Ethylene and Nitric Oxide Involvement in the Regulation of Fe and P Deficiency Responses in Dicotyledonous Plants
Abstract
:1. Introduction
2. Fe Acquisition by Dicotyledonous Plants
3. P Acquisition by Dicotyledonous Plants
4. Fe and P Nutrition Interactions. A Great Opportunity to Improve the Nutrition of Both Elements
5. Role of ET and NO in the Regulation of Fe and P Deficiency Responses. Similarities and Differences
5.1. ET and NO Involvement in the Regulation of Physiological and Morphological Responses to Fe Deficiency
5.2. ET and NO Involvement in the Regulation of Physiological and Morphological Responses to P Deficiency
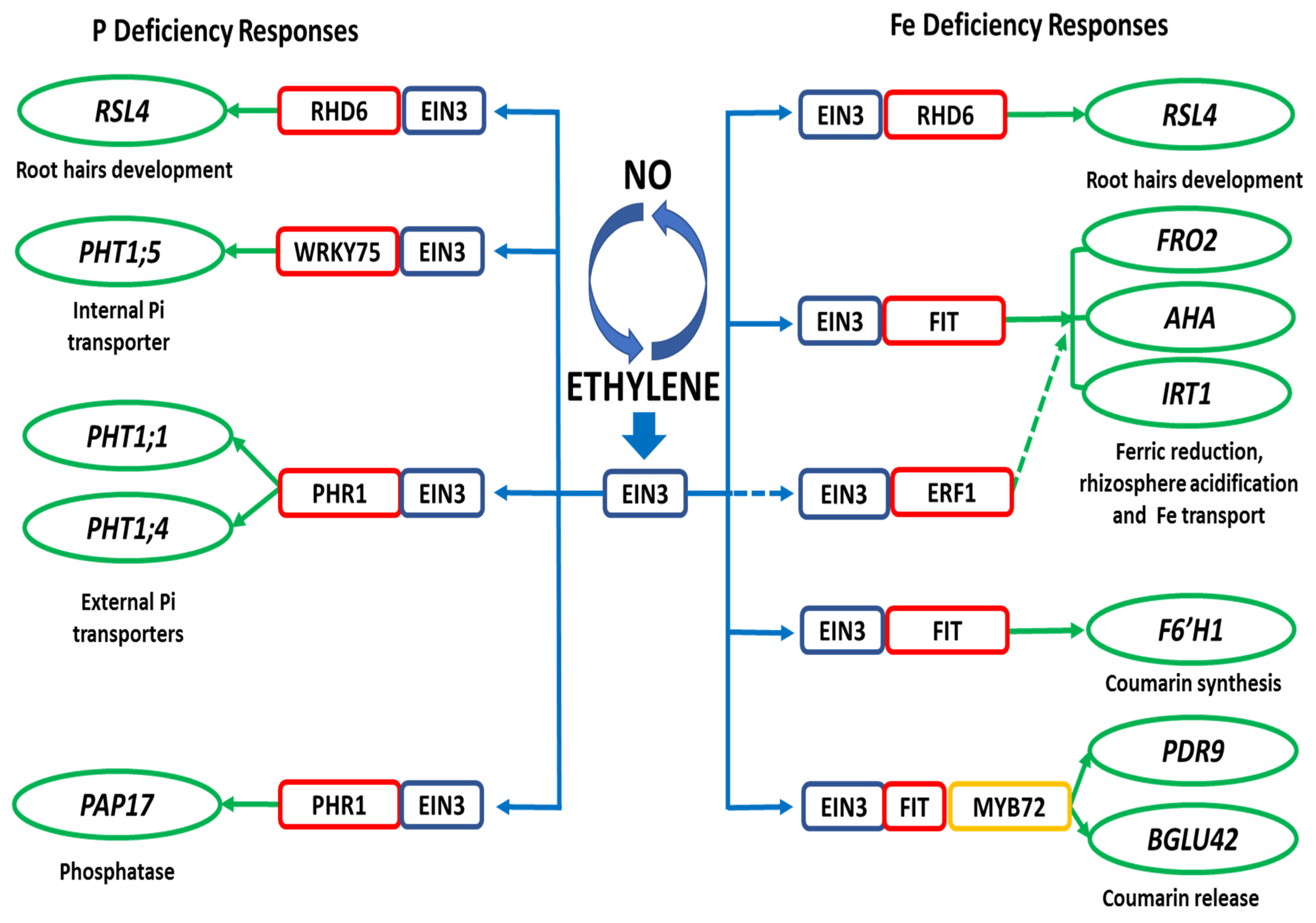
6. Mechanisms for the Induction of Fe- and P-Related Genes by ET and NO
7. Interactions with Other Signals in the Regulation of Fe and P Deficiency Responses
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, J.; Ibrikci, H.; Delgado, A.; Torrent, J.; Sommer, R.; Rashid, A. Significance of Phosphorus for Agriculture and the Environment in the West Asia and North Africa Region. Adv. Agron. 2012, 114, 91–153. [Google Scholar] [CrossRef]
- Foy, R.H.; Sims, J.T.; Sharpley, A.N. The Return of the Phosphorus Paradigm: Agricultural Phosphorus and Eutrophication. In Agronomy Monographs; Wiley: Hoboken, NJ, USA, 2015; pp. 909–939. [Google Scholar]
- Delgado, A.; Scalenghe, R. Aspects of phosphorus transfer from soils in Europe. J. Plant. Nutr. Soil Sci. 2008, 171, 552–575. [Google Scholar] [CrossRef]
- Torrent, J.; Barberis, E.; Gil-Sotres, F. Agriculture as a source of phosphorus for eutrophication in southern Europe. Soil Use Manag. 2007, 23, 25–35. [Google Scholar] [CrossRef]
- Macdonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [Green Version]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Marschner, H. Mineral. Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant. Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Abadía, J.; Álvarez-Fernández, A.; Rombolà, A.D.; Sanz, M.; Tagliavini, M.; Abadía, A. Technologies for the diagnosis and remediation of Fe deficiency. Soil Sci. Plant. Nutr. 2004, 50, 965–971. [Google Scholar] [CrossRef]
- Sanz, M.; Cavero, J.; Abadía, J. Iron chlorosis in the Ebro River basin, Spain. J. Plant. Nutr. 1992, 15, 1971–1981. [Google Scholar] [CrossRef]
- Álvarez-Fernández, A.; Paniagua, P.; Abadía, J.; Abadia, A. Effects of Fe Deficiency Chlorosis on Yield and Fruit Quality in Peach (Prunus persica L. Batsch). J. Agric. Food Chem. 2003, 51, 5738–5744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Escobar, R.; Barranco, D.; Benlloch, M. Overcoming Iron Chlorosis in Olive and Peach Trees Using a Low-pressure Trunk-injection Method. HortScience 1993, 28, 192–194. [Google Scholar] [CrossRef]
- Hansen, N.; Jolley, V.; Naeve, S.; Goos, R. Iron deficiency of soybean in the North Central U.S. and associated soil properties. Soil Sci. Plant. Nutr. 2004, 50, 983–987. [Google Scholar] [CrossRef]
- Kim, S.A.; Guerinot, M.L. Mining iron: Iron uptake and transport in plants. FEBS Lett. 2007, 581, 2273–2280. [Google Scholar] [CrossRef] [Green Version]
- Crombez, H.; Motte, H.; Beeckman, T. Tackling Plant Phosphate Starvation by the Roots. Dev. Cell 2019, 48, 599–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Nishizawa, N.K. Iron Uptake, Translocation, and Regulation in Higher Plants. Annu. Rev. Plant. Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, N.J.; Procter, C.M.; Connolly, E.L.; Guerinot, M.L. A ferric-chelate reductase for iron uptake from soils. Nature 1999, 397, 694–697. [Google Scholar] [CrossRef]
- Eide, D.; Broderius, M.; Fett, J.; Guerinot, M.L. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. USA 1996, 93, 5624–5628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schikora, A.; Schmidt, W. Formation of transfer cells and H + -ATPase expression in tomato roots under P and Fe deficiency. Planta 2002, 215, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhu, Y.; Müller, C.; Zörb, C.; Schubert, S. Adaptation of H+-Pumping and Plasma Membrane H+ ATPase Activity in Proteoid Roots of White Lupin under Phosphate Deficiency. Plant. Physiol. 2002, 129, 50–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santi, S.; Cesco, S.; Varanini, Z.; Pinton, R. Two plasma membrane H+-ATPase genes are differentially expressed in iron-deficient cucumber plants. Plant. Physiol. Biochem. 2005, 43, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; Lucena, C.; Romera, F.J.; Jester, G.G.; Wynn, A.N.; Rojas, C.L.; Alcántara, E.; Pérez-Vicente, R. Ethylene involvement in the regulation of the H+-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron trasnporter CsIRT1 genes in cucumber plants. Plant Physiol. Biochem. 2007, 45, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, N.B.; Giehl, R.F.; Döll, S.; Mock, H.-P.; Strehmel, N.; Scheel, D.; Kong, X.; Hider, R.C.; von Wirén, N. Feruloyl-CoA 6’-Hydroxylase1-Dependent Coumarins Mediate Iron Acquisition from Alkaline Substrates in Arabidopsis. Plant. Physiol. 2014, 164, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, S.; Weber, M. The essential role of coumarin secretion for Fe acquisition from alkaline soil. Plant. Signal. Behav. 2016, 11, e1114197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisó-Terraza, P.; Rios, J.J.; Abadía, J.; Abadía, A.; Álvarez-Fernández, A. Flavins secreted by roots of iron-deficient Beta vulgaris enable mining of ferric oxide via reductive mechanisms. New Phytol. 2016, 209, 733–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisó-Terraza, P.; Luis-Villarroya, A.; Fourcroy, P.; Briat, J.-F.; Abadía, A.; Gaymard, F.; Abadía, J.; Álvarez-Fernández, A. Accumulation and Secretion of Coumarinolignans and other Coumarins in Arabidopsis thaliana Roots in Response to Iron Deficiency at High pH. Front. Plant. Sci. 2016, 7, 1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.; Schmidt, W. One way. Or another? Iron uptake in plants. New Phytol. 2017, 214, 500–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kai, K.; Mizutani, M.; Kawamura, N.; Yamamoto, R.; Tamai, M.; Yamaguchi, H.; Sakata, K.; Shimizu, B.-I. Scopoletin is biosynthesized viaortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana. Plant. J. 2008, 55, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Siwinska, J.; Siatkowska, K.; Olry, A.; Grosjean, J.; Hehn, A.; Bourgaud, F.; Meharg, A.A.; Carey, M.; Lojkowska, E.; Ihnatowicz, A. Scopoletin 8-hydroxylase: A novel enzyme involved in coumarin biosynthesis and iron-deficiency responses in Arabidopsis. J. Exp. Bot. 2018, 69, 1735–1748. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.-H.; Rodríguez-Celma, J.; Lan, P.; Wu, Y.-C.; Vélez-Bermúdez, I.C.; Schmidt, W. Scopoletin 8-Hydroxylase-Mediated Fraxetin Production Is Crucial for Iron Mobilization. Plant. Physiol. 2018, 177, 194–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, J.; Schmidt, S.; Strehmel, N.; Scheel, D.; Abel, S. Arabidopsis Transporter ABCG37/PDR9 contributes primarily highly oxygenated Coumarins to Root Exudation. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Marschner, H.; Romheld, V.; Kissel, M. Different strategies in higher plants in mobilization and uptake of iron. J. Plant. Nutr. 1986, 9, 695–713. [Google Scholar] [CrossRef]
- Lucena, C.; Romera, F.J.; García, M.J.; Alcántara, E.; Pérez-Vicente, R. Ethylene Participates in the Regulation of Fe Deficiency Responses in Strategy I Plants and in Rice. Front. Plant. Sci. 2015, 6, 1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brumbarova, T.; Bauer, P.; Ivanov, R. Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant. Sci. 2015, 20, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Robe, K.; Gaymard, F.; Izquierdo, E.; Dubos, C. The Transcriptional Control of Iron Homeostasis in Plants: A Tale of bHLH Transcription Factors? Front. Plant. Sci. 2019, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, B.; Bauer, P. FIT, a regulatory hub for iron deficiency and stress signaling in roots, and FIT-dependent and -independent gene signatures. J. Exp. Bot. 2020, 71, 1694–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.A.; Lacroix, I.S.; Gerber, S.A.; Guerinot, M.L. The iron deficiency response in Arabidopsis thaliana requires the phosphorylated transcription factor URI. Proc. Natl. Acad. Sci. USA 2019, 116, 24933–24942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, T.A.; Tsukagoshi, H.; Busch, W.; Lahner, B.; Salt, D.E.; Benfey, P.N. The bHLH Transcription Factor POPEYE Regulates Response to Iron Deficiency in Arabidopsis Roots. Plant. Cell 2010, 22, 2219–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selote, D.; Samira, R.; Matthiadis, A.; Gillikin, J.W.; Long, T.A. Iron-Binding E3 Ligase Mediates Iron Response in Plants by Targeting Basic Helix-Loop-Helix Transcription Factors. Plant. Physiol. 2014, 167, 273–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lingam, S.; Mohrbacher, J.; Brumbarova, T.; Potuschak, T.; Fink-Straube, C.; Blondet, E.; Genschik, P.; Bauer, P. Interaction between the bHLH Transcription Factor FIT and ETHYLENE INSENSITIVE3/ETHYLENE INSENSITIVE3-LIKE1 Reveals Molecular Linkage between the Regulation of Iron Acquisition and Ethylene Signaling in Arabidopsis. Plant. Cell 2011, 23, 1815–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Ou, B.; Zhang, J.; Si, W.; Gu, H.; Qin, G.; Qu, L.-J. The Arabidopsis Mediator subunit MED16 regulates iron homeostasis by associating with EIN3/EIL1 through subunit MED25. Plant. J. 2014, 77, 838–851. [Google Scholar] [CrossRef]
- Colangelo, E.P.; Guerinot, M.L. The Essential Basic Helix-Loop-Helix Protein FIT1 Is Required for the Iron Deficiency Response. Plant. Cell 2004, 16, 3400–3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakoby, M.; Wang, H.-Y.; Reidt, W.; Weisshaar, B.; Bauer, P. FRU(BHLH029) is required for induction of iron mobilization genes in Arabidopsis thaliana. FEBS Lett. 2004, 577, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, R.; Brumbarova, T.; Bauer, P. Fitting into the Harsh Reality: Regulation of Iron-deficiency Responses in Dicotyledonous Plants. Mol. Plant. 2012, 5, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Chutia, R.; Abel, S.; Ziegler, J. Iron and Phosphate Deficiency Regulators Concertedly Control Coumarin Profiles in Arabidopsis thaliana Roots During Iron, Phosphate, and Combined Deficiencies. Front. Plant. Sci. 2019, 10, 113. [Google Scholar] [CrossRef] [Green Version]
- Curie, C.; Mari, S. New routes for plant iron mining. New Phytol. 2017, 214, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Lei, G.J.; Wang, Z.W.; Shi, Y.Z.; Braam, J.; Li, G.X.; Zheng, S.J. Coordination between Apoplastic and Symplastic Detoxification Confers Plant Aluminum Resistance. Plant. Physiol. 2013, 162, 1947–1955. [Google Scholar] [CrossRef] [Green Version]
- Lei, G.J.; Zhu, X.F.; Wang, Z.W.; Dong, F.; Dong, N.Y.; Zheng, S.J. Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in Arabidopsis. Plant Cell Environ. 2014, 37, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.Q.; Jin, C.W.; Fan, S.K.; Mao, Q.Q.; Sun, C.L.; Yu, Y.; Lin, X.Y. Elevation of NO production increases Fe immobilization in the Fe-deficiency roots apoplast by decreasing pectin methylation of cell wall. Sci. Rep. 2015, 5, 10746. [Google Scholar] [CrossRef] [Green Version]
- Barberon, M.; Vermeer, J.E.M.; de Bellis, D.; Wang, P.; Naseer, S.; Andersen, T.G.; Humbel, B.M.; Nawrath, C.; Takano, J.; Salt, D.E.; et al. Adaptation of Root Function by Nutrient-Induced Plasticity of Endodermal Differentiation. Cell 2016, 164, 447–459. [Google Scholar] [CrossRef] [Green Version]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate Nutrition: Improving Low-Phosphate Tolerance in Crops. Annu. Rev. Plant. Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef]
- Briat, J.-F.; Rouached, H.; Tissot, N.; Gaymard, F.; Dubos, C. Integration of P, S, Fe, and Zn nutrition signals in Arabidopsis thaliana: Potential involvement of PHOSPHATE STARVATION RESPONSE 1 (PHR1). Front. Plant. Sci. 2015, 6, 290. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, J.; Marin, E.; Floriani, M.; Chiarenza, S.; Richaud, P.; Nussaume, L.; Thibaud, M. Phosphate deficiency promotes modification of iron distribution in Arabidopsis plants. Biochimie 2006, 88, 1767–1771. [Google Scholar] [CrossRef]
- Matar, A.; Torrent, J.; Ryan, J. Soil and Fertilizer Phosphorus and Crop Responses in the Dryland Mediterranean Zone. Adv. Soil Sci. 12 1992, 18, 81–146. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Delhaize, E.; Frommer, W.B.; Guerinot, M.L.; Harrison, M.J.; Herrera-Estrella, L.; Horie, T.; Kochian, L.V.; Munns, R.; Nishizawa, N.K.; et al. Using membrane transporters to improve crops for sustainable food production. Nature 2013, 497, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, R.; Schachtman, D.P. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA 2004, 101, 8827–8832. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, V.K.; Smith, A.P. Ethylene’s Role in Phosphate Starvation Signaling: More than Just a Root Growth Regulator. Plant. Cell Physiol. 2011, 53, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Lucena, C.; Porras, R.; Romera, F.J.; Alcántara, E.; García, M.J.; Pérez-Vicente, R. Similarities and Differences in the Acquisition of Fe and P by Dicot Plants. Agronomy 2018, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Lucena, C.; Porras, R.; García, M.J.; Alcántara, E.; Pérez-Vicente, R.; Zamarreño-Ángel, M.; Bacaicoa, E.; García-Mina, J.M.; Smith, A.P.; Romera, F.J. Ethylene and Phloem Signals Are Involved in the Regulation of Responses to Fe and P Deficiencies in Roots of Strategy I Plants. Front. Plant. Sci. 2019, 10, 1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, A.S.; Varadarajan, D.K.; Mukatira, U.T.; D’Urzo, M.P.; Damsz, B.; Raghothama, K.G. Regulated Expression of Arabidopsis Phosphate Transporters. Plant. Physiol. 2002, 130, 221–233. [Google Scholar] [CrossRef] [Green Version]
- Mudge, S.R.; Rae, A.L.; Diatloff, E.; Smith, F.W. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant. J. 2002, 31, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhang, D.; Song, T.; Xu, F.; Lin, S.; Xu, W.; Li, Q.; Zhu, Y.; Liang, J.; Zhang, J. Arabidopsis plasma membrane H+-ATPase genes AHA2 and AHA7 have distinct and overlapping roles in the modulation of root tip H+ efflux in response to low-phosphorus stress. J. Exp. Bot. 2017, 68, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Bucciarelli, B.; Liu, J.; Zinn, K.; Miller, S.; Patton-Vogt, J.; Allan, D.; Shen, J.; Vance, C.P. White Lupin Cluster Root Acclimation to Phosphorus Deficiency and Root Hair Development Involve Unique Glycerophosphodiester Phosphodiesterases. Plant. Physiol. 2011, 156, 1131–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Bucciarelli, B.; Shen, J.; Allan, D.; Vance, C.P. Update on White Lupin Cluster Root Acclimation to Phosphorus Deficiency Update on Lupin Cluster Roots. Plant. Physiol. 2011, 156, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Y.; Tian, J.; Lim, B.L.; Yan, X.; Liao, H. Overexpressing AtPAP15 Enhances Phosphorus Efficiency in Soybean. Plant. Physiol. 2009, 151, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Robinson, W.D.; Park, J.; Tran, H.T.; del Vecchio, H.A.; Ying, S.; Zins, J.L.; Patel, K.; McKnight, T.D.; Plaxton, W.C. The secreted purple acid phosphatase isozymes AtPAP12 and AtPAP26 play a pivotal role in extracellular phosphate-scavenging by Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 6531–6542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Liu, Y.; Ni, J.; Wang, Y.; Bai, Y.; Shi, J.; Gan, J.; Wu, Z.; Wu, P. OsPHF1 Regulates the Plasma Membrane Localization of Low- and High-Affinity Inorganic Phosphate Transporters and Determines Inorganic Phosphate Uptake and Translocation in Rice. Plant. Physiol. 2011, 157, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Bustos, R.; Castrillo, G.; Linhares, F.; Puga, M.I.; Rubio, V.; Pérez-Pérez, J.; Solano, R.; Leyva, A.; Paz-Ares, J. A Central Regulatory System Largely Controls Transcriptional Activation and Repression Responses to Phosphate Starvation in Arabidopsis. PLoS Genet. 2010, 6, e1001102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Xie, Y.; Wang, H.; Ma, X.; Yao, W.; Wang, H. Light and Ethylene Coordinately Regulate the Phosphate Starvation Response through Transcriptional Regulation of PHOSPHATE STARVATION RESPONSE1. Plant. Cell 2017, 29, 2269–2284. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-H.; Nimmo, G.A.; Jenkins, G.I.; Nimmo, H.G. BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis. Biochem. J. 2007, 405, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Puga, M.I.; Mateos, I.; Charukesi, R.; Wang, Z.; Franco-Zorrilla, J.M.; de Lorenzo, L.; Irigoyen, M.L.; Masiero, S.; Bustos, R.; Rodríguez, J.; et al. SPX1 is a phosphate-dependent inhibitor of PHOSPHATE STARVATION RESPONSE 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14947–14952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Straub, D.; Yang, H.; Kania, A.; Shen, J.; Ludewig, U.; Neumann, G. The regulatory network of cluster-root function and development in phosphate-deficient white lupin (Lupinus albus) identified by transcriptome sequencing. Physiol. Plant. 2014, 151, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Schmidt, S.; Chutia, R.; Müller, J.; Böttcher, C.; Strehmel, N.; Scheel, D.; Abel, S. Non-targeted profiling of semi-polar metabolites in Arabidopsis root exudates uncovers a role for coumarin secretion and lignification during the local response to phosphate limitation. J. Exp. Bot. 2016, 67, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Fourcroy, P.; Sisó-Terraza, P.; Sudre, D.; Savirón, M.; Reyt, G.; Gaymard, F.; Abadía, A.; Abadia, J.; Álvarez-Fernández, A.; Briat, J.-F. Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol. 2014, 201, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Gopu, V.; Meena, C.K.; Shetty, P.H. Quercetin influences quorum sensing in food borne bacteria: In-vitro and in-silico evi-dence. PLoS ONE 2015, 10, e0134684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, J.; Toev, T.; Heisters, M.; Teller, J.; Moore, K.L.; Hause, G.; Dinesh, D.C.; Bürstenbinder, K.; Abel, S. Iron-Dependent Callose Deposition Adjusts Root Meristem Maintenance to Phosphate Availability. Dev. Cell 2015, 33, 216–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Bucio, J.S.; Salmeron-Barrera, G.J.; Ravelo-Ortega, G.; Raya-Gonzalez, J.; Leon, P.; Reyes de la Cruz, H.; Campos-Garcia, J.; Lopez-Bucio, J.; Guevara-Garcia, A.A. Mitogen-activated protein kinase 6 integrates phosphate and iron responses for indeterminate root growth in Arabidopsis thaliana. Planta 2019, 250, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Dörmann, P.; Benning, C. Galactolipids rule in seed plants. Trends Plant. Sci. 2002, 7, 112–118. [Google Scholar] [CrossRef]
- Nagarajan, V.K.; Jain, A.; Poling, M.D.; Lewis, A.J.; Raghothama, K.G.; Smith, A.P. Arabidopsis Pht1;5 Mobilizes Phosphate between Source and Sink Organs and Influences the Interaction between Phosphate Homeostasis and Ethylene Signaling. Plant. Physiol. 2011, 156, 1149–1163. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant. Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- García, M.J.; Romera, F.J.; Lucena, C.; Alcántara, E.; Pérez-Vicente, R. Ethylene and the Regulation of Physiological and Morphological Responses to Nutrient Deficiencies. Plant. Physiol. 2015, 169, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Neumann, G. The Role of Ethylene in Plant Adaptations for Phosphate Acquisition in Soils—A Review. Front. Plant. Sci. 2016, 6, 1224. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J. Root Architecture and Plant Productivity. Plant. Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.; Brown, K.M. Ethylene and plant responses to nutritional stress. Physiol. Plant. 1997, 100, 613–619. [Google Scholar] [CrossRef]
- Dinkelaker, B.; Hengeler, C.; Marschner, H. Distribution and Function of Proteoid Roots and other Root Clusters. Bot. Acta 1995, 108, 183–200. [Google Scholar] [CrossRef]
- Adams, M.A.; Bell, T.L.; Pate, J.S. Phosphorus sources and availability modify growth and distribution of root clusters and nodules of native Australian legumes. Plant Cell Environ. 2002, 25, 837–850. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [Green Version]
- Romera, F.J.; Alcántara, E.; de la Guardia, M.D. Effects of bicarbonate, phosphate and high pH on the reducing capacity of Fe-deficient sunflower and cucumber plants. J. Plant. Nutr. 1992, 15, 1519–1530. [Google Scholar] [CrossRef]
- Misson, J.; Raghothama, K.G.; Jain, A.; Jouhet, J.; Block, M.A.; Bligny, R.; Ortet, P.; Creff, A.; Somerville, S.; Rolland, N.; et al. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. USA 2005, 102, 11934–11939. [Google Scholar] [CrossRef] [Green Version]
- Bournier, M.; Tissot, N.; Mari, S.; Boucherez, J.; Lacombe, E.; Briat, J.-F.; Gaymard, F. Arabidopsis Ferritin 1 (AtFer1) Gene Regulation by the Phosphate Starvation Response 1 (AtPHR1) Transcription Factor Reveals a Direct Molecular Link between Iron and Phosphate Homeostasis. J. Biol. Chem. 2013, 288, 22670–22680. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.T.; Lahner, B.; Yakubova, E.; Salt, D.E.; Raghothama, K.G. The Effect of Iron on the Primary Root Elongation of Arabidopsis during Phosphate Deficiency. Plant. Physiol. 2008, 147, 1181–1191. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Rodríguez, A.R.; del Campillo, M.C.; Torrent, J. Phosphate aggravates iron chlorosis in sensitive plants grown on model calcium carbonate−iron oxide systems. Plant. Soil 2013, 373, 31–42. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; del Campillo, M.C.; Torrent, J. The severity of iron chlorosis in sensitive plants is related to soil phosphorus levels. J. Sci. Food Agric. 2014, 94, 2766–2773. [Google Scholar] [CrossRef]
- Venuti, S.; Zanin, L.; Marroni, F.; Franco, A.; Morgante, M.; Pinton, R.; Tomasi, N. Physiological and transcriptomic data highlight common features between iron and phosphorus acquisition mechanisms in white lupin roots. Plant. Sci. 2019, 285, 110–121. [Google Scholar] [CrossRef]
- Zaid, H.; El Morabet, R.; Diem, H.G.; Arahou, M. Does ethylene mediate cluster root formation under iron deficiency? Ann. Bot. 2003, 92, 673–677. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Yu, H.; Dong, J.; Che, X.; Jiao, Y.; Liu, D. The Molecular Mechanism of Ethylene-Mediated Root Hair Development Induced by Phosphate Starvation. PLoS Genet. 2016, 12, e1006194. [Google Scholar] [CrossRef]
- Wang, B.L.; Tang, X.Y.; Cheng, L.Y.; Zhang, A.Z.; Zhang, W.H.; Zhang, F.S.; Liu, J.Q.; Cao, Y.; Allan, D.L.; Vance, C.P.; et al. Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytol. 2010, 187, 1112–1123. [Google Scholar] [CrossRef]
- García, M.J.; Angulo, M.; García, C.; Lucena, C.; Alcántara, E.; Pérez-Vicente, R.; Romera, F.J. Influence of Ethylene Signaling in the Crosstalk Between Fe, S, and P Deficiency Responses in Arabidopsis thaliana. Front. Plant. Sci. 2021, 12, 643585. [Google Scholar] [CrossRef]
- Song, L.; Liu, D. Ethylene and plant responses to phosphate deficiency. Front. Plant. Sci. 2015, 6, 796. [Google Scholar] [CrossRef] [Green Version]
- Buet, A.; Galatro, A.; Ramos-Artuso, F.; Simontacchi, M. Nitric oxide and plant mineral nutrition: Current knowledge. J. Exp. Bot. 2019, 70, 4461–4476. [Google Scholar] [CrossRef]
- Galatro, A.; Ramos-Artuso, F.; Luquet, M.; Buet, A.; Simontacchi, M. An Update on Nitric Oxide Production and Role Under Phosphorus Scarcity in Plants. Front. Plant. Sci. 2020, 11, 413. [Google Scholar] [CrossRef] [Green Version]
- Romera, F.J.; Alcantara, E. Iron-Deficiency Stress Responses in Cucumber (Cucumis sativus L.) Roots (A Possible Role for Ethylene?). Plant. Physiol. 1994, 105, 1133–1138. [Google Scholar] [CrossRef]
- Romera, F.J.; Alcantara, E.; de la Guardia, M.D. Ethylene Production by Fe-deficient Roots and its Involvement in the Regulation of Fe-deficiency Stress Responses by Strategy I Plants. Ann. Bot. 1999, 83, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Romera, F.J.; Alcántara, E. Ethylene involvement in the regulation of Fe-deficiency stress responses by Strategy I plants. Funct. Plant. Biol. 2004, 31, 315–328. [Google Scholar] [CrossRef]
- Lucena, C.; Waters, B.M.; Romera, F.J.; García, M.J.; Morales, M.; Alcántara, E.; Pérez-Vicente, R. Ethylene could influence ferric reductase, iron transporter and H+-ATPase gene expression by affecting FER (or FER-like) gene activity. J. Exp. Bot. 2006, 57, 4145–4154. [Google Scholar] [CrossRef] [Green Version]
- Graziano, M.; LaMattina, L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant. J. 2007, 52, 949–960. [Google Scholar] [CrossRef]
- García, M.J.; Lucena, C.; Romera, F.J.; Alcántara, E.; Pérez-Vicente, R. Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. J. Exp. Bot. 2010, 61, 3885–3899. [Google Scholar] [CrossRef] [PubMed]
- García, M.J.; Suárez, V.; Romera, F.J.; Alcántara, E.; Pérez-Vicente, R. A new model involving ethylene, nitric oxide and Fe to explain the regulation of Fe-acquisition genes in Strategy I plants. Plant. Physiol. Biochem. 2011, 49, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Plant Signaling & Behavior. Plant. Signal. Behav. 2015, 6, 167–170. [CrossRef]
- Shanmugam, V.; Wang, Y.-W.; Tsednee, M.; Karunakaran, K.; Yeh, K.-C. Glutathione plays an essential role in nitric oxide-mediated iron-deficiency signaling and iron-deficiency tolerance in Arabidopsis. Plant. J. 2015, 84, 464–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kailasam, S.; Wang, Y.; Lo, J.-C.; Chang, H.-F.; Yeh, K.-C. S-Nitrosoglutathione works downstream of nitric oxide to mediate iron-deficiency signaling in Arabidopsis. Plant. J. 2018, 94, 157–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.J.; Corpas, F.J.; Lucena, C.; Alcántara, E.; Pérez-Vicente, R.; Zamarreño, Á.M.; Bacaicoa, E.; García-Mina, J.M.; Bauer, P.; Romera, F.J. A Shoot Fe Signaling Pathway Requiring the OPT3 Transporter Controls GSNO Reductase and Ethylene in Arabidopsis thaliana Roots. Front. Plant. Sci. 2018, 9, 1325. [Google Scholar] [CrossRef]
- Corpas, F.J.; Alché, J.D.D.; Barroso, J.B. Current overview of S-nitrosoglutathione (GSNO) in higher plants. Front. Plant. Sci. 2013, 4, 126. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.W.; Yang, J.L.; Qin, C.; Jin, C.W.; Mo, J.H.; Ye, T.; Zheng, S.J. Nitric Oxide Acts Downstream of Auxin to Trigger Root Ferric-Chelate Reductase Activity in Response to Iron Deficiency in Arabidopsis. Plant. Physiol. 2010, 154, 810–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, L.; Simontacchi, M.; Murgia, I.; Zabaleta, E.; Lamattina, L. Nitric oxide, nitrosyl iron complexes, ferritin and frataxin: A well equipped team to preserve plant iron homeostasis. Plant. Sci. 2011, 181, 582–592. [Google Scholar] [CrossRef]
- Koen, E.; Szymańska, K.; Klinguer, A.; Dobrowolska, G.; Besson-Bard, A.; Wendehenne, D. Nitric oxide and glutathione impact the expression of iron uptake- and iron transport-related genes as well as the content of metals in A. thalianaplants grown under iron deficiency. Plant. Signal. Behav. 2012, 7, 1246–1250. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.F.; Wang, B.; Song, W.F.; Zheng, S.J.; Shen, R.F. Putrescine Alleviates Iron Deficiency via NO-Dependent Reutilization of Root Cell-Wall Fe in Arabidopsis. Plant. Physiol. 2015, 170, 558–567. [Google Scholar] [CrossRef]
- López-Bucio, J.; Hernández-Abreu, E.; Sánchez-Calderón, L.; Nieto-Jacobo, M.F.; Simpson, J.; Herrera-Estrella, L. Phosphate Availability Alters Architecture and Causes Changes in Hormone Sensitivity in the Arabidopsis Root System. Plant. Physiol. 2002, 129, 244–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Baskin, T.I.; Brown, K.M.; Lynch, J.P. Regulation of Root Elongation under Phosphorus Stress Involves Changes in Ethylene Responsiveness. Plant. Physiol. 2003, 131, 1381–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lynch, J.P.; Brown, K.M. Ethylene and phosphorus availability have interacting yet distinct effects on root hair development. J. Exp. Bot. 2003, 54, 2351–2361. [Google Scholar] [CrossRef] [Green Version]
- Lei, M.; Zhu, C.; Liu, Y.; Karthikeyan, A.S.; Bressan, R.A.; Raghothama, K.G.; Liu, D. Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis. New Phytol. 2010, 189, 1084–1095. [Google Scholar] [CrossRef]
- Wang, L.; Dong, J.; Gao, Z.; Liu, N. The Arabidopsis gene HYPERSENSITIVE TO PHOSPHATE STARVATION 3 encodes ETHYLENE OVERPRODUCTION 1. Plant. Cell Physiol. 2012, 53, 1093–1105. [Google Scholar] [CrossRef] [Green Version]
- Roldan, M.; Dinh, P.; Leung, S.; McManus, M.T. Ethylene and the responses of plants to phosphate deficiency. AoB Pants 2013, 5, plt013. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.L.-C.; Li, H.; Ecker, J.R. Ethylene Biosynthesis and Signaling Networks. Plant. Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-S.; Gao, Y.; Tian, Q.-Y.; Shi, F.-L.; Li, L.-H.; Zhang, W.-H. Stimulation of root acid phosphatase by phosphorus deficiency is regulated by ethylene in Medicago falcata. Environ. Exp. Bot. 2011, 71, 114–120. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhu, C.Q.; Wang, C.; Dong, X.Y.; Shen, R.F. Nitric oxide acts upstream of ethylene in cell wall phosphorus reutilization in phosphorus-deficient rice. J. Exp. Bot. 2017, 68, 753–760. [Google Scholar] [CrossRef]
- Bin Meng, Z.; Chen, L.Q.; Suo, D.; Li, G.X.; Tang, C.X.; Zheng, S.J. Nitric oxide is the shared signalling molecule in phosphorus- and iron-deficiency-induced formation of cluster roots in white lupin (Lupinus albus). Ann. Bot. 2012, 109, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Angulo, M.; García, M.; Alcántara, E.; Pérez-Vicente, R.; Romera, F. Comparative Study of Several Fe Deficiency Responses in the Arabidopsis thaliana Ethylene Insensitive Mutants ein2-1 and ein2-5. Plants 2021, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Piqueras, R.; Sánchez-Serrano, J.J.; Solano, R. ETHYLENE RESPONSE FACTOR1 Integrates Signals from Ethylene and Jasmonate Pathways in Plant Defense. Plant Cell 2003, 15, 165–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.-C.; Liao, P.-M.; Kuo, W.-W.; Lin, T.-P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 Regulates Abiotic Stress-Responsive Gene Expression by Binding to Different cis-Acting Elements in Response to Different Stress Signals. Plant. Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balparda, M.; Armas, A.M.; Estavillo, G.M.; Roschzttardtz, H.; Pagani, M.A.; Gomez-Casati, D.F. The PAP/SAL1 retrograde signaling pathway is involved in iron homeostasis. Plant. Mol. Biol. 2020, 102, 323–337. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Wang, Y.; Wu, T.; Yang, Y.; Zhang, X.; Han, Z.; Xu, X. Ethylene response factor AtERF72 negatively regulates Arabidopsis thaliana response to iron deficiency. Biochem. Biophys. Res. Commun. 2017, 491, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Karemera, N.J.U.; Wu, T.; Yang, Y.; Zhang, X.; Xu, X.; Wang, Y.; Han, Z. The ethylene response factor AtERF4 negatively regulates the iron deficiency response in Arabidopsis thaliana. PLoS ONE 2017, 12, e0186580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Wu, T.; Li, Q.; Zhang, X.; Xu, X.; Li, T.; Han, Z.; Wang, Y. An ethylene response factor (MxERF4) functions as a repressor of Fe acquisition in Malus xiaojinensis. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Li, Z.; Huang, P.; Li, B.; Fang, S.; Chu, J.; Guo, H. A Tripartite Amplification Loop Involving the Transcription Factor WRKY75, Salicylic Acid, and Reactive Oxygen Species Accelerates Leaf Senescence. Plant. Cell 2017, 29, 2854–2870. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xu, P.; Li, B.; Li, P.; Wen, X.; An, F.; Gong, Y.; Xin, Y.; Zhu, Z.; Wang, Y.; et al. Ethylene promotes root hair growth through coordinated EIN3/EIL1 and RHD6/RSL1 activity in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 13834–13839. [Google Scholar] [CrossRef] [Green Version]
- Rubio, V.; Linhares, F.; Solano, R.; Martín, A.C.; Iglesias, J.; Leyva, A.; Paz-Ares, J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 2001, 15, 2122–2133. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhang, H.; Fang, X.; Zhang, Y.; Jin, C. Auxin Acts Downstream of Ethylene and Nitric Oxide to Regulate Magnesium Deficiency-Induced Root Hair Development in Arabidopsis thaliana. Plant. Cell Physiol. 2018, 2018, 591452. [Google Scholar] [CrossRef]
- Malik, S.I.; Hussain, A.; Yun, B.-W.; Spoel, S.H.; Loake, G.J. GSNOR-mediated de-nitrosylation in the plant defence response. Plant. Sci. 2011, 181, 540–544. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Mata-Pérez, C.; Valderrama, R.; Padilla, M.N.; López-Jaramillo, J.; Luque, F.; Corpas, F.J.; Barroso, J.B. Differential molecular response of monodehydroascorbate reductase and glutathione reductase by nitration and S-nitrosylation. J. Exp. Bot. 2015, 66, 5983–5996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaffagnini, M.; de Mia, M.; Morisse, S.; di Giacinto, N.; Marchand, C.; Maes, A.; Lemaire, S.; Trost, P. Protein S-nitrosylation in photosynthetic organisms: A comprehensive overview with future perspectives. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 952–966. [Google Scholar] [CrossRef]
- Sauter, M.; Moffatt, B.; Saechao, M.C.; Hell, R.; Wirtz, M. Methionine salvage and S-adenosylmethionine: Essential links between sulfur, ethylene and polyamine biosynthesis. Biochem. J. 2013, 451, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindermayr, C.; Saalbach, G.; Bahnweg, G.; Durner, J. Differential Inhibition of Arabidopsis Methionine Adenosyl transferases by Protein S-Nitrosylation. J. Biol. Chem. 2006, 281, 4285–4291. [Google Scholar] [CrossRef] [Green Version]
- Efreschi, L. Nitric oxide and phytohormone interactions: Current status and perspectives. Front. Plant. Sci. 2013, 4, 398. [Google Scholar] [CrossRef] [Green Version]
- La Rosa, N.M.-D.; Sotillo, B.; Miskolczi, P.; Gibbs, D.J.; Vicente, J.; Carbonero, P.; Oñate-Sánchez, L.; Holdsworth, M.J.; Bhalerao, R.; Alabadí, D.; et al. Large-Scale Identification of Gibberellin-Related Transcription Factors Defines Group VII ETHYLENE RESPONSE FACTORS as Functional DELLA Partners. Plant. Physiol. 2014, 166, 1022–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, D.J.; Isa, N.M.; Movahedi, M.; Lozano-Juste, J.; Mendiondo, G.M.; Berckhan, S.; la Rosa, N.M.-D.; Conde, J.V.; Correia, C.S.; Pearce, S.P.; et al. Nitric Oxide Sensing in Plants Is Mediated by Proteolytic Control of Group VII ERF Transcription Factors. Mol. Cell 2014, 53, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Wild, M.; Davière, J.-M.; Regnault, T.; Sakvarelidze-Achard, L.; Carrera, E.; Diaz, I.L.; Cayrel, A.; Dubeaux, G.; Vert, G.; Achard, P. Tissue-Specific Regulation of Gibberellin Signaling Fine-Tunes Arabidopsis Iron-Deficiency Responses. Dev. Cell 2016, 37, 190–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.J.; Romera, F.J.; Stacey, M.G.; Stacey, G.; Villar, E.; Alcántara, E.; Pérez-Vicente, R. Shoot to root communication is necessary to control the expression of iron-acquisition genes in Strategy I plants. Planta 2012, 237, 65–75. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.G.; Xie, Q.; Akmakjian, G.Z.; Jobe, T.O.; Patel, A.; Stacey, M.G.; Song, L.; Demoin, D.W.; Jurisson, S.S.; Stacey, G.; et al. OPT3 Is a Component of the Iron-Signaling Network between Leaves and Roots and Misregulation of OPT3 Leads to an Over-Accumulation of Cadmium in Seeds. Mol. Plant. 2014, 7, 1455–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Z.; Gayomba, S.R.; Jung, H.-I.; Vimalakumari, N.K.; Piñeros, M.; Craft, E.; Rutzke, M.A.; Danku, J.; Lahner, B.; Punshon, T.; et al. OPT3 Is a Phloem-Specific Iron Transporter That Is Essential for Systemic Iron Signaling and Redistribution of Iron and Cadmium in Arabidopsis. Plant. Cell 2014, 26, 2249–2264. [Google Scholar] [CrossRef] [Green Version]
- Stacey, M.G.; Patel, A.; McClain, W.E.; Mathieu, M.; Remley, M.; Rogers, E.E.; Gassmann, W.; Blevins, D.G.; Stacey, G. The Arabidopsis AtOPT3 Protein Functions in Metal Homeostasis and Movement of Iron to Developing Seeds. Plant. Physiol. 2008, 146, 323–324. [Google Scholar] [CrossRef] [Green Version]
- Estavillo, G.M.; Crisp, P.A.; Pornsiriwong, W.; Wirtz, M.; Collinge, D.; Carrie, C.; Giraud, E.; Whelan, J.; David, P.; Javot, H.; et al. Evidence for a SAL1-PAP Chloroplast Retrograde Pathway That Functions in Drought and High Light Signaling in Arabidopsis. Plant. Cell 2011, 23, 3992–4012. [Google Scholar] [CrossRef] [Green Version]
- Phua, S.Y.; Yan, D.; Chan, K.X.; Estavillo, G.M.; Nambara, E.; Pogson, B.J. The Arabidopsis SAL1-PAP Pathway: A Case Study for Integrating Chloroplast Retrograde, Light and Hormonal Signaling in Modulating Plant Growth and Development? Front. Plant. Sci. 2018, 9, 1171. [Google Scholar] [CrossRef]
- Xiong, L.; Lee, B.-H.; Ishitani, M.; Lee, H.; Zhang, C.; Zhu, J.-K. FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev. 2001, 15, 1971–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, J.; Misson, J.; Crisp, P.A.; David, P.; Bayle, V.; Estavillo, G.M.; Javot, H.; Chiarenza, S.; Mallory, A.C.; Maizel, A.; et al. A Novel fry1 Allele Reveals the Existence of a Mutant Phenotype Unrelated to 5′->3′ Exoribonuclease (XRN) Activities in Arabidopsis thaliana Roots. PLoS ONE 2011, 6, e16724. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Vanneste, S.; Brewer, P.B.; Michniewicz, M.; Grones, P.; Kleine-Vehn, J.; Löfke, C.; Teichmann, T.; Bielach, A.; Cannoot, B.; et al. Inositol Trisphosphate-Induced Ca2+ Signaling Modulates Auxin Transport and PIN Polarity. Dev. Cell 2011, 20, 855–866. [Google Scholar] [CrossRef]
- Olmedo, G.; Guo, H.; Gregory, B.D.; Nourizadeh, S.D.; Aguilar-Henonin, L.; Li, H.; An, F.; Guzman, P.; Ecker, J.R. ETHYLENE-INSENSITIVE5 encodes a 5’->3’ exoribonuclease required for regulation of the EIN3-targeting F-box proteins EBF1/2. Proc. Natl. Acad. Sci. USA 2006, 103, 13286–13293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.-R.; Huseby, S.; Koprivova, A.; Chételat, A.; Wirtz, M.; Mugford, S.T.; Navid, E.; Brearley, C.; Saha, S.; Mithen, R.; et al. Effects of fou8/fry1 Mutation on Sulfur Metabolism: Is Decreased Internal Sulfate the Trigger of Sulfate Starvation Response? PLoS ONE 2012, 7, e39425. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-Y.; Huang, T.-K.; Leong, S.J.; Chiou, T.-J. Long-distance call from phosphate: Systemic regulation of phosphate starvation responses. J. Exp. Bot. 2013, 65, 1817–1827. [Google Scholar] [CrossRef]
- Delhaize, E.; Randall, P.J. Characterization of a Phosphate-Accumulator Mutant of Arabidopsis thaliana. Plant. Physiol. 1995, 107, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Bari, R.; Pant, B.D.; Stitt, M.; Scheible, W.-R. PHO2, MicroRNA399, and PHR1 Define a Phosphate-Signaling Pathway in Plants. Plant. Physiol. 2006, 141, 988–999. [Google Scholar] [CrossRef] [Green Version]
- Buhtz, A.; Pieritz, J.; Springer, F.; Kehr, J. Phloem small RNAs, nutrient stress responses, and systemic mobility. BMC Plant. Biol. 2010, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.K.; Chu, H.-H.; Abundis, C.; Vasques, K.; Rodriguez, D.C.; Chia, J.-C.; Huang, R.; Vatamaniuk, O.K.; Walker, E.L. Iron-Nicotianamine Transporters Are Required for Proper Long Distance Iron Signaling. Plant. Physiol. 2017, 175, 1254–1268. [Google Scholar] [CrossRef] [Green Version]
- Fujii, H.; Chiou, T.-J.; Lin, S.-I.; Aung, K.; Zhu, J.-K. A miRNA Involved in Phosphate-Starvation Response in Arabidopsis. Curr. Biol. 2005, 15, 2038–2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, K.; Rus, A.; Sharkhuu, A.; Yokoi, S.; Karthikeyan, A.S.; Raghothama, K.G.; Baek, D.; Koo, Y.D.; Jin, J.B.; Bressan, R.A.; et al. The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proc. Natl. Acad. Sci. USA 2005, 102, 7760–7765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiou, T.-J.; Aung, K.; Lin, S.-I.; Wu, C.-C.; Chiang, S.-F.; Su, C.-L. Regulation of Phosphate Homeostasis by MicroRNA in Arabidopsis. Plant. Cell 2006, 18, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Chiou, T.-J. The role of microRNAs in sensing nutrient stress. Plant Cell Environ. 2007, 30, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liao, H.; Lucas, W.J. Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. J. Integr. Plant. Biol. 2014, 56, 192–220. [Google Scholar] [CrossRef] [PubMed]
- Pagnussat, G.C.; Simontacchi, M.; Puntarulo, S.; la Mattina, L. Nitric Oxide Is Required for Root Organogenesis. Plant. Physiol. 2002, 129, 954–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa-Aragunde, N.; Graziano, M.; Chevalier, C.; la Mattina, L. Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J. Exp. Bot. 2006, 57, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandonadi, D.B.; Santos, M.P.; Dobbss, L.B.; Olivares, F.L.; Canellas, L.P.; Binzel, M.L.; Okorokova-Façanha, A.L.; Façanha, A.R. Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation. Planta 2010, 231, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; Graziano, M.; la Mattina, L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004, 218, 900–905. [Google Scholar] [CrossRef]
- Wu, A.P.; Gong, L.; Chen, X.; Wang, J.X. Interactions between nitric oxide, gibberellic acid, and phosphorus regulate primary root growth in Arabidopsis. Biol. Plant. 2014, 58, 335–340. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, X.; Liao, L.; Harberd, N.P.; Fu, X. Phosphate Starvation Root Architecture and Anthocyanin Accumulation Responses Are Modulated by the Gibberellin-DELLA Signaling Pathway in Arabidopsis. Plant. Physiol. 2007, 145, 1460–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardo, M.C.; Graziano, M.; Polacco, J.C.; la Mattina, L. Nitric Oxide Functions as a Positive Regulator of Root Hair Development. Plant. Signal. Behav. 2006, 1, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.-Y.; Ried, M.K.; Hothorn, M.; Poirier, Y. Control of plant phosphate homeostasis by inositol pyrophosphates and the SPX domain. Curr. Opin. Biotechnol. 2018, 49, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Grillet, L.; Lan, P.; Li, W.; Mokkapati, G.; Schmidt, W. IRON MAN is a ubiquitous family of peptides that control iron transport in plants. Nat. Plants 2018, 4, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Hindt, M.N.; Akmakjian, G.Z.; Pivarski, K.L.; Punshon, T.; Baxter, I.; Salt, D.E.; Guerinot, M.L. BRUTUS and its paralogs, BTS LIKE1 and BTS LIKE2, encode important negative regulators of the iron deficiency response in Arabidopsis thaliana. Metallomic 2017, 9, 876–890. [Google Scholar] [CrossRef]
- Romera, F.J.; Lucena, C.; García, M.J.; Alcántara, E.; Pérez-Vicente, R. The Role of Ethylene and Other Signals in the Regulation of Fe Deficiency Responses by Dicot Plants. In Stress Signaling in Plants: Genomics and Proteomics Perspective; Springer Science and Business Media LLC: Berlin/Heildeberg, Germany, 2016; Volume 2, pp. 277–300. [Google Scholar]
- Romera, F.J.; Lucena, C.; García, M.J.; Alcántara, E.; Angulo, M.; Aparicio, M.A.; Pérez-Vicente, R. Plant hormones and nutrient deficiency responses. In Hormones and Plant Response; Gupta, D., Corpas, F.J., Eds.; Springer: Dordrecht, The Netherlands, 2021; in press. [Google Scholar]
- Huang, K.-L.; Ma, G.-J.; Zhang, M.-L.; Xiong, H.; Wu, H.; Zhao, C.-Z.; Liu, C.-S.; Jia, H.-X.; Chen, L.; Kjorven, J.O.; et al. The ARF7 and ARF19 Transcription Factors Positively Regulate PHOSPHATE STARVATION RESPONSE1 in Arabidopsis Roots. Plant. Physiol. 2018, 178, 413–427. [Google Scholar] [CrossRef] [Green Version]
- Franco-Zorrilla, J.M.; González, E.; Bustos, R.; Linhares, F.; Leyva, A.; Paz-Ares, J. The transcriptional control of plant responses to phosphate limitation. J. Exp. Bot. 2004, 55, 285–293. [Google Scholar] [CrossRef]
- Séguéla, M.; Briat, J.; Vert, G.; Curie, C. Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway. Plant. J. 2008, 55, 289–300. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, M.J.; Lucena, C.; Romera, F.J. Ethylene and Nitric Oxide Involvement in the Regulation of Fe and P Deficiency Responses in Dicotyledonous Plants. Int. J. Mol. Sci. 2021, 22, 4904. https://doi.org/10.3390/ijms22094904
García MJ, Lucena C, Romera FJ. Ethylene and Nitric Oxide Involvement in the Regulation of Fe and P Deficiency Responses in Dicotyledonous Plants. International Journal of Molecular Sciences. 2021; 22(9):4904. https://doi.org/10.3390/ijms22094904
Chicago/Turabian StyleGarcía, María José, Carlos Lucena, and Francisco Javier Romera. 2021. "Ethylene and Nitric Oxide Involvement in the Regulation of Fe and P Deficiency Responses in Dicotyledonous Plants" International Journal of Molecular Sciences 22, no. 9: 4904. https://doi.org/10.3390/ijms22094904
APA StyleGarcía, M. J., Lucena, C., & Romera, F. J. (2021). Ethylene and Nitric Oxide Involvement in the Regulation of Fe and P Deficiency Responses in Dicotyledonous Plants. International Journal of Molecular Sciences, 22(9), 4904. https://doi.org/10.3390/ijms22094904





