Hyperthermia Enhances Doxorubicin Therapeutic Efficacy against A375 and MNT-1 Melanoma Cells
Abstract
:1. Introduction
2. Results
2.1. DOX Decreases A375 and MNT-1 Viability
2.2. Combination of DOX and 43 °C Hyperthermia Decreases Cell Viability in A375 and MNT-1 Cell Lines
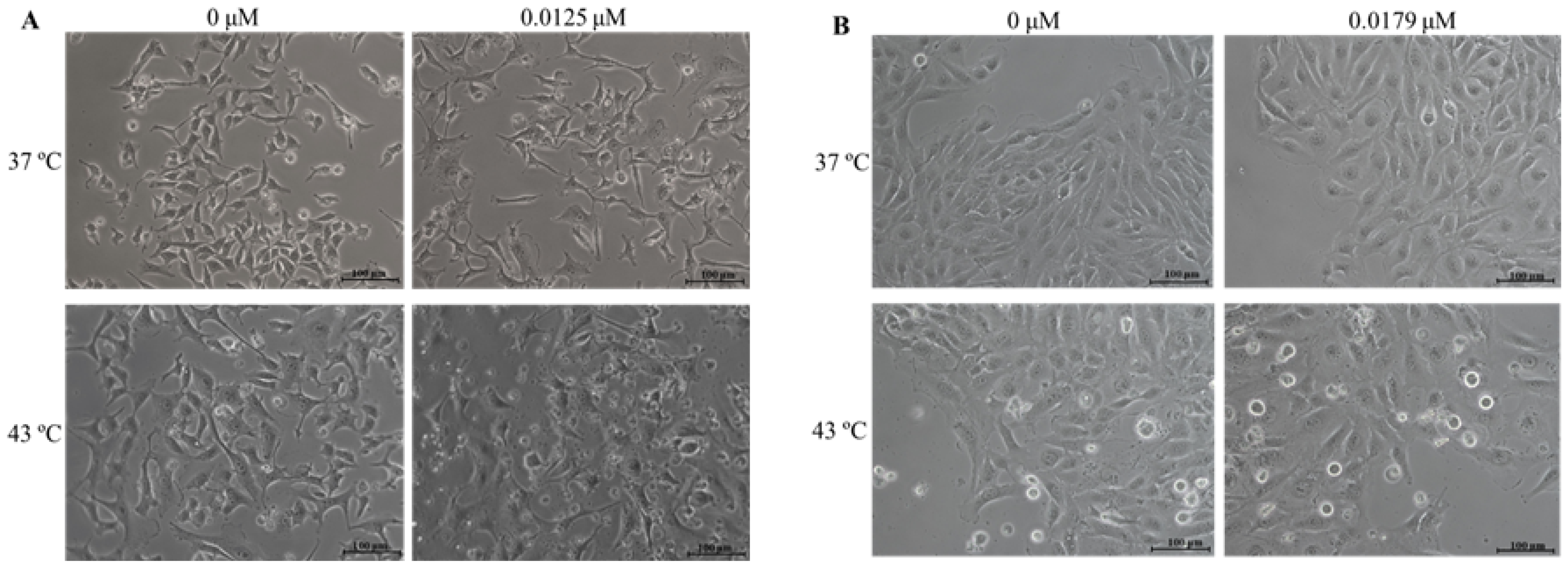
2.3. Combination of DOX and 43 °C Hyperthermia Alters Cell Morphology
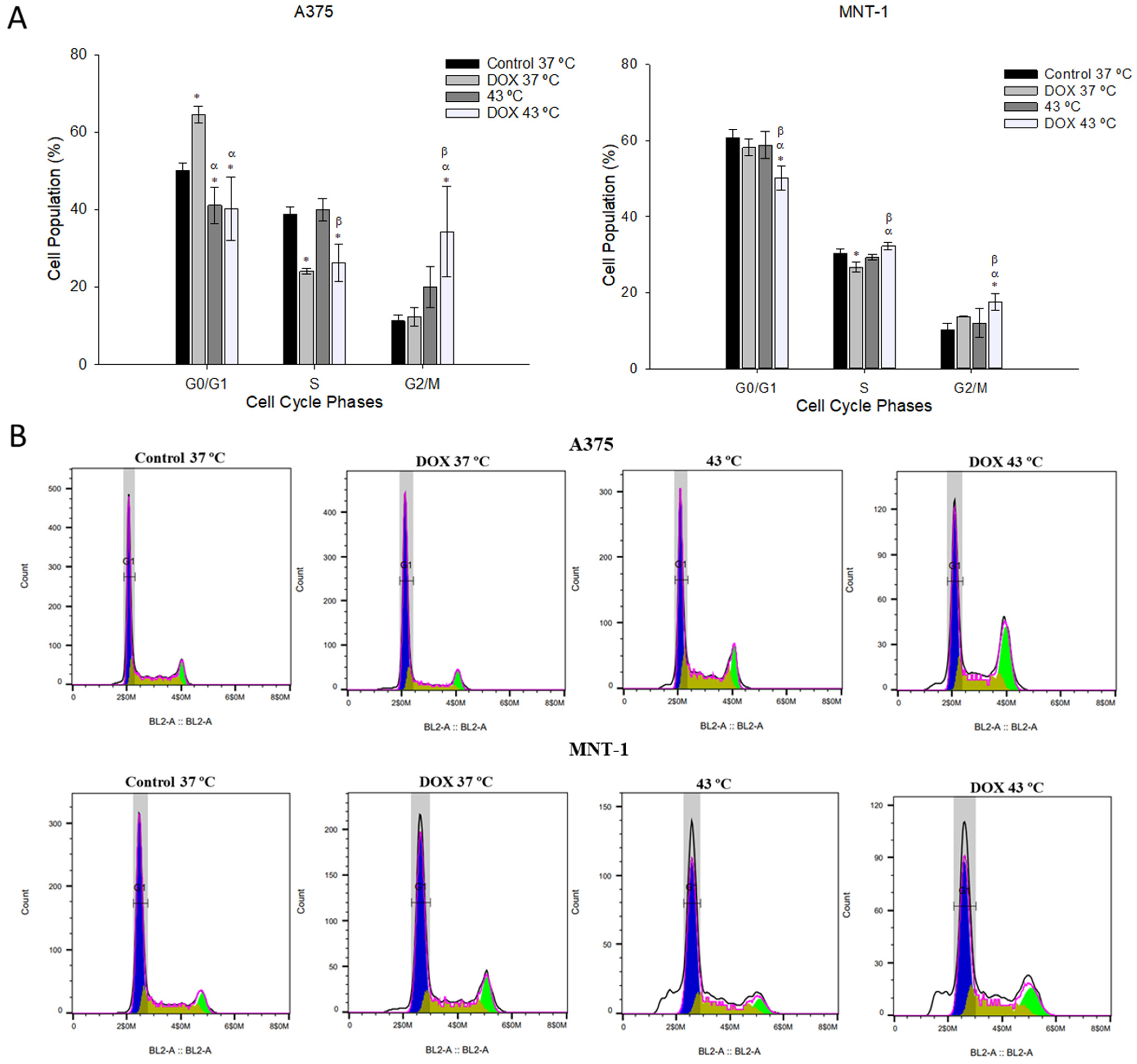
2.4. Combination of DOX and 43 °C Hyperthermia Induces Cell Cycle Arrest at G2/M Phase
2.5. Combination of DOX and 43 °C Hyperthermia Increases Intracellular ROS Levels
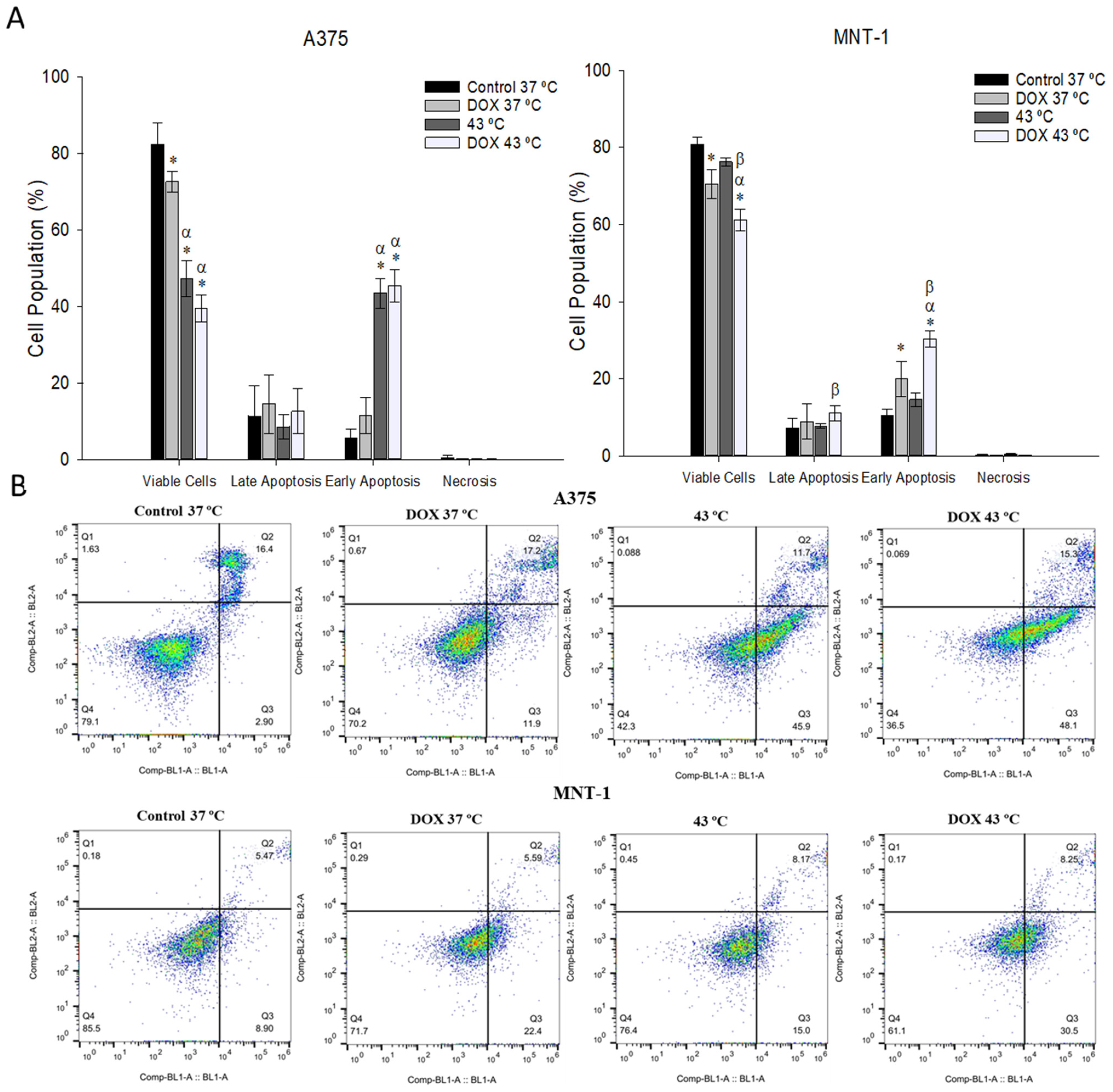
2.6. Combination of DOX and 43 °C Hyperthermia Induces Apoptosis in MNT-1 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Determination of Cell Viability
4.2.1. Exposure to DOX
4.2.2. Exposure to Hyperthermia Combined with DOX
4.2.3. Cell Viability Measurements
4.3. Cell Morphology
4.4. Cell Cycle Analysis
4.5. Analysis of Intracellular ROS
4.6. Cell Apoptosis Assay
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Olaku, O.O.; Taylor, E.A. Cancer in the Medically Underserved Population. Prim. Care 2017, 44, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezes, A.C.; Raposo, S.; Simões, S.; Ribeiro, H.; Oliveira, H.; Ascenso, A. Prevention of Photocarcinogenesis by Agonists of 5-HT1A and Antagonists of 5-HT2A Receptors. Mol. Neurobiol. 2016, 53, 1145–1164. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, F.; Rajotte, V.; Drobetsky, E.A. A majority of human melanoma cell lines exhibits an S phase-specific defect in excision of UV-induced DNA photoproducts. PLoS ONE 2014, 9, e85294. [Google Scholar] [CrossRef] [Green Version]
- Kibbi, N.; Kluger, H.; Choi, J.N. Melanoma: Clinical presentations. Cancer Treat. Res. 2016, 167, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, N.; Della Corte, M.; Pelaia, C.; Piazzetta, G.; Lobello, N.; Del Duca, E.; Bennardo, L.; Nisticò, S.P. Primary Mucosal Melanoma Presenting with a Unilateral Nasal Obstruction of the Left Inferior Turbinate. Medicina 2021, 57, 359. [Google Scholar] [CrossRef] [PubMed]
- Yde, S.S.; Sjoegren, P.; Heje, M.; Stolle, L.B. Mucosal Melanoma: A Literature Review. Curr. Oncol. Rep. 2018, 20, 28. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannan, B.; Perego, M.; Somasundaram, R.; Herlyn, M. Heterogeneity in melanoma. Melanoma 2016, 167, 1–15. [Google Scholar]
- Yoncheva, K.; Merino, M.; Shenol, A.; Daskalov, N.; Petkov, P.; Vayssilov, G.; Garrido, M. Optimization and in-vitro/in-vivo evaluation of doxorubicin-loaded chitosan-alginate nanoparticles using a melanoma mouse model. Int. J. Pharm. 2019, 556, 1–8. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, Q.; Shen, S. Enhanced antitumor efficacy and attenuated cardiotoxicity of doxorubicin in combination with lycopene liposomes. J. Liposome Res. 2020, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Rui, M.; Shen, H.; Xin, Y.; Zhang, J.; Li, J.; Yue, L.; Lai, W.; Xu, X. Tumor-specific delivery of doxorubicin through conjugation of pH-responsive peptide for overcoming drug resistance in cancer. Int. J. Pharm. 2017, 528, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Takemura, G.; Fujiwara, H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 2007, 49, 330–352. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.; Cardoso, S.; Correia, S.; Oliveira, P.; Santos, M.; Moreira, P. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Kumar, A.; Bhatkar, D.; Sharma, N.K. Molecular avenues in targeted doxorubicin cancer therapy. Future Oncol. 2020, 16, 687–700. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologie developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, N.; Poulton, J. Mitochondrial DNA is a direct target of anti-cancer anthracycline drugs. Biochem. Biophys. Res. Commun. 2009, 378, 450–455. [Google Scholar] [CrossRef]
- Petznek, H.; Kleiter, M.; Tichy, A.; Fuchs-Baumgartinger, A.; Hohenadl, C. Murine xenograft model demonstrates significant radio-sensitising effect of liposomal doxorubicin in a combination therapy for Feline Injection Site Sarcoma. Res. Vet. Sci. 2014, 97, 386–390. [Google Scholar] [CrossRef]
- Rocconi, R.; Straughn, J.; Leath, C.; Kilgore, L.; Huh, W.; Barnes, M.; Partridge, E.; Alvarez, R. Pegylated liposomal doxorubicin consolidation therapy after platinum/paclitaxel-based chemotherapy for suboptimally debulked, advanced-stage epithelial ovarian cancer patients. Oncologist 2006, 11, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Szwed, M.; Laroche-Clary, A.; Robert, J.; Jozwiak, Z. Induction of apoptosis by doxorubicin-transferrin conjugate compared to free doxorubicin in the human leukemia cell lines. Chem. Biol. Interact. 2014, 220, 140–148. [Google Scholar] [CrossRef]
- Yuan, M.; Qiu, Y.; Zhang, L.; Gao, H.; He, Q. Targeted delivery of transferrin and TAT co-modified liposomes encapsulating both paclitaxel and doxorubicin for melanoma. Drug Deliv. 2016, 23, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.; McBride, R.; Eisenberger, A.; Tsai, W.; Grann, V.; Jacobson, J. Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 2008, 26, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Wadler, S.; Yang, C.P. Reversal of doxorubicin resistance by hydrophobic, but not hydrophilic, forskolins. Mol. Pharmacol. 1991, 40, 960–964. [Google Scholar]
- Abushouk, A.; Ismail, A.; Salem, A.; Afifi, A.; Abdel-Daim, M. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2017, 90, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; El-Azem, N.; Pamplona, R.; Ramirez-Tortosa, C.; Pulido-Moran, M.; Vera-Ramirez, L.; Quiles, J.; Sanchez-Rovira, P.; Naudí, A.; Portero-Otin, M.; et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem. Pharmacol. 2014, 90, 25–33. [Google Scholar] [CrossRef]
- Harima, Y.; Ohguri, T.; Imada, H.; Sakurai, H.; Ohno, T.; Hiraki, Y.; Tuji, K.; Tanaka, M.; Terashima, H. A multicentre randomised clinical trial of chemoradiotherapy plus hyperthermia versus chemoradiotherapy alone in patients with locally advanced cervical cancer. Int. J. Hyperth. 2016, 32, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Özayral, S.; Caserto, J.S.; ten Cate, R.; Anders, N.M.; Barnett, J.D.; Kandala, S.K.; Henderson, E.; Stewart, J.; Liapi, E.; et al. Increased uptake of doxorubicin by cells undergoing heat stress does not explain its synergistic cytotoxicity with hyperthermia. Int. J. Hyperth. 2019, 36, 712–720. [Google Scholar] [CrossRef]
- Krawczyk, P.; Eppink, B.; Essers, J.; Stap, J.; Rodermond, H.; Odijk, H.; Zelensky, A.; van Bree, C.; Stalpers, L.; Buist, M.; et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc. Natl. Acad. Sci. USA 2011, 108, 9851–9856. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, S.; Choi, B.-H.; Park, M.-T.; Lee, J.; Jeong, S.-Y.; Choi, E.K.; Lim, B.-U.; Kim, C.; Park, H.J. Hyperthermia improves therapeutic efficacy of doxorubicin carried by mesoporous silica nanocontainers in human lung cancer cells. Int. J. Hyperth. 2011, 27, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.L.; Vriend, L.E.M.; Crezee, J.; Franken, N.A.P.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaupel, P.; Horsman, M.R. Tumour perfusion and associated physiology: Characterization and significance for hyperthermia. Int. J. Hyperth. 2010, 26, 209–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Susa, M.; Iyer, A.K.; Ryu, K.; Hornicek, F.J.; Mankin, H.; Amiji, M.M.; Duan, Z. Doxorubicin loaded Polymeric Nanoparticulate Delivery System to overcome drug resistance in osteosarcoma. BMC Cancer 2009, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Smylie, M.; Wong, R.; Mihalcioiu, C.; Lee, C.; Pouliot, J. A phase II, open label, monotherapy study of liposomal doxorubicin in patients with metastatic malignant melanoma. Investig. New Drugs 2007, 25, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Vorobiof, D.; Rapoport, B.; Mahomed, R.; Karime, M. Phase II study of pegylated liposomal doxorubicin in patients with metastatic malignant melanoma failing standard chemotherapy treatment. Melanoma Res. 2003, 13, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Teodoro, J.G.; Nadeau, J.L. Intratumoral gold-doxorubicin is effective in treating melanoma in mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1365–1375. [Google Scholar] [CrossRef]
- Cox, J.; Weinman, S. Mechanisms of doxorubicin resistance in hepatocellular carcinoma. Hepat. Oncol. 2016, 3, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; He, Q.; Gao, Y.; Shi, J.; Li, Y. Mesoporous silica nanoparticles loading doxorubicin reverse multidrug resistance: Performance and mechanism. Nanoscale 2011, 3, 4314–4322. [Google Scholar] [CrossRef]
- Terasaki, A.; Kurokawa, H.; Ito, H.; Komatsu, Y.; Matano, D.; Terasaki, M.; Bando, H.; Hara, H.; Matsui, H. Elevated Production of Mitochondrial Reactive Oxygen Species via Hyperthermia Enhanced Cytotoxic Effect of Doxorubicin in Human Breast Cancer Cell Lines MDA-MB-453 and MCF-7. Int. J. Mol. Sci. 2020, 21, 9522. [Google Scholar] [CrossRef]
- Blasiak, J.; Widera, K.; Pertyński, T. Hyperthermia can differentially modulate the repair of doxorubicin-damaged DNA in normal and cancer cells. Acta Biochim. Pol. 2003, 50, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Guy, G.P.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C.; Centers for Disease Control and Prevention (CDC). Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982–2030. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. [Google Scholar]
- Li, J.; Wang, Y.; Liang, R.; An, X.; Wang, K.; Shen, G.; Tu, Y.; Zhu, J.; Tao, J. Recent advances in targeted nanoparticles drug delivery to melanoma. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 769–794. [Google Scholar] [CrossRef] [PubMed]
- Svensson, S.P.S.; Lindgren, S.; Powell, W.; Green, H. Melanin Inhibits Cytotoxic Effects of Doxorubicin and Daunorubicin in MOLT 4 Cells. Pigment Cell Res. 2003, 16, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Ohnoshi, T.; Ohnuma, T.; Beranek, J.T.; Holland, J.F. Combined cytotoxicity effect of hyperthermia and anthracycline antibiotics on human tumor cells. J. Natl. Cancer Inst. 1985, 74, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Maehara, Y.; Emi, Y.; Kohnoe, S.; Sugimachi, K. Adriamycin combined with hyperthermia and dipyridamole is cytotoxic both in vitro and in vivo. Eur. Surg. Res. 1992, 24, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Supino, R.; Bardella, L.; Gibelli, N.; Cairo, G.; Schiaffonati, L. Interaction of heat with chemotherapy in vitro: Effect on cell viability and protein synthesis in human and murine cell lines. Tumori J. 1987, 73, 109–116. [Google Scholar] [CrossRef]
- Fang, H.-S.; Lang, M.-F.; Sun, J. New Methods for Cell Cycle Analysis. Chin. J. Anal. Chem. 2019, 47, 1293–1301. [Google Scholar] [CrossRef]
- Sánchez, I.; Dynlacht, B. New insights into cyclins, CDKs, and cell cycle control. Semin. Cell Dev. Biol. 2005, 16, 311–321. [Google Scholar] [CrossRef]
- Pavey, S.; Spoerri, L.; Haass, N.; Gabrielli, B. DNA repair and cell cycle checkpoint defects as drivers and therapeutic targets in melanoma. Pigment Cell Melanoma Res. 2013, 26, 805–816. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Moralli, S.; Tarrado-Castellarnau, M.; Miranda, A.; Cascante, M. Targeting cell cycle regulation in cancer therapy. Pharmacol. Ther. 2013, 138, 255–271. [Google Scholar] [CrossRef]
- Mittal, A.; Tabasum, S.; Singh, R.P. Berberine in combination with doxorubicin suppresses growth of murine melanoma B16F10 cells in culture and xenograft. Phytomedicine 2014, 21, 340–347. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kotcherlakota, R.; Haque, S.; Bhattacharya, D.; Kumar, J.M.; Chakravarty, S.; Patra, C.R. Improved delivery of doxorubicin using rationally designed PEGylated platinum nanoparticles for the treatment of melanoma. Mater. Sci. Eng. C 2020, 108, 110375. [Google Scholar] [CrossRef]
- Khaki-khatibi, F.; Ghorbani, M.; Sabzichi, M.; Ramezani, F.; Mohammadian, J. Adjuvant therapy with stattic enriches the anti-proliferative effect of doxorubicin in human ZR-75-1 breast cancer cells via arresting cell cycle and inducing apoptosis. Biom. Pharmacother. 2019, 109, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Vancsik, T.; Forika, G.; Balogh, A.; Kiss, E.; Krenacs, T. Modulated electro-hyperthermia induced p53 driven apoptosis and cell cycle arrest additively support doxorubicin chemotherapy of colorectal cancer in vitro. Cancer Med. 2019, 8, 4292–4303. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, H.; Ren, Q.; Ye, T.; Liu, Y.; Zheng, C.; Zhou, G.; Xia, X. Sublethal hyperthermia enhances anticancer activity of doxorubicin in chronically hypoxic HepG2 cells through ROS-dependent mechanism. Biosci. Rep. 2021, 41, BSR20210442. [Google Scholar] [CrossRef] [PubMed]
- Zaffaroni, N.; Villa, R.; Daidone, M.G.; Vaglini, M.; Santinami, M.; Silvestrini, R. Antitumor activity of hyperthermia alone or in combination with cisplatin and melphalan in primary cultures of human malignant melanoma. Int. J. Cell Cloning 1989, 7, 385–394. [Google Scholar] [CrossRef]
- Kusumoto, T.; Holden, S.; Ara, G.; Teicher, B. Hyperthermia and platinum complexes: Time between treatments and synergy in vitro and in vivo. Int. J. Hyperth. 1995, 11, 575–586. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- de Sá Junior, P.L.; Câmara, D.A.D.; Porcacchia, A.S.; Fonseca, P.M.M.; Jorge, S.D.; Araldi, R.P.; Ferreira, A.K. The Roles of ROS in Cancer Heterogeneity and Therapy. Oxid. Med. Cell. Longev. 2017, 2017, 2467940. [Google Scholar] [CrossRef]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinogen. 2006, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraja, A.T. Role of Reactive Oxygen Species (ROS) in Therapeutics and Drug Resistance in Cancer and Bacteria. J. Med. Chem. 2017, 60, 3221–3240. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-H.; Lin, F.-L.; Hou, S.-M.; Liu, J.-F. Hyperthermia Induces Apoptosis through Endoplasmic Reticulum and Reactive Oxygen Species in Human Osteosarcoma Cells. Int. J. Mol. Sci. 2014, 15, 17380. [Google Scholar] [CrossRef] [Green Version]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative Stress and Cellular Response to Doxorubicin: A Common Factor in the Complex Milieu of Anthracycline Cardiotoxicity. Oxid. Med. Cell. Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Xiang, Y.; Luo, G.; Zhao, X.; Xiao, B.; Cheng, Y.; Feng, C.; Duan, C.; Xia, X.; Wong, V.K.W.; et al. Salubrinal Enhances Doxorubicin Sensitivity in Human Cholangiocarcinoma Cells Through Promoting DNA Damage. Cancer Biother. Radiopharm. 2018, 33, 258–265. [Google Scholar] [CrossRef]
- Zhou, S.; Palmeira, C.M.; Wallace, K.B. Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol. Lett. 2001, 121, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, R.; Doerr, V.; Min, K.; Szeto, H.; Smuder, A. Doxorubicin-induced oxidative stress differentially regulates proteolytic signaling in cardiac and skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, 227–233. [Google Scholar] [CrossRef]
- Asensio-López, M.C.; Soler, F.; Pascual-Figal, D.; Fernández-Belda, F.; Lax, A. Doxorubicin-induced oxidative stress: The protective effect of nicorandil on HL-1 cardiomyocytes. PLoS ONE 2017, 12, e0172803. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-Y.; Kim, S.-J.; Kim, B.-J.; Rah, S.-Y.; Chung, S.M.; Im, M.-J.; Kim, U.-H. Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+increase are reciprocally modulated in rat cardiomyocytes. Exp. Mol. Med. 2006, 38, 535–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swalwell, H.; Latimer, J.; Haywood, R.M.; Birch-Machin, M.A. Investigating the role of melanin in UVA/UVB- and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic. Biol. Med. 2012, 52, 626–634. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Helmbach, H.; Rossmann, E.; Kern, M.A.; Schadendorf, D. Drug-resistance in human melanoma. Int. J. Cancer 2001, 93, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidanian, S.; Mentelle, M.; Meyskens, F.L.; Farmer, P.J. Melanosomal Damage in Normal Human Melanocytes Induced by UVB and Metal Uptake-A Basis for the Pro-oxidant State of Melanoma. Photochem. Photobiol. 2009, 84, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Engeland, M.; Nieland, L.; Ramaekers, F.; Schutte, B.; Reutelingsperger, C. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Mantso, T.; Vasleiads, S.; Lampri, E.; Botaitis, S.; Perente, S.; Simopoulos, C.; Chlichlia, K.; Pappa, A.; Panayiotidis, M. Hyperthermia Suppresses Post—In Vitro Proliferation and Tumor Growth in Murine Malignant Melanoma and Colon Carcinoma. Anticancer Res. 2019, 39, 2307–2315. [Google Scholar] [CrossRef] [Green Version]
- Shellman, Y.G.; Howe, W.R.; Miller, L.A.; Goldstein, N.B.; Pacheco, T.R.; Mahajan, R.L.; Larue, S.M.; Norris, D.A. Hyperthermia Induces Endoplasmic Reticulum-Mediated Apoptosis in Melanoma and Non-Melanoma Skin Cancer Cells. J. Investig. Dermatol. 2008, 128, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Licarete, E.; Rauca, V.F.; Luput, L.; Patras, L.; Sesarman, A.; Banciu, M. The prednisolone phosphate-induced suppression of the angiogenic function of tumor-associated macrophages enhances the antitumor effects of doxorubicin on B16.F10 murine melanoma cells in vitro. Oncol. Rep. 2019, 42, 2694–2705. [Google Scholar] [CrossRef]
- Park, K.; Lee, G.Y.; Park, R.W.; Kim, I.S.; Kim, S.Y.; Byun, Y. Combination Therapy of Heparin–Deoxycholic Acid Conjugate and Doxorubicin against Squamous Cell Carcinoma and B16F10 Melanoma. Pharm. Res. 2008, 25, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Ghussen, F.; Nagel, K.; Growth, W.; Müller, J.M.; Stützer, H. A prospective randomized study of regional extremity perfusion in patients with malignant melanoma. Ann. Surg. 1984, 200, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Stehlin, J.S.; Giovanella, B.C.; de Ipolyi, P.D.; Anderson, R.F. Eleven years’ experience with hyperthermic perfusion for melanoma of the extremities. World J. Surg. 1979, 3, 305–307. [Google Scholar] [CrossRef]
- Fraker, D.L.; Alexander, H.R.; Andrich, M.; Rosenberg, S.A. Treatment of patients with melanoma of the extremity using hyperthermic isolated limb perfusion with melphalan, tumor necrosis factor, and interferon gamma: Results of a tumor necrosis factor dose-escalation study. J. Clinic. Oncol. 1996, 14, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, R.T.; Galt, J.M.; Ludgate, C.M.; Smith, A.N. Clinical effects of whole-body hyperthermia in advanced malignancy. Br. Med. J. 1974, 4, 679–682. [Google Scholar] [CrossRef] [Green Version]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef]
- Basel, M.T.; Balivada, S.; Wang, H.; Shrestha, T.B.; Seo, G.M.; Pyle, M.; Abayaweera, G.; Dani, R.; Koper, O.B.; Tamura, M.; et al. Cell-delivered magnetic nanoparticles caused hyperthermia-mediated increased survival in a murine pancreatic cancer model. Int. J. Nanomed. 2012, 7, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Rachakatla, R.S.; Balivada, S.; Seo, G.M.; Myers, C.B.; Wang, H.; Samarakoon, T.N.; Dani, R.; Pyle, M.; Kroh, F.O.; Walker, B.; et al. Attenuation of mouse melanoma by A/C magnetic field after delivery of bi-magnetic nanoparticles by neural progenitor cells. ACS Nano 2010, 4, 7093–7104. [Google Scholar] [CrossRef]
- Shevtsov, M.; Kaesler, S.; Posch, C.; Multhoff, G.; Biedermann, T. Magnetic nanoparticles in theranostics of malignant melanoma. EJNMMI Res. 2021, 11, 127. [Google Scholar] [CrossRef]
- Khaledian, M.; Nourbakhsh, M.S.; Saber, R.; Hashemzadeh, H.; Darvishi, M.H. Preparation and Evaluation of Doxorubicin-Loaded PLA-PEG-FA Copolymer Containing Superparamagnetic Iron Oxide Nanoparticles (SPIONs) for Cancer Treatment: Combination Therapy with Hyperthermia and Chemotherapy. Int. J. Nanomed. 2020, 15, 6167–6182. [Google Scholar] [CrossRef]



| Cell Line | IC | 24 H | 48 H | 72 H |
|---|---|---|---|---|
| A375 | IC10 | 0.012 | 0.0056 | 0.0012 |
| IC20 | 0.043 | 0.0125 | 0.0026 | |
| IC50 | 0.45 | 0.052 | 0.0111 | |
| MNT-1 | IC10 | 0.68 | 0.0066 | 0.0042 |
| IC20 | 1.38 | 0.0179 | 0.0092 | |
| IC50 | 4.88 | 0.102 | 0.0391 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador, D.; Bastos, V.; Oliveira, H. Hyperthermia Enhances Doxorubicin Therapeutic Efficacy against A375 and MNT-1 Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 35. https://doi.org/10.3390/ijms23010035
Salvador D, Bastos V, Oliveira H. Hyperthermia Enhances Doxorubicin Therapeutic Efficacy against A375 and MNT-1 Melanoma Cells. International Journal of Molecular Sciences. 2022; 23(1):35. https://doi.org/10.3390/ijms23010035
Chicago/Turabian StyleSalvador, Diana, Verónica Bastos, and Helena Oliveira. 2022. "Hyperthermia Enhances Doxorubicin Therapeutic Efficacy against A375 and MNT-1 Melanoma Cells" International Journal of Molecular Sciences 23, no. 1: 35. https://doi.org/10.3390/ijms23010035
APA StyleSalvador, D., Bastos, V., & Oliveira, H. (2022). Hyperthermia Enhances Doxorubicin Therapeutic Efficacy against A375 and MNT-1 Melanoma Cells. International Journal of Molecular Sciences, 23(1), 35. https://doi.org/10.3390/ijms23010035







