterium, Synechocystis Sp. PCC6803. Plant Cell Physiol. 2006, 47, 1612–1621. [Google Scholar] [CrossRef] [Green Version]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does It Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kong, L.; Zhang, Y.; Liao, Y. Gene and Metabolite Integration Analysis through Transcriptome and Metabolome Brings New Insight into Heat Stress Tolerance in Potato (Solanum Tuberosum L.). Plants 2021, 10, 103. [Google Scholar] [CrossRef]
- Roháček, K. Chlorophyll Fluorescence Parameters: The Definitions, Photosynthetic Meaning, and Mutual Relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, X.; Lv, T.; Bai, M.; Wang, Z.; Niu, J. Effects of Water Stress on Fluorescence Parameters and Photosynthetic Characteristics of Drip Irrigation in Rice. Water 2020, 12, 289. [Google Scholar] [CrossRef] [Green Version]
- de Melo, H.F.; de Souza, E.R.; Cunha, J.C.; de Melo, H.F.; de Souza, E.R.; Cunha, J.C. Fluorescence of Chlorophyll a and Photosynthetic Pigments in Atriplex Nummularia under Abiotic Stresses. Rev. Bras. Eng. Agrícola Ambient. 2017, 21, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf Chlorophyll Fluorescence, Hyperspectral Reflectance, Pigments Content, Malondialdehyde and Proline Accumulation Responses of Castor Bean (Ricinus Communis L.) Seedlings to Salt Stress Levels. Ind. Crop. Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Yamada, M.; Hidaka, T.; Fukamachi, H. Heat Tolerance in Leaves of Tropical Fruit Crops as Measured by Chlorophyll Fluorescence. Sci. Hortic. 1996, 67, 39–48. [Google Scholar] [CrossRef]
- Rahimzadeh-Bajgiran, P.; Tubuxin, B.; Omasa, K. Estimating Chlorophyll Fluorescence Parameters Using the Joint Fraunhofer Line Depth and Laser-Induced Saturation Pulse (FLD-LISP) Method in Different Plant Species. Remote Sens. 2017, 9, 599. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, J.; Zhang, X.; Wei, H.; Cui, L. Effects of Heat Acclimation Pretreatment on Changes of Membrane Lipid Peroxidation, Antioxidant Metabolites, and Ultrastructure of Chloroplasts in Two Cool-Season Turfgrass Species under Heat Stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Heat Stress Injury in Relation to Membrane Lipid Peroxidation in Creeping Bentgrass. Crop. Sci. 2000, 40, 503–510. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Drought and Heat Stress Injury to Two Cool-Season Turfgrasses in Relation to Antioxidant Metabolism and Lipid Peroxidation. Crop. Sci. 2001, 41, 436–442. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.-J.; Dai, X.; Tian, M.; Li, Z.-G. Involvement of Calcium and Calmodulin in the Acquisition of Heat-Shock Induced Thermotolerance in Maize Seedlings. J. Plant Physiol. 1997, 150, 615–621. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When Defense Pathways Collide. The Response of Arabidopsis to a Combination of Drought and Heat Stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [Green Version]
- Harsh, A.; Sharma, Y.K.; Joshi, U.; Rampuria, S.; Singh, G.; Kumar, S.; Sharma, R. Effect of Short-Term Heat Stress on Total Sugars, Proline and Some Antioxidant Enzymes in Moth Bean (Vigna Aconitifolia). Ann. Agric. Sci. 2016, 61, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Gall, H.L.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Dresler, S.; Wójciak-Kosior, M.; Sowa, I.; Stanisławski, G.; Bany, I.; Wójcik, M. Effect of Short-Term Zn/Pb or Long-Term Multi-Metal Stress on Physiological and Morphological Parameters of Metallicolous and Nonmetallicolous Echium Vulgare L. Populations. Plant Physiol. Bioch. 2017, 115, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to Cold and Heat Stress: Accumulation of Phenolic Compounds in Tomato and Watermelon Plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A.; Krzaczek, T. The Furanocoumarins in the Roots of Heracleum Sibiricum L. Acta Pol. Pharm. Drug Res. 2003, 60, 391–393. [Google Scholar]
- Walker, D.J.; Martínez-Fernández, D.; Correal, E.; Romero-Espinar, P.; Antonio del Río, J. Accumulation of Furanocoumarins by Bituminaria Bituminosa in Relation to Plant Development and Environmental Stress. Plant Physiol. Biochem. 2012, 54, 133–139. [Google Scholar] [CrossRef]
- Bourgaud, F.; Brunel, M.C.; Guckert, A.; Forlot, P. Effect of Nitrogen Nutrition and Environmental Conditions on the Production of Pharmaceutically Useful Metabolites by Psoralea Cinerea. Eur. J. Agron. 1992, 1, 37–43. [Google Scholar] [CrossRef]
- Ali, S.T.; Mahmooduzzafar, M.Z.; Iqbal, M. Ontogenetic Changes in Foliar Features and Psoralen Content of Psoralea Corylifolia Linn. Exposed to SO2 Stress. J. Environ. Biol. 2008, 29, 661–668. [Google Scholar]
- Cai, J.; Ma, Y.; Hu, P.; Zhang, Y.; Chen, J.; Li, X. Elicitation of Furanocoumarins in Changium Smyrnioides Suspension Cells. Plant Cell Tissue Organ Cult. 2017, 130, 1–12. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of Flavonols over Hydroxycinnamic Acids Favors Oxidative Damage Protection under Abiotic Stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- Złotek, U.; Szymanowska, U.; Baraniak, B.; Karaś, M. Antioxidant Activity of Polyphenols of Adzuki Bean (Vigna Angularis) Germinated in Abiotic Stress Conditions. Acta Sci. Pol. Technol. Aliment. 2015, 14, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shvarts, M.; Borochov, A.; Weiss, D. Low Temperature Enhances Petunia Flower Pigmentation and Induced Chalcone Synthase Gene Expression. Physiol. Plant. 1997, 99, 67–72. [Google Scholar] [CrossRef]
- Shaked-Sachray, L.; Weiss, D.; Reuveni, M.; Nissim-Levi, A.; Oren-Shamir, M. Increased Anthocyanin Accumulation in Aster Flowers at Elevated Temperatures Due to Magnesium Treatment. Physiol. Plant. 2002, 114, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Chalker-Scott, L. Do Anthocyanins Function as Osmoregulators in Leaf Tissues? In Advances in Botanical Research; Academic Press: Amsterdam, The Netherlands, 2002; Volume 37, pp. 103–127. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Hanaka, A.; Maksymiec, W.; Bednarek, W. The Effect of Methyl Jasmonate on Selected Physiological Parameters of Copper-Treated Phaseolus Coccineus Plants. Plant Growth Regul. 2015, 77, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dresler, S.; Bogucka-Kocka, A.; Kováčik, J.; Kubrak, T.; Strzemski, M.; Wójciak-Kosior, M.; Rysiak, A.; Sowa, I. Separation and Determination of Coumarins Including Furanocoumarins Using Micellar Electrokinetic Capillary Chromatography. Talanta 2018, 187, 120–124. [Google Scholar] [CrossRef]
- Strzemski, M.; Płachno, B.J.; Mazurek, B.; Kozłowska, W.; Sowa, I.; Lustofin, K.; Załuski, D.; Rydzik, Ł.; Szczepanek, D.; Sawicki, J.; et al. Morphological, Anatomical, and Phytochemical Studies of Carlina Acaulis L. Cypsela. Int. J. Mol. Sci. 2020, 21, 9230. [Google Scholar] [CrossRef]
- Dresler, S.; Strzemski, M.; Kováčik, J.; Sawicki, J.; Staniak, M.; Wójciak, M.; Sowa, I.; Hawrylak-Nowak, B. Tolerance of Facultative Metallophyte Carlina Acaulis to Cadmium Relies on Chelating and Antioxidative Metabolites. Int. J. Mol. Sci. 2020, 21, 2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dresler, S.; Bednarek, W.; Wójcik, M. Effect of Cadmium on Selected Physiological and Morphological Parameters in Metallicolous and Non-Metallicolous Populations of Echium Vulgare L. Ecotoxicol. Environ. Saf. 2014, 104, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, A.L.; Yang, C.-P.H.; Lindquist, P.; Anderson, O.R.; Rabino, I. Photocontrol of Anthocyanin Synthesis: III. The Action of Streptomycin on the Synthesis of Chlorophyll and Anthocyanin 1. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Berkel, G.J.V. Electrospray Mass Spectrometry and UV/Visible Spectrophotometry Studies of Aluminum(III)–Flavonoid Complexes. J. Mass Spectrom. 1998, 33, 1080–1087. [Google Scholar] [CrossRef]
- Kováčik, J.; Bačkor, M.; Kaduková, J. Physiological Responses of Matricaria Chamomilla to Cadmium and Copper Excess. Environ. Toxicol. 2008, 23, 123–130. [Google Scholar] [CrossRef]
- Dresler, S.; Rutkowska, E.; Bednarek, W.; Stanisławski, G.; Kubrak, T.; Bogucka-Kocka, A.; Wójcik, M. Selected Secondary Metabolites in Echium Vulgare L. Populations from Nonmetalliferous and Metalliferous Areas. Phytochemistry 2017, 133, 4–14. [Google Scholar] [CrossRef]





| Soluble Flavonols (mg QUE g−1 DW) | Soluble Phenols (mg GAE g−1 DW) | Antioxidant Capacity (mg TE g−1 DW) | ||
|---|---|---|---|---|
| DPPH | ABTS | |||
| Control | 0.56 ± 0.04 b | 7.97 ± 1.20 a | 5.08 ± 0.35 a | 10.31 ± 0.61 a |
| 35 °C/1 d | 0.85 ± 0.12 a | 6.91 ± 0.72 ab | 2.96 ± 0.68 b | 8.45 ± 0.63 ab |
| 35 °C/2 d | 0.62 ± 0.03 b | 7.97 ± 0.54 a | 3.81 ± 0.26 ab | 9.86 ± 0.30 a |
| 30 °C/7 d | 0.52 ± 0.07 b | 5.27 ± 0.19 b | 2.64 ± 0.34 b | 7.46 ± 0.30 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rysiak, A.; Dresler, S.; Hanaka, A.; Hawrylak-Nowak, B.; Strzemski, M.; Kováčik, J.; Sowa, I.; Latalski, M.; Wójciak, M. High Temperature Alters Secondary Metabolites and Photosynthetic Efficiency in Heracleum sosnowskyi. Int. J. Mol. Sci. 2021, 22, 4756. https://doi.org/10.3390/ijms22094756
Rysiak A, Dresler S, Hanaka A, Hawrylak-Nowak B, Strzemski M, Kováčik J, Sowa I, Latalski M, Wójciak M. High Temperature Alters Secondary Metabolites and Photosynthetic Efficiency in Heracleum sosnowskyi. International Journal of Molecular Sciences. 2021; 22(9):4756. https://doi.org/10.3390/ijms22094756
Chicago/Turabian StyleRysiak, Anna, Sławomir Dresler, Agnieszka Hanaka, Barbara Hawrylak-Nowak, Maciej Strzemski, Jozef Kováčik, Ireneusz Sowa, Michał Latalski, and Magdalena Wójciak. 2021. "High Temperature Alters Secondary Metabolites and Photosynthetic Efficiency in Heracleum sosnowskyi" International Journal of Molecular Sciences 22, no. 9: 4756. https://doi.org/10.3390/ijms22094756







