Modulation of Mesenchymal Stem Cells for Enhanced Therapeutic Utility in Ischemic Vascular Diseases
Abstract
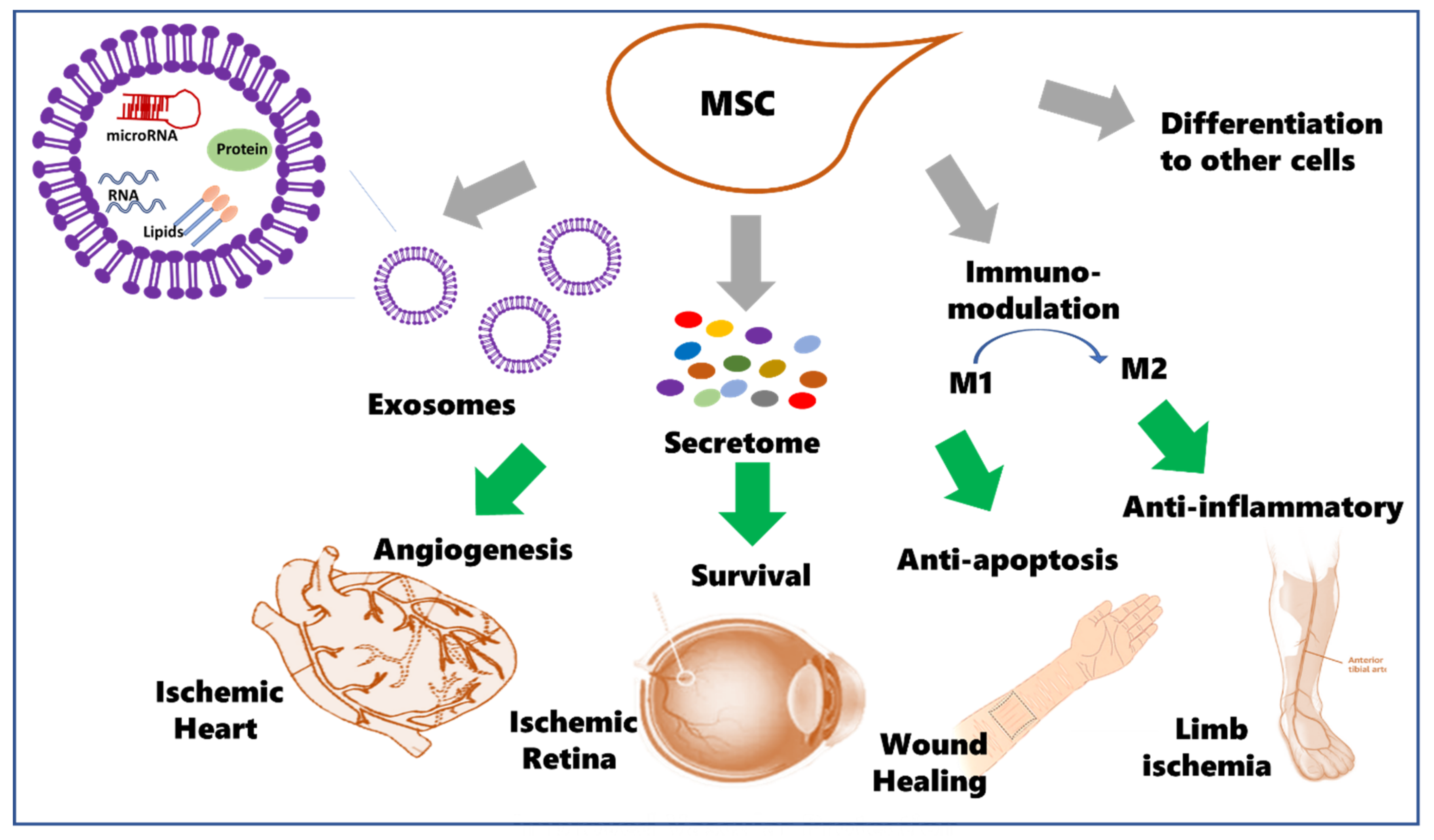
:1. Introduction
1.1. Potential Therapeutic Use of Mesenchymal Stem Cells (MSCs)
1.2. MSCs Secretome
1.3. The Potential Use for MSCs and Cell-Free Therapy in Ischemic Diseases
2. Ischemic Heart Diseases
2.1. Clinical Scope and Need for New Therapeutics for Ischemic Heart Diseases
2.2. Preclinical Studies of Modulated MSCs in IHD Animal Models
2.3. Preclinical Studies of MSCs Exosomes in IHD Animal Models
2.4. Clinical Studies of MSCs in IHD
| Study | Clinical Trials Identifier: | Intervention | Delivery Method | Disease | Phase | Status | Reference |
|---|---|---|---|---|---|---|---|
| A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human MSC (prochymal) after acute myocardial infarction | NCT00114452 | Allogeneic hMSCs | IV | myocardial infarction | Phase I | Completed | [59] |
| Safety Study of Adult Mesenchymal Stem Cells (MSC) to Treat Acute Myocardial Infarction | NCT00114452 | Provacel ex vivo cultured adult MSCs | IV | Myocardial Infarction | Phase I | Completed | [59] |
| Intramyocardial Injection of Autologous Bone Marrow-Derived Ex Vivo Expanded Mesenchymal Stem Cells in Acute MI Patients is Feasible and Safe up to 5 Years of Follow-up | Dutch trial registry (NTR1553) | Autologous BM-MSCs | Intramyocardial injection | Acute ST-segment elevation MI | Completed | [60] | |
| Intracoronary cardiosphere-derived cells for heart regeneration after MI (CADUCEUS): a prospective, randomized phase I trial | NCT00893360 | Autologous stem cell infusion | Intracoronary infusion | Recent Myocardial Infarction Ventricular Dysfunction | Phase I | Completed | [64] |
| Comparison of allogeneic vs. autologous BM-MSC delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial | NCT01087996 | Auto-hMSCs Allo-hMSCs | Transendocardial Injection | LV dysfunction due to ICM | Phase I Phase II | Completed | [65] |
| Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) | NCT00587990 | MSC injection | Intramyocardial injection | Left Ventricular Dysfunction | Phase I Phase II | Terminated (Difficulty in recruitment.) | [66] |
| Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial | NCT00426868 | Direct injection of (ADRCs) | Direct injection into the Left Ventricle | coronary artery disease refractory to revascularization | Phase I | Completed | [67] |
| Rationale and design of the first randomized, double-blind, placebo-controlled trial of intramyocardial injection of autologous BM-MSCs in chronic ischemic Heart Failure (MSC-HF Trial) | NCT00644410 | Autologous BM-MSC | Intramyocardial injection | Congestive Heart Failure | Phase I Phase II | Completed | [68] |
| Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) | NCT00810238 | BM- cardiopoietic cells | LV endocardial injection | heart failure Class II or III of ischemic origin | Phase II Phase III | Completed | [69] |
| Prochymal® (Human Adult Stem Cells) Intravenous Infusion Following Acute Myocardial Infarction (AMI) | NCT00877903 | Prochymal® (Human Adult Stem Cells) | IV infusion | Myocardial Infarction | Phase II | Completed | - |
| Safety Study of Allogeneic Mesenchymal Precursor Cell Infusion in MyoCardial Infarction (AMICI) | NCT01781390 | Mesenchymal Precursor Cells (MPC) | Intracoronary infusion | Acute Myocardial Infarction | Phase II | Active, not recruiting | - |
3. Ischemic Retinal Diseases
3.1. Clinical Scope and Need for MSC Therapeutics
3.2. Preclinical Studies of MSCs in Ischemic Retina Animal Models
3.3. Preclinical Studies of MSCs-Exosomes in Ischemic Retina Models
3.4. Clinical Studies of MSCs in Ischemic Retinopathy
| Study | Clinical Trials Identifier | Intervention | Delivery Method | Disease | Phase | Status | Reference |
|---|---|---|---|---|---|---|---|
| Stem Cell Ophthalmology Treatment Study (SCOTS) | NCT01920867 | Autologous BM-MSC | Retrobulbar, subtenon, Intravenous, intravitreal, intraocular injections | Degenerative, ischemic or physical damage | N/A | Completed | [106] |
| Stem Cell Ophthalmology Treatment Study II (SCOTS2) | NCT03011541 | Autologous BM-MSCs | Retrobulbar, subtenon, Intravenous, intravitreal, intraocular injections | Degenerative, ischemic or physical damage | N/A | Recruiting | [103] |
| Randomized trial for patients with neuromyelitis optica | NCT02249676 | BM-MSC | IV infusion | neuromyelitis optica | phase II | Completed |
4. Wound Healing
4.1. Clinical Scope and Need for MSC Therapeutics
4.2. Preclinical Studies of Modulated MSCs in Wound Healing Models
4.3. Preclinical Studies of MSCs-Exosomes in Wound Healing Models
4.4. Clinical Studies of MSCs in Wound Healing
| Study | Clinical Trials Identifier | Intervention | Delivery Method | Disease | Phase | Status | Reference |
|---|---|---|---|---|---|---|---|
| Stem Cell Therapy to Improve Burn Wound Healing | NCT02104713 | Allogeneic MSCs | Topical Application | Skin burns 2nd degree | Phase I | Completed | |
| Safety and Exploratory Efficacy Study of Collagen Membrane with Mesenchymal Stem Cells in the Treatment of Skin Defects (SEESCMMSCTSD) | NCT02672280 | MSC with Medical Collagen Membrane | Topical application | Wounds Diabetic Foot Ulcers Burns | Phase I Phase II | Unknown | |
| Human Placental Mesenchymal Stem Cells Treatment on Diabetic Foot Ulcer | NCT04464213 | Human placental MSC gel | Topical application | Diabetic Foot Ulcer | Phase I Phase II | Recruiting | |
| Phase I, open-label safety study of umbilical cord lining mesenchymal stem cells (corlicyte®) to heal chronic diabetic foot ulcers | NCT04104451 | Expanded UC-MSC (Corlicyte®) | Chronic Diabetic Foot Ulcers | Phase I | Recruiting | ||
| Clinical Application of MSC Seeded in Chitosan Scaffold for Diabetic Foot Ulcers | NCT03259217 | ASC seeded in Curcumin loaded into collagen-alginate | Topical application | Diabetic Foot Ulcers | Phase I | Unknown | |
| Comparison of Autologous MSC and Mononuclear Cells on Diabetic Critical Limb Ischemia and Foot Ulcer | NCT00955669 | MSCs or MNCs transplantation | Intramuscular injection | Diabetic Foot | Phase I | Completed | [136] |
| Allogeneic ABCB5-positive Stem Cells for Treatment of DFU “Malum Perforans | NCT03267784 | allogeneic ABCB5-positive MSCs | Topical application | Diabetic Neuropathic Ulcer | Phase I Phase II | Completed | [137] |
| Therapeutic Potential of Stem Cell Conditioned Medium on Chronic Ulcer Wounds | NCT04134676 | hWJ-MSC conditioned media | Topical application | chronic skin ulcers | Phase I | Completed | |
| Safety of MSC Extracellular Vesicles (BM-MSC-EVs) for the Treatment of Burn Wounds | NCT05078385 | AGLE-102 (BM-MSCs- derived EVs) | Direct application | 2nd degree burn | Phase I | Not yet recruiting |
5. Peripheral/Critical Limb Ischemia (PLI/CLI)
5.1. Clinical Scope and Need for Therapeutics
5.2. Preclinical Studies of Modulated MSCs in Critical Limb Ischemic Models
5.3. Preclinical Studies of Paracrine Factors of MSCs in Critical Limb Ischemic Models
5.4. Clinical Studies of MSCs in PLI
6. Limitation of MSC Therapeutics and Future Perspectives
| Study | Clinical Trial Identifier | Intervention | Delivery Method | Disease | Phase | Status | Reference |
|---|---|---|---|---|---|---|---|
| Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: RESTORE-CLI trial | BM-MSC | Intramuscular injections | PLI patients with no options for revascularization | Phase II | Completed | [182] | |
| Comparison of BM-MSC for treatment of diabetic critical limb ischemia and foot ulcer: | IM BM-MSCs IM BM-MNCs | Intramuscular injection | Diabetic patients with bilateral CLI and foot ulcer | [183] | |||
| The safety and efficacy of allogeneic BM-MSC in critical limb ischemia | NCT00883870 | BM-MSCs | Intramuscular injection | Critical limb Ischemia | Phase I Phase II | Completed | [184] |
| The use of autologous cultured ASC- to treat patients with non-revascularizable CLI | NCT01211028 | Adipose-derived stroma/stem cells | Intramuscular injection | Non-revascularizable critical limb ischemia | Phase I | Completed | [185] |
| BM-derived Cell Therapy in Critical Limb Ischemia | Bone marrow-derived stem cells | - | Critical limb ischemia | Meta-analysis | [189] | ||
| Autologous Cell Therapy for Peripheral Arterial Disease: | Randomized, Nonrandomized, and Noncontrolled Studies | - | Critical limb ischemia Intractable peripheral arterial disease | Systematic Review and Meta-Analysis | [190] | ||
| Safety and Efficacy of Allogeneic Adipose Tissue-MSCs in Diabetic Patients with CLI | NCT04466007 | Low and high dose IM Allogeneic ASC | Intramuscular injection | Limb Ischemia Diabetic Foot | Phase II | Recruiting |
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α-SMA | Alpha-smooth muscle actin |
| ABCB5 | Adenosine triphosphate-binding cassette transporter |
| ABI | Ankle brachial index |
| AD-MSCs | Adipose tissue derived mesenchymal stem cells |
| ASC | Adipose stem cells |
| ATP | Adenosine triphosphate |
| Bax | B-cell lymphoma associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| bFGF | Basic fibroblast growth factors |
| BMP-2 | Bone morphogenetic protein 2 |
| BMSCs | Bone marrow stromal cells |
| BM-MNCs | Bone marrow-derived mononuclear cells |
| BM-MSCs | Bone marrow-derived mesenchymal stem cell |
| CCL | Chemokine Ligand |
| CCR | C-C Chemokine receptor |
| CD | Cluster of differentiation |
| CircRNA | Circular RNA |
| CLI | Critical Limb Ischemia |
| CM | Conditioned medium |
| CRP | C-Reactive Protein |
| CXCR | C-X-C chemokine receptor |
| DFU | Diabetic foot ulcers |
| DM | Diabetes mellitus |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| ERK | Extracellular-signal-regulated kinase |
| EV | Extracellular vesicles |
| FasL | Fas ligand |
| FGF-2 | Fibroblast Growth Factor-2 |
| G-CSF | Granulocyte-colony stimulating factor |
| GFAP | Glial fibrillary acidic protein |
| GSK3β | Glycogen synthase kinase 3 beta |
| hAD-MSCs | Human adipose tissue-derived mesenchymal stem cells |
| hBMSCs | Human bone marrow-derived mesenchymal stem cells |
| HGF | Hepatocyte growth factor |
| HLI | Hindlimb ischemia |
| HO-1 | Heme oxygenase-1 |
| hUC-MSC | Human umbilical cord-derived mesenchymal stem cell |
| ICAM1 | Intercellular adhesion molecule |
| IDO | Indoleamine 2,3 dioxygenase |
| IGF-1 | Insulin-like growth factor-1 |
| IHD | Ischemic heart disease |
| IFN-γ | Interferon- gamma |
| IL | Interleukin |
| IL-1RA | Interleukin-1 receptor antagonists |
| IM | Intramuscular |
| iMSCs | Induced mesenchymal stem cells |
| iNOS | Induced |
| ITGA1 | Integrin alpha 1 |
| IV | Intravenous |
| JAK | Just another kinase (Janus kinase) |
| LAD | Left anterior descending artery |
| lncRNA | Long non-coding RNA |
| LPS | Lipopolysaccharides |
| LV | Left Ventricular |
| MCP | Monocyte chemoattractant protein |
| MI | Myocardial infarction |
| MIP | Macrophage inflammatory protein |
| miRNA | Micro Ribonucleic Acid |
| MMP11 | Matrix metalloproteinase-11 |
| MNC | Mononuclear cells |
| MPCs | Mesenchymal precursor cells |
| mRNA | Messenger Ribonucleic Acid |
| MSC-exo | Mesenchymal stem cell derived exosomes |
| MSCs | Mesenchymal stem cells |
| mTOR | Mammalian target of rapamycin |
| NFkB | Nuclear Factor kappa B |
| NGF-β | Nerve growth factor-beta |
| OGD | Oxygen-glucose deprivation |
| OIR | Oxygen-induced retinopathy |
| P-p44/42 MAPK | Phospho-p44/42 mitogen-activated protein kinase |
| p-SMAD2 | Phosphorylated small mothers against decapentaplegic protein |
| PDCD4 | Programmed Cell Death Protein 4 |
| PDGF | Platelet derived growth factor |
| PDGFR | Platelet derived growth factor receptor |
| Peli1 | Pellino1 |
| PI3K | Phosphatidylinositol 3-kinase |
| PIGF | Placental growth factor |
| PLI | Peripheral leg ischemia |
| PTEN | Phosphatase and tensin homolog |
| ROS | Reactive oxygen species |
| SC | Subcutaneous |
| SCF | Stem cell factor |
| SDF-1 | Stromal-derived factor 1 |
| Sh-H19 | Short hairpin-H19 |
| STAT | Signal transducer and activator of transcription |
| STEMI | ST-elevated myocardial infarction |
| TGF-β | Transforming growth factor-beta |
| Th2 | T- helper 2 |
| THP-1 | Tamm-Horsfall Protein 1. |
| TIMP-1 | Tissue Inhibitor of Metalloproteinase-1 |
| TLR | Toll-like receptors |
| TNF-α | Tumor necrosis factor-alpha |
| TNFR | Tumor necrosis factor receptor |
| TSG 101 | Tumor susceptibility gene |
| TSG-6 | TNF stimulated gene-6 |
| UC-MSCs | Umbilical cord mesenchymal stem cells |
| VCAM1 | Vascular cell adhesion protein 1 |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless-related integration site |
References
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, O.F.; Alini, M.; Stoddart, M.J. Mesenchymal Stem Cells Derived from Human Bone Marrow. Methods Mol. Biol. 2015, 1340, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2020, 11, 591065. [Google Scholar] [CrossRef]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef] [Green Version]
- Koç, O.N.; Day, J.; Nieder, M.; Gerson, S.L.; Lazarus, H.M.; Krivit, W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant. 2002, 30, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.L.; Tang, K.C.; Patel, A.P.; Bonilla, L.M.; Pierobon, N.; Ponzio, N.M.; Rameshwar, P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood 2006, 107, 4817–4824. [Google Scholar] [CrossRef]
- Eliopoulos, N.; Stagg, J.; Lejeune, L.; Pommey, S.; Galipeau, J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood 2005, 106, 4057–4065. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [Green Version]
- English, K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2013, 91, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Stock, P.; Brückner, S.; Winkler, S.; Dollinger, M.M.; Christ, B. Human bone marrow mesenchymal stem cell-derived hepatocytes improve the mouse liver after acute acetaminophen intoxication by preventing progress of injury. Int. J. Mol. Sci. 2014, 15, 7004–7028. [Google Scholar] [CrossRef] [Green Version]
- Bai, C.; Gao, Y.; Li, Q.; Feng, Y.; Yu, Y.; Meng, G.; Zhang, M.; Guan, W. Differentiation of chicken umbilical cord mesenchymal stem cells into beta-like pancreatic islet cells. Artif. Cells Nanomed. Biotechnol. 2015, 43, 106–111. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Zhao, J.; Zhang, Z.; Yang, R.; Xie, J.; Liu, X.; Qi, S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS ONE 2014, 9, e96161. [Google Scholar] [CrossRef] [Green Version]
- Zimmerlin, L.; Park, T.S.; Zambidis, E.T.; Donnenberg, V.S.; Donnenberg, A.D. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 2013, 95, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.G.; Salgado, A.J. Mesenchymal stem cells secretome: Current trends and future challenges. Neural Regen. Res. 2020, 15, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.X.; Zhang, X.W.; Sun, Q.; Yang, L.; Liu, A.; Hu, S.; Guo, F.; Liu, S.; Huang, Y.; et al. Chemokine receptor 7 overexpression promotes mesenchymal stem cell migration and proliferation via secreting Chemokine ligand 12. Sci. Rep. 2018, 8, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assuncao-Silva, R.C.; Mendes-Pinheiro, B.; Patricio, P.; Behie, L.A.; Teixeira, F.G.; Pinto, L.; Salgado, A.J. Exploiting the impact of the secretome of MSCs isolated from different tissue sources on neuronal differentiation and axonal growth. Biochimie 2018, 155, 83–91. [Google Scholar] [CrossRef]
- Serra, S.C.; Costa, J.C.; Assuncao-Silva, R.C.C.; Teixeira, F.G.; Silva, N.A.; Anjo, S.I.; Manadas, B.; Gimble, J.M.; Behie, L.A.; Salgado, A.J. Influence of passage number on the impact of the secretome of adipose tissue stem cells on neural survival, neurodifferentiation and axonal growth. Biochimie 2018, 155, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Koniusz, S.; Andrzejewska, A.; Muraca, M.; Srivastava, A.K.; Janowski, M.; Lukomska, B. Extracellular Vesicles in Physiology, Pathology, and Therapy of the Immune and Central Nervous System, with Focus on Extracellular Vesicles Derived from Mesenchymal Stem Cells as Therapeutic Tools. Front. Cell Neurosci. 2016, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.P.; Breakefield, X.O. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front. Physiol. 2012, 3, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabin, K.; Kikyo, N. Microvesicles as mediators of tissue regeneration. Transl. Res. 2014, 163, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, H.K.; Kim, T.M.; Leem, S.H.; Oh, I.H. Regulation of mesenchymal stromal cells through fine tuning of canonical Wnt signaling. Stem Cell Res. 2015, 14, 356–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Balla, C.; Pavasini, R.; Ferrari, R. Treatment of Angina: Where Are We? Cardiology 2018, 140, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Golpanian, S.; Wolf, A.; Hatzistergos, K.E.; Hare, J.M. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol. Rev. 2016, 96, 1127–1168. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, Y.; Zhong, Z.; Wu, Y.; Zhao, J.; Wang, Y.; Cheng, H.; Kong, M.; Zhang, F.; Chen, Q.; et al. A Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in Nonhuman Primates: Paracrine Activity Without Remuscularization. Circ. Res. 2016, 118, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Z.; Guo, J.; Ni, A.; Deb, A.; Zhang, L.; Mirotsou, M.; Pratt, R.E.; Dzau, V.J. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ. Res. 2010, 106, 1753–1762. [Google Scholar] [CrossRef]
- Kelly, M.L.; Wang, M.; Crisostomo, P.R.; Abarbanell, A.M.; Herrmann, J.L.; Weil, B.R.; Meldrum, D.R. TNF receptor 2, not TNF receptor 1, enhances mesenchymal stem cell-mediated cardiac protection following acute ischemia. Shock 2010, 33, 602–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Mal, N.; Kiedrowski, M.; Chacko, M.; Askari, A.T.; Popovic, Z.B.; Koc, O.N.; Penn, M.S. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007, 21, 3197–3207. [Google Scholar] [CrossRef]
- Li, W.; Ma, N.; Ong, L.L.; Nesselmann, C.; Klopsch, C.; Ladilov, Y.; Furlani, D.; Piechaczek, C.; Moebius, J.M.; Lützow, K.; et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 2007, 25, 2118–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Lin, C.; Guo, Y.; Chen, Y.; Du, Y.; Lau, W.B.; Xia, Y.; Zhang, F.; Su, R.; Gao, E.; et al. N-Cadherin Overexpression Mobilizes the Protective Effects of Mesenchymal Stromal Cells Against Ischemic Heart Injury Through a β-Catenin-Dependent Manner. Circ. Res. 2020, 126, 857–874. [Google Scholar] [CrossRef]
- Guo, J.; Lin, G.; Bao, C.; Hu, Z.; Chu, H.; Hu, M. Insulin-like growth factor 1 improves the efficacy of mesenchymal stem cells transplantation in a rat model of myocardial infarction. J. Biomed. Sci. 2008, 15, 89–97. [Google Scholar] [CrossRef]
- Hahn, J.Y.; Cho, H.J.; Kang, H.J.; Kim, T.S.; Kim, M.H.; Chung, J.H.; Bae, J.W.; Oh, B.H.; Park, Y.B.; Kim, H.S. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J. Am. Coll. Cardiol. 2008, 51, 933–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisel, S.; Khan, M.; Kuppusamy, M.L.; Mohan, I.K.; Chacko, S.M.; Rivera, B.K.; Sun, B.C.; Hideg, K.; Kuppusamy, P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J. Pharmacol. Exp. Ther. 2009, 329, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Fan, L.; Lin, C.; Zhuo, S.; Chen, L.; Liu, N.; Luo, Y.; Fang, J.; Huang, Z.; Lin, Y.; Chen, J. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur. J. Heart Fail. 2009, 11, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Nasser, M.I.; Masood, M.; Adlat, S.; Gang, D.; Zhu, S.; Li, G.; Li, N.; Chen, J.; Zhu, P. Mesenchymal stem cell-derived exosome microRNA as therapy for cardiac ischemic injury. Biomed. Pharm. 2021, 143, 112118. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, S.; Wang, S.; Li, W.; Liu, J. BMSCs-derived exosomal microRNA-150-5p attenuates myocardial infarction in mice. Int. Immunopharmacol. 2021, 93, 107389. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, Y.; Lan, B.; Wang, J.; Zhang, Z.; Zhang, L.; Xiao, P.; Meng, Q.; Geng, Y.J.; Yu, X.Y.; et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. Biomed. Res. Int. 2017, 2017, 4150705. [Google Scholar] [CrossRef]
- Luther, K.M.; Haar, L.; McGuinness, M.; Wang, Y.; Lynch Iv, T.L.; Phan, A.; Song, Y.; Shen, Z.; Gardner, G.; Kuffel, G.; et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J. Mol. Cell Cardiol. 2018, 119, 125–137. [Google Scholar] [CrossRef]
- Mayourian, J.; Ceholski, D.K.; Gorski, P.A.; Mathiyalagan, P.; Murphy, J.F.; Salazar, S.I.; Stillitano, F.; Hare, J.M.; Sahoo, S.; Hajjar, R.J.; et al. Exosomal microRNA-21-5p Mediates Mesenchymal Stem Cell Paracrine Effects on Human Cardiac Tissue Contractility. Circ. Res. 2018, 122, 933–944. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef]
- Lai, T.C.; Lee, T.L.; Chang, Y.C.; Chen, Y.C.; Lin, S.R.; Lin, S.W.; Pu, C.M.; Tsai, J.S.; Chen, Y.L. MicroRNA-221/222 Mediates ADSC-Exosome-Induced Cardioprotection Against Ischemia/Reperfusion by Targeting PUMA and ETS-1. Front. Cell Dev. Biol. 2020, 8, 569150. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Zhang, O.; Wu, X.; Guan, X.; Xue, Y.; Li, S.; Zhuang, X.; Zhou, B.; Miao, G.; et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-185 represses ventricular remolding of mice with myocardial infarction by inhibiting SOCS2. Int. Immunopharmacol. 2020, 80, 106156. [Google Scholar] [CrossRef]
- Park, H.; Park, H.; Mun, D.; Kang, J.; Kim, H.; Kim, M.; Cui, S.; Lee, S.H.; Joung, B. Extracellular Vesicles Derived from Hypoxic Human Mesenchymal Stem Cells Attenuate GSK3β Expression via miRNA-26a in an Ischemia-Reperfusion Injury Model. Yonsei Med. J. 2018, 59, 736–745. [Google Scholar] [CrossRef]
- Yu, B.; Kim, H.W.; Gong, M.; Wang, J.; Millard, R.W.; Wang, Y.; Ashraf, M.; Xu, M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 2015, 182, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669. [Google Scholar] [CrossRef]
- Mirotsou, M.; Zhang, Z.; Deb, A.; Zhang, L.; Gnecchi, M.; Noiseux, N.; Mu, H.; Pachori, A.; Dzau, V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc. Natl. Acad. Sci. USA 2007, 104, 1643–1648. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.H.; Liu, H.; Wang, S.J.; Liang, S.W.; Wang, G.G. Exosomes derived from SDF1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. J. Cell Physiol. 2019, 234, 13878–13893. [Google Scholar] [CrossRef]
- Zeng, B.; Ren, X.; Lin, G.; Zhu, C.; Chen, H.; Yin, J.; Jiang, H.; Yang, B.; Ding, D. Paracrine action of HO-1-modified mesenchymal stem cells mediates cardiac protection and functional improvement. Cell Biol. Int. 2008, 32, 1256–1264. [Google Scholar] [CrossRef]
- Nah, J.; Fernández, Á.F.; Kitsis, R.N.; Levine, B.; Sadoshima, J. Does Autophagy Mediate Cardiac Myocyte Death During Stress? Circ. Res. 2016, 119, 893–895. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Wang, K.; Xu, Y.; Hu, H.; Zhang, N.; Wang, Y.; Zhong, Z.; Zhao, J.; Li, Q.; Zhu, D.; et al. Transplanted Mesenchymal Stem Cells Reduce Autophagic Flux in Infarcted Hearts via the Exosomal Transfer of miR-125b. Circ. Res. 2018, 123, 564–578. [Google Scholar] [CrossRef]
- Li, J.; Rohailla, S.; Gelber, N.; Rutka, J.; Sabah, N.; Gladstone, R.A.; Wei, C.; Hu, P.; Kharbanda, R.K.; Redington, A.N. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res. Cardiol. 2014, 109, 423. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef] [Green Version]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, S.F.; van Ramshorst, J.; Hoogslag, G.E.; Boden, H.; Velders, M.A.; Cannegieter, S.C.; Roelofs, H.; Al Younis, I.; Dibbets-Schneider, P.; Fibbe, W.E.; et al. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J. Cardiovasc. Transl. Res. 2013, 6, 816–825. [Google Scholar] [CrossRef] [Green Version]
- Makkar, R.R.; Price, M.J.; Lill, M.; Frantzen, M.; Takizawa, K.; Kleisli, T.; Zheng, J.; Kar, S.; McClelan, R.; Miyamota, T.; et al. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J. Cardiovasc. Pharmacol. Ther. 2005, 10, 225–233. [Google Scholar] [CrossRef]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marbán, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gyöngyösi, M.; Wojakowski, W.; Lemarchand, P.; Lunde, K.; Tendera, M.; Bartunek, J.; Marban, E.; Assmus, B.; Henry, T.D.; Traverse, J.H.; et al. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ. Res. 2015, 116, 1346–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; DiFede Velazquez, D.L.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef]
- Karantalis, V.; DiFede, D.L.; Gerstenblith, G.; Pham, S.; Symes, J.; Zambrano, J.P.; Fishman, J.; Pattany, P.; McNiece, I.; Conte, J.; et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ. Res. 2014, 114, 1302–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perin, E.C.; Sanz-Ruiz, R.; Sánchez, P.L.; Lasso, J.; Pérez-Cano, R.; Alonso-Farto, J.C.; Pérez-David, E.; Fernández-Santos, M.E.; Serruys, P.W.; Duckers, H.J.; et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am. Heart J. 2014, 168, 88–95. [Google Scholar] [CrossRef]
- Mathiasen, A.B.; Jorgensen, E.; Qayyum, A.A.; Haack-Sorensen, M.; Ekblond, A.; Kastrup, J. Rationale and design of the first randomized, double-blind, placebo-controlled trial of intramyocardial injection of autologous bone-marrow derived Mesenchymal Stromal Cells in chronic ischemic Heart Failure (MSC-HF Trial). Am. Heart J. 2012, 164, 285–291. [Google Scholar] [CrossRef]
- Bartunek, J.; Behfar, A.; Dolatabadi, D.; Vanderheyden, M.; Ostojic, M.; Dens, J.; El Nakadi, B.; Banovic, M.; Beleslin, B.; Vrolix, M.; et al. Cardiopoietic stem cell therapy in heart failure: The C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J. Am. Coll. Cardiol. 2013, 61, 2329–2338. [Google Scholar] [CrossRef] [Green Version]
- Park, S.S. Cell Therapy Applications for Retinal Vascular Diseases: Diabetic Retinopathy and Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFj1–ORSFj10. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.C.; Dabouz, R.; Noueihed, B.; Omri, S.; Tahiri, H.; Chemtob, S. Ischemic Retinopathies: Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2017, 2017, 3940241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertelli, P.M.; Pedrini, E.; Guduric-Fuchs, J.; Peixoto, E.; Pathak, V.; Stitt, A.W.; Medina, R.J. Vascular Regeneration for Ischemic Retinopathies: Hope from Cell Therapies. Curr. Eye Res. 2020, 45, 372–384. [Google Scholar] [CrossRef] [Green Version]
- Blair, K.; Czyz, C.N. Central Retinal Vein Occlusion. In StatPearls; Treasure Island, FL, USA, 2021.
- Coucha, M.; Elshaer, S.L.; Eldahshan, W.S.; Mysona, B.A.; El-Remessy, A.B. Molecular mechanisms of diabetic retinopathy: Potential therapeutic targets. Middle East Afr. J. Ophthalmol. 2015, 22, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Poston, J.N.; Dreixler, J.C.; Torres, L.; Lopez, J.; Zelkha, R.; Balyasnikova, I.; Lesniak, M.S.; Roth, S. Bone-marrow mesenchymal stem-cell administration significantly improves outcome after retinal ischemia in rats. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1581–1592. [Google Scholar] [CrossRef]
- Li, L.; Du, G.; Wang, D.; Zhou, J.; Jiang, G.; Jiang, H. Overexpression of Heme Oxygenase-1 in Mesenchymal Stem Cells Augments Their Protection on Retinal Cells In Vitro and Attenuates Retinal Ischemia/Reperfusion Injury In Vivo against Oxidative Stress. Stem Cells Int. 2017, 2017, 4985323. [Google Scholar] [CrossRef] [PubMed]
- Kicic, A.; Shen, W.Y.; Wilson, A.S.; Constable, I.J.; Robertson, T.; Rakoczy, P.E. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J. Neurosci. 2003, 23, 7742–7749. [Google Scholar] [CrossRef] [Green Version]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Di Lauro, S.; Garcia-Gutierrez, M.T.; Srivastava, G.K.; Pastor, J.C.; Fernandez-Bueno, I. Mesenchymal stem cells provide paracrine neuroprotective resources that delay degeneration of co-cultured organotypic neuroretinal cultures. Exp. Eye Res. 2019, 185, 107671. [Google Scholar] [CrossRef]
- Elshaer, S.L.; Park, H.S.; Pearson, L.; Hill, W.D.; Longo, F.M.; El-Remessy, A.B. Modulation of p75(NTR) on Mesenchymal Stem Cells Increases Their Vascular Protection in Retinal Ischemia-Reperfusion Mouse Model. Int. J. Mol. Sci. 2021, 22, 829. [Google Scholar] [CrossRef] [PubMed]
- Kohli, N.; Al-Delfi, I.R.T.; Snow, M.; Sakamoto, T.; Miyazaki, T.; Nakajima, H.; Uchida, K.; Johnson, W.E.B. CD271-selected mesenchymal stem cells from adipose tissue enhance cartilage repair and are less angiogenic than plastic adherent mesenchymal stem cells. Sci. Rep. 2019, 9, 3194. [Google Scholar] [CrossRef] [Green Version]
- Rajashekhar, G. Mesenchymal stem cells: New players in retinopathy therapy. Front. Endocrinol. 2014, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Gaddam, S.; Periasamy, R.; Gangaraju, R. Adult Stem Cell Therapeutics in Diabetic Retinopathy. Int. J. Mol. Sci. 2019, 20, 4876. [Google Scholar] [CrossRef] [Green Version]
- Periasamy, R.; Elshaer, S.L.; Gangaraju, R. CD140b (PDGFRbeta) signaling in adipose-derived stem cells mediates angiogenic behavior of retinal endothelial cells. Regen. Eng. Transl. Med. 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rajashekhar, G.; Jonna, Y.; Gomaa, A.; Jayagopal, A. Adipose stromal cells enriched for CD146+ display increased migration and adhesion and ameliorate retinal ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci. 2015, 56, 921. [Google Scholar]
- Chen, J.; Luo, Y.; Huang, H.; Wu, S.; Feng, J.; Zhang, J.; Yan, X. CD146 is essential for PDGFRβ-induced pericyte recruitment. Protein Cell 2018, 9, 743–747. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, Y.; Kong, J.; Dong, M.; Duan, H.; Chen, S. Therapeutic efficacy of neural stem cells originating from umbilical cord-derived mesenchymal stem cells in diabetic retinopathy. Sci. Rep. 2017, 7, 408. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Rasiah, P.K.; Bajwa, A.; Rajasingh, J.; Gangaraju, R. Mesenchymal Stem Cell Induced Foxp3(+) Tregs Suppress Effector T Cells and Protect against Retinal Ischemic Injury. Cells 2021, 10, 3006. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, S.L.; El-Remessy, A.B. Deletion of p75(NTR) prevents vaso-obliteration and retinal neovascularization via activation of Trk—A receptor in ischemic retinopathy model. Sci. Rep. 2018, 8, 12490. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Dreixler, J.C.; Mathew, B.; Balyasnikova, I.; Mann, J.R.; Boddapati, V.; Xue, L.; Lesniak, M.S. Hypoxic-Preconditioned Bone Marrow Stem Cell Medium Significantly Improves Outcome After Retinal Ischemia in Rats. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3522–3532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noueihed, B.; Rivera, J.C.; Dabouz, R.; Abram, P.; Omri, S.; Lahaie, I.; Chemtob, S. Mesenchymal Stromal Cells Promote Retinal Vascular Repair by Modulating Sema3E and IL-17A in a Model of Ischemic Retinopathy. Front. Cell Dev. Biol. 2021, 9, 630645. [Google Scholar] [CrossRef]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.-C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, Y.; Chen, J.; Wang, T.; Li, Z.; Fu, Y.; Zhai, A.; Bi, C. Long noncoding RNA H19 acts as a miR-29b sponge to promote wound healing in diabetic foot ulcer. FASEB J. 2021, 35, e20526. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Gao, Y. Adipose mesenchymal stem cells-secreted extracellular vesicles containing microRNA-192 delays diabetic retinopathy by targeting ITGA1. J. Cell. Physiol. 2021, 236, 5036–5051. [Google Scholar] [CrossRef] [PubMed]
- Moisseiev, E.; Anderson, J.D.; Oltjen, S.; Goswami, M.; Zawadzki, R.J.; Nolta, J.A.; Park, S.S. Protective Effect of Intravitreal Administration of Exosomes Derived from Mesenchymal Stem Cells on Retinal Ischemia. Curr. Eye Res. 2017, 42, 1358–1367. [Google Scholar] [CrossRef]
- Mead, B.; Ahmed, Z.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in a Genetic DBA/2J Mouse Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5473–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, K.A.; Gentry, J.; Del Mar, N.A.; Reiner, A.; Sohl, N.; Gangaraju, R. Adipose Tissue-Derived Mesenchymal Stem Cell Concentrated Conditioned Medium Alters the Expression Pattern of Glutamate Regulatory Proteins and Aquaporin-4 in the Retina after Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1702–1716. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Klaic, L.; Elshaer, S.L.; Gentry, J.; Russell, J.M.; Beland, A.; Reiner, A.; Jotterand, V.; et al. Concentrated Conditioned Media from Adipose Tissue Derived Mesenchymal Stem Cells Mitigates Visual Deficits and Retinal Inflammation Following Mild Traumatic Brain Injury. Int. J. Mol. Sci. 2018, 19, 2016. [Google Scholar] [CrossRef] [Green Version]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Gentry, J.; Klaic, L.; Del Mar, N.; Reiner, A.; Yang, C.H.; Pfeffer, L.M.; Sohl, N.; et al. TSG-6 in conditioned media from adipose mesenchymal stem cells protects against visual deficits in mild traumatic brain injury model through neurovascular modulation. Stem Cell Res. Ther. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Shao, H.; Wang, H.; Zhang, Z.; Su, C.; Dong, L.; Yu, B.; Chen, X.; Li, X.; Zhang, X. Effects of Mesenchymal Stem Cell-Derived Exosomes on Experimental Autoimmune Uveitis. Sci. Rep. 2017, 7, 4323. [Google Scholar] [CrossRef] [Green Version]
- Ritter, M.R.; Banin, E.; Moreno, S.K.; Aguilar, E.; Dorrell, M.I.; Friedlander, M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J. Clin. Invest. 2006, 116, 3266–3276. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Zhang, Y.; Zhang, J.; Zhang, H.K.; Wu, X.; Zhang, Y.; Lam, F.F.; Kang, S.; Xia, J.C.; Lai, W.H.; et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 2010, 121, 1113–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, J.N.; Levy, S.; Benes, S.C. Stem Cell Ophthalmology Treatment Study: Bone marrow derived stem cells in the treatment of non-arteritic ischemic optic neuropathy (NAION). Stem Cell Investig. 2017, 4, 94. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone marrow derived stem cells in the treatment of Dominant Optic Atrophy. Stem Cell Investig. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Landázuri, N.; Levit, R.D.; Joseph, G.; Ortega-Legaspi, J.M.; Flores, C.A.; Weiss, D.; Sambanis, A.; Weber, C.J.; Safley, S.A.; Taylor, W.R. Alginate microencapsulation of human mesenchymal stem cells as a strategy to enhance paracrine-mediated vascular recovery after hindlimb ischaemia. J. Tissue Eng. Regen. Med. 2016, 10, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Levy, S.; Malkin, A. Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: A preliminary report. Neural Regen. Res. 2015, 10, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Benes, S.C.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Improvement in serpiginous choroidopathy following autologous bone marrow derived stem cell treatment. Neural Regen. Res. 2016, 11, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Levy, S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone Marrow-Derived Stem Cells in the Treatment of Stargardt Disease. Medicines 2021, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Jarbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Dumville, J.C.; Soares, M.O.; O’Meara, S.; Cullum, N. Systematic review and mixed treatment comparison: Dressings to heal diabetic foot ulcers. Diabetologia 2012, 55, 1902–1910. [Google Scholar] [CrossRef] [Green Version]
- Kranke, P.; Bennett, M.H.; Martyn-St James, M.; Schnabel, A.; Debus, S.E.; Weibel, S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst. Rev. 2015, 2015, CD004123. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Gou, M.; Da, L.C.; Zhang, W.Q.; Xie, H.Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part. B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Su, W.R.; Shi, S.H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Yang, Y.; Yan, X.; Zhang, K. Abnormalities in cytokine secretion from mesenchymal stem cells in psoriatic skin lesions. Eur. J. Derm. 2013, 23, 600–607. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Hou, Y.; Chai, J.; Duan, H.; Chu, W.; Zhang, H.; Hu, Q.; Du, J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS ONE 2014, 9, e88348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, R.; Lian, W.; Jin, Y.; Cao, C.; Han, S.; Yang, X.; Zhao, S.; Li, M.; Zhao, H. Role and effect of vein-transplanted human umbilical cord mesenchymal stem cells in the repair of diabetic foot ulcers in rats. Acta Biochim. Biophys. Sin. 2020, 52, 620–630. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- Xie, F.; Teng, L.; Xu, J.; Lu, J.; Zhang, C.; Yang, L.; Ma, X.; Zhao, M. Interleukin-10 modified bone marrow mesenchymal stem cells prevent hypertrophic scar formation by inhibiting inflammation. Pharmazie 2020, 75, 571–575. [Google Scholar] [CrossRef]
- Munir, S.; Basu, A.; Maity, P.; Krug, L.; Haas, P.; Jiang, D.; Strauss, G.; Wlaschek, M.; Geiger, H.; Singh, K.; et al. TLR4-dependent shaping of the wound site by MSCs accelerates wound healing. EMBO Rep. 2020, 21, e48777. [Google Scholar] [CrossRef]
- Huang, S.; Wu, Y.; Gao, D.; Fu, X. Paracrine action of mesenchymal stromal cells delivered by microspheres contributes to cutaneous wound healing and prevents scar formation in mice. Cytotherapy 2015, 17, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.K.; Chang, Y.S.; Sung, S.I.; Ahn, S.Y.; Park, W.S. Thrombin Preconditioning of Extracellular Vesicles Derived from Mesenchymal Stem Cells Accelerates Cutaneous Wound Healing by Boosting Their Biogenesis and Enriching Cargo Content. J. Clin. Med. 2019, 8, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, W.; Feng, Z.; Song, Q.; Niu, H.; Miao, G. Modulation of mesenchymal stem cells with miR-375 to improve their therapeutic outcome during scar formation. Am. J. Transl. Res. 2016, 8, 2079–2087. [Google Scholar]
- Li, B.; Zhou, Y.; Chen, J.; Wang, T.; Li, Z.; Fu, Y.; Bi, C.; Zhai, A. Long non-coding RNA H19 contributes to wound healing of diabetic foot ulcer. J. Mol. Endocrinol. 2020, 65, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.R.; Yin, L.; Chen, Y.Q.; Jin, X.J.; Zhou, X.; Zhu, N.N.; Liu, X.Q.; Wei, H.W.; Duan, L.S. Autologous blood transfusion augments impaired wound healing in diabetic mice by enhancing lncRNA H19 expression via the HIF-1alpha signaling pathway. Cell Commun. Signal. 2018, 16, 84. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Luan, S.; Chen, J.; Zhou, Y.; Wang, T.; Li, Z.; Fu, Y.; Zhai, A.; Bi, C. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol. Ther. Nucleic Acids 2020, 19, 814–826. [Google Scholar] [CrossRef]
- Abbaszadeh-Goudarzi, K.; Radbakhsh, S.; Pourhanifeh, M.H.; Khanbabaei, H.; Davoodvandi, A.; Fathizadeh, H.; Sahebkar, A.; Shahrzad, M.K.; Mirzaei, H. Circular RNA and Diabetes: Epigenetic Regulator with Diagnostic Role. Curr. Mol. Med. 2020, 20, 516–526. [Google Scholar] [CrossRef]
- Zaiou, M. circRNAs Signature as Potential Diagnostic and Prognostic Biomarker for Diabetes Mellitus and Related Cardiovascular Complications. Cells 2020, 9, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, N.R.; Dash, S.N.; Routray, P.; Mohapatra, S.; Mohapatra, P.C. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009, 12, 359–366. [Google Scholar] [CrossRef]
- Li, X.Y.; Zheng, Z.H.; Li, X.Y.; Guo, J.; Zhang, Y.; Li, H.; Wang, Y.W.; Ren, J.; Wu, Z.B. Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: Response and correction of immunological anomalies. Curr. Pharm. Des. 2013, 19, 4893–4899. [Google Scholar] [CrossRef] [PubMed]
- Nojehdehi, S.; Soudi, S.; Hesampour, A.; Rasouli, S.; Soleimani, M.; Hashemi, S.M. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell Biochem. 2018, 119, 9433–9443. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.L.; Zhu, X.H.; Zhang, B.; Zhou, L.; Wang, W.Y. Clinical Evaluation of Human Umbilical Cord Mesenchymal Stem Cell Transplantation after Angioplasty for Diabetic Foot. Exp. Clin. Endocrinol. Diabetes 2016, 124, 497–503. [Google Scholar] [CrossRef]
- Vojtassak, J.; Danisovic, L.; Kubes, M.; Bakos, D.; Jarabek, L.; Ulicna, M.; Blasko, M. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol. Lett. 2006, 27 (Suppl. S2), 134–137. [Google Scholar] [PubMed]
- Lafosse, A.; Desmet, C.; Aouassar, N.; Andre, W.; Hanet, M.S.; Beauloye, C.; Vanwijck, R.; Poirel, H.A.; Gallez, B.; Dufrane, D. Autologous Adipose Stromal Cells Seeded onto a Human Collagen Matrix for Dermal Regeneration in Chronic Wounds: Clinical Proof of Concept. Plast. Reconstr. Surg. 2015, 136, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Rehou, S.; McCann, M.R.; Shahrokhi, S. Allogeneic mesenchymal stem cells for treatment of severe burn injury. Stem Cell Res. Ther. 2019, 10, 337. [Google Scholar] [CrossRef]
- Rasulov, M.F.; Vasilchenkov, A.V.; Onishchenko, N.A.; Krasheninnikov, M.E.; Kravchenko, V.I.; Gorshenin, T.L.; Pidtsan, R.E.; Potapov, I.V. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull. Exp. Biol. Med. 2005, 139, 141–144. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, M.S.; Pomposelli, F.B. Society for Vascular Surgery Practice guidelines for atherosclerotic occlusive disease of the lower extremities management of asymptomatic disease and claudication. Introduction. J. Vasc. Surg. 2015, 61, 1S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.L.; Halperin, J.L.; Albert, N.M.; Bozkurt, B.; Brindis, R.G.; Curtis, L.H.; DeMets, D.; Guyton, R.A.; Hochman, J.S.; Kovacs, R.J.; et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, 1425–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Vázquez, M.D.; Herrero de la Parte, B.; García-Alonso, I.; Morales, M.C. Analysis of Biological Properties of Human Adult Mesenchymal Stem Cells and Their Effect on Mouse Hind Limb Ischemia. J. Vasc. Res. 2019, 56, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Qadura, M.; Terenzi, D.C.; Verma, S.; Al-Omran, M.; Hess, D.A. Concise Review: Cell Therapy for Critical Limb Ischemia: An Integrated Review of Preclinical and Clinical Studies. Stem Cells 2018, 36, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Shou, M.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef]
- Nammian, P.; Asadi-Yousefabad, S.L.; Daneshi, S.; Sheikhha, M.H.; Tabei, S.M.B.; Razban, V. Comparative analysis of mouse bone marrow and adipose tissue mesenchymal stem cells for critical limb ischemia cell therapy. Stem Cell Res. Ther. 2021, 12, 58. [Google Scholar] [CrossRef]
- Yao, Z.; Liu, H.; Yang, M.; Bai, Y.; Zhang, B.; Wang, C.; Yan, Z.; Niu, G.; Zou, Y.; Li, Y. Bone marrow mesenchymal stem cell-derived endothelial cells increase capillary density and accelerate angiogenesis in mouse hindlimb ischemia model. Stem Cell Res. Ther. 2021, 11, 221. [Google Scholar] [CrossRef]
- Laurila, J.P.; Laatikainen, L.; Castellone, M.D.; Trivedi, P.; Heikkila, J.; Hinkkanen, A.; Hematti, P.; Laukkanen, M.O. Human embryonic stem cell-derived mesenchymal stromal cell transplantation in a rat hind limb injury model. Cytotherapy 2009, 11, 726–737. [Google Scholar] [CrossRef]
- Du, W.; Li, X.; Chi, Y.; Ma, F.; Li, Z.; Yang, S.; Song, B.; Cui, J.; Ma, T.; Li, J.; et al. VCAM-1+ placenta chorionic villi-derived mesenchymal stem cells display potent pro-angiogenic activity. Stem Cell Res. Ther. 2016, 7, 49. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zheng, L.; Lian, C.; Qi, Y.; Li, W.; Wang, S. Human Umbilical Cord-Derived Mesenchymal Stem Cells Relieve Hind Limb Ischemia by Promoting Angiogenesis in Mice. Stem Cells Dev. 2019, 28, 1384–1397. [Google Scholar] [CrossRef]
- Gao, W.H.; Gao, H.Y.; Li, Y.T.; Huang, P.P. Effectiveness of umbilical cord mesenchymal stem cells in patients with critical limb ischemia. Med. Clin. 2019, 153, 341–346. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, T.J.; Li, Y.; Gao, Y. Mesenchymal Stem Cells Decrease M1/M2 Ratio and Alleviate Inflammation to Improve Limb Ischemia in Mice. Med. Sci. Monit. 2020, 26, e923287. [Google Scholar] [CrossRef] [PubMed]
- Chute, J.P.; Muramoto, G.G.; Whitesides, J.; Colvin, M.; Safi, R.; Chao, N.J.; McDonnell, D.P. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 11707–11712. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Liu, C.; Wang, Y.; Ma, J.; Mao, X.; Li, Q. Netrin-1 promotes mesenchymal stem cell revascularization of limb ischaemia. Diab. Vasc. Dis. Res. 2016, 13, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, S.C.; Kwon, Y.W.; Park, G.T.; Kwon, S.M.; Bae, S.S.; Park, B.J.; Kim, J.H. Mesenchymal Stem Cell-Mediated Therapy of Peripheral Artery Disease Is Stimulated by a Lamin A-Progerin Binding Inhibitor. J. Lipid Atheroscler. 2020, 9, 460–473. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, Y.S.; Lee, S.H. Melatonin-Induced PGC-1α Improves Angiogenic Potential of Mesenchymal Stem Cells in Hindlimb Ischemia. Biomol. Ther. 2020, 28, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Pan, W.Y.; Tseng, M.T.; Lin, K.J.; Yang, Y.P.; Tsai, H.W.; Hwang, S.M.; Chang, Y.; Wei, H.J.; Sung, H.W. Enhancement of cell adhesion, retention, and survival of HUVEC/cbMSC aggregates that are transplanted in ischemic tissues by concurrent delivery of an antioxidant for therapeutic angiogenesis. Biomaterials 2016, 74, 53–63. [Google Scholar] [CrossRef]
- Park, W.S.; Heo, S.C.; Jeon, E.S.; Hong, D.H.; Son, Y.K.; Ko, J.H.; Kim, H.K.; Lee, S.Y.; Kim, J.H.; Han, J. Functional expression of smooth muscle-specific ion channels in TGF-β(1)-treated human adipose-derived mesenchymal stem cells. Am. J. Physiol. Cell Physiol. 2013, 305, C377–C391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.H.; Kim, A.K.; Kim, M.H.; Kim, D.H.; Go, H.N.; Kim, D.I. Enhancement of angiogenic effects by hypoxia-preconditioned human umbilical cord-derived mesenchymal stem cells in a mouse model of hindlimb ischemia. Cell Biol. Int. 2016, 40, 27–35. [Google Scholar] [CrossRef]
- Yin, T.; He, S.; Su, C.; Chen, X.; Zhang, D.; Wan, Y.; Ye, T.; Shen, G.; Wang, Y.; Shi, H.; et al. Genetically modified human placenta-derived mesenchymal stem cells with FGF-2 and PDGF-BB enhance neovascularization in a model of hindlimb ischemia. Mol. Med. Rep. 2015, 12, 5093–5099. [Google Scholar] [CrossRef] [Green Version]
- Ishii, M.; Shibata, R.; Numaguchi, Y.; Kito, T.; Suzuki, H.; Shimizu, K.; Ito, A.; Honda, H.; Murohara, T. Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arter. Thromb. Vasc. Biol. 2011, 31, 2210–2215. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Yue, Z.; Cui, J.; Yao, Y.; Song, X.; Cui, B.; Qi, X.; Han, Z.; Han, Z.C.; Guo, Z.; et al. IGF-1C domain-modified hydrogel enhances therapeutic potential of mesenchymal stem cells for hindlimb ischemia. Stem Cell Res. Ther. 2019, 10, 129. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, Y.S.; Lee, S.H. Long-Duration Three-Dimensional Spheroid Culture Promotes Angiogenic Activities of Adipose-Derived Mesenchymal Stem Cells. Biomol. Ther. 2016, 24, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Poitevin, S.; Cussac, D.; Leroyer, A.S.; Albinet, V.; Sarlon-Bartoli, G.; Guillet, B.; Hubert, L.; Andrieu-Abadie, N.; Couderc, B.; Parini, A.; et al. Sphingosine kinase 1 expressed by endothelial colony-forming cells has a critical role in their revascularization activity. Cardiovasc. Res. 2014, 103, 121–130. [Google Scholar] [CrossRef]
- Hu, G.W.; Li, Q.; Niu, X.; Hu, B.; Liu, J.; Zhou, S.M.; Guo, S.C.; Lang, H.L.; Zhang, C.Q.; Wang, Y.; et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 2015, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016, 34, 601–613. [Google Scholar] [CrossRef] [Green Version]
- Yan, B.; Zhang, Y.; Liang, C.; Liu, B.; Ding, F.; Wang, Y.; Zhu, B.; Zhao, R.; Yu, X.Y.; Li, Y. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/ FOXO3a pathway. Theranostics 2020, 10, 6728–6742. [Google Scholar] [CrossRef]
- Zhang, H.C.; Liu, X.B.; Huang, S.; Bi, X.Y.; Wang, H.X.; Xie, L.X.; Wang, Y.Q.; Cao, X.F.; Lv, J.; Xiao, F.J.; et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012, 21, 3289–3297. [Google Scholar] [CrossRef]
- Kang, T.; Jones, T.M.; Naddell, C.; Bacanamwo, M.; Calvert, J.W.; Thompson, W.E.; Bond, V.C.; Chen, Y.E.; Liu, D. Adipose-Derived Stem Cells Induce Angiogenesis via Microvesicle Transport of miRNA-31. Stem Cells Transl. Med. 2016, 5, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Furth, M.E.; Atala, A.; Van Dyke, M.E. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials 2007, 28, 5068–5073. [Google Scholar] [CrossRef]
- Dahl, S.L.; Blum, J.L.; Niklason, L.E. Bioengineered vascular grafts: Can we make them off-the-shelf? Trends Cardiovasc. Med. 2011, 21, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, P.E.; Driessen-Mol, A.; Frese, L.; Hoerstrup, S.P.; Baaijens, F.P. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 2012, 33, 4545–4554. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Bianco, J.; Brown, C.; Fuetterer, L.; Watkins, J.F.; Samani, A.; Flynn, L.E. Porous decellularized adipose tissue foams for soft tissue regeneration. Biomaterials 2013, 34, 3290–3302. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef]
- Guiducci, S.; Porta, F.; Saccardi, R.; Guidi, S.; Ibba-Manneschi, L.; Manetti, M.; Mazzanti, B.; Dal Pozzo, S.; Milia, A.F.; Bellando-Randone, S.; et al. Autologous mesenchymal stem cells foster revascularization of ischemic limbs in systemic sclerosis: A case report. Ann. Intern. Med. 2010, 153, 650–654. [Google Scholar] [CrossRef]
- Powell, R.J.; Marston, W.A.; Berceli, S.A.; Guzman, R.; Henry, T.D.; Longcore, A.T.; Stern, T.P.; Watling, S.; Bartel, R.L. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: The randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol. Ther. 2012, 20, 1280–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.; Chen, B.; Liang, Z.; Deng, W.; Jiang, Y.; Li, S.; Xu, J.; Wu, Q.; Zhang, Z.; Xie, B.; et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res. Clin. Pract. 2011, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chullikana, A.; Parakh, R.; Desai, S.; Das, A.; Gottipamula, S.; Krishnamurthy, S.; Anthony, N.; Pherwani, A.; Majumdar, A.S. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J. Transl. Med. 2013, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Bura, A.; Planat-Benard, V.; Bourin, P.; Silvestre, J.S.; Gross, F.; Grolleau, J.L.; Saint-Lebese, B.; Peyrafitte, J.A.; Fleury, S.; Gadelorge, M.; et al. Phase I trial: The use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy 2014, 16, 245–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizilay Mancini, Ö.; Lora, M.; Shum-Tim, D.; Nadeau, S.; Rodier, F.; Colmegna, I. A Proinflammatory Secretome Mediates the Impaired Immunopotency of Human Mesenchymal Stromal Cells in Elderly Patients with Atherosclerosis. Stem Cells Transl. Med. 2017, 6, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Gremmels, H.; Teraa, M.; Quax, P.H.; den Ouden, K.; Fledderus, J.O.; Verhaar, M.C. Neovascularization capacity of mesenchymal stromal cells from critical limb ischemia patients is equivalent to healthy controls. Mol. Ther. 2014, 22, 1960–1970. [Google Scholar] [CrossRef] [Green Version]
- Brewster, L.; Robinson, S.; Wang, R.; Griffiths, S.; Li, H.; Peister, A.; Copland, I.; McDevitt, T. Expansion and angiogenic potential of mesenchymal stem cells from patients with critical limb ischemia. J. Vasc. Surg. 2017, 65, 826–838. [Google Scholar] [CrossRef] [Green Version]
- Peeters Weem, S.M.; Teraa, M.; de Borst, G.J.; Verhaar, M.C.; Moll, F.L. Bone Marrow derived Cell Therapy in Critical Limb Ischemia: A Meta-analysis of Randomized Placebo Controlled Trials. Eur J. Vasc. Endovasc. Surg. 2015, 50, 775–783. [Google Scholar] [CrossRef]
- Rigato, M.; Monami, M.; Fadini, G.P. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ. Res. 2017, 120, 1326–1340. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.B.; Looi, Q.H.; How, C.W.; Lee, S.H.; Al-Masawa, M.E.; Chong, P.P.; Law, J.X. Mesenchymal Stem Cell-Derived Exosomes and MicroRNAs in Cartilage Regeneration: Biogenesis, Efficacy, miRNA Enrichment and Delivery. Pharmaceuticals 2021, 14, 1093. [Google Scholar] [CrossRef]
- Munoz-Perez, E.; Gonzalez-Pujana, A.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Mesenchymal Stromal Cell Secretome for the Treatment of Immune-Mediated Inflammatory Diseases: Latest Trends in Isolation, Content Optimization and Delivery Avenues. Pharmaceutics 2021, 13, 1802. [Google Scholar] [CrossRef]
- Aneesh, A.; Liu, A.; Moss, H.E.; Feinstein, D.; Ravindran, S.; Mathew, B.; Roth, S. Emerging concepts in the treatment of optic neuritis: Mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2021, 12, 594. [Google Scholar] [CrossRef]
- Johnson, J.; Shojaee, M.; Mitchell Crow, J.; Khanabdali, R. From Mesenchymal Stromal Cells to Engineered Extracellular Vesicles: A New Therapeutic Paradigm. Front. Cell Dev. Biol. 2021, 9, 705676. [Google Scholar] [CrossRef]
- Tan, T.T.; Lai, R.C.; Padmanabhan, J.; Sim, W.K.; Choo, A.B.H.; Lim, S.K. Assessment of Tumorigenic Potential in Mesenchymal-Stem/Stromal-Cell-Derived Small Extracellular Vesicles (MSC-sEV). Pharmaceutics 2021, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Gregory, C.A.; Lee, R.H.; Reger, R.L.; Qin, L.; Hai, B.; Park, M.S.; Yoon, N.; Clough, B.; McNeill, E.; et al. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proc. Natl. Acad. Sci. USA 2015, 112, 530–535. [Google Scholar] [CrossRef] [Green Version]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef] [Green Version]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marban, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Jiang, Y.; Deng, W.; Zhang, Y.; Liang, Z.; Wu, Q.; Jiang, X.; Zhang, L.; Gao, F.; Cao, Y.; et al. Long-Term Outcomes of BMMSC Compared with BMMNC for Treatment of Critical Limb Ischemia and Foot Ulcer in Patients with Diabetes. Cell Transplant. 2019, 28, 645–652. [Google Scholar] [CrossRef]
- Kerstan, A.; Niebergall-Roth, E.; Esterlechner, J.; Schroder, H.M.; Gasser, M.; Waaga-Gasser, A.M.; Goebeler, M.; Rak, K.; Schrufer, P.; Endres, S.; et al. Ex vivo-expanded highly pure ABCB5(+) mesenchymal stromal cells as Good Manufacturing Practice-compliant autologous advanced therapy medicinal product for clinical use: Process validation and first in-human data. Cytotherapy 2021, 23, 165–175. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshaer, S.L.; Bahram, S.H.; Rajashekar, P.; Gangaraju, R.; El-Remessy, A.B. Modulation of Mesenchymal Stem Cells for Enhanced Therapeutic Utility in Ischemic Vascular Diseases. Int. J. Mol. Sci. 2022, 23, 249. https://doi.org/10.3390/ijms23010249
Elshaer SL, Bahram SH, Rajashekar P, Gangaraju R, El-Remessy AB. Modulation of Mesenchymal Stem Cells for Enhanced Therapeutic Utility in Ischemic Vascular Diseases. International Journal of Molecular Sciences. 2022; 23(1):249. https://doi.org/10.3390/ijms23010249
Chicago/Turabian StyleElshaer, Sally L., Salma H. Bahram, Pranav Rajashekar, Rajashekhar Gangaraju, and Azza B. El-Remessy. 2022. "Modulation of Mesenchymal Stem Cells for Enhanced Therapeutic Utility in Ischemic Vascular Diseases" International Journal of Molecular Sciences 23, no. 1: 249. https://doi.org/10.3390/ijms23010249
APA StyleElshaer, S. L., Bahram, S. H., Rajashekar, P., Gangaraju, R., & El-Remessy, A. B. (2022). Modulation of Mesenchymal Stem Cells for Enhanced Therapeutic Utility in Ischemic Vascular Diseases. International Journal of Molecular Sciences, 23(1), 249. https://doi.org/10.3390/ijms23010249







