Ly6c as a New Marker of Mouse Blood Vessels: Qualitative and Quantitative Analyses on Intact and Ischemic Retinas
Abstract
:1. Introduction
2. Results
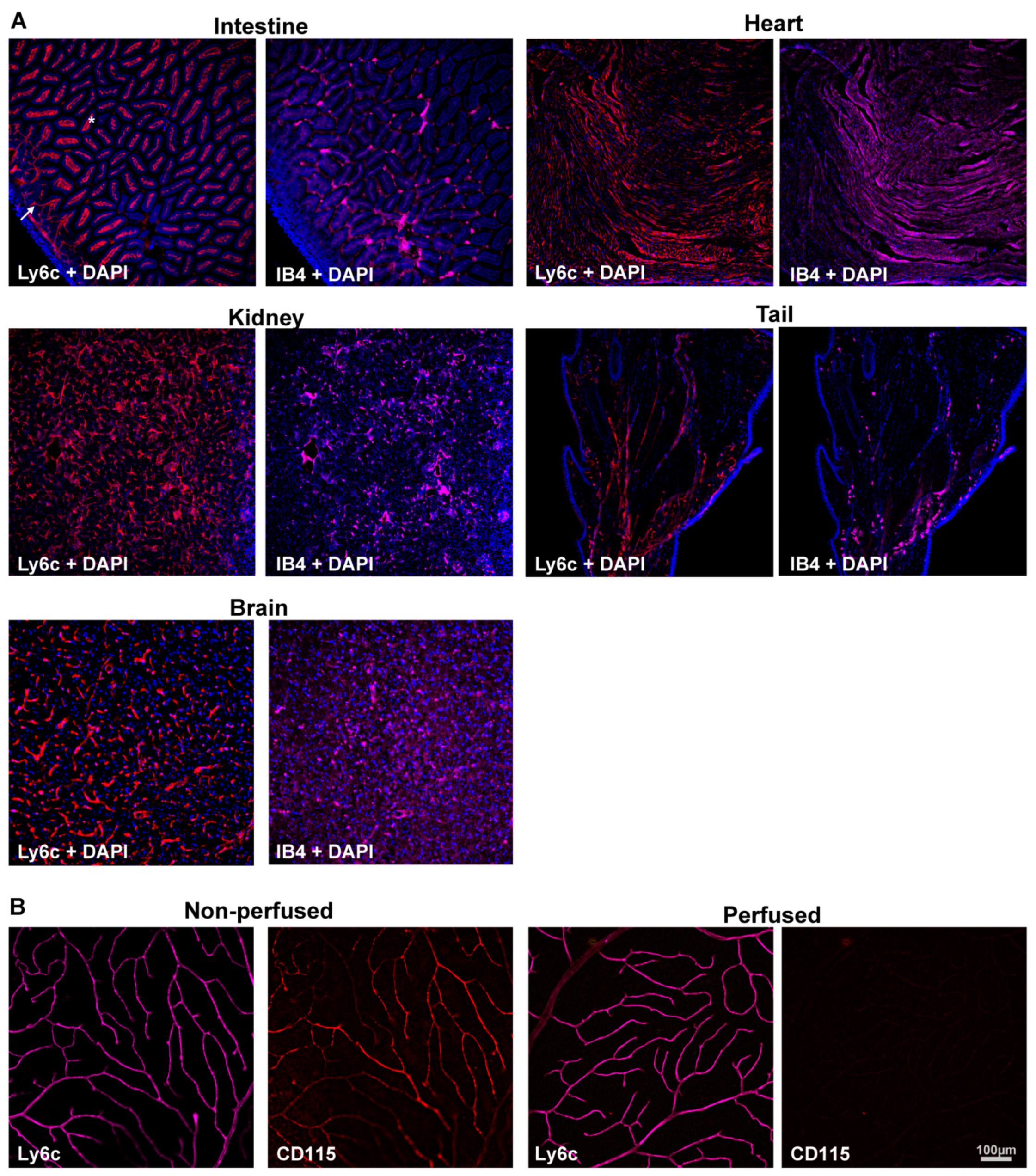
2.1. Determination of the Ly6c Value as a Vasculature Marker
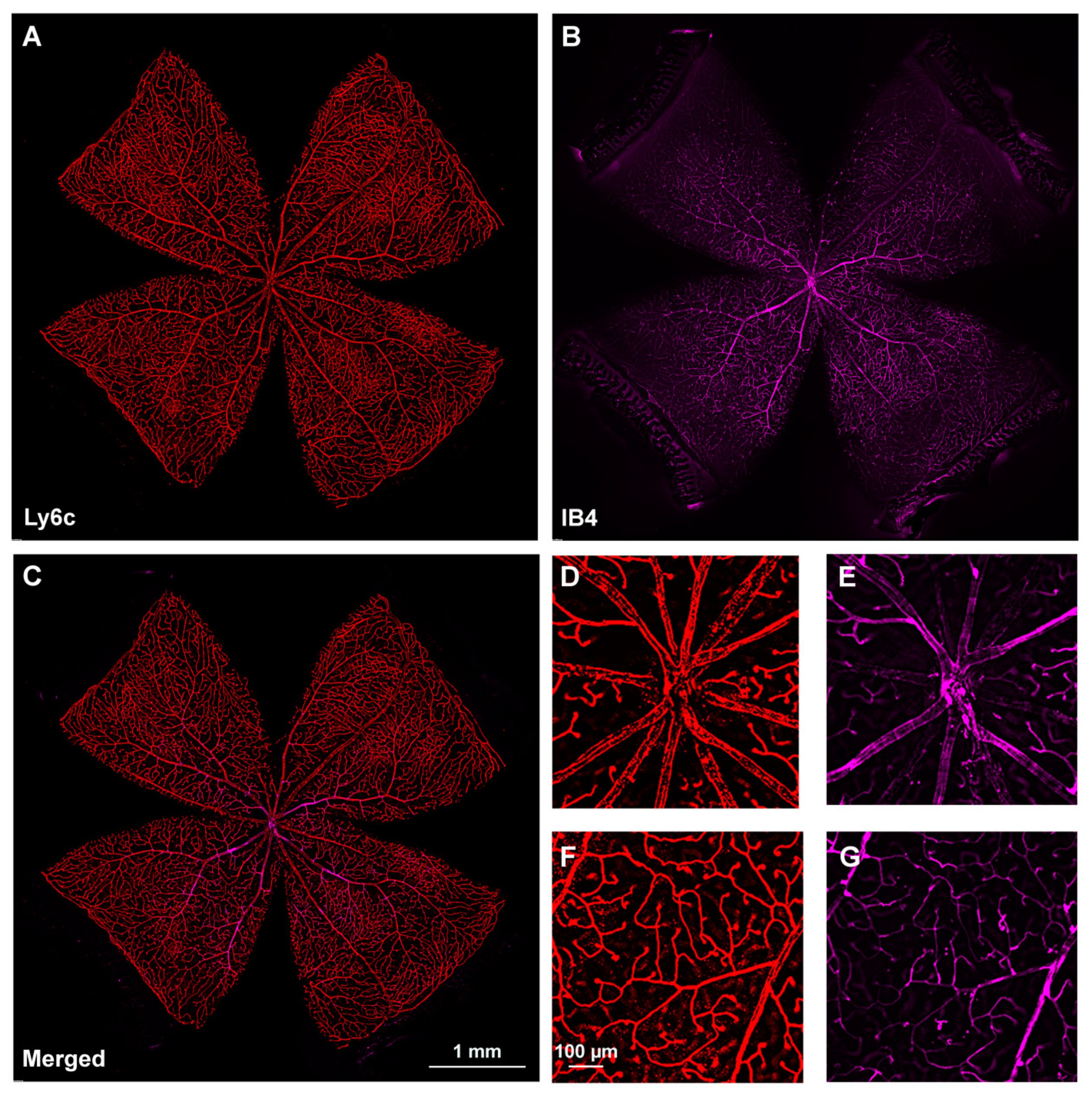
2.2. Retinal Vasculature Visualized with Ly6c
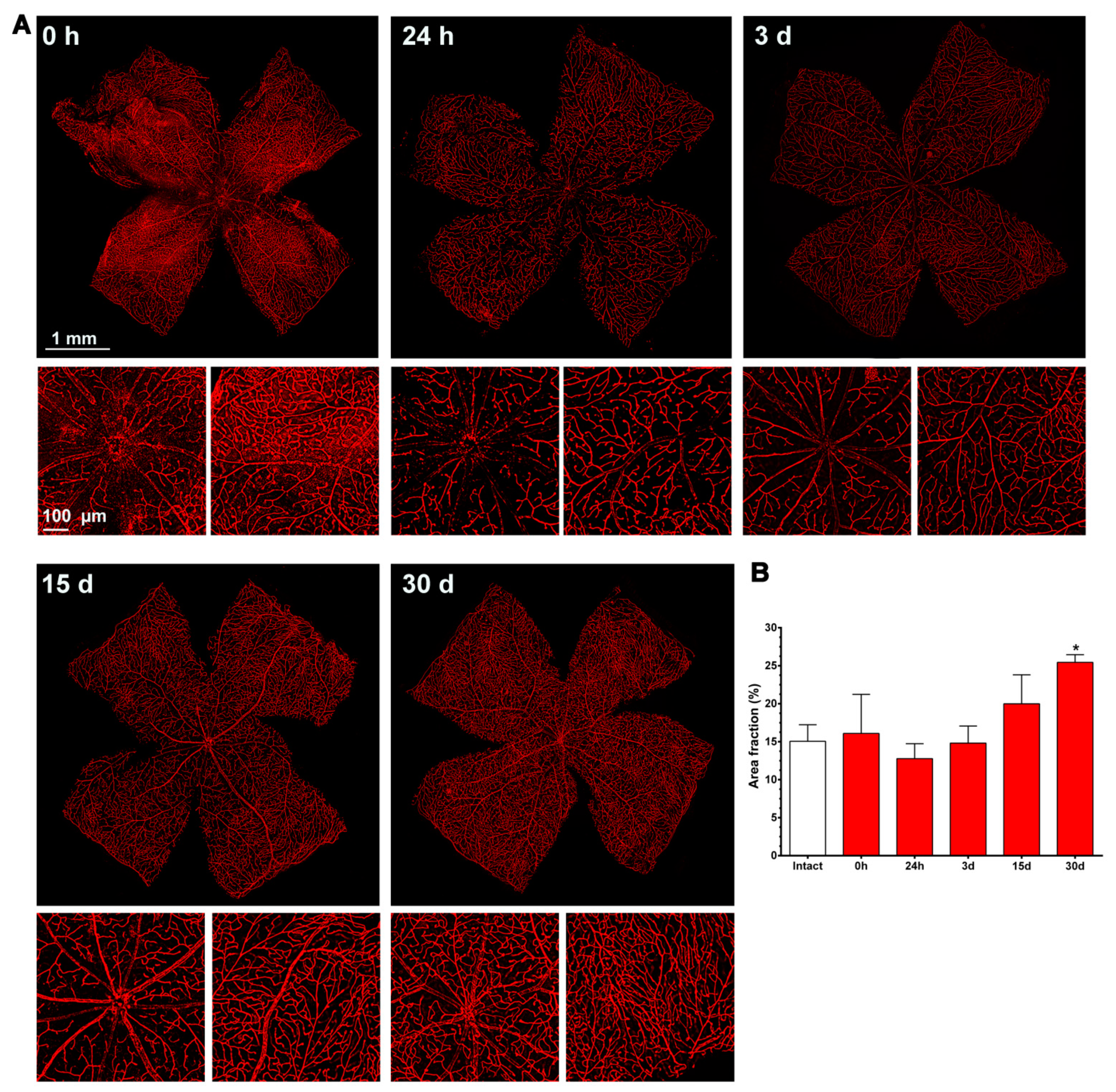
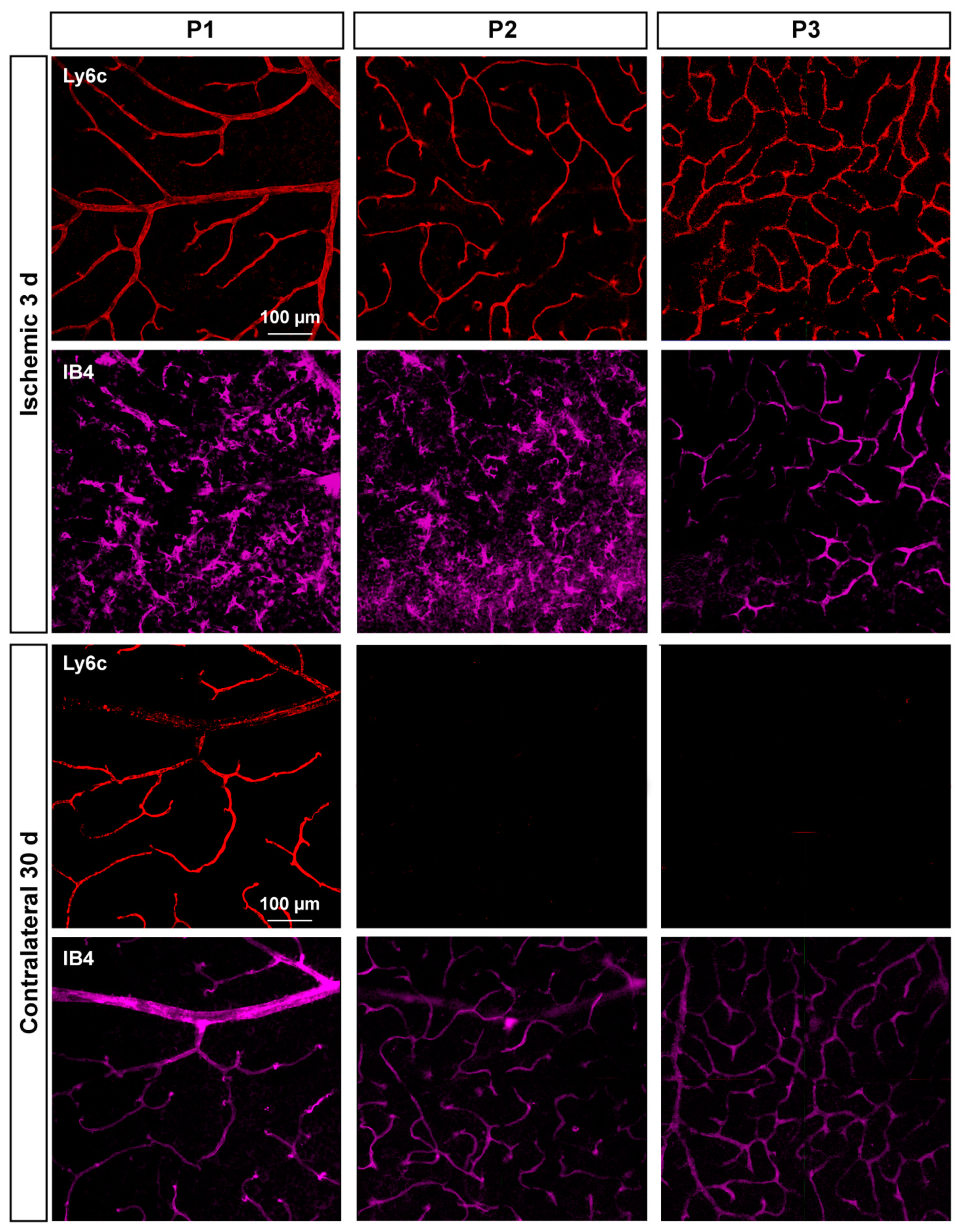
2.3. Ly6c Identifies Vascular Changes in a Model of Retinal Ischemia
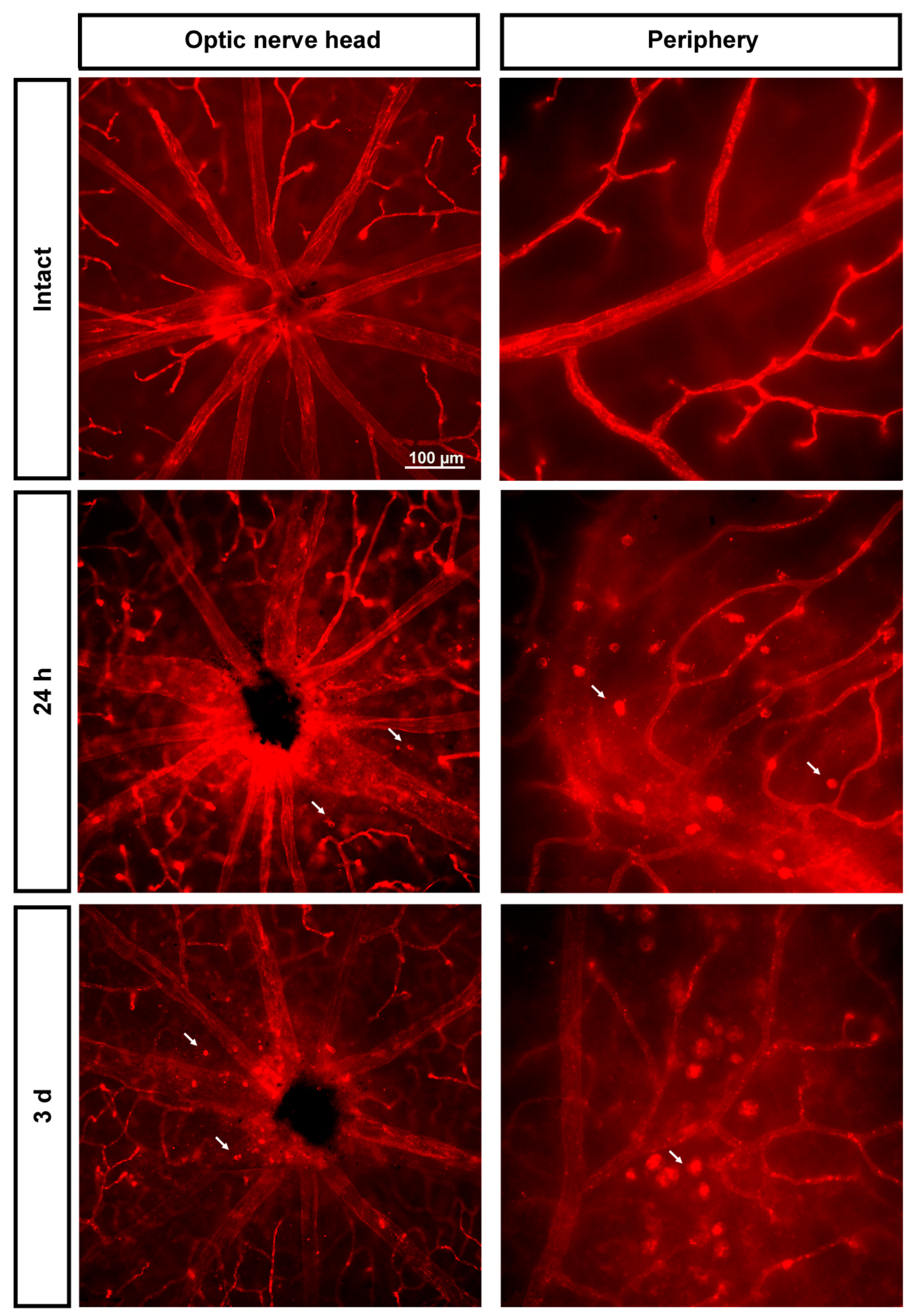
2.3.1. Injured Retinas
2.3.2. Contralateral Retinas
3. Discussion
4. Materials and Methods
4.1. Animal Handling
4.2. Acute Ocular Hypertension (AOHT) Induction
4.3. Tissue Processing
4.4. Immunodetection
4.5. Image Acquisition and Analysis
4.6. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ly6c | Lymphocyte antigen 6 complex |
| CNS | Central nervous system |
| RGC | Retinal ganglion cells |
| CD31 | Cluster of differentiation 31 |
| ICAM2 | Intercellular adhesion molecule 2 |
| CLDN5 | Claudin 5 |
| Col4 | Collagen 4 |
| ENG | Endoglin |
| ZO-1 | Zonula occludens-1 |
| CDH5 | Cadherin 5 |
| Erg | ETS-related gene |
| BSA | Bovine serum albumin |
| P (1–3) | Vascular plexus |
| IOP | Intraocular pressure |
| OHT | Ocular hypertension |
| AOHT | Acute ocular hypertension |
| PFA | Paraformaldehyde |
| OCT | Optimal cutting temperature compound |
| PBS | Phosphate buffered saline |
| SD | Standard deviation |
References
- Lendahl, U.; Nilsson, P.; Betsholtz, C. Emerging Links between Cerebrovascular and Neurodegenerative Diseases-a Special Role for Pericytes. EMBO Rep. 2019, 20, e48070. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The Role of Brain Vasculature in Neurodegenerative Disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Cortes-Canteli, M.; Iadecola, C. Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 942–951. [Google Scholar] [CrossRef]
- Sun, Y.; Smith, L.E.H. Retinal Vasculature in Development and Diseases. Annu. Rev. Vis. Sci. 2018, 4, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; MacIntyre, J.N.; Pouliot, M.; Casanova, C.; Vaucher, E.; Kelly, M.E.M.; Di Polo, A. Acetylcholinesterase Inhibition Promotes Retinal Vasoprotection and Increases Ocular Blood Flow in Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3171–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colucciello, M. Retinal Vascular Disease in Hypertension. Postgrad. Med. 2005, 117, 33–42. [Google Scholar] [CrossRef]
- Schuster, A.K.; Erb, C.; Hoffmann, E.M.; Dietlein, T.; Pfeiffer, N. The Diagnosis and Treatment of Glaucoma. Dtsch. Arztebl. Int. 2020, 117, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Namekata, K.; Guo, X.; Noro, T.; Harada, C.; Harada, T. Targeting Oxidative Stress for Treatment of Glaucoma and Optic Neuritis. Oxid. Med. Cell. Longev. 2017, 2017, 2817252. [Google Scholar] [CrossRef]
- Harder, J.M.; Williams, P.A.; Braine, C.E.; Yang, H.S.; Thomas, J.M.; Foxworth, N.E.; John, S.W.M.; Howell, G.R. Complement Peptide C3a Receptor 1 Promotes Optic Nerve Degeneration in DBA/2J Mice. J. Neuroinflamm. 2020, 17, 336. [Google Scholar] [CrossRef]
- He, Z.; Vingrys, A.J.; Armitage, J.A.; Bui, B.V. The Role of Blood Pressure in Glaucoma. Clin. Exp. Optom. 2011, 94, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.A.; Jones, M.L.; Bernabeu, M.O.; Geudens, I.; Mathivet, T.; Rosa, A.; Lopes, F.M.; Lima, A.P.; Ragab, A.; Collins, R.T.; et al. Dynamic Endothelial Cell Rearrangements Drive Developmental Vessel Regression. PLoS Biol. 2015, 13, e1002125. [Google Scholar] [CrossRef]
- Rust, R.; Grönnert, L.; Dogançay, B.; Schwab, M.E. A Revised View on Growth and Remodeling in the Retinal Vasculature. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Löfqvist, C.A.; Liegl, R.; Wang, Z.; Sun, Y.; Gong, Y.; Liu, C.; Meng, S.S.; Burnim, S.B.; Arellano, I.; et al. Photoreceptor Glucose Metabolism Determines Normal Retinal Vascular Growth. EMBO Mol. Med. 2018, 10, 76–90. [Google Scholar] [CrossRef]
- Dudiki, T.; Meller, J.; Mahajan, G.; Liu, H.; Zhevlakova, I.; Stefl, S.; Witherow, C.; Podrez, E.; Kothapalli, C.R.; Byzova, T.V. Microglia Control Vascular Architecture via a TGFβ1 Dependent Paracrine Mechanism Linked to Tissue Mechanics. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Fruttiger, M.; Zhu, L.; Chung, S.H.; Barnett, N.L.; Kirk, J.K.; Lee, S.R.; Coorey, N.J.; Killingsworth, M.; Sherman, L.S.; et al. Conditional Müller Cell Ablation Causes Independent Neuronal and Vascular Pathologies in a Novel Transgenic Model. J. Neurosci. 2012, 32, 15715–15727. [Google Scholar] [CrossRef]
- Franco, C.A.; Blanc, J.; Parlakian, A.; Blanco, R.; Aspalter, I.M.; Kazakova, N.; Diguet, N.; Mylonas, E.; Gao-Li, J.; Vaahtokari, A.; et al. SRF Selectively Controls Tip Cell Invasive Behavior in Angiogenesis. Development 2013, 140, 2321–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Kim, S.A.; Choi, Y.A.; Park, D.Y.; Lee, J. Alpha-Smooth Muscle Actin-Positive Perivascular Cells in Diabetic Retina and Choroid. Int. J. Mol. Sci. 2020, 21, 2158. [Google Scholar] [CrossRef] [Green Version]
- Halai, K.; Whiteford, J.; Ma, B.; Nourshargh, S.; Woodfin, A. ICAM-2 Facilitates Luminal Interactions between Neutrophils and Endothelial Cells in Vivo. J. Cell Sci. 2014, 127, 620–629. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Perovic, T.; Fechner, I.; Edgar, L.T.; Hoskins, P.R.; Gerhardt, H.; Krüger, T.; Bernabeu, M.O. Association between Erythrocyte Dynamics and Vessel Remodelling in Developmental Vascular Networks. J. R. Soc. Interface 2021, 18, 20210113. [Google Scholar] [CrossRef]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight Junction Proteins at the Blood–Brain Barrier: Far More than Claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002. [Google Scholar] [CrossRef]
- Scott, A.; Powner, M.B.; Fruttiger, M. Quantification of Vascular Tortuosity as an Early Outcome Measure in Oxygen Induced Retinopathy (OIR). Exp. Eye Res. 2014, 120, 55–60. [Google Scholar] [CrossRef]
- Tual-chalot, S.; Mahmoud, M.; Allinson, K.R.; Redgrave, R.E.; Zhai, Z.; Oh, S.P.; Fruttiger, M.; Arthur, H.M. Endothelial Depletion of Acvrl1 in Mice Leads to Arteriovenous Malformations Associated with Reduced Endoglin Expression. PLoS ONE 2014, 9, e98646. [Google Scholar] [CrossRef] [Green Version]
- Rabelink, T.J.; Lebrin, F. Thresholds of Endoglin Expression in Endothelial Cells Explains Vascular Etiology in Hereditary Hemorrhagic Telangiectasia Type 1. Int. J. Mol. Sci. 2021, 22, 8948. [Google Scholar] [CrossRef]
- Barnett, J.M.; Suarez, S.; McCollum, G.W.; Penn, J.S. Endoglin Promotes Angiogenesis in Cell- and Animal-Based Models of Retinal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6490–6498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 Controls Endothelial Adherens Junctions, Cell–Cell Tension, Angiogenesis, and Barrier Formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.V.; Birdsey, G.M.; Peghaire, C.; Pitulescu, M.E.; Dufton, N.P.; Yang, Y.; Weinberg, I.; Osuna Almagro, L.; Payne, L.; Mason, J.C.; et al. The Endothelial Transcription Factor ERG Mediates Angiopoietin-1-Dependent Control of Notch Signalling and Vascular Stability. Nat. Commun. 2017, 8, 16002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Li, T.; Luo, Y.; Yu, H.; Sun, Y.; Zhou, H.; Liang, X.; Huang, J.; Tang, S. Retro-Orbital Injection of FITC-Dextran Is an Effective and Economical Method for Observing Mouse Retinal Vessels. Mol. Vis. 2011, 17, 3566–3573. [Google Scholar]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of Blood Vessels and Tissues by a Population of Monocytes with Patrolling Behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef] [Green Version]
- Di Giovanna, A.P.; Tibo, A.; Silvestri, L.; Müllenbroich, M.C.; Costantini, I.; Allegra Mascaro, A.L.; Sacconi, L.; Frasconi, P.; Pavone, F.S. Whole-Brain Vasculature Reconstruction at the Single Capillary Level. Sci. Rep. 2018, 8, 12573. [Google Scholar] [CrossRef] [Green Version]
- Alliot, F.; Rutin, J.; Pessac, B. Ly-6C Is Expressed in Brain Vessels Endothelial Cells but Not in Microgila of the Mouse. Neurosci. Lett. 1998, 251, 37–40. [Google Scholar] [CrossRef]
- Trost, A.; Motloch, K.; Bruckner, D.; Schroedl, F.; Bogner, B.; Kaser-Eichberger, A.; Runge, C.; Strohmaier, C.; Klein, B.; Aigner, L.; et al. Time-Dependent Retinal Ganglion Cell Loss, Microglial Activation and Blood-Retina-Barrier Tightness in an Acute Model of Ocular Hypertension. Exp. Eye Res. 2015, 136, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Duijvestijn, A.M.; van Goor, H.; Klatter, F.; Majoor, G.D.; van Bussel, E.; van Breda Vriesman, P.J. Antibodies Defining Rat Endothelial Cells: RECA-1, a Pan-Endothelial Cell-Specific Monoclonal Antibody. Lab. Investig. 1992, 66, 459–466. [Google Scholar] [PubMed]
- Alkhani, A.; Levy, C.S.; Tsui, M.; Rosenberg, K.A.; Polovina, K.; Mattis, A.N.; Mack, M.; Van Dyken, S.; Wang, B.M.; Maher, J.J.; et al. Ly6cLo Non-Classical Monocytes Promote Resolution of Rhesus Rotavirus-Mediated Perinatal Hepatic Inflammation. Sci. Rep. 2020, 10, 7165. [Google Scholar] [CrossRef]
- Teh, Y.C.; Ding, J.L.; Ng, L.G.; Chong, S.Z. Capturing the Fantastic Voyage of Monocytes Through Time and Space. Front. Immunol. 2019, 10, 834. [Google Scholar] [CrossRef]
- Rovere, G.; Nadal-Nicolás, F.M.; Wang, J.; Bernal-Garro, J.M.; García-Carrillo, N.; Villegas-Pérez, M.P.; Agudo-Barriuso, M.; Vidal-Sanz, M. Melanopsin-Containing or Non-Melanopsin–Containing Retinal Ganglion Cells Response to Acute Ocular Hypertension With or Without Brain-Derived Neurotrophic Factor Neuroprotection. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6652–6661. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Valiente-Soriano, F.J.; Nadal-Nicolás, F.M.; Rovere, G.; Chen, S.; Huang, W.; Agudo-Barriuso, M.; Jonas, J.B.; Vidal-Sanz, M.; Zhang, X. MicroRNA Regulation in an Animal Model of Acute Ocular Hypertension. Acta Ophthalmol. 2017, 95, e10–e21. [Google Scholar] [CrossRef] [Green Version]
- Gallego-Ortega, A.; Norte-Muñoz, M.; Miralles de Imperial-Ollero, J.A.; Bernal-Garro, J.M.; Valiente-Soriano, F.J.; de la Villa Polo, P.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Functional and Morphological Alterations in a Glaucoma Model of Acute Ocular Hypertension. Prog. Brain Res. 2020, 256, 1–29. [Google Scholar] [CrossRef]
- Honda, M.; Surewaard, B.G.J.; Watanabe, M.; Hedrick, C.C.; Lee, W.-Y.; Brown, K.; McCoy, K.D.; Kubes, P. Perivascular Localization of Macrophages in the Intestinal Mucosa Is Regulated by Nr4a1 and the Microbiome. Nat. Commun. 2020, 11, 1329. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roussel, M.F.; Rettenmier, C.W. Colony-Stimulating Factor-1 Receptor (c-Fms). J. Cell. Biochem. 1988, 38, 179–187. [Google Scholar] [CrossRef]
- Hallmann, R.; Horn, N.; Selg, M.; Wendler, O.; Pausch, F.; Sorokin, L.M. Expression and Function of Laminins in the Embryonic and Mature Vasculature. Physiol. Rev. 2005, 85, 979–1000. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.B.; Lynch, J.J.; Cooper, J.A. Endothelial Barrier Function. J. Investig. Dermatol. 1989, 93, 62S–67S. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Smith, F.M.; Watson, L.; Lu, D.Y.; Goldstein, I. Griffonia Simplicifolia I: Fluorescent Tracer for Microcirculatory Vessels in Nonperfused Thin Muscles and Sectioned Muscle. Microvasc. Res. 1988, 36, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Di Pierdomenico, J.; García-Ayuso, D.; Pinilla, I.; Cuenca, N.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Villegas-Pérez, M.P. Early Events in Retinal Degeneration Caused by Rhodopsin Mutation or Pigment Epithelium Malfunction: Differences and Similarities. Front. Neuroanat. 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitulescu, M.E.; Schmidt, I.; Benedito, R.; Adams, R.H. Inducible Gene Targeting in the Neonatal Vasculature and Analysis of Retinal Angiogenesis in Mice. Nat. Protoc. 2010, 5, 1518–1534. [Google Scholar] [CrossRef]
- Selvam, S.; Kumar, T.; Fruttiger, M. Retinal Vasculature Development in Health and Disease. Prog. Retin. Eye Res. 2018, 63, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.L.; Amato, R.; Lazzara, F.; Porciatti, V.; Chou, T.-H.; Drago, F.; Bucolo, C. P2X7 Receptor Antagonism Preserves Retinal Ganglion Cells in Glaucomatous Mice. Biochem. Pharmacol. 2020, 180, 114199. [Google Scholar] [CrossRef]
- Vidal-Sanz, M.; Valiente-Soriano, F.J.; Ortín-Martínez, A.; Nadal-Nicolás, F.M.; Jiménez-López, M.; Salinas-Navarro, M.; Alarcón-Martínez, L.; García-Ayuso, D.; Avilés-Trigueros, M.; Agudo-Barriuso, M.; et al. Retinal Neurodegeneration in Experimental Glaucoma. Prog. Brain Res. 2015, 220, 1–35. [Google Scholar] [PubMed]
- Sarvari, S.; Moakedi, F.; Hone, E.; Simpkins, J.W.; Ren, X. Mechanisms in Blood-Brain Barrier Opening and Metabolism-Challenged Cerebrovascular Ischemia with Emphasis on Ischemic Stroke. Metab. Brain Dis. 2020, 35, 851–868. [Google Scholar] [CrossRef]
- Gariano, R.F.; Gardner, T.W. Retinal Angiogenesis in Development and Disease. Nature 2005, 438, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Ruiz, F.; Galindo-Romero, C.; Albaladejo-García, V.; Vidal-Sanz, M.; Agudo-Barriuso, M. Mechanisms Implicated in the Contralateral Effect in the Central Nervous System after Unilateral Injury: Focus on the Visual System. Neural Regen. Res. 2021, 16, 2125–2131. [Google Scholar] [CrossRef]
- Valiente-Soriano, F.J.; Salinas-Navarro, M.; Jiménez-López, M.; Alarcón-Martínez, L.; Ortín-Martínez, A.; Bernal-Garro, J.M.; Avilés-Trigueros, M.; Agudo-Barriuso, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Effects of Ocular Hypertension in the Visual System of Pigmented Mice. PLoS ONE 2015, 10, e0121134. [Google Scholar] [CrossRef]
- Dekeyster, E.; Aerts, J.; Valiente-Soriano, F.J.; De Groef, L.; Vreysen, S.; Salinas-Navarro, M.; Vidal-Sanz, M.; Arckens, L.; Moons, L. Ocular Hypertension Results in Retinotopic Alterations in the Visual Cortex of Adult Mice. Curr. Eye Res. 2015, 40, 1269–1283. [Google Scholar] [CrossRef]
- Lucas-Ruiz, F.; Galindo-Romero, C.; Rodríguez-Ramírez, K.T.; Vidal-Sanz, M.; Agudo-Barriuso, M. Neuronal Death in the Contralateral Un-Injured Retina after Unilateral Axotomy: Role of Microglial Cells. Int. J. Mol. Sci. 2019, 20, 5733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, A.I.; Fernández-Albarral, J.A.; de Hoz, R.; López-Cuenca, I.; Salobrar-García, E.; Rojas, P.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; et al. Microglial Changes in the Early Aging Stage in a Healthy Retina and an Experimental Glaucoma Model. Prog. Brain Res. 2020, 256, 125–149. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Albarral, J.A.; Salazar, J.J.; de Hoz, R.; Marco, E.M.; Martín-Sánchez, B.; Flores-Salguero, E.; Salobrar-García, E.; López-Cuenca, I.; Barrios-Sabador, V.; Avilés-Trigueros, M.; et al. Retinal Molecular Changes Are Associated with Neuroinflammation and Loss of RGCs in an Experimental Model of Glaucoma. Int. J. Mol. Sci. 2021, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Di Pierdomenico, J.; García-Ayuso, D.; Jiménez-López, M.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Different Ipsi- and Contralateral Glial Responses to Anti-VEGF and Triamcinolone Intravitreal Injections in Rats. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3533–3544. [Google Scholar] [CrossRef]
- González-Riquelme, M.J.; Galindo-Romero, C.; Lucas-Ruiz, F.; Martínez-Carmona, M.; Rodríguez-Ramírez, K.T.; Cabrera-Maqueda, J.M.; Norte-Muñoz, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Axonal Injuries Cast Long Shadows: Long Term Glial Activation in Injured and Contralateral Retinas after Unilateral Axotomy. Int. J. Mol. Sci. 2021, 22, 8517. [Google Scholar] [CrossRef]
- Sánchez-Migallón, M.C.; Valiente-Soriano, F.J.; Salinas-Navarro, M.; Nadal-Nicolás, F.M.; Jiménez-López, M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Nerve Fibre Layer Degeneration and Retinal Ganglion Cell Loss Long Term after Optic Nerve Crush or Transection in Adult Mice. Exp. Eye Res. 2018, 170, 40–50. [Google Scholar] [CrossRef]
- Galindo-Romero, C.; Valiente-Soriano, F.J.; Jiménez-López, M.; García-Ayuso, D.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Effect of Brain-Derived Neurotrophic Factor on Mouse Axotomized Retinal Ganglion Cells and Phagocytic Microglia. Investig. Ophthalmol. Vis. Sci. 2013, 54, 974–985. [Google Scholar] [CrossRef] [Green Version]
- Valiente-Soriano, F.J.; Nadal-Nicolás, F.M.; Salinas-Navarro, M.; Jiménez-López, M.; Bernal-Garro, J.M.; Villegas-Pérez, M.P.; Agudo-Barriuso, M.; Vidal-Sanz, M. BDNF Rescues RGCs But Not Intrinsically Photosensitive RGCs in Ocular Hypertensive Albino Rat Retinas. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1924–1936. [Google Scholar] [CrossRef] [Green Version]
- Shenker, N.; Haigh, R.; Roberts, E.; Mapp, P.; Harris, N.; Blake, D. A Review of Contralateral Responses to a Unilateral Inflammatory Lesion. Rheumatology 2003, 42, 1279–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, A.I.; Salazar, J.J.; de Hoz, R.; Rojas, B.; Gallego, B.I.; Salinas-Navarro, M.; Alarcón-Martínez, L.; Ortín-Martínez, A.; Avilés-Trigueros, M.; Vidal-Sanz, M.; et al. Quantification of the Effect of Different Levels of IOP in the Astroglia of the Rat Retina Ipsilateral and Contralateral to Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5690–5696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Hoz, R.; Gallego, B.I.; Ramírez, A.I.; Rojas, B.; Salazar, J.J.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Triviño, A.; et al. Rod-like Microglia Are Restricted to Eyes with Laser-Induced Ocular Hypertension but Absent from the Microglial Changes in the Contralateral Untreated Eye. PLoS ONE 2013, 8, e83733. [Google Scholar] [CrossRef] [Green Version]




| Marker | Target | Characteristics | References |
|---|---|---|---|
| Isolectin-IB4 | Terminal α-d-galactosyl residues | Binds to blood vessels and to activated microglial cells | [5,11,12,13,14,15,16] |
| CD31 (cluster of differentiation 31) also known as PECAM-1 (platelet endothelial cell adhesion molecule) | Adhesion molecule that constitutes a large part of the intercellular junctions of endothelial cells | It is found on endothelial cells, platelets, Kupffer cells, macrophages, granulocytes, lymphocytes, megakaryocytes, osteoclasts, and in certain tumors | [12,16,17,18] |
| ICAM2 (intercellular adhesion molecule 2) also known as CD102 (cluster of differentiation 102) | Type I transmembrane glycoprotein present in the apical/luminal endothelial cell membrane | ICAM2 masks highlight vessel segments undergoing remodeling. It mediates adhesive interactions important for antigen-specific immune response | [11,18,19] |
| CLDN5 (claudin 5) | It is one of the six high abundant tight junction proteins in the blood–brain barrier in vivo and the dominant one in vitro | Transiently expressed in the retinal pigment epithelium (RPE) during development, where its expression correlates with permeability changes in the developing RPE | [12,20] |
| ColIV or Col4 (collagen IV) | One of the main components of the basement membrane (BM), a specialized extracellular matrix that compartmentalizes tissues, provides structural support, and influences cell behavior and signaling | Collagen IV is the most abundant structural BM component and is essential for BM integrity but not initial BM formation | [11,19,21] |
| Endoglin (ENG) also known as CD105 | Transmembrane glycoprotein that functions as a coreceptor for ligands of the transforming growth factor-β superfamily. It is predominantly expressed by activated endothelial cells | It is a facilitator of ligand binding and has a crucial role in angiogenesis. It is also a marker of mesenchymal stem cells and it is expressed in progenitor cells involved in vascular remodeling in animal models | [22,23,24] |
| ZO-1 (zonula occludens-1) also known as TJP1 (tight junction protein-1) | One of the proteins that create intercellular boundaries between the plasma membrane domains of epithelial and endothelial cells (endothelial cell–cell junctions) | Is thought to have both structural and signaling roles. It can also associate with claudin, occludin, and F-actin, at tight junction stands, where it provides a linkage between the actin cytoskeleton and the tight junction | [11,16,25] |
| CDH5 (cadherin 5) also known as VE-cadherin | It is a strictly endothelial specific adhesion molecule located at junctions between endothelial cells and promotes homotypic cell-to-cell interaction | It is vital for the maintenance and control of endothelial cell contacts. It is relevant for the control of vascular permeability and leukocyte extravasation and regulates various cellular processes such as cell proliferation and apoptosis and modulates vascular endothelial growth factor receptor functions. It is essential during embryonic angiogenesis | [11,16] |
| Erg (ETS-related gene) | It is expressed in the nuclei of endothelial cells | It is a transcription factor that has been linked to angiogenesis and to the promotion of vascular stability | [11,26] |
| Dextran-fluorophore (complex branched glucan labelled with a fluorophore) | Blood vessel lumen | Used for intravascular perfusion. High-molecular-weight FITC-dextran remains in the vasculature without diffusion | [27,28] |
| gel-BSA-FITC (gel-bovine serum albumin-fluorescein isothiocyanate) | Blood vessel lumen | Used for intravascular perfusion. The high molecular weight of albumin prevents the marker from crossing blood vessel walls, which ensures the confinement of the fluorescent signal within the blood vessels | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Carmona, M.; Lucas-Ruiz, F.; Gallego-Ortega, A.; Galindo-Romero, C.; Norte-Muñoz, M.; González-Riquelme, M.J.; Valiente-Soriano, F.J.; Vidal-Sanz, M.; Agudo-Barriuso, M. Ly6c as a New Marker of Mouse Blood Vessels: Qualitative and Quantitative Analyses on Intact and Ischemic Retinas. Int. J. Mol. Sci. 2022, 23, 19. https://doi.org/10.3390/ijms23010019
Martínez-Carmona M, Lucas-Ruiz F, Gallego-Ortega A, Galindo-Romero C, Norte-Muñoz M, González-Riquelme MJ, Valiente-Soriano FJ, Vidal-Sanz M, Agudo-Barriuso M. Ly6c as a New Marker of Mouse Blood Vessels: Qualitative and Quantitative Analyses on Intact and Ischemic Retinas. International Journal of Molecular Sciences. 2022; 23(1):19. https://doi.org/10.3390/ijms23010019
Chicago/Turabian StyleMartínez-Carmona, Marina, Fernando Lucas-Ruiz, Alejandro Gallego-Ortega, Caridad Galindo-Romero, María Norte-Muñoz, María José González-Riquelme, Francisco J. Valiente-Soriano, Manuel Vidal-Sanz, and Marta Agudo-Barriuso. 2022. "Ly6c as a New Marker of Mouse Blood Vessels: Qualitative and Quantitative Analyses on Intact and Ischemic Retinas" International Journal of Molecular Sciences 23, no. 1: 19. https://doi.org/10.3390/ijms23010019
APA StyleMartínez-Carmona, M., Lucas-Ruiz, F., Gallego-Ortega, A., Galindo-Romero, C., Norte-Muñoz, M., González-Riquelme, M. J., Valiente-Soriano, F. J., Vidal-Sanz, M., & Agudo-Barriuso, M. (2022). Ly6c as a New Marker of Mouse Blood Vessels: Qualitative and Quantitative Analyses on Intact and Ischemic Retinas. International Journal of Molecular Sciences, 23(1), 19. https://doi.org/10.3390/ijms23010019








