Glial Cell Activation and Oxidative Stress in Retinal Degeneration Induced by β-Alanine Caused Taurine Depletion and Light Exposure
Abstract
:1. Introduction
2. Results
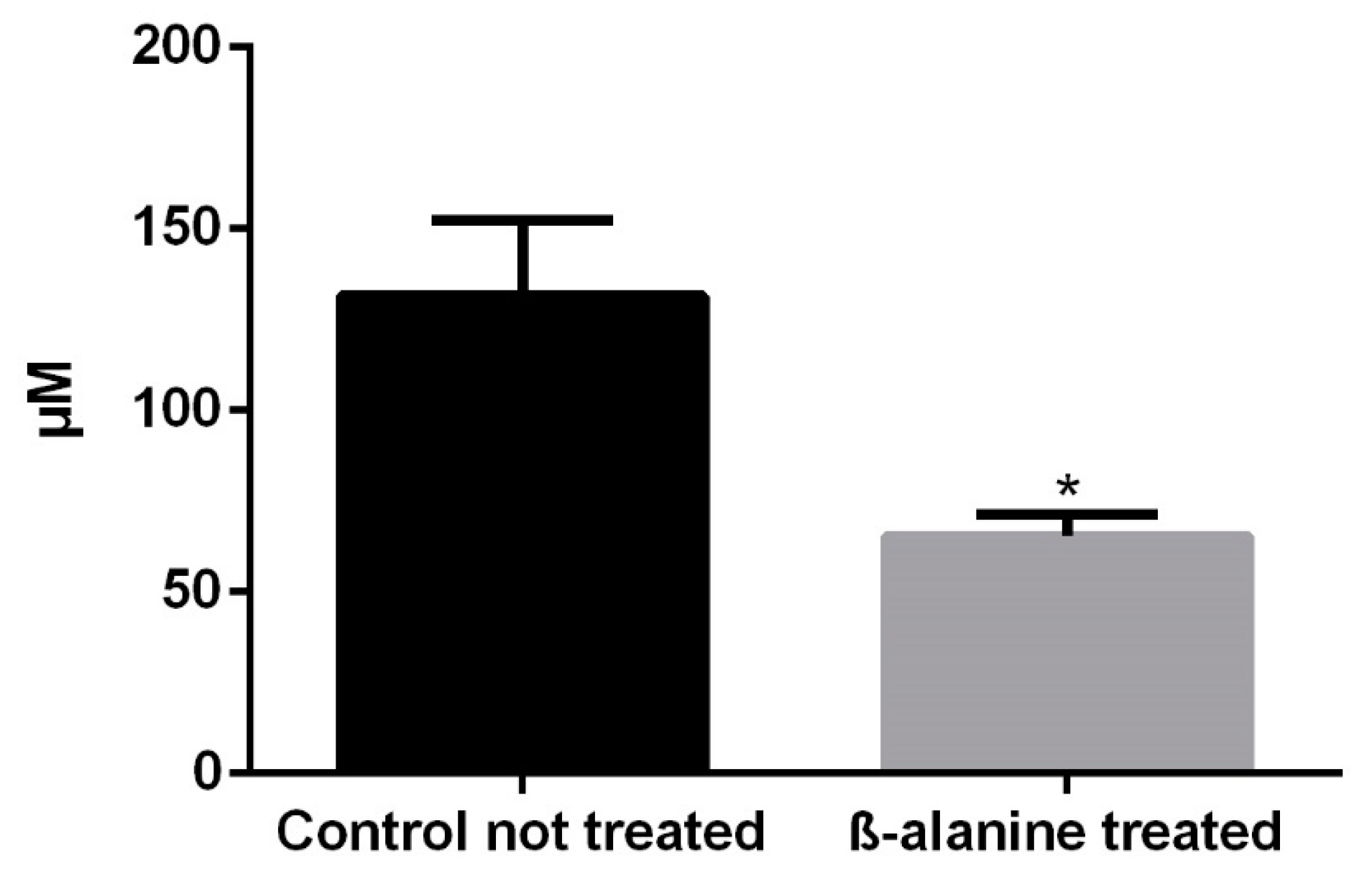
2.1. Taurine Plasma Levels
2.2. SD-OCT
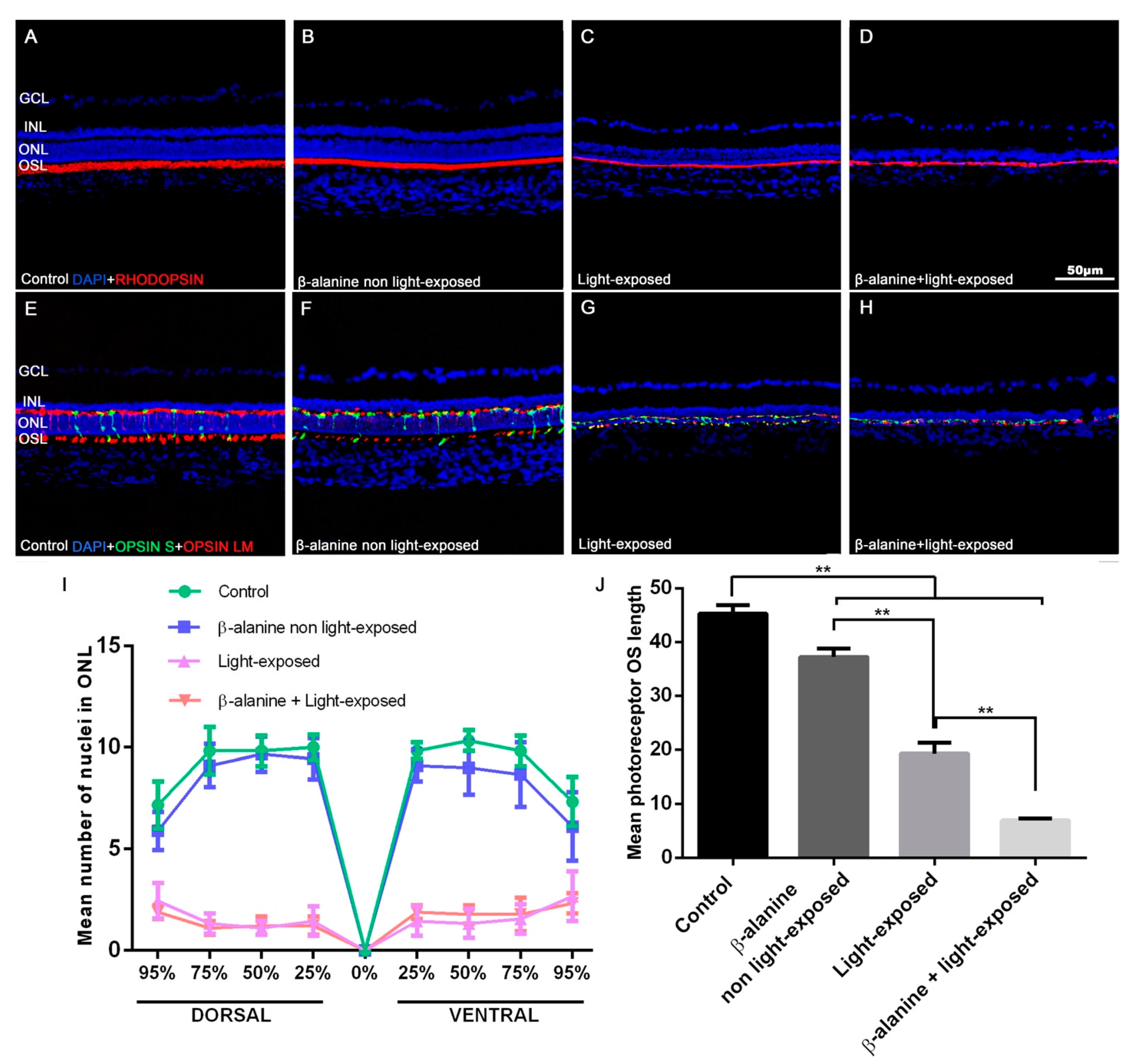
2.3. Outer Nuclear Layer Thickness, Photoreceptor Survival and Morphology
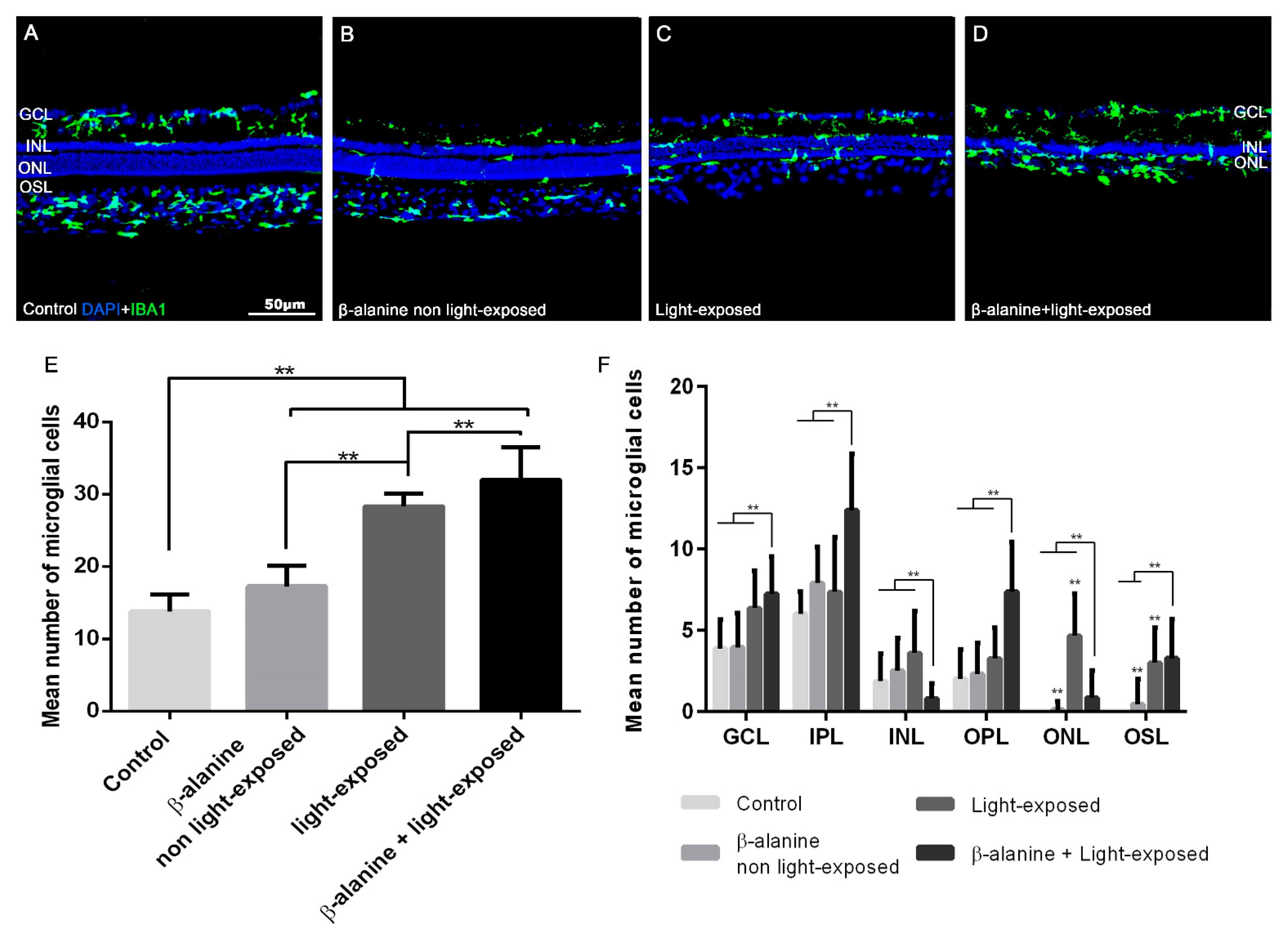
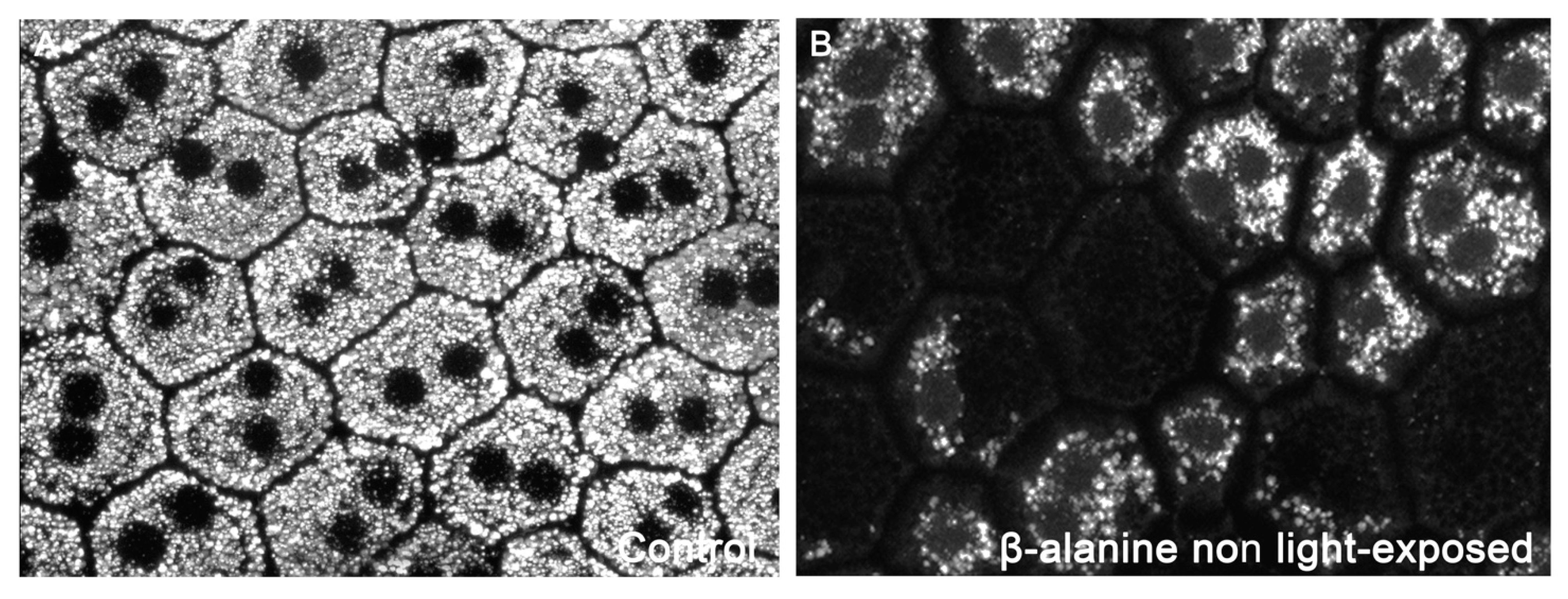
2.4. Microglial Cell Numbers
2.5. Macroglial Cell Reactivity
2.6. Oxidative Stress
2.7. Synaptic Connections
2.8. Retinal Pigment Epithelium Function and Morphology
3. Discussion
3.1. Photoreceptor Outer Segment Degeneration Caused by Taurine Depletion Is Exacerbated by Light Exposure
3.2. Glial Cell Activation Due to Taurine Depletion Is Exacerbated by Light Exposure
4. Materials and Methods
4.1. Animal Handling
4.2. Light Exposure
4.3. Analysis of Taurine Plasma Levels
4.4. Spectral-Domain Optical Coherence Tomography
4.5. Retinal Pigment Epithelium Labeling
4.6. Tissue Processing and Immunohistofluorescence
4.6.1. Primary Antibodies
4.6.2. Secondary Antibodies
4.7. Image Analysis of Retinal Cross Sections and Quantification of Photoreceptors and Microglial Cells
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Froger, N.; Moutsimilli, L.; Cadetti, L.; Jammoul, F.; Wang, Q.P.; Fan, Y.; Gaucher, D.; Rosolen, S.G.; Neveux, N.; Cynober, L.; et al. Taurine: The comeback of a neutraceutical in the prevention of retinal degenerations. Prog. Retin. Eye Res. 2014, 41, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Castelli, V.; Paladini, A.; d’Angelo, M.; Allegretti, M.; Mantelli, F.; Brandolini, L.; Cocchiaro, P.; Cimini, A.; Varrassi, G. Taurine and oxidative stress in retinal health and disease. CNS Neurosci. Ther. 2021, 27, 403–412. [Google Scholar] [CrossRef]
- Rascher, K.; Servos, G.; Berthold, G.; Hartwig, H.G.; Warskulat, U.; Heller-Stilb, B.; Haussinger, D. Light deprivation slows but does not prevent the loss of photoreceptors in taurine transporter knockout mice. Vision Res. 2004, 44, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ayuso, D.; Di Pierdomenico, J.; Hadj-Said, W.; Marie, M.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Picaud, S.; Villegas-Perez, M.P. Taurine Depletion Causes ipRGC Loss and Increases Light-Induced Photoreceptor Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Said, W.; Froger, N.; Ivkovic, I.; Jimenez-Lopez, M.; Dubus, E.; Degardin-Chicaud, J.; Simonutti, M.; Quenol, C.; Neveux, N.; Villegas-Perez, M.P.; et al. Quantitative and Topographical Analysis of the Losses of Cone Photoreceptors and Retinal Ganglion Cells Under Taurine Depletion. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4692–4703. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Ayuso, D.; Di Pierdomenico, J.; Valiente-Soriano, F.J.; Martinez-Vacas, A.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Picaud, S.; Villegas-Perez, M.P. beta-alanine supplementation induces taurine depletion and causes alterations of the retinal nerve fiber layer and axonal transport by retinal ganglion cells. Exp. Eye Res. 2019, 188, 107781. [Google Scholar] [CrossRef] [PubMed]
- Lake, N.; Malik, N. Retinal morphology in rats treated with a taurine transport antagonist. Exp. Eye Res. 1987, 44, 331–346. [Google Scholar] [CrossRef]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar]
- Preising, M.N.; Gorg, B.; Friedburg, C.; Qvartskhava, N.; Budde, B.S.; Bonus, M.; Toliat, M.R.; Pfleger, C.; Altmuller, J.; Herebian, D.; et al. Biallelic mutation of human SLC6A6 encoding the taurine transporter TAUT is linked to early retinal degeneration. FASEB J. 2019, 33, 11507–11527. [Google Scholar] [CrossRef] [PubMed]
- Ansar, M.; Ranza, E.; Shetty, M.; Paracha, S.A.; Azam, M.; Kern, I.; Iwaszkiewicz, J.; Farooq, O.; Pournaras, C.J.; Malcles, A.; et al. Taurine treatment of retinal degeneration and cardiomyopathy in a consanguineous family with SLC6A6 taurine transporter deficiency. Hum. Mol. Genet. 2020, 29, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yang, J.; Ma, Z.; Yan, Z.; Liu, C.; Ma, J.; Wang, Y.; Yang, Z.; Huang, Y.F. The Vigabatrin Induced Retinal Toxicity is Associated with Photopic Exposure and Taurine Deficiency: An In Vivo Study. Cell Physiol. Biochem. 2016, 40, 831–846. [Google Scholar] [CrossRef]
- Jammoul, F.; Wang, Q.; Nabbout, R.; Coriat, C.; Duboc, A.; Simonutti, M.; Dubus, E.; Craft, C.M.; Ye, W.; Collins, S.D.; et al. Taurine deficiency is a cause of vigabatrin-induced retinal phototoxicity. Ann. Neurol. 2009, 65, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef]
- Trouillet, A.; Dubus, E.; Degardin, J.; Estivalet, A.; Ivkovic, I.; Godefroy, D.; Garcia-Ayuso, D.; Simonutti, M.; Sahly, I.; Sahel, J.A.; et al. Cone degeneration is triggered by the absence of USH1 proteins but prevented by antioxidant treatments. Sci. Rep. 2018, 8, 1968. [Google Scholar] [CrossRef] [PubMed]
- Micera, A.; Balzamino, B.O.; Di Zazzo, A.; Dinice, L.; Bonini, S.; Coassin, M. Biomarkers of Neurodegeneration and Precision Therapy in Retinal Disease. Front. Pharmacol. 2020, 11, 601647. [Google Scholar] [CrossRef] [PubMed]
- Militante, J.; Lombardini, J.B. Age-related retinal degeneration in animal models of aging: Possible involvement of taurine deficiency and oxidative stress. Neurochem. Res. 2004, 29, 151–160. [Google Scholar] [CrossRef]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuenca, N.; Fernandez-Sanchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; Garcia-Ayuso, D.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Role of microglial cells in photoreceptor degeneration. Neural. Regen. Res. 2019, 14, 1186–1190. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; Garcia-Ayuso, D.; Pinilla, I.; Cuenca, N.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Villegas-Perez, M.P. Early Events in Retinal Degeneration Caused by Rhodopsin Mutation or Pigment Epithelium Malfunction: Differences and Similarities. Front. Neuroanat. 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pierdomenico, J.; Martinez-Vacas, A.; Hernandez-Munoz, D.; Gomez-Ramirez, A.M.; Valiente-Soriano, F.J.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Perez, M.P.; Garcia-Ayuso, D. Coordinated Intervention of Microglial and Muller Cells in Light-Induced Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogino, N.; Matsumura, M.; Shirakawa, H.; Tsukahara, I. Phagocytic activity of cultured retinal pigment epithelial cells from chick embryo: Inhibition by melatonin and cyclic AMP, and its reversal by taurine and cyclic GMP. Ophthalmic Res. 1983, 15, 72–89. [Google Scholar] [CrossRef]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Tucker, J.; Marc, R.E. Retinal Remodeling and Metabolic Alterations in Human AMD. Front. Cell Neurosci. 2016, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Okada, M.; Okuma, Y.; Osumi, Y.; Nishihara, M.; Yokotani, K.; Ueno, H. Neurotransmitter contents in the retina of RCS rat. Graefes. Arch. Clin. Exp. Ophthalmol. 2000, 238, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Audo, I.; Mohand-Said, S.; Boulanger-Scemama, E.; Zanlonghi, X.; Condroyer, C.; Demontant, V.; Boyard, F.; Antonio, A.; Mejecase, C.; El Shamieh, S.; et al. MERTK mutation update in inherited retinal diseases. Hum. Mutat. 2018, 39, 887–913. [Google Scholar] [CrossRef]
- D’Cruz, P.M.; Yasumura, D.; Weir, J.; Matthes, M.T.; Abderrahim, H.; LaVail, M.M.; Vollrath, D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000, 9, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Ayuso, D.; Di Pierdomenico, J.; Vidal-Sanz, M.; Villegas-Perez, M.P. Retinal Ganglion Cell Death as a Late Remodeling Effect of Photoreceptor Degeneration. Int. J. Mol. Sci. 2019, 20, 4649. [Google Scholar] [CrossRef] [Green Version]
- Di Pierdomenico, J.; Garcia-Ayuso, D.; Rodriguez Gonzalez-Herrero, M.E.; Garcia-Bernal, D.; Blanquer, M.; Bernal-Garro, J.M.; Garcia-Hernandez, A.M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Bone Marrow-Derived Mononuclear Cell Transplants Decrease Retinal Gliosis in Two Animal Models of Inherited Photoreceptor Degeneration. Int. J. Mol. Sci. 2020, 21, 7252. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; Scholz, R.; Valiente-Soriano, F.J.; Sanchez-Migallon, M.C.; Vidal-Sanz, M.; Langmann, T.; Agudo-Barriuso, M.; Garcia-Ayuso, D.; Villegas-Perez, M.P. Neuroprotective Effects of FGF2 and Minocycline in Two Animal Models of Inherited Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4392–4403. [Google Scholar] [CrossRef] [Green Version]
- Noailles, A.; Maneu, V.; Campello, L.; Gomez-Vicente, V.; Lax, P.; Cuenca, N. Persistent inflammatory state after photoreceptor loss in an animal model of retinal degeneration. Sci. Rep. 2016, 6, 33356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanos, S. Sick photoreceptors attract activated microglia from the ganglion cell layer: A model to study the inflammatory cascades in rats with inherited retinal dystrophy. Brain Res. 1992, 588, 21–28. [Google Scholar] [CrossRef]
- Valiente-Soriano, F.J.; Ortin-Martinez, A.; Di Pierdomenico, J.; Garcia-Ayuso, D.; Gallego-Ortega, A.; Miralles de Imperial-Ollero, J.A.; Jimenez-Lopez, M.; Villegas-Perez, M.P.; Wheeler, L.A.; Vidal-Sanz, M. Topical Brimonidine or Intravitreal BDNF, CNTF, or bFGF Protect Cones Against Phototoxicity. Transl. Vis. Sci. Technol. 2019, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeiffer, R.L.; Marc, R.E.; Jones, B.W. Persistent remodeling and neurodegeneration in late-stage retinal degeneration. Prog. Retin. Eye Res. 2020, 74, 100771. [Google Scholar] [CrossRef] [PubMed]
- Grotegut, P.; Perumal, N.; Kuehn, S.; Smit, A.; Dick, H.B.; Grus, F.H.; Joachim, S.C. Minocycline reduces inflammatory response and cell death in a S100B retina degeneration model. J. Neuroinflammation 2020, 17, 375. [Google Scholar] [CrossRef]
- Todd, L.; Finkbeiner, C.; Wong, C.K.; Hooper, M.J.; Reh, T.A. Microglia Suppress Ascl1-Induced Retinal Regeneration in Mice. Cell Rep. 2020, 33, 108507. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lai, J.; Yuan, Y.; Wang, L.; Wang, Q.; Yuan, F. Taurine Protects Retinal Cells and Improves Synaptic Connections in Early Diabetic Rats. Curr. Eye Res. 2020, 45, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; He, M.; Yang, Q.; Ma, Z.; Qu, Y.; Chen, W.; Peng, G.; Teng, D. Systemic taurine treatment provides neuroprotection against retinal photoreceptor degeneration and visual function impairments. Drug Des. Devel. Ther. 2019, 13, 2689–2702. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Ayuso, D.; Salinas-Navarro, M.; Agudo-Barriuso, M.; Alarcon-Martinez, L.; Vidal-Sanz, M.; Villegas-Perez, M.P. Retinal ganglion cell axonal compression by retinal vessels in light-induced retinal degeneration. Mol. Vis. 2011, 17, 1716–1733. [Google Scholar]
- Parildar-Karpuzoglu, H.; Dogru-Abbasoglu, S.; Balkan, J.; Aykac-Toker, G.; Uysal, M. Decreases in taurine levels induced by beta-alanine treatment did not affect the susceptibility of tissues to lipid peroxidation. Amino Acids 2007, 32, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Khimsuksri, S.; Wyss, J.M.; Thaeomor, A.; Paphangkorakit, J.; Jirakulsomchok, D.; Roysommuti, S. Perinatal taurine exposure programs patterns of autonomic nerve activity responses to tooth pulp stimulation in adult male rats. Adv. Exp. Med. Biol. 2013, 775, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Roysommuti, S.; Lerdweeraphon, W.; Michael Wyss, J. Perinatal Taurine Imbalance Followed by High Sugar Intake Alters the Effects of Estrogen on Renal Excretory Function in Adult Female Rats. Adv. Exp. Med. Biol. 2017, 2, 769–787. [Google Scholar] [CrossRef]
- Ament, M.E.; Geggel, H.S.; Heckenlively, J.R.; Martin, D.A.; Kopple, J. Taurine supplementation in infants receiving long-term total parenteral nutrition. J. Am. Coll. Nutr. 1986, 5, 127–135. [Google Scholar] [CrossRef]
- Garcia-Ayuso, D.; Di Pierdomenico, J.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death. Neural Regen. Res. 2018, 13, 1885–1886. [Google Scholar] [CrossRef]
- Hou, X.; Sun, G.; Guo, L.; Gong, Z.; Han, Y.; Bai, X. Cardioprotective effect of taurine and β-alanine against cardiac disease in myocardial ischemia and reperfusion-induced rats. Electron. J. Biotechnol. 2020, 45, 46–52. [Google Scholar] [CrossRef]
- Saunders, B.; Elliott-Sale, K.; Artioli, G.G.; Swinton, P.A.; Dolan, E.; Roschel, H.; Sale, C.; Gualano, B. β-alanine supplementation to improve exercise capacity and performance: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Swinton, P.A.; Painelli, V.S.; Stephens Hemingway, B.; Mazzolani, B.; Infante Smaira, F.; Saunders, B.; Artioli, G.G.; Gualano, B. A Systematic Risk Assessment and Meta-Analysis on the Use of Oral β-Alanine Supplementation. Adv. Nutr. 2019, 10, 452–463. [Google Scholar] [CrossRef]
- Sobrado-Calvo, P.; Vidal-Sanz, M.; Villegas-Perez, M.P. Rat retinal microglial cells under normal conditions, after optic nerve section, and after optic nerve section and intravitreal injection of trophic factors or macrophage inhibitory factor. J. Comp. Neurol. 2007, 501, 866–878. [Google Scholar] [CrossRef]
- Li, F.; Jiang, D.; Samuel, M.A. Microglia in the developing retina. Neural Dev. 2019, 14, 12. [Google Scholar] [CrossRef] [Green Version]
- Picard, K.; St-Pierre, M.K.; Vecchiarelli, H.A.; Bordeleau, M.; Tremblay, M.E. Neuroendocrine, neuroinflammatory and pathological outcomes of chronic stress: A story of microglial remodeling. Neurochem. Int. 2021, 145, 104987. [Google Scholar] [CrossRef]
- Garcia-Ayuso, D.; Salinas-Navarro, M.; Agudo, M.; Cuenca, N.; Pinilla, I.; Vidal-Sanz, M.; Villegas-Perez, M.P. Retinal ganglion cell numbers and delayed retinal ganglion cell death in the P23H rat retina. Exp. Eye Res. 2010, 91, 800–810. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.Y.; Berson, E.L.; Watson, G.; Huang, C. Retinal degeneration in cats fed casein. III. Taurine deficiency and ERG amplitudes. Investig. Ophthalmol. Vis. Sci. 1977, 16, 673–678. [Google Scholar]
- Leon, A.; Levick, W.R.; Sarossy, M.G. Lesion topography and new histological features in feline taurine deficiency retinopathy. Exp. Eye Res. 1995, 61, 731–741. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Meadows, N.J.; Keeling, P.W.; Mitchell, W.D.; Thompson, R.P. Rod mediated retinal dysfunction in cats with zinc depletion: Comparison with taurine depletion. Clin. Sci. (Lond.) 1986, 71, 559–564. [Google Scholar] [CrossRef]
- Gaucher, D.; Arnault, E.; Husson, Z.; Froger, N.; Dubus, E.; Gondouin, P.; Dherbecourt, D.; Degardin, J.; Simonutti, M.; Fouquet, S.; et al. Taurine deficiency damages retinal neurones: Cone photoreceptors and retinal ganglion cells. Amino Acids 2012, 43, 1979–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jammoul, F.; Degardin, J.; Pain, D.; Gondouin, P.; Simonutti, M.; Dubus, E.; Caplette, R.; Fouquet, S.; Craft, C.M.; Sahel, J.A.; et al. Taurine deficiency damages photoreceptors and retinal ganglion cells in vigabatrin-treated neonatal rats. Mol. Cell Neurosci. 2010, 43, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szel, A.; Rohlich, P. Two cone types of rat retina detected by anti-visual pigment antibodies. Exp. Eye Res. 1992, 55, 47–52. [Google Scholar] [CrossRef]
- Kutsyr, O.; Sanchez-Saez, X.; Martinez-Gil, N.; de Juan, E.; Lax, P.; Maneu, V.; Cuenca, N. Gradual Increase in Environmental Light Intensity Induces Oxidative Stress and Inflammation and Accelerates Retinal Neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Tejedor, J.; Marchena, M.; Ramírez, L.; García-Ayuso, D.; Gómez-Vicente, V.; Sánchez-Ramos, C.; de la Villa, P.; Germain, F. Removal of the blue component of light significantly decreases retinal damage after high intensity exposure. PLoS ONE 2018, 13, e0194218. [Google Scholar] [CrossRef]
- Marco-Gomariz, M.A.; Hurtado-Montalban, N.; Vidal-Sanz, M.; Lund, R.D.; Villegas-Perez, M.P. Phototoxic-induced photoreceptor degeneration causes retinal ganglion cell degeneration in pigmented rats. J. Comp. Neurol. 2006, 498, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ayuso, D.; Galindo-Romero, C.; Di Pierdomenico, J.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Villegas Perez, M.P. Light-induced retinal degeneration causes a transient downregulation of melanopsin in the rat retina. Exp. Eye Res. 2017, 161, 10–16. [Google Scholar] [CrossRef]
- Cocker, S.E.; Lake, N. Effects of dark maintenance on retinal biochemistry and function during taurine depletion in the adult rat. Vis. Neurosci. 1989, 3, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Quesada, O.; Picones, A.; Pasantes-Morales, H. Effect of light deprivation on the ERG responses of taurine-deficient rats. Exp. Eye Res. 1988, 46, 13–20. [Google Scholar] [CrossRef]
- Rapp, L.M.; Thum, L.A.; Anderson, R.E. Synergism between environmental lighting and taurine depletion in causing photoreceptor cell degeneration. Exp. Eye Res. 1988, 46, 229–238. [Google Scholar] [CrossRef]
- Kim, H.J.; Zhao, J.; Sparrow, J.R. Vitamin A aldehyde-taurine adduct and the visual cycle. Proc. Natl. Acad. Sci. USA 2020, 117, 24867–24875. [Google Scholar] [CrossRef] [PubMed]
- Kjellstrom, U.; Andreasson, S.; Ponjavic, V. Attenuation of the retinal nerve fibre layer and reduced retinal function assessed by optical coherence tomography and full-field electroretinography in patients exposed to vigabatrin medication. Acta Ophthalmol. 2014, 92, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Mackay, M.T.; Weiss, S.K.; Adams-Webber, T.; Ashwal, S.; Stephens, D.; Ballaban-Gill, K.; Baram, T.Z.; Duchowny, M.; Hirtz, D.; Pellock, J.M.; et al. Practice parameter: Medical treatment of infantile spasms: Report of the American Academy of Neurology and the Child Neurology Society. Neurology 2004, 62, 1668–1681. [Google Scholar] [CrossRef] [Green Version]
- Pellock, J.M.; Hrachovy, R.; Shinnar, S.; Baram, T.Z.; Bettis, D.; Dlugos, D.J.; Gaillard, W.D.; Gibson, P.A.; Holmes, G.L.; Nordl, D.R.; et al. Infantile spasms: A U.S. consensus report. Epilepsia 2010, 51, 2175–2189. [Google Scholar] [CrossRef]
- Horvath, G.A.; Hukin, J.; Stockler-Ipsiroglu, S.G.; Aroichane, M. Eye Findings on Vigabatrin and Taurine Treatment in Two Patients with Succinic Semialdehyde Dehydrogenase Deficiency. Neuropediatrics 2016, 47, 263–267. [Google Scholar] [CrossRef]
- Dayang, W.; Dongbo, P. Taurine reduces blue light-induced retinal neuronal cell apoptosis in vitro. Cutan. Ocul. Toxicol. 2018, 37, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Pasantes-Morales, H.; Ademe, R.M.; Quesada, O. Protective effect of taurine on the light-induced disruption of isolated frog rod outer segments. J. Neurosci. Res. 1981, 6, 337–348. [Google Scholar] [CrossRef]
- Pasantes-Morales, H.; Cruz, C. Taurine and hypotaurine inhibit light-induced lipid peroxidation and protect rod outer segment structure. Brain Res. 1985, 330, 154–157. [Google Scholar] [CrossRef]
- Yu, X.; Chen, K.; Wei, N.; Zhang, Q.; Liu, J.; Mi, M. Dietary taurine reduces retinal damage produced by photochemical stress via antioxidant and anti-apoptotic mechanisms in Sprague-Dawley rats. Br. J. Nutr. 2007, 98, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, M.K.; Saraswathy, S.; Parikh, J.G.; Rao, N.A. The role of TLR4 activation in photoreceptor mitochondrial oxidative stress. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5824–5835. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Soriano, F.J.; Salinas-Navarro, M.; Di Pierdomenico, J.; Garcia-Ayuso, D.; Lucas-Ruiz, F.; Pinilla, I.; Cuenca, N.; Vidal-Sanz, M.; Villegas-Perez, M.P.; Agudo-Barriuso, M. Tracing the retina to analyze the integrity and phagocytic capacity of the retinal pigment epithelium. Sci. Rep. 2020, 10, 7273. [Google Scholar] [CrossRef]
- Garcia-Ayuso, D.; Di Pierdomenico, J.; Esquiva, G.; Nadal-Nicolas, F.M.; Pinilla, I.; Cuenca, N.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Villegas-Perez, M.P. Inherited Photoreceptor Degeneration Causes the Death of Melanopsin-Positive Retinal Ganglion Cells and Increases Their Coexpression of Brn3a. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4592–4604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Ayuso, D.; Salinas-Navarro, M.; Nadal-Nicolas, F.M.; Ortin-Martinez, A.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Sectorial loss of retinal ganglion cells in inherited photoreceptor degeneration is due to RGC death. Br. J. Ophthalmol. 2014, 98, 396–401. [Google Scholar] [CrossRef] [Green Version]
- Vugler, A.; Semo, M.; Ortín-Martínez, A.; Rojanasakul, A.; Nommiste, B.; Valiente-Soriano, F.J.; García-Ayuso, D.; Coffey, P.; Vidal-Sanz, M.; Gias, C. A role for the outer retina in development of the intrinsic pupillary light reflex in mice. Neuroscience 2015, 286, 60–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandstatter, J.H.; Fletcher, E.L.; Garner, C.C.; Gundelfinger, E.D.; Wassle, H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur. J. Neurosci. 1999, 11, 3683–3693. [Google Scholar] [CrossRef]
- Che, Y.; Hou, L.; Sun, F.; Zhang, C.; Liu, X.; Piao, F.; Zhang, D.; Li, H.; Wang, Q. Taurine protects dopaminergic neurons in a mouse Parkinson’s disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018, 9, 435. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, L.; Lucius, R.; Roider, J.; Klettner, A. Interaction of inflammatorily activated retinal pigment epithelium with retinal microglia and neuronal cells. Exp. Eye Res. 2020, 199, 108167. [Google Scholar] [CrossRef]
- Murakami, Y.; Nakabeppu, Y.; Sonoda, K.H. Oxidative Stress and Microglial Response in Retinitis Pigmentosa. Int. J. Mol. Sci. 2020, 21, 7170. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kambhampati, S.P.; Bhutto, I.A.; McLeod, D.S.; Lutty, G.A.; Kannan, R.M. Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model. Exp. Eye Res. 2021, 203, 108391. [Google Scholar] [CrossRef]
- Ramirez, A.I.; de Hoz, R.; Fernandez-Albarral, J.A.; Salobrar-Garcia, E.; Rojas, B.; Valiente-Soriano, F.J.; Aviles-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Trivino, A.; et al. Time course of bilateral microglial activation in a mouse model of laser-induced glaucoma. Sci. Rep. 2020, 10, 4890. [Google Scholar] [CrossRef] [Green Version]
- De Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Gallego, B.I.; Trivino, A.; Ramirez, J.M. Retinal Macroglial Responses in Health and Disease. Biomed. Res. Int. 2016, 2016, 2954721. [Google Scholar] [CrossRef] [Green Version]
- Di Pierdomenico, J.; Garcia-Ayuso, D.; Jimenez-Lopez, M.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Different Ipsi- and Contralateral Glial Responses to Anti-VEGF and Triamcinolone Intravitreal Injections in Rats. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3533–3544. [Google Scholar] [CrossRef]
- DiLoreto, D.A., Jr.; Martzen, M.R.; del Cerro, C.; Coleman, P.D.; del Cerro, M. Muller cell changes precede photoreceptor cell degeneration in the age-related retinal degeneration of the Fischer 344 rat. Brain Res. 1995, 698, 1–14. [Google Scholar] [CrossRef]
- Ortin-Martinez, A.; Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Alburquerque-Bejar, J.J.; Nieto-Lopez, L.; Garcia-Ayuso, D.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Number and distribution of mouse retinal cone photoreceptors: Differences between an albino (Swiss) and a pigmented (C57/BL6) strain. PLoS ONE 2014, 9, e102392. [Google Scholar] [CrossRef]
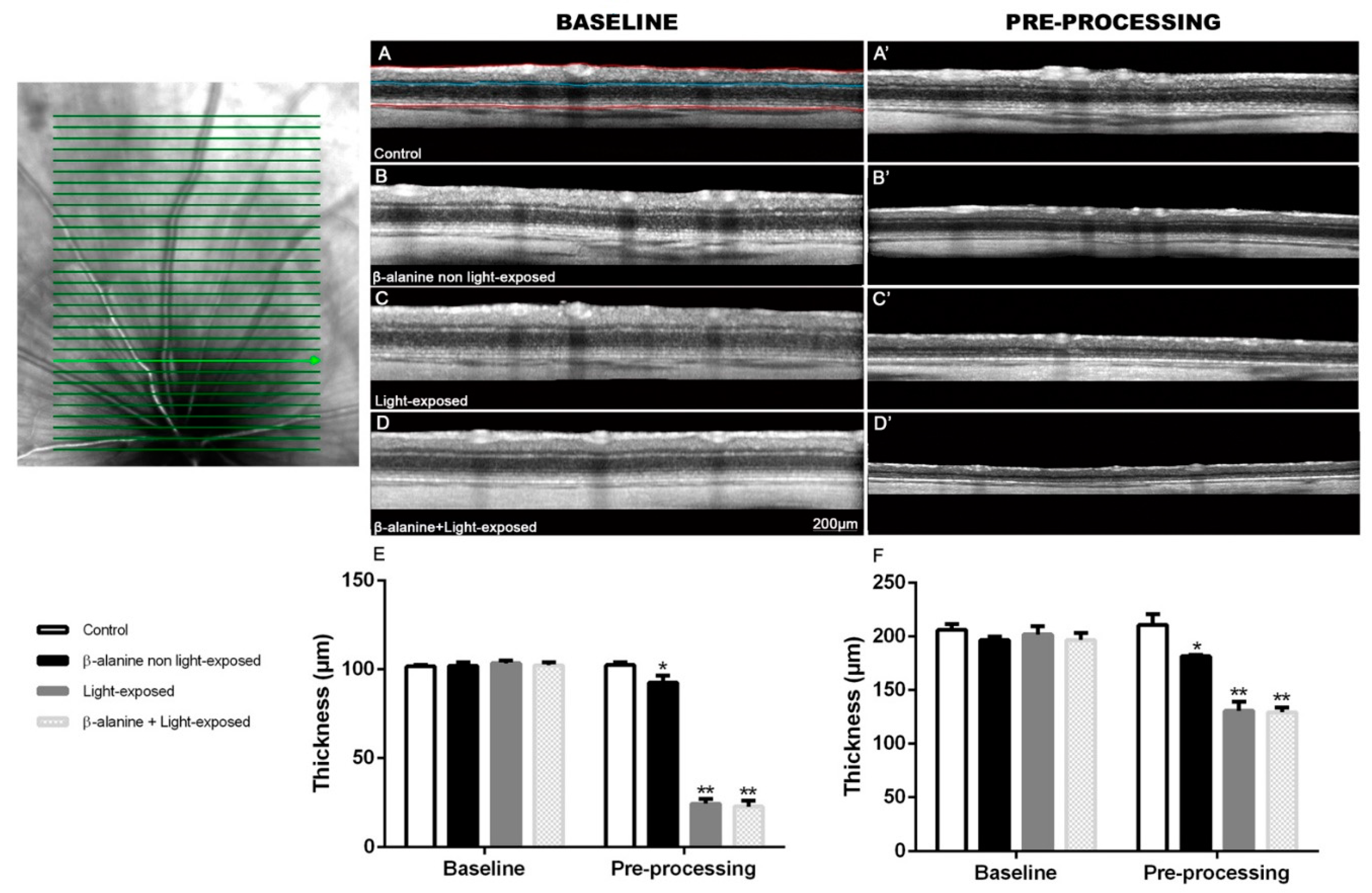


| Control | β-Alanine Non Light-Exposed | Light-Exposed | β-Alanine + Light-Exposed | ||
|---|---|---|---|---|---|
| OUTERRETINA | Baseline | 101.6 ± 0.9 µm | 102.1 ± 1.9 µm | 101.6 ± 0.9 µm | 102.1 ± 1.9 µm |
| Pre-processing | 102.4 ± 1.5 µm | 92.5 ± 4.1 µm *,† | 24.3 ± 2.6 µm *,† | 22.7 ± 3.3 µm *,† | |
| TOTAL RETINA | Baseline | 206.2 ± 5.4 µm | 196.7 ± 3.4 µm | 201.9 ± 7.7 µm | 196.8 ± 6.3 µm |
| Pre-processing | 210.8 ± 9.9 µm | 181.6 ± 1.1 *,† | 130.7 ± 8.5 µm *,† | 129.3 ± 4.5 µm *,† |
| DORSAL | VENTRAL | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% | 75% | 50% | 25% | 25% | 50% | 75% | 95% | |
| Control | 7.2 ± 1.2 | 9.8 ± 1.2 | 9.8 ± 0.8 | 10 ± 0.6 | 9.8 ± 0.4 | 10.3 ± 0.5 | 9.8 ± 0.8 | 7.3 ± 1.2 |
| β-alanine non light-exposed | 5.9 ± 0.9 * | 9.1 ± 1 | 1.9 ± 0.9 | 9 ± 1 | 9.1 ± 0.8 | 9 ± 1.3 * | 8.7 ± 1.6 | 6.1 ± 1.7 |
| Light-exposed | 2.4 ± 0.9 *,† | 1.3 ± 0.5 *,† | 1.1 ± 0.3 *,† | 1 ± 0.7 *,† | 1.4 ± 0.7 *,† | 1.3 ± 0.7 *,† | 1.6 ± 0.7 *,† | 2.7 ± 1.2 *,† |
| β-alanine + Light exposed | 1.9 ± 0.3 *,† | 1.1 ± 0.3 *,† | 1.2 ± 0.4 *,† | 1.2 ± 0.4 *,† | 1.9 ± 0.4 *,† | 1.8 ± 0.4 *,† | 1.8 ± 0.8 *,† | 2.3 ± 0.5 *,† |
| Control | β-Alanine Non Light-Exposed | Light-Exposed | β-Alanine + Light Exposed | |
|---|---|---|---|---|
| GCL | 3.9 ± 1.8 | 4 ± 2.1 | 6.4 ± 2.3 * | 7.2 ± 2.3 *,† |
| IPL | 6 ± 1.4 | 7.9 ± 2.2 | 7.4 ± 3.4 | 12.4 ± 3.5 *,†,Ŧ |
| INL | 1.9 ± 1.7 | 2.5 ± 2 | 3.6 ± 2.6 | 0.8 ± 0.9 Ŧ |
| OPL | 2 ± 1.8 | 2.3 ± 1.9 | 3.3 ± 1.9 | 7.4 ± 3 *,†,Ŧ |
| ONL | 0 | 0.1 ± 0.5 | 4.7 ± 2.6 *,† | 0.8 ± 1.7 Ŧ |
| OS | 0 | 0.5 ± 1.5 | 3 ± 2.2 *,† | 3.3 ± 2.4 *,† |
| All layers | 13.8 ± 2.3 | 17.3 ± 2.8 * | 28.3 ± 1.8 *,† | 31.9 ± 4.5 *,†,Ŧ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Vacas, A.; Di Pierdomenico, J.; Valiente-Soriano, F.J.; Vidal-Sanz, M.; Picaud, S.; Villegas-Pérez, M.P.; García-Ayuso, D. Glial Cell Activation and Oxidative Stress in Retinal Degeneration Induced by β-Alanine Caused Taurine Depletion and Light Exposure. Int. J. Mol. Sci. 2022, 23, 346. https://doi.org/10.3390/ijms23010346
Martínez-Vacas A, Di Pierdomenico J, Valiente-Soriano FJ, Vidal-Sanz M, Picaud S, Villegas-Pérez MP, García-Ayuso D. Glial Cell Activation and Oxidative Stress in Retinal Degeneration Induced by β-Alanine Caused Taurine Depletion and Light Exposure. International Journal of Molecular Sciences. 2022; 23(1):346. https://doi.org/10.3390/ijms23010346
Chicago/Turabian StyleMartínez-Vacas, Ana, Johnny Di Pierdomenico, Francisco J. Valiente-Soriano, Manuel Vidal-Sanz, Serge Picaud, María Paz Villegas-Pérez, and Diego García-Ayuso. 2022. "Glial Cell Activation and Oxidative Stress in Retinal Degeneration Induced by β-Alanine Caused Taurine Depletion and Light Exposure" International Journal of Molecular Sciences 23, no. 1: 346. https://doi.org/10.3390/ijms23010346







