Exercise-Induced Plasticity in Signaling Pathways Involved in Motor Recovery after Spinal Cord Injury
Abstract
:1. Introduction
2. Effect of Rehabilitation on Spinal Networks below the Injury
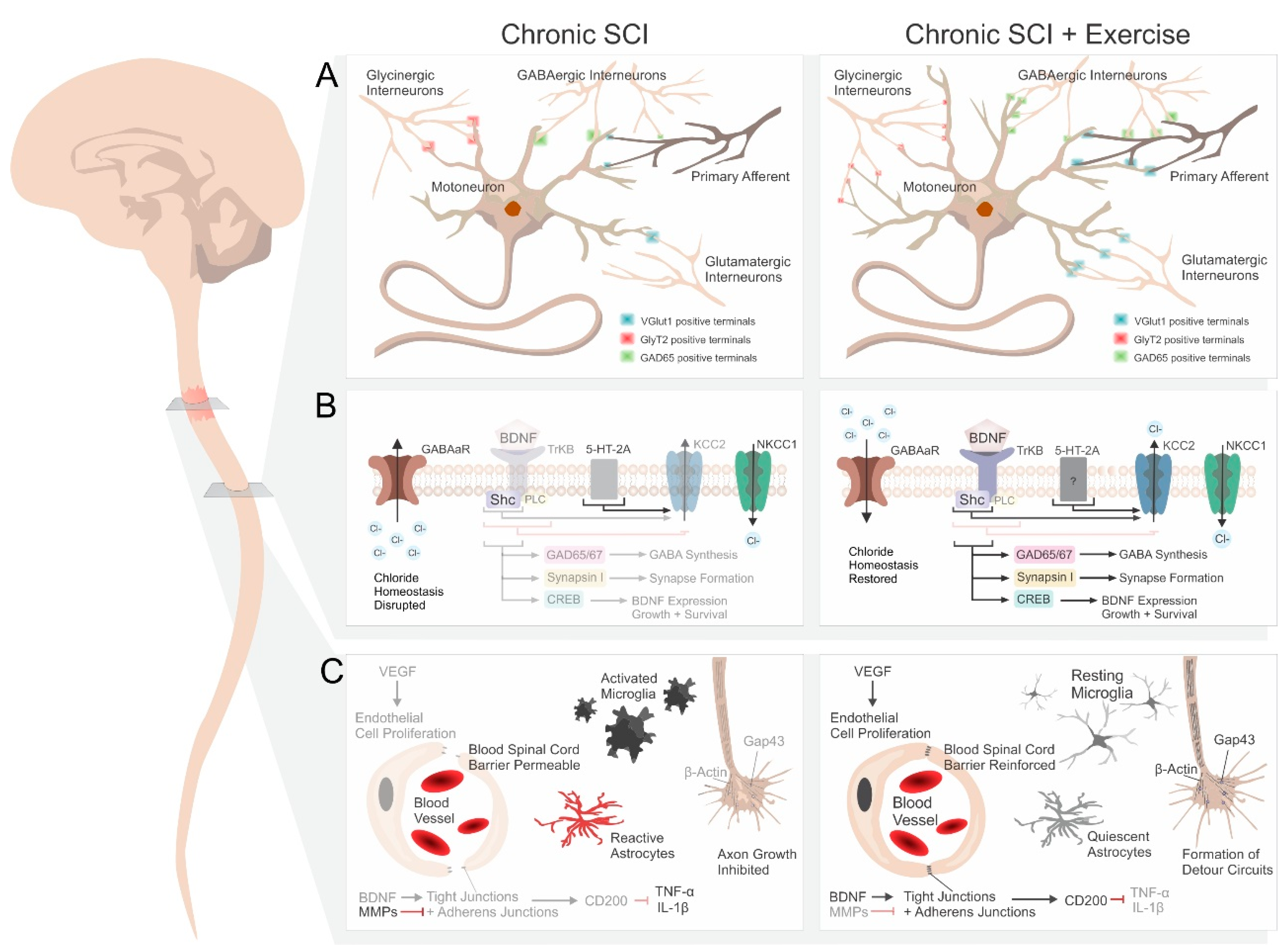
2.1. Synaptic Plasticity and Synapse Formation
2.2. Neurotrophins
2.3. Serotonin Receptors
2.4. Inhibitory Neurotransmitters
2.5. Chloride Homeostasis
3. Rehabilitation Promotes Sprouting and Regeneration
3.1. Serotoninergic Fiber Sprouting
3.2. Markers of Regeneration
3.3. Bypassing the Lesion
4. Other Considerations
5. Potential for Translation and Limitations
| Section | Chronic SCI | References | Chronic SCI + Exercise | References |
|---|---|---|---|---|
| 2.1. Synaptic Plasticity | ↓ CREB | [19,23] | ↑ CREB | [19,23,54] |
| ↓ Synapsin I | [19,54] | ↑ Synapsin I | [19,22,54] | |
| ↓ Synaptophysin | [18,19,183] | ↑ Synaptophysin | [17,28] | |
| ↓ PSD-95 | [17] | ↑ PSD-95 | [17] | |
| ↓ PNN | [20,27] | ↑ PNN | [20,27] | |
| 2.2. Neurotrophins | ↓ BDNF | [18,19,23,45,51,54] | ↑ BDNF | [18,19,23,44,45,46,47,51,54] |
| 2.3. Serotonin Receptors | ↑ 5-HT1A | [35,86] | ↑ 5-HT1A | [35] |
| ↑ 5-HT2A | [35,87,88,89,90,91,92] | ↑ 5-HT2A | [35,87] | |
| ↑ 5-HT2C | [79,81,93] | = 5-HT2C | [87] | |
| 2.4. Markers of in inhibition | ↑↓ GlyR | ↑ [37] = [36] ↓ [114] | ↓↑ GlyR | ↓ [37] ↑ [36] |
| ↑↓ GABAAR | ↑↓ [107] ↓ [114] | ↓↑ GABAAR | ↑↓ [107] | |
| ↑↓ GAD67 | ↑ [106] ↓ [23] | ↓↑ GAD67 | [25,45] | |
| ↑↓ GAD65 | = [36,106] ↓ [23] | ↑↓ GAD65 | ↑ [23] = [43] ↑↓ [36] | |
| 2.5. Chloride Homeostasis | ↓ KCC2 | [20,36,51,62,73,87] | ↑ KCC2 | [20,36,46,51,62,87] |
| ↓ PLCγ | [46] | = PLCγ | [46] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Spinal Cord Injury Statistical Center. Facts and Figures at a Glance; University of Alabama at Birmingham: Birmingham, AL, USA, 2021. [Google Scholar]
- Legg Ditterline, B.E.; Aslan, S.C.; Randall, D.C.; Harkema, S.J.; Castillo, C.; Ovechkin, A.V. Effects of Respiratory Training on Heart Rate Variability and Baroreflex Sensitivity in Individuals With Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2018, 99, 423–432. [Google Scholar] [CrossRef]
- Terson de Paleville, D.; McKay, W.; Aslan, S.; Folz, R.; Sayenko, D.; Ovechkin, A. Locomotor step training with body weight support improves respiratory motor function in individuals with chronic spinal cord injury. Respir. Physiol. Neurobiol. 2013, 189, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Mueller, G.; Hopman, M.T.; Perret, C. Comparison of respiratory muscle training methods in individuals with motor and sensory complete tetraplegia: A randomized controlled trial. J. Rehabil. Med. 2013, 45, 248–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onushko, T.; Mahtani, G.B.; Brazg, G.; Hornby, T.G.; Schmit, B.D. Exercise-Induced Alterations in Sympathetic-Somatomotor Coupling in Incomplete Spinal Cord Injury. J. Neurotrauma 2019, 36, 2688–2697. [Google Scholar] [CrossRef]
- Ginis, K.A.; Hicks, A.L.; Latimer, A.E.; Warburton, D.E.; Bourne, C.; Ditor, D.S.; Goodwin, D.L.; Hayes, K.C.; McCartney, N.; McIlraith, A.; et al. The developMent. of evidence-inforMed. physical activity guidelines for adults with spinal cord injury. Spinal Cord 2011, 49, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Hubscher, C.H.; Herrity, A.N.; Williams, C.S.; Montgomery, L.R.; Willhite, A.M.; Angeli, C.A.; Harkema, S.J. Improvements in bladder, bowel and sexual outcomes following task-specific locomotor training in human spinal cord injury. PLoS ONE 2018, 13, e0190998. [Google Scholar] [CrossRef] [Green Version]
- Morrison, S.A.; Lorenz, D.; Eskay, C.P.; Forrest, G.F.; Basso, D.M. Longitudinal Recovery and Reduced Costs After 120 Sessions of Locomotor Training for Motor Incomplete Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2018, 99, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Harkema, S.J.; Hillyer, J.; Schmidt-Read, M.; Ardolino, E.; Sisto, S.A.; Behrman, A.L. Locomotor training: As a treatMent. of spinal cord injury and in the progression of neurologic rehabilitation. Arch. Phys. Med. Rehabil. 2012, 93, 1588–1597. [Google Scholar] [CrossRef]
- Kaiser, A.; Chan, K.; Pakosh, M.; Musselman, K.E. Characteristics of activity-based therapy interventions for people living with spinal cord injury or disease across the continuum of care: A scoping review protocol. BMJ. Open 2020, 10, e040014. [Google Scholar] [CrossRef]
- Tse, C.M.; Chisholm, A.E.; Lam, T.; Eng, J.J.; Team, S.R. A systematic review of the effectiveness of task-specific rehabilitation interventions for improving independent sitting and standing function in spinal cord injury. J. Spinal Cord Med. 2018, 41, 254–266. [Google Scholar] [CrossRef]
- Rejc, E.; Angeli, C.A.; Atkinson, D.; Harkema, S.J. Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci. Rep. 2017, 7, 13476. [Google Scholar] [CrossRef] [Green Version]
- Rejc, E.; Angeli, C.A.; Bryant, N.; Harkema, S.J. Effects of Stand and Step Training with Epidural Stimulation on Motor Function for Standing in Chronic Complete Paraplegics. J. Neurotrauma 2017, 34, 1787–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harkema, S.; Behrman, A.; Barbeau, H. Evidence-based therapy for recovery of function after spinal cord injury. Handb. Clin. Neurol. 2012, 109, 259–274. [Google Scholar] [CrossRef]
- Côté, M.-P.; Murray, M.; Lemay, M.A. Rehabilitation Strategies after Spinal Cord Injury: Inquiry into the Mechanisms of Success and Failure. J. Neurotrauma 2017, 34, 1841–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin Ginis, K.A.; van der Scheer, J.W.; Latimer-Cheung, A.E.; Barrow, A.; Bourne, C.; Carruthers, P.; Bernardi, M.; Ditor, D.S.; Gaudet, S.; de Groot, S.; et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: An update and a new guideline. Spinal Cord 2018, 56, 308–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldshmit, Y.; Lythgo, N.; Galea, M.P.; Turnley, A.M. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J. Neurotrauma 2008, 25, 449–465. [Google Scholar] [CrossRef]
- Wang, H.; Liu, N.K.; Zhang, Y.P.; Deng, L.; Lu, Q.B.; Shields, C.B.; Walker, M.J.; Li, J.; Xu, X.M. Treadmill training induced lumbar motoneuron dendritic plasticity and behavior recovery in adult rats after a thoracic contusive spinal cord injury. Exp. Neurol. 2015, 271, 368–378. [Google Scholar] [CrossRef]
- Ying, Z.; Roy, R.R.; Edgerton, V.R.; Gomez-Pinilla, F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 2005, 193, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ventura, J.; Gimenez-Llort, L.; Penas, C.; Udina, E. Voluntary wheel running preserves lumbar perineuronal nets, enhances motor functions and prevents hyperreflexia after spinal cord injury. Exp. Neurol. 2021, 336, 113533. [Google Scholar] [CrossRef]
- Petruska, J.C.; Ichiyama, R.M.; Jindrich, D.L.; Crown, E.D.; Tansey, K.E.; Roy, R.R.; Edgerton, V.R.; Mendell, L.M. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J. Neurosci. 2007, 27, 4460–4471. [Google Scholar] [CrossRef]
- Gallegos, C.; Carey, M.; Zheng, Y.; He, X.; Cao, Q.L. Reaching and Grasping Training Improves Functional Recovery After Chronic Cervical Spinal Cord Injury. Front. Cell Neurosci. 2020, 14, 110. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Ding, J.; Wang, S.; Dong, C.; Wu, Q. Exercise training modulates glutamic acid decarboxylase-65/67 expression through TrkB signaling to ameliorate neuropathic pain in rats with spinal cord injury. Mol. Pain 2020, 16, 1744806920924511. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.W.; Futter, M.; Rosenblum, K.; Webber, M.J.; Hunt, S.P.; Bliss, T.V.; Bramham, C.R. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: RequireMent. for ERK activation coupled to CREB and upregulation of Arc synthesis. J. Neurosci. 2002, 22, 1532–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walton, M.; Connor, B.; Lawlor, P.; Young, D.; Sirimanne, E.; Gluckman, P.; Cole, G.; Dragunow, M. Neuronal death and survival in two models of hypoxic-ischemic brain damage. Brain Res. Brain Res. Rev. 1999, 29, 137–168. [Google Scholar] [CrossRef]
- Smith, C.C.; Mauricio, R.; Nobre, L.; Marsh, B.; Wust, R.C.; Rossiter, H.B.; Ichiyama, R.M. Differential regulation of perineuronal nets in the brain and spinal cord with exercise training. Brain Res. Bull. 2015, 111, 20–26. [Google Scholar] [CrossRef]
- Wang, D.; Ichiyama, R.M.; Zhao, R.; Andrews, M.R.; Fawcett, J.W. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J. Neurosci. 2011, 31, 9332–9344. [Google Scholar] [CrossRef]
- Kwok, J.C.; Afshari, F.; Garcia-Alias, G.; Fawcett, J.W. Proteoglycans in the central nervous system: Plasticity, regeneration and their stimulation with chondroitinase ABC. Restor. Neurol. Neurosci. 2008, 26, 131–145. [Google Scholar]
- Fawcett, J.W.; Oohashi, T.; Pizzorusso, T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat. Rev. Neurosci. 2019, 20, 451–465. [Google Scholar] [CrossRef]
- Pizzorusso, T.; Medini, P.; Berardi, N.; Chierzi, S.; Fawcett, J.W.; Maffei, L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 2002, 298, 1248–1251. [Google Scholar] [CrossRef] [Green Version]
- Arbat-Plana, A.; Torres-Espin, A.; Navarro, X.; Udina, E. Activity dependent therapies modulate the spinal changes that motoneurons suffer after a peripheral nerve injury. Exp. Neurol. 2015, 263, 293–305. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Ying, Z.; Roy, R.R.; Molteni, R.; Edgerton, V.R. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 2002, 88, 2187–2195. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, A.; Okada, M.; Tamada, A.; Okuda, S.; Nozumi, M.; Ito, Y.; Kobayashi, D.; Yamasaki, T.; Yokoyama, R.; Shibata, T.; et al. Growth Cone Phosphoproteomics Reveals that GAP-43 Phosphorylated by JNK Is a Marker of Axon Growth and Regeneration. Science 2018, 4, 190–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandaru, S.P.; Liu, S.; Waxman, S.G.; Tan, A.M. Dendritic spine dysgenesis contributes to hyperreflexia after spinal cord injury. J. Neurophysiol. 2015, 113, 1598–1615. [Google Scholar] [CrossRef] [Green Version]
- Ganzer, P.D.; Beringer, C.R.; Shumsky, J.S.; Nwaobasi, C.; Moxon, K.A. Serotonin receptor and dendritic plasticity in the spinal cord mediated by chronic serotonergic pharmacotherapy combined with exercise following complete SCI. in the adult rat. Exp. Neurol. 2018, 304, 132–142. [Google Scholar] [CrossRef]
- Khalki, L.; Sadlaoud, K.; Lerond, J.; Coq, J.O.; Brezun, J.M.; Vinay, L.; Coulon, P.; Bras, H. Changes in innervation of lumbar motoneurons and organization of premotor network following training of transected adult rats. Exp. Neurol. 2018, 299, 1–14. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Leon, R.D.; Harkema, S.J.; Hodgson, J.A.; London, N.; Reinkensmeyer, D.J.; Roy, R.R.; Talmadge, R.J.; Tillakaratne, N.J.; Timoszyk, W.; et al. Retraining the injured spinal cord. J. Physiol. 2001, 533, 15–22. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal developMent. and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keefe, K.M.; Sheikh, I.S.; Smith, G.M. Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for TreatMent. of Spinal Cord Injury. Int. J. Mol. Sci. 2017, 18, 548. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Huie, J.R.; Ying, Z.; Ferguson, A.R.; Crown, E.D.; Baumbauer, K.M.; Edgerton, V.R.; Grau, J.W. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience 2007, 148, 893–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 2003, 10, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Neeper, S.A.; Gomez-Pinilla, F.; Choi, J.; Cotman, C. Exercise and brain neurotrophins. Nature 1995, 373, 109. [Google Scholar] [CrossRef] [PubMed]
- Tillakaratne, N.J.; De Leon, R.D.; Hoang, T.X.; Roy, R.R.; Edgerton, V.R.; Tobin, A.J. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J. Neurosci. 2002, 22, 3130–3143. [Google Scholar] [CrossRef]
- Côté, M.-P.; Azzam, G.A.; Lemay, M.A.; Zhukareva, V.; Houle, J.D. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J. Neurotrauma 2011, 28, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, K.J.; Gomez-Pinilla, F.; Crowe, M.J.; Ying, Z.; Basso, D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain 2004, 127, 1403–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashiro, S.; Shinozaki, M.; Mukaino, M.; Renault-Mihara, F.; Toyama, Y.; Liu, M.; Nakamura, M.; Okano, H. BDNF Induced by Treadmill Training Contributes to the Suppression of Spasticity and Allodynia After Spinal Cord Injury via Upregulation of KCC2. Neurorehabil. Neural Repair 2015, 29, 677–689. [Google Scholar] [CrossRef]
- Ying, X.; Xie, Q.; Yu, X.; Li, S.; Wu, Q.; Chen, X.; Yue, J.; Zhou, K.; Tu, W.; Jiang, S. Water treadmill training protects the integrity of the blood-spinal cord barrier following SCI. via the BDNF/TrkB-CREB signalling pathway. Neurochem. Int. 2021, 143, 104945. [Google Scholar] [CrossRef]
- Goldhardt, M.G.; Andreia, A.; Dorneles, G.P.; da Silva, I.R.; Pochmann, D.; Peres, A.; Rostirola Elsner, V. Does a single bout of exercise impacts BDNF, oxidative stress and epigenetic markers in spinal cord injury patients? Funct. Neurol. 2019, 34, 158–166. [Google Scholar]
- Leech, K.A.; Hornby, T.G. High-Intensity Locomotor Exercise Increases Brain-Derived Neurotrophic Factor in Individuals with Incomplete Spinal Cord Injury. J. Neurotrauma 2017, 34, 1240–1248. [Google Scholar] [CrossRef] [Green Version]
- Rojas Vega, S.; Abel, T.; Lindschulten, R.; Hollmann, W.; Bloch, W.; Struder, H.K. Impact of exercise on neuroplasticity-related proteins in spinal cord injured humans. Neuroscience 2008, 153, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Côté, M.-P.; Gandhi, S.; Zambrotta, M.; Houle, J.D. Exercise modulates chloride homeostasis after spinal cord injury. J. Neurosci. 2014, 34, 8976–8987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Pinilla, F.; Ying, Z.; Roy, R.R.; Hodgson, J.; Edgerton, V.R. Afferent input modulates neurotrophins and synaptic plasticity in the spinal cord. J. Neurophysiol. 2004, 92, 3423–3432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont-Versteegden, E.E.; Houle, J.D.; Dennis, R.A.; Zhang, J.; Knox, M.; Wagoner, G.; Peterson, C.A. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve 2004, 29, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Roy, R.R.; Zhong, H.; Zdunowski, S.; Edgerton, V.R.; Gomez-Pinilla, F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience 2008, 155, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Beaumont, E.; Kaloustian, S.; Rousseau, G.; Cormery, B. Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci. Res. 2008, 62, 147–154. [Google Scholar] [CrossRef]
- Boyce, V.S.; Mendell, L.M. Neurotrophic factors in spinal cord injury. Handb. Exp. Pharmacol. 2014, 220, 443–460. [Google Scholar] [CrossRef]
- Weishaupt, N.; Blesch, A.; Fouad, K. BDNF: The career of a multifaceted neurotrophin in spinal cord injury. Exp. Neurol. 2012, 238, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Thoenen, H. Neurotrophins and activity-dependent plasticity. Prog. Brain Res. 2000, 128, 183–191. [Google Scholar] [PubMed]
- Jovanovic, J.N.; Czernik, A.J.; Fienberg, A.A.; Greengard, P.; Sihra, T.S. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat. Neurosci. 2000, 3, 323–329. [Google Scholar] [CrossRef]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience 2003, 122, 647–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wu, Q.; Xie, C.; Wang, C.; Wang, Q.; Dong, C.; Fang, L.; Ding, J.; Wang, T. Blocking of BDNF-TrkB signaling inhibits the promotion effect of neurological function recovery after treadmill training in rats with spinal cord injury. Spinal Cord 2019, 57, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Beverungen, H.; Klaszky, S.C.; Klaszky, M.; Cote, M.P. Rehabilitation Decreases Spasticity by Restoring Chloride Homeostasis through the Brain-Derived Neurotrophic Factor-KCC2 Pathway after Spinal Cord Injury. J. Neurotrauma 2020, 37, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife 2016, 5. [Google Scholar] [CrossRef]
- Tao, X.; Finkbeiner, S.; Arnold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 1998, 20, 709–726. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; West, A.E.; Chen, W.G.; Corfas, G.; Greenberg, M.E. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron 2002, 33, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.G.; West, A.E.; Tao, X.; Corfas, G.; Szentirmay, M.N.; Sawadogo, M.; Vinson, C.; Greenberg, M.E. Upstream stimulatory factors are mediators of Ca2+-responsive transcription in neurons. J. Neurosci. 2003, 23, 2572–2581. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Fujita, Y.; Matsuzaki, R.; Yamashita, T. Class I histone deacetylase (HDAC) inhibitor CI-994 promotes functional recovery following spinal cord injury. Cell Death Dis. 2018, 9, 460. [Google Scholar] [CrossRef] [Green Version]
- Ying, Z.; Roy, R.R.; Edgerton, V.R.; Gomez-Pinilla, F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003, 987, 93–99. [Google Scholar] [CrossRef]
- Skup, M.; Dwornik, A.; Macias, M.; Sulejczak, D.; Wiater, M.; Czarkowska-Bauch, J. Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp. Neurol. 2002, 176, 289–307. [Google Scholar] [CrossRef]
- Bregman, B.S.; Coumans, J.V.; Dai, H.N.; Kuhn, P.L.; Lynskey, J.; McAtee, M.; Sandhu, F. Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. Prog. Brain Res. 2002, 137, 257–273. [Google Scholar]
- Ollivier-Lanvin, K.; Fischer, I.; Tom, V.; Houle, J.D.; Lemay, M.A. Either brain-derived neurotrophic factor or neurotrophin-3 only neurotrophin-producing grafts promote locomotor recovery in untrained spinalized cats. Neurorehabil. Neural Repair 2015, 29, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Boyce, V.S.; Park, J.; Gage, F.H.; Mendell, L.M. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur. J. Neurosci. 2012, 35, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010, 16, 302–307. [Google Scholar] [CrossRef]
- Garraway, S.M.; Woller, S.A.; Huie, J.R.; Hartman, J.J.; Hook, M.A.; Miranda, R.C.; Huang, Y.J.; Ferguson, A.R.; Grau, J.W. Peripheral noxious stimulation reduces withdrawal threshold to mechanical stimuli after spinal cord injury: Role of tumor necrosis factor alpha and apoptosis. Pain 2014, 155, 2344–2359. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Blesch, A.; Graham, L.; Wang, Y.; Samara, R.; Banos, K.; Haringer, V.; Havton, L.; Weishaupt, N.; Bennett, D.; et al. Motor axonal regeneration after partial and complete spinal cord transection. J. Neurosci. 2012, 32, 8208–8218. [Google Scholar] [CrossRef]
- Fouad, K.; Bennett, D.J.; Vavrek, R.; Blesch, A. Long-term viral brain-derived neurotrophic factor delivery promotes spasticity in rats with a cervical spinal cord hemisection. Front. Neurol. 2013, 4, 187. [Google Scholar] [CrossRef] [Green Version]
- de Leon, R.D.; See, P.A.; Chow, C.H. Differential effects of low versus high amounts of weight supported treadmill training in spinally transected rats. J. Neurotrauma 2011, 28, 1021–1033. [Google Scholar] [CrossRef]
- Cha, J.; Heng, C.; Reinkensmeyer, D.J.; Roy, R.R.; Edgerton, V.R.; De Leon, R.D. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J. Neurotrauma 2007, 24, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.C.; Nakae, A.; Stephens, M.J.; Rank, M.; D’Amico, J.; Harvey, P.J.; Li, X.; Harris, R.L.; Ballou, E.W.; Anelli, R.; et al. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat. Med. 2010, 16, 694–700. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.J.; Jordan, L.M. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res.Bull. 2000, 53, 689–710. [Google Scholar] [CrossRef]
- Hayashi, Y.; Jacob-Vadakot, S.; Dugan, E.A.; McBride, S.; Olexa, R.; Simansky, K.; Murray, M.; Shumsky, J.S. 5-HT precursor loading, but not 5-HT receptor agonists, increases motor function after spinal cord contusion in adult rats. Exp. Neurol. 2010, 221, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, P.J.; Li, X.; Li, Y.; Bennett, D.J. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J. Neurophysiol. 2006, 96, 1158–1170. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Li, Y.; Yu, B.; Zhang, Z.; Brommer, B.; Williams, P.R.; Liu, Y.; Hegarty, S.V.; Zhou, S.; Zhu, J.; et al. Reactivation of Dormant Relay Pathways in Injured Spinal Cord by KCC2 Manipulations. Cell 2018, 174, 521–535.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M. Normal Distribution and Plasticity of Serotonin Receptors After Spinal Cord Injury and Their Impacts on Motor Outputs. In Recovery of Motor Function Following Spinal Cord Injury; Fuller, H., Gates, M., Eds.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Slawinska, U.; Jordan, L.M. Serotonergic influences on locomotor circuits. Curr. Opin. Physiol. 2019, 8, 63–69. [Google Scholar] [CrossRef]
- Otoshi, C.K.; Walwyn, W.M.; Tillakaratne, N.J.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Distribution and localization of 5-HT(1A) receptors in the rat lumbar spinal cord after transection and deafferentation. J. Neurotrauma 2009, 26, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Chopek, J.W.; Sheppard, P.C.; Gardiner, K.; Gardiner, P.F. Serotonin receptor and KCC2 gene expression in lumbar flexor and extensor motoneurons posttransection with and without passive cycling. J. Neurophysiol. 2015, 113, 1369–1376. [Google Scholar] [CrossRef]
- Ryu, Y.; Ogata, T.; Nagao, M.; Sawada, Y.; Nishimura, R.; Fujita, N. Effects of Treadmill Training Combined with Serotonergic Interventions on Spasticity after Contusive Spinal Cord Injury. J. Neurotrauma 2018, 35, 1358–1366. [Google Scholar] [CrossRef]
- Lee, J.K.; Johnson, C.S.; Wrathall, J.R. Up-regulation of 5-HT2 receptors is involved in the increased H-reflex amplitude after contusive spinal cord injury. Exp. Neurol. 2007, 203, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarrett, S.; Collier, L.; Cardozo, C.; Dracheva, S. Alterations of serotonin 2C and 2A receptors in response to T10 spinal cord transection in rats. Neurosci. Lett. 2012, 506, 74–78. [Google Scholar] [CrossRef]
- Kong, X.Y.; Wienecke, J.; Hultborn, H.; Zhang, M. Robust upregulation of serotonin 2A receptors after chronic spinal transection of rats: An immunohistochemical study. Brain Res. 2010, 1320, 60–68. [Google Scholar] [CrossRef]
- Kong, X.Y.; Wienecke, J.; Chen, M.; Hultborn, H.; Zhang, M. The time course of serotonin 2A receptor expression after spinal transection of rats: An immunohistochemical study. Neuroscience 2011, 177, 114–126. [Google Scholar] [CrossRef]
- Ren, L.Q.; Wienecke, J.; Chen, M.; Moller, M.; Hultborn, H.; Zhang, M. The time course of serotonin 2C receptor expression after spinal transection of rats: An immunohistochemical study. Neuroscience 2013, 236, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Fouad, K.; Rank, M.M.; Vavrek, R.; Murray, K.C.; Sanelli, L.; Bennett, D.J. Locomotion after spinal cord injury depends on constitutive activity in serotonin receptors. J. Neurophysiol. 2010, 104, 2975–2984. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Reggie Edgerton, V. Transcutaneous electrical spinal-cord stimulation in humans. Ann. Phys. Rehabil. Med. 2015, 58, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gad, P.; Gerasimenko, Y.; Zdunowski, S.; Turner, A.; Sayenko, D.; Lu, D.C.; Edgerton, V.R. Weight Bearing Over-ground Stepping in an Exoskeleton with Non-invasive Spinal Cord Neuromodulation after Motor Complete Paraplegia. Front. Neurosci. 2017, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Moshonkina, T.R.; Shapkova, E.Y.; Sukhotina, I.A.; Emeljannikov, D.V.; Gerasimenko, Y.P. Effect of Combination of Non-Invasive Spinal Cord Electrical Stimulation and Serotonin Receptor Activation in Patients with Chronic Spinal Cord Lesion. Bull. Exp. Biol. Med. 2016, 161, 749–754. [Google Scholar] [CrossRef]
- Radhakrishna, M.; Steuer, I.; Prince, F.; Roberts, M.; Mongeon, D.; Kia, M.; Dyck, S.; Matte, G.; Vaillancourt, M.; Guertin, P.A. Double-Blind, Placebo-Controlled, Randomized Phase I/IIa Study (Safety and Efficacy) with Buspirone/Levodopa/Carbidopa (SpinalonTM) in Subjects with Complete AIS A or Motor-Complete AIS B Spinal Cord Injury. Curr. Pharm. Des. 2017, 23, 1789–1804. [Google Scholar] [CrossRef]
- Takeoka, A.; Vollenweider, I.; Courtine, G.; Arber, S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 2014, 159, 1626–1639. [Google Scholar] [CrossRef] [Green Version]
- Manohar, A.; Foffani, G.; Ganzer, P.D.; Bethea, J.R.; Moxon, K.A. Cortex-dependent recovery of unassisted hindlimb locomotion after complete spinal cord injury in adult rats. Elife 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Foffani, G.; Shumsky, J.; Knudsen, E.B.; Ganzer, P.D.; Moxon, K.A. Interactive Effects Between Exercise and Serotonergic Pharmacotherapy on Cortical Reorganization After Spinal Cord Injury. Neurorehabil. Neural Repair 2016, 30, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Maeso, J.; Sealfon, S.C. Psychedelics and schizophrenia. Trends Neurosci. 2009, 32, 225–232. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Kim, Y.S.; Yoon, B.E. Altered GABAergic Signaling in Brain Disease at Various Stages of Life. Exp. Neurobiol. 2017, 26, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, R.W.; Thompson, M.L. Glycinergic signaling in the human nervous system: An overview of therapeutic drug targets and clinical effects. Ment. Health Clin. 2016, 6, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Tillakaratne, N.J.; Mouria, M.; Ziv, N.B.; Roy, R.R.; Edgerton, V.R.; Tobin, A.J. Increased expression of glutamate decarboxylase (GAD(67)) in feline lumbar spinal cord after complete thoracic spinal cord transection. J. Neurosci. Res. 2000, 60, 219–230. [Google Scholar] [CrossRef]
- Khristy, W.; Ali, N.J.; Bravo, A.B.; de, L.R.; Roy, R.R.; Zhong, H.; London, N.J.; Edgerton, V.R.; Tillakaratne, N.J. Changes in GABA(A) receptor subunit gamma 2 in extensor and flexor motoneurons and astrocytes after spinal cord transection and motor training. Brain Res. 2009, 1273, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichiyama, R.M.; Broman, J.; Roy, R.R.; Zhong, H.; Edgerton, V.R.; Havton, L.A. Locomotor training maintains normal inhibitory influence on both alpha- and gamma-motoneurons after neonatal spinal cord transection. J. Neurosci. 2011, 31, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Edgerton, V.R.; De Leon, R.D.; Tillakaratne, N.; Recktenwald, M.R.; Hodgson, J.A.; Roy, R.R. Use-dependent plasticity in spinal stepping and standing. Adv. Neurol. 1997, 72, 233–247. [Google Scholar] [PubMed]
- Lim, B.V.; Shin, M.S.; Lee, J.M.; Seo, J.H. Treadmill exercise prevents GABAergic neuronal loss with suppression of neuronal activation in the pilocarpine-induced epileptic rats. J. Exerc. Rehabil. 2015, 11, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kami, K.; Taguchi Ms, S.; Tajima, F.; Senba, E. Improvements in impaired GABA and GAD65/67 production in the spinal dorsal horn contribute to exercise-induced hypoalgesia in a mouse model of neuropathic pain. Mol. Pain 2016, 12. [Google Scholar] [CrossRef] [Green Version]
- Farzad, B.; Rajabi, H.; Gharakhanlou, R.; Allison, D.J.; Hayat, P.; Jameie, S.B. Swimming Training Attenuates Allodynia and Hyperalgesia Induced by Peripheral Nerve Injury in an Adult Male Rat Neuropathic Model: Effects on Irisin and GAD65. Pain Med. 2018, 19, 2236–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, L.E.; Droste, S.K.; Nutt, D.J.; Linthorst, A.C.; Reul, J.M. Voluntary exercise alters GABA(A) receptor subunit and glutamic acid decarboxylase-67 gene expression in the rat forebrain. J. Psychopharmacol. 2010, 24, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Sadlaoud, K.; Khalki, L.; Brocard, F.; Vinay, L.; Boulenguez, P.; Bras, H. Alteration of glycinergic receptor expression in lumbar spinal motoneurons is involved in the mechanisms underlying spasticity after spinal cord injury. J. Chem. Neuroanat. 2020, 106, 101787. [Google Scholar] [CrossRef] [PubMed]
- Caron, G.; Bilchak, J.N.; Cote, M.P. Direct evidence for decreased presynaptic inhibition evoked by PBSt group I muscle afferents after chronic SCI. and recovery with step-training in rats. J. Physiol. 2020, 598, 4621–4642. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Lee, K.H.; Grau, J.W. Complete spinal cord injury (SCI) transforms how brain derived neurotrophic factor (BDNF) affects nociceptive sensitization. Exp. Neurol. 2017, 288, 38–50. [Google Scholar] [CrossRef]
- Bos, R.; Sadlaoud, K.; Boulenguez, P.; Buttigieg, D.; Liabeuf, S.; Brocard, C.; Haase, G.; Bras, H.; Vinay, L. Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proc. Natl. Acad. Sci. USA 2013, 110, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Jean-Xavier, C.; Pflieger, J.F.; Liabeuf, S.; Vinay, L. Inhibitory postsynaptic potentials in lumbar motoneurons remain depolarizing after neonatal spinal cord transection in the rat. J. Neurophysiol. 2006, 96, 2274–2281. [Google Scholar] [CrossRef]
- Liabeuf, S.; Stuhl-Gourmand, L.; Gackiere, F.; Mancuso, R.; Sanchez Brualla, I.; Marino, P.; Brocard, F.; Vinay, L. Prochlorperazine Increases KCC2 Function and Reduces Spasticity after Spinal Cord Injury. J. Neurotrauma 2017, 34, 3397–3406. [Google Scholar] [CrossRef]
- Delpire, E.; Mount, D.B. Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu. Rev. Physiol. 2002, 64, 803–843. [Google Scholar] [CrossRef]
- Huang, Y.J.; Grau, J.W. Ionic plasticity and pain: The loss of descending serotonergic fibers after spinal cord injury transforms how GABA affects pain. Exp. Neurol. 2018, 306, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Côté, M.-P. Role of chloride cotransporters in the development. of spasticity and neuropathic pain after spinal cord injury. In Neuronal Chloride Transporters in Health and Disease; Tang, X., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 463–516. [Google Scholar] [CrossRef]
- Sanchez-Brualla, I.; Boulenguez, P.; Brocard, C.; Liabeuf, S.; Viallat-Lieutaud, A.; Navarro, X.; Udina, E.; Brocard, F. Activation of 5-HT2A Receptors Restores KCC2 Function and Reduces Neuropathic Pain after Spinal Cord Injury. Neuroscience 2018, 387, 48–57. [Google Scholar] [CrossRef]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. ManageMent. of spasticity after spinal cord injury: Current techniques and future directions. Neurorehabil. Neural Repair 2010, 24, 23–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulou, K.B.; Panourias, I.G.; Rapidi, C.A.; Sakas, D.E. The importance of neurorehabilitation to the outcome of neuromodulation in spasticity. Acta Neurochir. Suppl. 2007, 97, 243–250. [Google Scholar]
- Bilchak, J.; Yeakle, K.; Caron, G.; Malloy, D.; Cote, M.P. Enhancing KCC2 activity decreases hyperreflexia and spasticity after chronic spinal cord injury. Exp. Neurol. 2021, 113605. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Voipio, J.; Thomas-Crusells, J.; Li, H.; Emri, Z.; Sipila, S.; Payne, J.A.; Minichiello, L.; Saarma, M.; Kaila, K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 2004, 24, 4683–4691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, C.; Voipio, J.; Kaila, K. Two developmental switches in GABAergic signalling: The K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J. Physiol. 2005, 562, 27–36. [Google Scholar] [CrossRef]
- Ferrini, F.; De Koninck, Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013, 2013, 429815. [Google Scholar] [CrossRef] [PubMed]
- Glykys, J.; Dzhala, V.; Egawa, K.; Balena, T.; Saponjian, Y.; Kuchibhotla, K.V.; Bacskai, B.J.; Kahle, K.T.; Zeuthen, T.; Staley, K.J. Local impermeant anions establish the neuronal chloride concentration. Science 2014, 343, 670–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Ari, Y.; Khalilov, I.; Kahle, K.T.; Cherubini, E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 2012, 18, 467–486. [Google Scholar] [CrossRef]
- Galtrey, C.M.; Kwok, J.C.; Carulli, D.; Rhodes, K.E.; Fawcett, J.W. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur. J. Neurosci. 2008, 27, 1373–1390. [Google Scholar] [CrossRef]
- Engesser-Cesar, C.; Ichiyama, R.M.; Nefas, A.L.; Hill, M.A.; Edgerton, V.R.; Cotman, C.W.; Anderson, A.J. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur. J. Neurosci. 2007, 25, 1931–1939. [Google Scholar] [CrossRef]
- Fouad, K.; Tetzlaff, W. Rehabilitative training and plasticity following spinal cord injury. Exp. Neurol. 2012, 235, 91–99. [Google Scholar] [CrossRef]
- Liu, Z.H.; Yip, P.K.; Priestley, J.V.; Michael-Titus, A.T. A Single Dose of Docosahexaenoic Acid Increases the Functional Recovery Promoted by Rehabilitation after Cervical Spinal Cord Injury in the Rat. J. Neurotrauma 2017, 34, 1766–1777. [Google Scholar] [CrossRef] [PubMed]
- Loy, K.; Schmalz, A.; Hoche, T.; Jacobi, A.; Kreutzfeldt, M.; Merkler, D.; Bareyre, F.M. Enhanced Voluntary Exercise Improves Functional Recovery following Spinal Cord Injury by Impacting the Local Neuroglial Injury Response and Supporting the Rewiring of Supraspinal Circuits. J. Neurotrauma 2018, 35, 2904–2915. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, H.; Rossignol, S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991, 546, 250–260. [Google Scholar] [CrossRef]
- Feraboli-Lohnherr, D.; Barthe, J.Y.; Orsal, D. Serotonin-induced activation of the network for locomotion in adult spinal rats. J. Neurosci. Res. 1999, 55, 87–98. [Google Scholar] [CrossRef]
- Antri, M.; Mouffle, C.; Orsal, D.; Barthe, J.Y. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur. J. Neurosci. 2003, 18, 1963–1972. [Google Scholar] [CrossRef]
- Leszczynska, A.N.; Majczynski, H.; Wilczynski, G.M.; Slawinska, U.; Cabaj, A.M. Thoracic Hemisection in Rats Results in Initial Recovery Followed by a Late DecreMent. in Locomotor Movements, with Changes in Coordination Correlated with Serotonergic Innervation of the Ventral Horn. PLoS ONE 2015, 10, e0143602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saruhashi, Y.; Young, W.; Perkins, R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp. Neurol. 1996, 139, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Saruhashi, Y.; Matsusue, Y.; Fujimiya, M. The recovery of 5-HT transporter and 5-HT immunoreactivity in injured rat spinal cord. Arch. Orthop. Trauma Surg. 2009, 129, 1279–1285. [Google Scholar] [CrossRef]
- Zuchner, M.; Kondratskaya, E.; Sylte, C.B.; Glover, J.C.; Boulland, J.L. Rapid recovery and altered neurochemical dependence of locomotor central pattern generation following lumbar neonatal spinal cord injury. J. Physiol. 2018, 596, 281–303. [Google Scholar] [CrossRef] [Green Version]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef] [PubMed]
- Beauparlant, J.; van den Brand, R.; Barraud, Q.; Friedli, L.; Musienko, P.; Dietz, V.; Courtine, G. Undirected compensatory plasticity contributes to neuronal dysfunction after severe spinal cord injury. Brain 2013, 136, 3347–3361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.Q.; Zaaimi, B.; Martin, J.H. Competition with Primary Sensory Afferents Drives Remodeling of Corticospinal Axons in Mature Spinal Motor Circuits. J. Neurosci. 2016, 36, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Torres-Espin, A.; Beaudry, E.; Fenrich, K.; Fouad, K. Rehabilitative Training in Animal Models of Spinal Cord Injury. J. Neurotrauma 2018, 35, 1970–1985. [Google Scholar] [CrossRef]
- Sachdeva, R.; Theisen, C.C.; Ninan, V.; Twiss, J.L.; Houle, J.D. Exercise dependent increase in axon regeneration into peripheral nerve grafts by propriospinal but not sensory neurons after spinal cord injury is associated with modulation of regeneration-associated genes. Exp. Neurol. 2016, 276, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, S.; Nishimura, S.; Iwai, H.; Sugai, K.; Zhang, L.; Shinozaki, M.; Iwanami, A.; Toyama, Y.; Liu, M.; Okano, H.; et al. Functional Recovery from Neural. Stem/Progenitor Cell Transplantation Combined with Treadmill Training in Mice with Chronic Spinal Cord Injury. Sci. Rep. 2016, 6, 30898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505. [Google Scholar] [CrossRef]
- Theisen, C.C.; Sachdeva, R.; Austin, S.; Kulich, D.; Kranz, V.; Houle, J.D. Exercise and Peripheral Nerve Grafts as a Strategy To Promote Regeneration after Acute or Chronic Spinal Cord Injury. J. Neurotrauma 2017, 34, 1909–1914. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, S.; Nishimura, S.; Shinozaki, M.; Takano, M.; Konomi, T.; Tsuji, O.; Nagoshi, N.; Toyama, Y.; Liu, M.; Okano, H.; et al. The Amelioration of Pain-Related Behavior in Mice with Chronic Spinal Cord Injury Treated with Neural. Stem/Progenitor Cell Transplantation Combined with Treadmill Training. J. Neurotrauma 2018, 35, 2561–2571. [Google Scholar] [CrossRef]
- Garcia-Alias, G.; Barkhuysen, S.; Buckle, M.; Fawcett, J.W. Chondroitinase ABC treatMent. opens a window of opportunity for task-specific rehabilitation. Nat. Neurosci. 2009, 12, 1145–1151. [Google Scholar] [CrossRef]
- Garcia-Alias, G.; Lin, R.; Akrimi, S.F.; Story, D.; Bradbury, E.J.; Fawcett, J.W. Therapeutic time window for the application of chondroitinase ABC after spinal cord injury. Exp. Neurol. 2008, 210, 331–338. [Google Scholar] [CrossRef]
- Kubasak, M.D.; Jindrich, D.L.; Zhong, H.; Takeoka, A.; McFarland, K.C.; Munoz-Quiles, C.; Roy, R.R.; Edgerton, V.R.; Ramon-Cueto, A.; Phelps, P.E. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain 2008, 131, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Quiles, C.; Santos-Benito, F.F.; Llamusi, M.B.; Ramon-Cueto, A. Chronic spinal injury repair by olfactory bulb ensheathing glia and feasibility for autologous therapy. J. Neuropathol. Exp. Neurol. 2009, 68, 1294–1308. [Google Scholar] [CrossRef] [Green Version]
- Takeoka, A.; Jindrich, D.L.; Munoz-Quiles, C.; Zhong, H.; van den, B.R.; Pham, D.L.; Ziegler, M.D.; Ramon-Cueto, A.; Roy, R.R.; Edgerton, V.R.; et al. Axon regeneration can facilitate or suppress hindlimb function after olfactory ensheathing glia transplantation. J. Neurosci. 2011, 31, 4298–4310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Zdunowski, S.; Edgerton, V.R.; Roy, R.R.; Zhong, H.; Hsiao, I.; Lin, V.W. ImproveMent. of gait patterns in step-trained, complete spinal cord-transected rats treated with a peripheral nerve graft and acidic fibroblast growth factor. Exp. Neurol. 2010, 224, 429–437. [Google Scholar] [CrossRef] [Green Version]
- van den Brand, R.; Heutschi, J.; Barraud, Q.; DiGiovanna, J.; Bartholdi, K.; Huerlimann, M.; Friedli, L.; Vollenweider, I.; Moraud, E.M.; Duis, S.; et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 2012, 336, 1182–1185. [Google Scholar] [CrossRef] [Green Version]
- Bareyre, F.M.; Kerschensteiner, M.; Raineteau, O.; Mettenleiter, T.C.; Weinmann, O.; Schwab, M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004, 7, 269–277. [Google Scholar] [CrossRef]
- Courtine, G.; Song, B.; Roy, R.R.; Zhong, H.; Herrmann, J.E.; Ao, Y.; Qi, J.; Edgerton, V.R.; Sofroniew, M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008, 14, 69–74. [Google Scholar] [CrossRef]
- Filli, L.; Engmann, A.K.; Zorner, B.; Weinmann, O.; Moraitis, T.; Gullo, M.; Kasper, H.; Schneider, R.; Schwab, M.E. Bridging the gap: A reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J. Neurosci. 2014, 34, 13399–13410. [Google Scholar] [CrossRef] [Green Version]
- Asboth, L.; Friedli, L.; Beauparlant, J.; Martinez-Gonzalez, C.; Anil, S.; Rey, E.; Baud, L.; Pidpruzhnykova, G.; Anderson, M.A.; Shkorbatova, P.; et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat. Neurosci. 2018, 21, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.B.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seanez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef]
- Fouad, K.; Pedersen, V.; Schwab, M.E.; Brosamle, C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr. Biol. 2001, 11, 1766–1770. [Google Scholar] [CrossRef] [Green Version]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Pan, J.Z.; Ni, L.; Sodhi, A.; Aguanno, A.; Young, W.; Hart, R.P. Cytokine activity contributes to induction of inflammatory cytokine mRNAs in spinal cord following contusion. J. Neurosci. Res. 2002, 68, 315–322. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.J.; Wu, Y.P.; Chen, W.S. Inflammation & apoptosis in spinal cord injury. Indian J. Med. Res. 2012, 135, 287–296. [Google Scholar]
- Sandrow-Feinberg, H.R.; Izzi, J.; Shumsky, J.S.; Zhukareva, V.; Houle, J.D. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J. Neurotrauma 2009, 26, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Dugan, E.A.; Jergova, S.; Sagen, J. Mutually beneficial effects of intensive exercise and GABAergic Neural. progenitor cell transplants in reducing neuropathic pain and spinal pathology in rats with spinal cord injury. Exp. Neurol. 2020, 327, 113208. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.D.S.; Dos Santos, R.V.T.; de Lira, F.S.; Almeida, A.A.; Edwards, K.; Benvenutti, M.; Tufik, S.; De Mello, M.T. Effects of intensity-matched exercise at different intensities on inflammatory responses in able-bodied and spinal cord injured individuals. J. Spinal Cord Med. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Morland, C.; Andersson, K.A.; Haugen, O.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef]
- Ying, X.; Xie, Q.; Li, S.; Yu, X.; Zhou, K.; Yue, J.; Chen, X.; Tu, W.; Yang, G.; Jiang, S. Water treadmill training attenuates blood-spinal cord barrier disruption in rats by promoting angiogenesis and inhibiting matrix metalloproteinase-2/9 expression following spinal cord injury. Fluids Barriers CNS 2020, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Q.; Zheng, G.; Wang, C.; Zhou, Y.; Wu, Y.; Xuan, J.; Tian, N.; Wang, X.; Wu, Y.; et al. Metformin ameliorates BSCB disruption by inhibiting neutrophil infiltration and MMP-9 expression but not direct TJ. proteins expression regulation. J. Cell Mol. Med. 2017, 21, 3322–3336. [Google Scholar] [CrossRef] [Green Version]
- Noble, L.J.; Donovan, F.; Igarashi, T.; Goussev, S.; Werb, Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J. Neurosci. 2002, 22, 7526–7535. [Google Scholar] [CrossRef]
- Cohen, M.; Ben-Yehuda, H.; Porat, Z.; Raposo, C.; Gordon, S.; Schwartz, M. Newly ForMed. Endothelial Cells Regulate Myeloid Cell Activity Following Spinal Cord Injury via Expression of CD200 Ligand. J. Neurosci. 2017, 37, 972–985. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Li, A.; Hou, T.; Tao, X.; Chen, M.; Wu, C.; Chen, S.; Zhu, L.; Liao, H. Neurogenesis promoted by the CD200/CD200R signaling pathway following treadmill exercise enhances post-stroke functional recovery in rats. Brain Behav. Immun. 2019, 82, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Schwab, M.E.; Popovich, P.G. Central nervous system regenerative failure: Role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb. Perspect. Biol. 2014, 7, a020602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhaya, S.J.; Quiros-Molina, D.; Tamashiro-Orrego, A.D.; Houle, J.D.; Detloff, M.R. Exercise-Induced Changes to the Macrophage Response in the Dorsal Root Ganglia Prevent Neuropathic Pain after Spinal Cord Injury. J. Neurotrauma 2019, 36, 877–890. [Google Scholar] [CrossRef]
- Duan, R.; Qu, M.; Yuan, Y.; Lin, M.; Liu, T.; Huang, W.; Gao, J.; Zhang, M.; Yu, X. Clinical Benefit of Rehabilitation Training in Spinal Cord Injury: A Systematic Review and Meta-Analysis. Spine 2021, 46, E398–E410. [Google Scholar] [CrossRef]
- Jones, M.L.; Evans, N.; Tefertiller, C.; Backus, D.; Sweatman, M.; Tansey, K.; Morrison, S. Activity-based therapy for recovery of walking in chronic spinal cord injury: Results from a secondary analysis to determine responsiveness to therapy. Arch. Phys. Med. Rehabil. 2014, 95, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Osman, J.; Cortes, M.; Guest, J.; Pascual-Leone, A. A Systematic Review of Experimental Strategies AiMed. at Improving Motor Function after Acute and Chronic Spinal Cord Injury. J. Neurotrauma 2016, 33, 425–438. [Google Scholar] [CrossRef] [Green Version]
- Nacimiento, W.; Sappok, T.; Brook, G.A.; Toth, L.; Schoen, S.W.; Noth, J.; Kreutzberg, G.W. Structural changes of anterior horn neurons and their synaptic input caudal to a low thoracic spinal cord hemisection in the adult rat: A light and electron microscopic study. Acta Neuropathol. 1995, 90, 552–564. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilchak, J.N.; Caron, G.; Côté, M.-P. Exercise-Induced Plasticity in Signaling Pathways Involved in Motor Recovery after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 4858. https://doi.org/10.3390/ijms22094858
Bilchak JN, Caron G, Côté M-P. Exercise-Induced Plasticity in Signaling Pathways Involved in Motor Recovery after Spinal Cord Injury. International Journal of Molecular Sciences. 2021; 22(9):4858. https://doi.org/10.3390/ijms22094858
Chicago/Turabian StyleBilchak, Jadwiga N., Guillaume Caron, and Marie-Pascale Côté. 2021. "Exercise-Induced Plasticity in Signaling Pathways Involved in Motor Recovery after Spinal Cord Injury" International Journal of Molecular Sciences 22, no. 9: 4858. https://doi.org/10.3390/ijms22094858






