Immunopathological Analysis of a Mouse Model of Arthritis-Associated Scleritis and Implications for Molecular Targeted Therapy for Severe Scleritis
Abstract
:1. Introduction
2. Results
2.1. Mouse Model of Collagen II (CII)-Induced Arthritis-Associated Scleritis (CIA-Scleritis)
2.1.1. Clinical Appearance and Histological Findings of CIA-Scleritis
2.1.2. Immune Cells, Complement, Immunoglobulin, and Hem- and Lymph-Angiogenesis in CIA-Scleritis
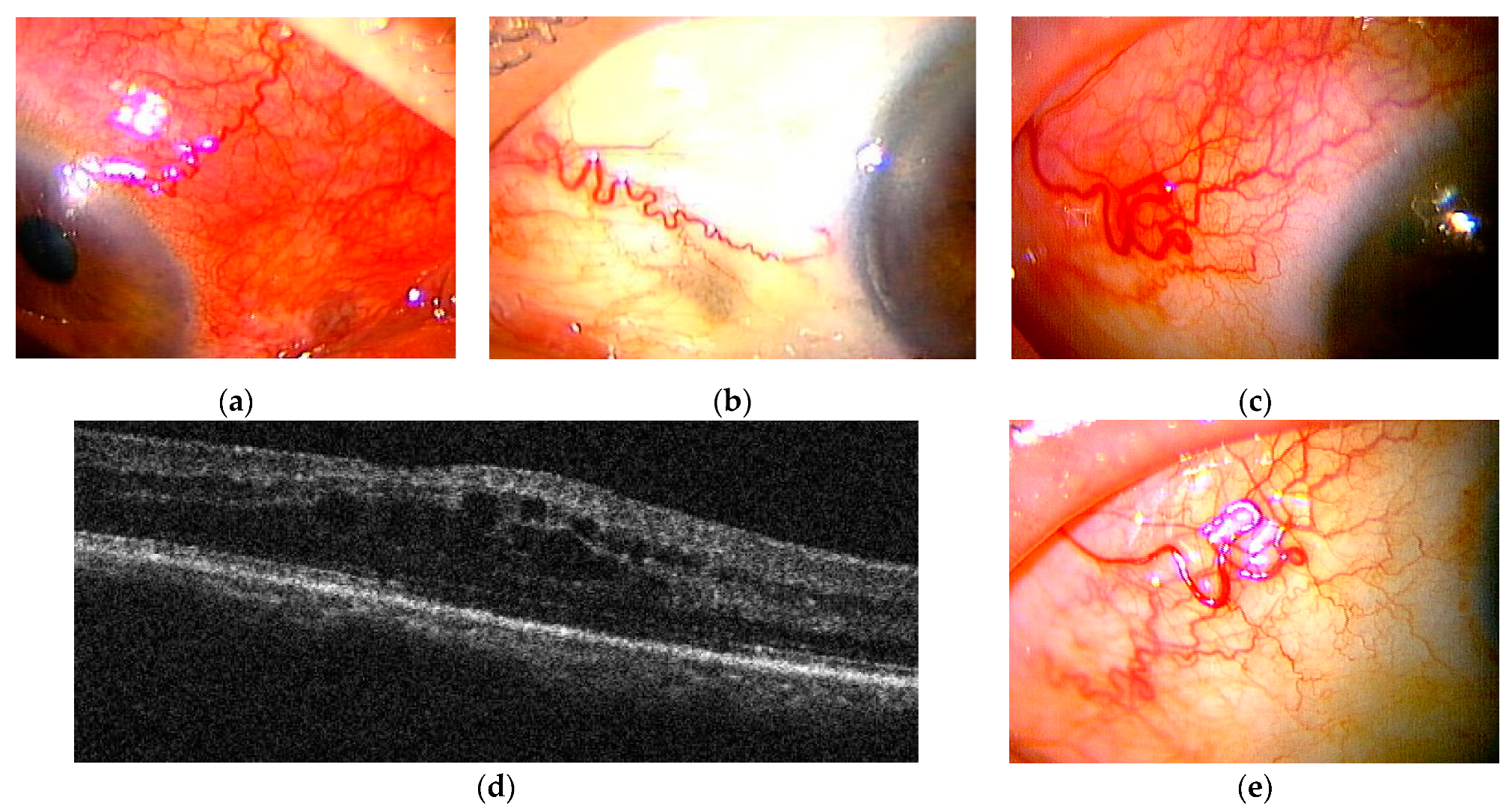
2.2. Case Series Study of Posterior Scletritis
2.2.1. Case Presentations of Severe Posterior Scleritis Treated with Molecularly Targeted Therapies
Case 1
Case 2
Case 3
3. Discussion
4. Materials and Methods
4.1. Mouse Model of CIA-Scleritis
Mice and Anesthesia
4.2. Abs
4.3. CIA-Scleritis
4.4. Evaluation of Arthritis
4.5. Evaluation of Scleritis
4.6. Histology and Immunohistochemistry
4.7. Statistical Analyses
4.8. Case Series Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Okhravi, N.; Odufuwa, B.; McCluskey, P.; Lightman, S. Scleritis. Surv. Ophthalmol. 2005, 50, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.J.; Sobol, E.K.; Gritz, D.C. Treatment and management of scleral disorders. Surv. Ophthalmol. 2016, 61, 702–717. [Google Scholar] [CrossRef]
- Wieringa, W.G.; Wieringa, J.E.; Loon, N.; Los, L.I. Visual outcome, treatment results, and prognostic factors in patients with scleritis. Ophthalmology 2013, 120, 379–386. [Google Scholar] [CrossRef]
- Smith, J.R.; Mackensen, F.; Rosenbaum, J.T. Therapy insight: Scleritis and its relationship to systemic autoimmune disease. Nat. Clin. Pract. Rheumatol. 2007, 3, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lavric, A.; Gonzalez-Lopez, J.J.; Majumder, P.D.; Bansal, N.; Biswas, J.; Pavesio, C.; Agrawal, R. Posterior Scleritis: Analysis of Epidemiology, Clinical Factors, and Risk of Recurrence in a Cohort of 114 Patients. Ocul. Immunol. Inflamm. 2016, 24, 6–15. [Google Scholar] [CrossRef]
- Akpek, E.K.; Thorne, J.E.; Qazi, F.A.; Do, D.V.; Jabs, D.A. Evaluation of patients with scleritis for systemic disease. Ophthalmology 2004, 111, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.G.; Hayreh, S.S. Scleritis and episcleritis. Br. J. Ophthalmol. 1976, 60, 163–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sainz-de-la-Maza, M.; Jabbur, N.S.; Foster, S.C. Severity of scleritis and episcleritis. Ophthalmology 1994, 101, 389–396. [Google Scholar] [CrossRef]
- Maza, M.S.; Molina, N.; Gonzalez-Gonzalez, L.A.; Doctor, P.P.; Tauber, J.; Foster, C.S. Clinical characteristics of a large cohort of patients with scleritis and episcleritis. Ophthalmology 2012, 119, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Keino, H.; Nakayama, M.; Watanabe, T.; Okada, A.A. Clinical Features, Treatment, and Visual Outcomes of Japanese Patients with Posterior Scleritis. Ocul. Immunol. Inflamm. 2020, 28, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Z.; Gan, Y.F.; Zhang, Y.N.; Zhang, Y.; Li, J.; Zheng, H.H. The clinical features of posterior scleritis with serous retinal detachment: A retrospective clinical analysis. Int. J. Ophthalmol. 2019, 18, 1151–1157. [Google Scholar] [CrossRef]
- Inan, S.; Ertan, E.; Inan, U.U. Multimodal imaging in a child with severe posterior scleritis. Rom. J. Ophthalmol. 2019, 63, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gonzalez, L.A.; Molina-Prat, N.; Doctor, P.; Tauber, J.; Sainz de la Maza, M.; Foster, C.S. Clinical features and presentation of posterior scleritis: A report of 31 cases. Ocul. Immunol. Inflamm. 2014, 22, 203–207. [Google Scholar] [CrossRef] [PubMed]
- McGavin, D.D.M.; Williamson, J.; Forrester, J.V.; Foulds, W.S.; Buchanan, W.W.; Dick, W.C.; Lee, P.; MacSween, R.N.; Whaley, K. Episcleritis and scleritis. A study of their clinical manifestation and association with rheumatoid arthritis. Br. J. Ophthalmol. 1976, 60, 192–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calthorpe, C.M.; Watson, P.G.; McCartney, A.C. Posterior scleritis: A clinical and histological survey. Eye 1988, 2, 267–277. [Google Scholar] [CrossRef]
- Keino, H.; Watanabe, T.; Taki, W.; Nakashima, C.; Okada, A.A. Clinical features and visual outcomes of Japanese patients with scleritis. Br. J. Ophthalmol. 2010, 94, 1459–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignesh, A.P.P.; Srinivasan, R. Ocular manifestations of rheumatoid arthritis and their correlation with anti-cyclic citrullinated peptide antibodies. Clin. Ophthalmol. 2015, 9, 393–397. [Google Scholar] [PubMed] [Green Version]
- Bettero, R.G.; Cebrian, R.F.M.; Skare, T.L. Prevalence of ocular manifestation in 198 patients with rheumatoid arthritis: A retrospective study. Arq. Bras. Ophtalmol. 2008, 71, 365–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlatanovic, G.; Veselinovic, D.; Cekic, S.; Zivkovic, M.; Dordevic-Jocic, J.; Zlatanovic, M. Ocular manifestation of rheumatoid arthritis-different forms and frequency. Bosn. J. Basic Med. Sci. 2010, 10, 323–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakayama, K.; Hori, J. Review of scleritis and Episcleritis at Nippon Medical School Hospital. J. Eye 2010, 27, 663–666. [Google Scholar]
- Benson, W.E. Posterior scleritis. Surv. Ophthalmol. 1988, 32, 297–316. [Google Scholar] [CrossRef]
- Yoshida, A.; Watanabe, M.; Okubo, A.; Kawashima, H. Clinical characteristics of scleritis patients with emphasized comparison of associated systemic diseases (anti-neutrophil cytoplasmic antibody-associated vasculitis and rheumatoid arthritis). Jpn. J. Ophthalmol. 2019, 63, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.; Li, Z.; Sen, H.N.; Lim, W.; Gill, F.; Perkins, K.; Rao, V.K.; Nussenblatt, R.B. Scleritis and multiple systemic Autoimmune manifestations in chronic natural killer cell lymphocytosis associated with elevated TCR alpha/beta+ CD3+CD4-CD8- double-negative T cells. Br. J. Ophthalmol. 2010, 94, 748–752. [Google Scholar] [CrossRef]
- McCluskey, P.J.; Watson, P.G.; Lightman, S.; Haybittle, J.; Restori, M.; Branley, M. Posterior scleritis: Clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology 1999, 106, 2380–2386. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Dick, A.D.; Brézin, A.P.; Nguyen, Q.D.; Thorne, J.E.; Kestelyn, P.; Barisani-Asenbauer, T.; Franco, P.; Heiligenhaus, A.; Scales, D.; et al. Adalimumab in Patients with Active Noninfectious Uveitis. N. Engl. J. Med. 2016, 375, 932–943. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.C.; Sy, A.; Cunningham, E.T., Jr. Rituximab for non-infectious Uveitis and Scleritis. J. Ophthalmic Inflamm. Infect. 2021, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, C.; Sota, J.; Sainz-de-la-Maza, M.; Pelegrin, L.; Emmi, G.; Lopalco, G.; Iannone, F.; Vannozzi, L.; Guerriero, S.; Frediani, B.; et al. Effectiveness of TNF-αblockade in the treatment of refractory non-infectious scleritis: A multicentre study. Clin. Exp. Rheumatol. 2020, 38, 1138–1144. [Google Scholar]
- Shenoy, R.; Suryawanshi, M.; Isaac, R.; Philip, S.K. Posterior scleritis in pediatric age group: A case report and Review of literature. Oman J. Ophthalmol. 2016, 9, 59–62. [Google Scholar] [CrossRef]
- RIono, W.P.; Hidayat, A.A.; Rao, N.A. Scleritis: A clinicopathologic study of 55 cases. Ophthalmology 1999, 106, 1328–1333. [Google Scholar] [CrossRef]
- Hankins, M.; Margo, C.E. Histopathological evaluation of scleritis. J. Clin. Pathol. 2019, 72, 386–390. [Google Scholar] [CrossRef]
- Watson, P.G.; Romano, A. The impact of new methods of investigation and treatment on the understanding of the pathology of scleral inflammation. Eye 2014, 28, 915–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergouwen, D.P.C.; Rothova, A.; Berge, J.C.T.; Verdijk, R.M.; Laar, J.A.M.; Vingerling, J.R.; Schreurs, M.W.J. Current insights in the pathogenesis of scleritis. Exp. Eye Res. 2020, 197, 108078. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.P.; Maza, M.S.; Beverly, A.; Rice, B.A.; Amy, E.; Kupferman, B.S.; Foster, C.S. Immunopathology of Scleritis. Ophthalmology 1991, 98, 472–479. [Google Scholar] [CrossRef]
- Wilhelmus, K.R.; Grierson, I.; Watson, P.G. Histopathologic and clinical associations of scleritis and glaucoma. Am. J. Ophthalmol. 1981, 91, 697–705. [Google Scholar] [CrossRef]
- Fraunfelder, F.T.; Watson, P.G. Evaluation of eyes enucleated for scleritis. Br. J. Ophthalmol. 1976, 60, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Fidelix, T.S.A.; Vieira, L.A.; Freitas, D.; Trevisani, V.F.M. Biologic therapy for refractory scleritis: A new treatment perspective. Int. Ophthalmol. 2015, 35, 903–912. [Google Scholar] [CrossRef]
- Silpa-Archa, S.; Oray, M.; Preble, J.M.; Foster, C.S. Outcome of tocilizumab treatment in refractory ocular inflammatory diseases. Acta Ophthalmol. 2016, 94, e400–e406. [Google Scholar] [CrossRef] [Green Version]
- Wakefield, D.; Di Girolamo, N.; Thurau, S.; Wildner, G.; McCluskey, P. Scleritis: Immunopathogenesis and molecular basis for therapy. Prog. Retin. Eye Res. 2013, 35, 44–62. [Google Scholar] [CrossRef]
- Lim, L.; Suhler, E.B.; Smith, J.R. Biologic therapies for inflammatory eye disease. Clin. Exp. Ophthalmol. 2006, 34, 365–374. [Google Scholar] [CrossRef]
- Suhler, E.B.; Lim, L.L.; Beardsley, R.M.; Giles, T.R.; Pasadhika, S.; Lee, S.T.; Sardos, A.S.; Butler, N.J.; Smith, J.R.; Rosenbaum, J.T. Rituximab in the treatment of refractory scleritis and non-infeccious orbitary inflammation: Preliminary results from a Phase I/II prospective, randomized, dose-ranging pilot study. JAMA Ophthalmol. 2014, 132, 572–578. [Google Scholar] [CrossRef]
- Usui, Y.; Parikh, J.; Goto, H. Immunopathology of necrotizing scleritis. Br. J. Ophthalmol. 2008, 92, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, N.; Lloyd, A.; McCluskey, P.; Filipic, M.; Wakefield, D. Imcreased expression of matrix metalloproteinases in vivo in scleritis tissue and in vitro in cultures human scleral fibroblasts. Am. J. Pathol. 1997, 150, 653–666. [Google Scholar]
- Skapenko, A.; Leipe, J.; Lipsky, P.E.; Schulze-Koops, H. The role of the T cell in autoimmune inflammation. Arthritis Res. 2005, 2, S4–S14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, L.; Jirholt, J.; Holmdahl, R.; Jansson, L. B cell deficient mice do not develop type II collagen-induced arthritis (CIA). Clin. Exp. Immunol. 1998, 111, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Wooley, P.H.; Luthra, H.S.; Griffiths, M.M.; Stuart, J.M.; Huse, A.; David, C.S. Type II collagen-induced arthritis in mice. IV. Variations in immunogenetic regulation provide evidence for multiple arthritogenic epitopes on the collagen molecule. J. Immunol. 1985, 135, 2443–2451. [Google Scholar] [PubMed]
- Ridgley, L.A.; Anderson, A.E.; Pratt, A.G. What are the dominant cytokines in early rheumatoid arthritis? Curr. Opin Rheumatol. 2018, 30, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Oishi, N.; Takeda, A.; Hori, J. Two Cases of Scleritis Induced as a Paradoxical Reaction to CTLA4Ig. J. Eye 2020, 37, 636–639. [Google Scholar]
- Doctor, P.; Sultan, A.; Syed, S.; Christen, W.; Bhat, P.; Foster, C.S. Infliximab for the treatment of refractory scleritis. Br. J. Ophthalmol. 2010, 94, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Sobrin, L.; Kim, E.C.; Christen, W.; Papadaki, T.; Letko, E.; Foster, C.S. Infliximab therapy for the treatment of refractory ocular inflammatory disease. Arch. Ophthalmol. 2007, 125, 895–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paley, M.A.; Karacal, H.; Rao, P.K.; Margolis, T.P.; Miner, J.J. Tofacitinib for refractory uveitis and scleritis. Am. J. Ophthalmol. Case Rep. 2018, 4, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Suhler, E.B.; Smith, J.R.; Wertheim, M.S.; Lauer, A.K.; Kurz, D.E.; Pickard, T.D.; Rosenbaum, J.T. A prospective Trial of infliximab therapy for refractory uveitis: Preliminary safety and efficacy outcomes. Arch. Ophthalmol. 2005, 123, 903–912. [Google Scholar] [CrossRef] [Green Version]
- Ragam, A.; Kolomeyer, A.M.; Fang, C.; Xu, Y.; Chu, D.S. Treatment of chronic, noninfectious, nonnecrotizing scleritis with tumor necrosis factor alpha inhibitors. Ocul. Immunol. Inflamm. 2014, 22, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.M.; Damato, E.; Hinchcliffe, A.E.; Andrews, C.D.; Myint, K.; Lee, R.; Dick, A.D. Long-term efficacy and tolerability of TNFα inhibitors in the treatment of non-infectious ocular inflammation: An 8-year prospective surveillance study. Br. J. Ophthalmol. 2021, 105, 1256–1262. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, A.; Seroogy, C.M.; Sandora, M.R.; Tarner, I.H.; Costa, G.L.; Taylor-Edwards, C.; Bachmann, M.H.; Contag, C.H.; Fathman, C.G. Antigen-specific T cell-mediated gene therapy in collagen-induced arthritis. J. Clin. Investig. 2001, 107, 1293–1301. [Google Scholar] [CrossRef]










| Case No. | Gender | Age at Ocular Inflammation Onset | Age of Posterior Scleritis Onset | Follow-Up (Month) | Laterality | Associated Systemic Disease |
|---|---|---|---|---|---|---|
| 1 | M | 81 | 82 | 23 | B | − |
| 2 | F | 76 | 76 | 19 | B | − |
| 3 | F | 31 | 37 | 165 | B | − |
| 4 | F | 85 | 85 | 25 | R | − |
| 5 | F | 36 | 36 | 52 | R | − |
| 6 | F | 75 | 80 | 29 | R | RA |
| 7 | F | 82 | 83 | 28 | L | AAV |
| Case No. | Ocular Pain | Associated Anterior Scleritis | Anterior Chamber Cells | Serous Retinal Detachment | RPE Folds | Optic disc Swelling/Hyperemia | Ocular Hypertension (>21 mmHg) |
|---|---|---|---|---|---|---|---|
| 1 | + | + | − | + | + | −/− | + |
| 2 | + | − | − | + | + | +/+ | − |
| 3 | + | + | − | + | + | −/− | + |
| 4 | + | + | + | + | + | −/− | + |
| 5 | + | − | − | + | + | −/− | − |
| 6 | + | + | − | + | + | −/− | − |
| 7 | + | + | + | + | + | −/− | − |
| Case No. | Topical Treatment | Systemic Treatment | Recurrences (Number) | Per- sistent | BCVA before Treatment OD, OS | BCVA after Treatment OD, OS | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eye Drops | Subconjunctival Injection of Steroid | Oral NSAIDs | Oral Steroid | Steroid Pulse Therapy | Immune Suppressant | Biologics | |||||
| 1 | + | − | + | + | + | − | − | 0 | − | 20/500, 20/32 | 20/12, 20/12 |
| 2 | + | − | + | + | + | + | − | 2 | − | 20/20, 20/32 | 20/16, 20/20 |
| 3 | + | + | + | + | − | + | − | 5 | − | 20/32, 20/25 | 20/12, 20/20 |
| 4 | + | + | + | + | − | − | − | 0 | − | 20/32, 20/25 | 20/40, 20/20 |
| 5 | + | − | + | + | + | +(CysA) | +(ADA) | 2 | − | 20/25, 20/16 | 20/12, 20/16 |
| 6 | + | + | + | + | − | +(MTX, TAC) | +(CTLA4Ig → GLM →SAR) | 1 | − | 20/1000, 20/32 | 20/600, 20/20 |
| 7 | + | + | + | + | + | − | +(RTX) | 1 | + | 20/25, 20/600 | 20/25, 20 cm HM |
| Total | 7/7 (100%) | 4/7 (57%) | 7/7 (100%) | 7/7 (100%) | 4/7 (57%) | 4/7 (57%) | 3/7 (43%) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishio, Y.; Taniguchi, H.; Takeda, A.; Hori, J. Immunopathological Analysis of a Mouse Model of Arthritis-Associated Scleritis and Implications for Molecular Targeted Therapy for Severe Scleritis. Int. J. Mol. Sci. 2022, 23, 341. https://doi.org/10.3390/ijms23010341
Nishio Y, Taniguchi H, Takeda A, Hori J. Immunopathological Analysis of a Mouse Model of Arthritis-Associated Scleritis and Implications for Molecular Targeted Therapy for Severe Scleritis. International Journal of Molecular Sciences. 2022; 23(1):341. https://doi.org/10.3390/ijms23010341
Chicago/Turabian StyleNishio, Yusuke, Hiroko Taniguchi, Ayaka Takeda, and Junko Hori. 2022. "Immunopathological Analysis of a Mouse Model of Arthritis-Associated Scleritis and Implications for Molecular Targeted Therapy for Severe Scleritis" International Journal of Molecular Sciences 23, no. 1: 341. https://doi.org/10.3390/ijms23010341






