Dopamine and Methamphetamine Differentially Affect Electron Transport Chain Complexes and Parkin in Rat Striatum: New Insight into Methamphetamine Neurotoxicity
Abstract
1. Introduction
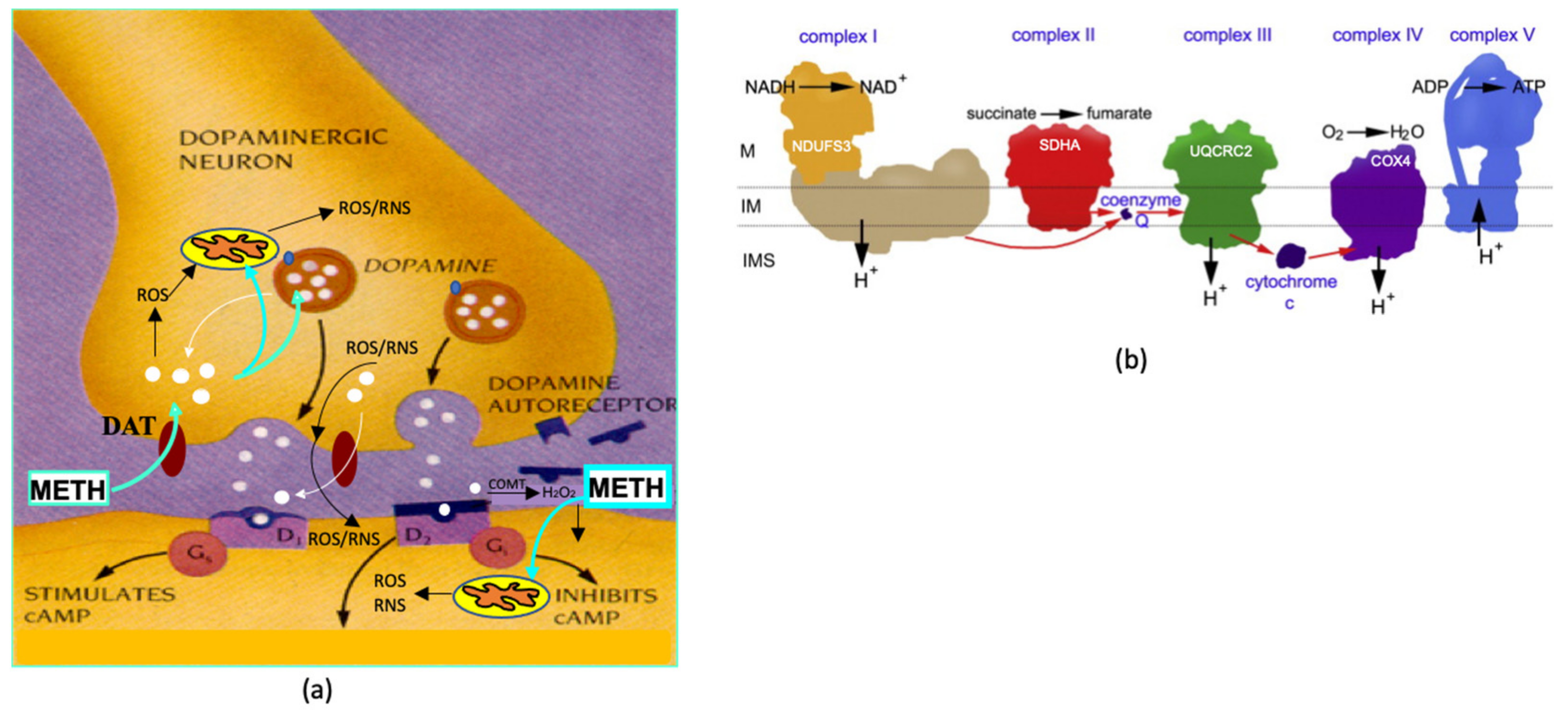
2. Results
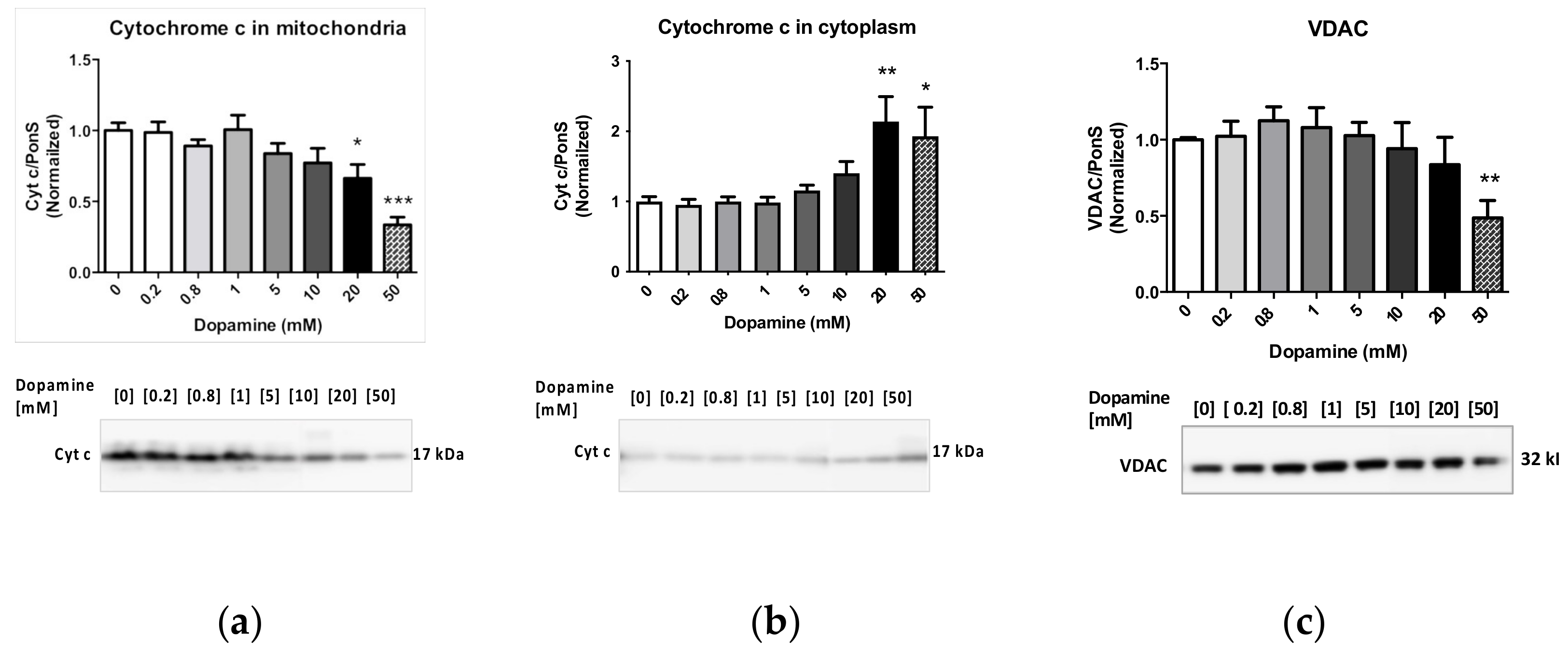
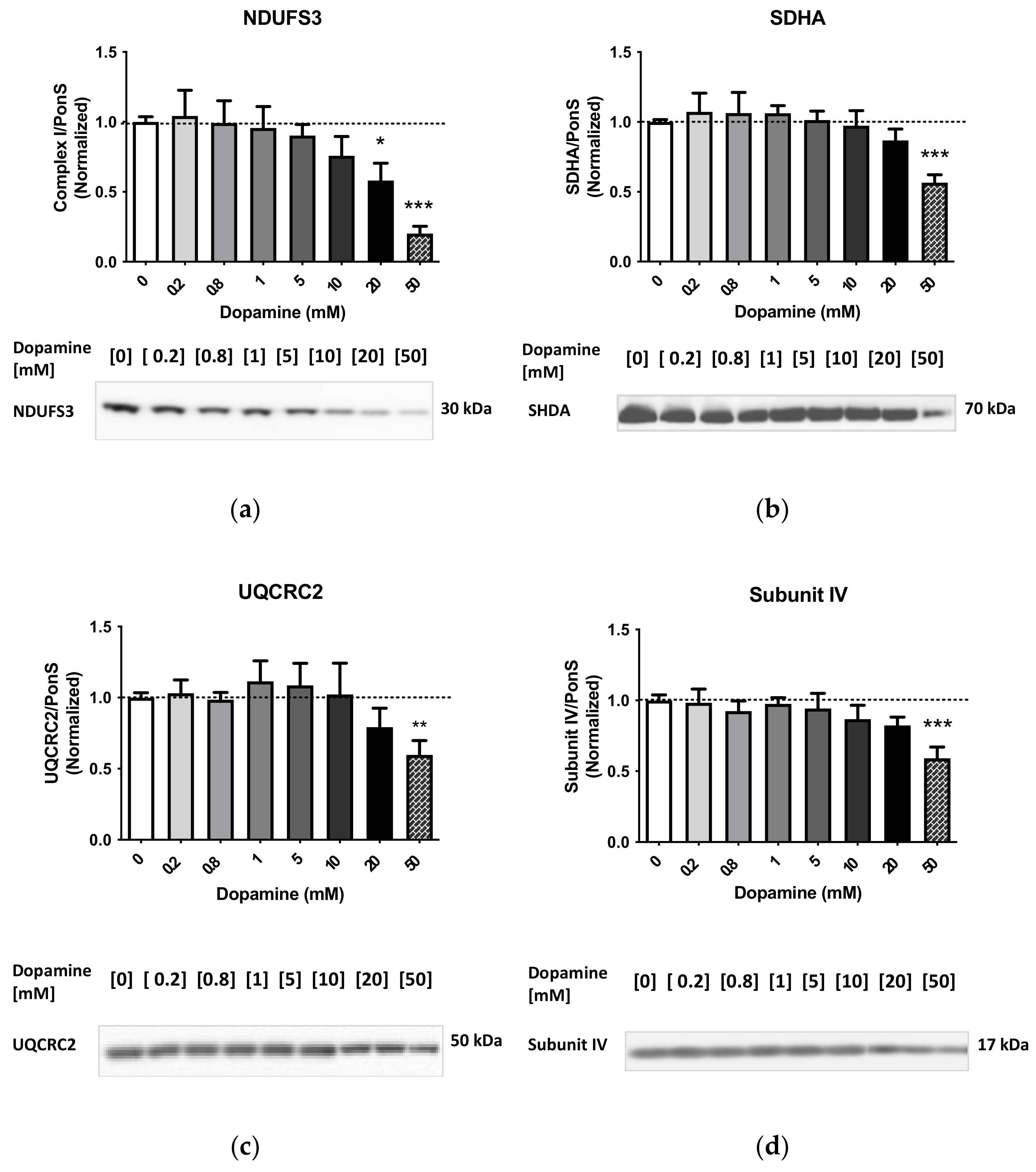
2.1. Effects of Dopamine on Cytoplasm-Suspended Striatal Mitochondria
2.2. Effects of Methamphetamine on Cytoplasm-Suspended Striatal Mitochondria
2.3. Effects of Dopamine or Methamphetamine on Isolation-Buffer-Suspended Striatal Mitochondria
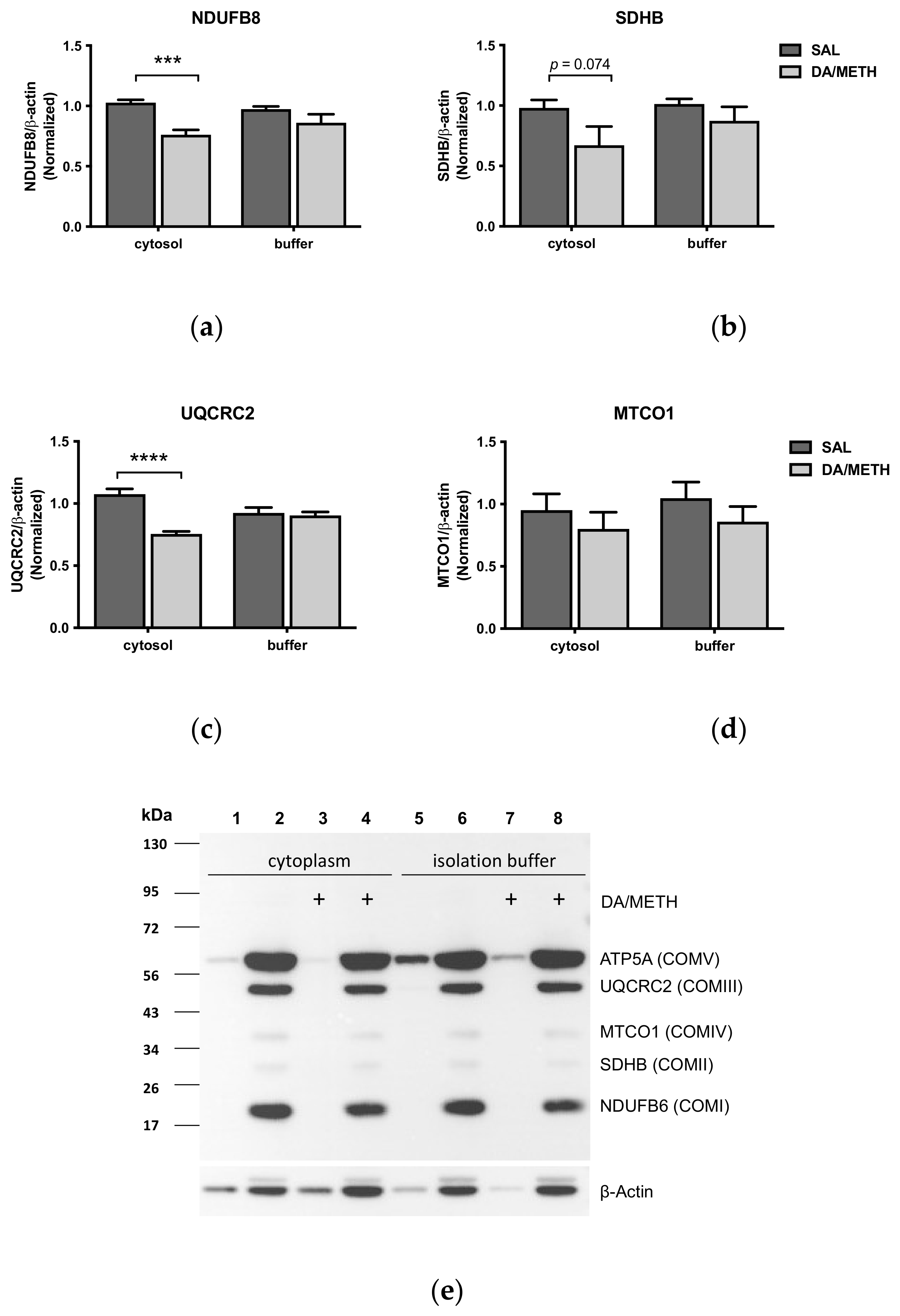
2.4. Effects of Dopamine and Methamphetamine Combination on Cytoplasm-Suspended and Isolation-Buffer-Suspended Striatal Mitochondria
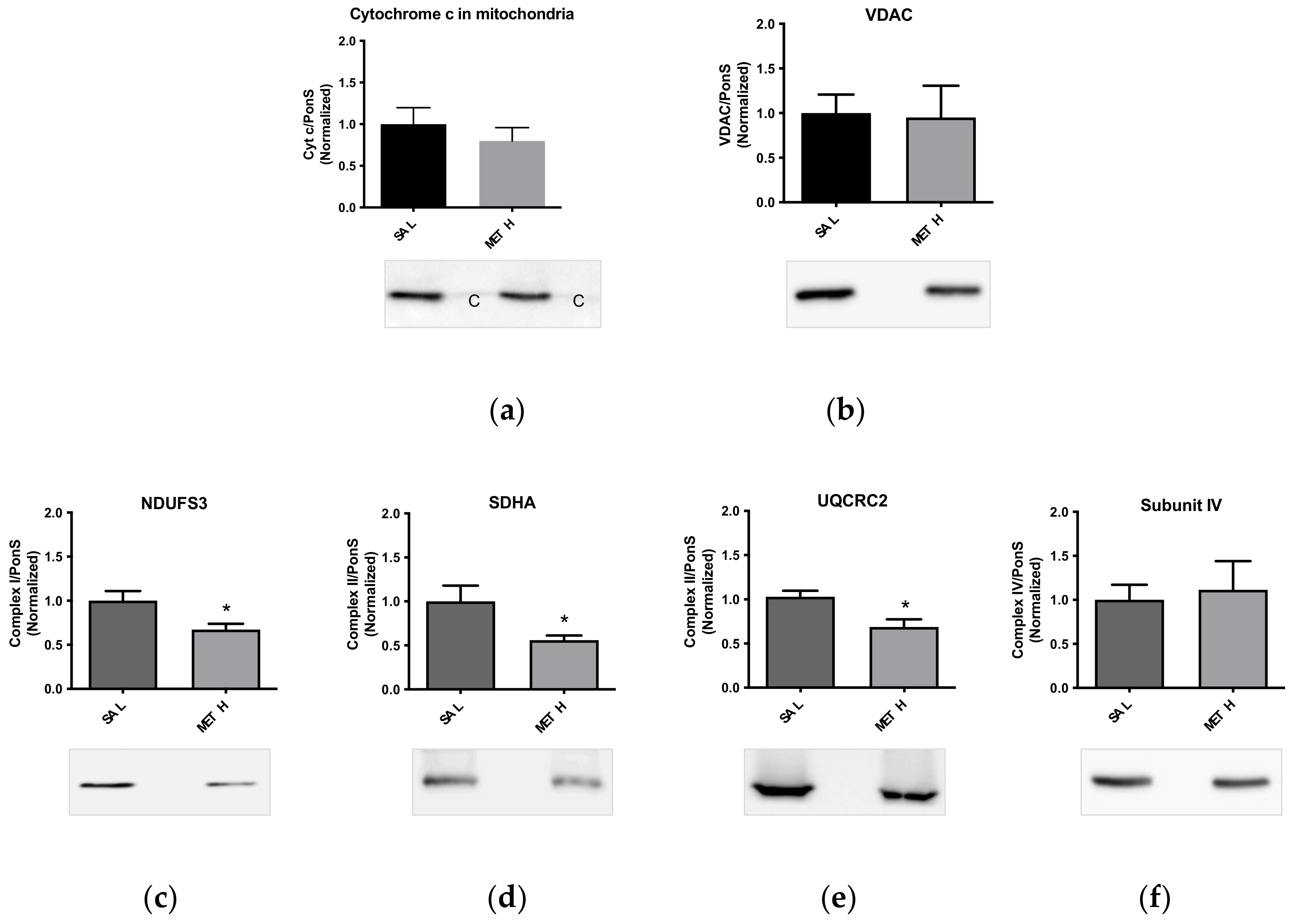
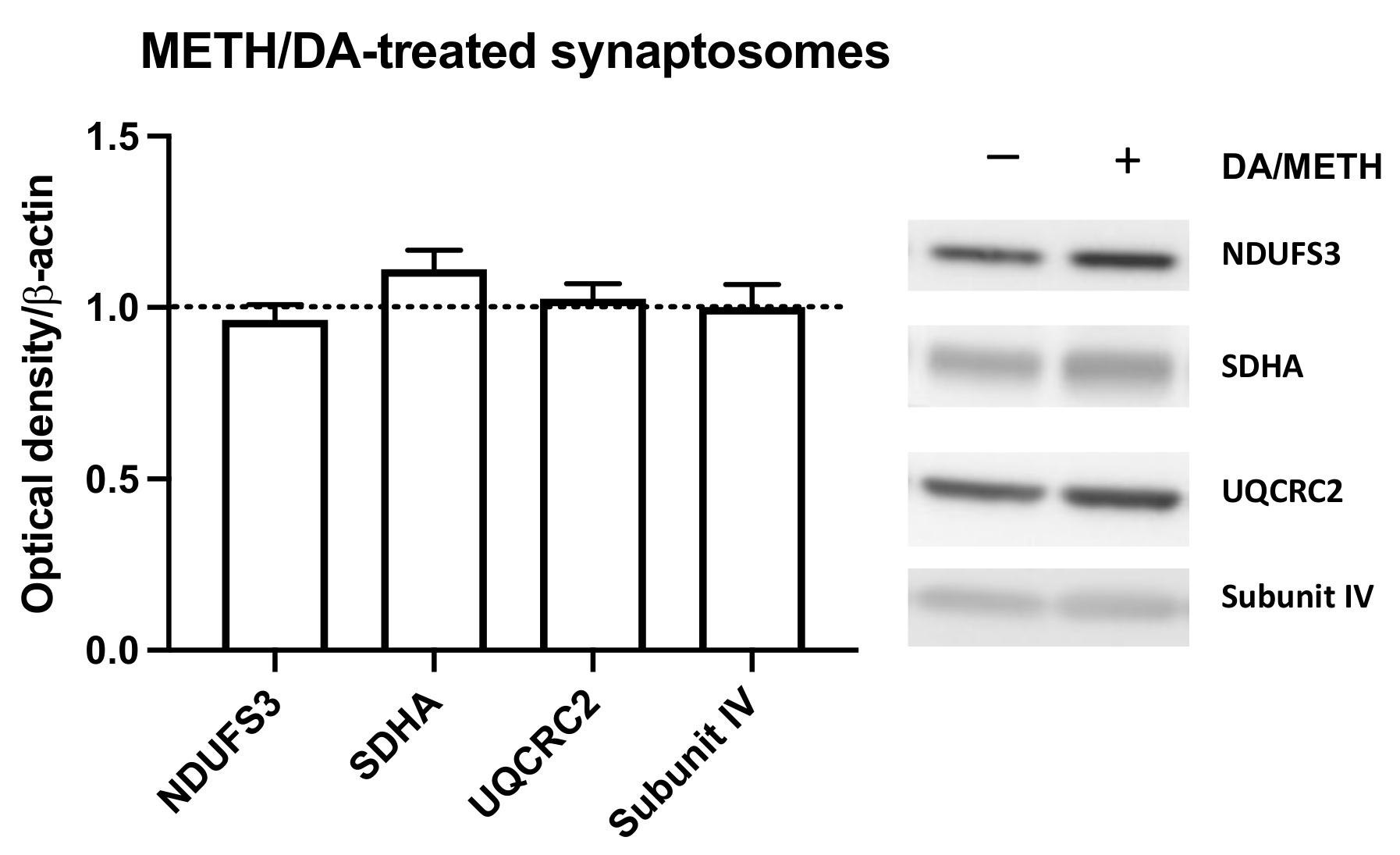
2.5. Effects of Dopamine and Methamphetamine Combination on Mitochondria from Striatal Synaptosomes
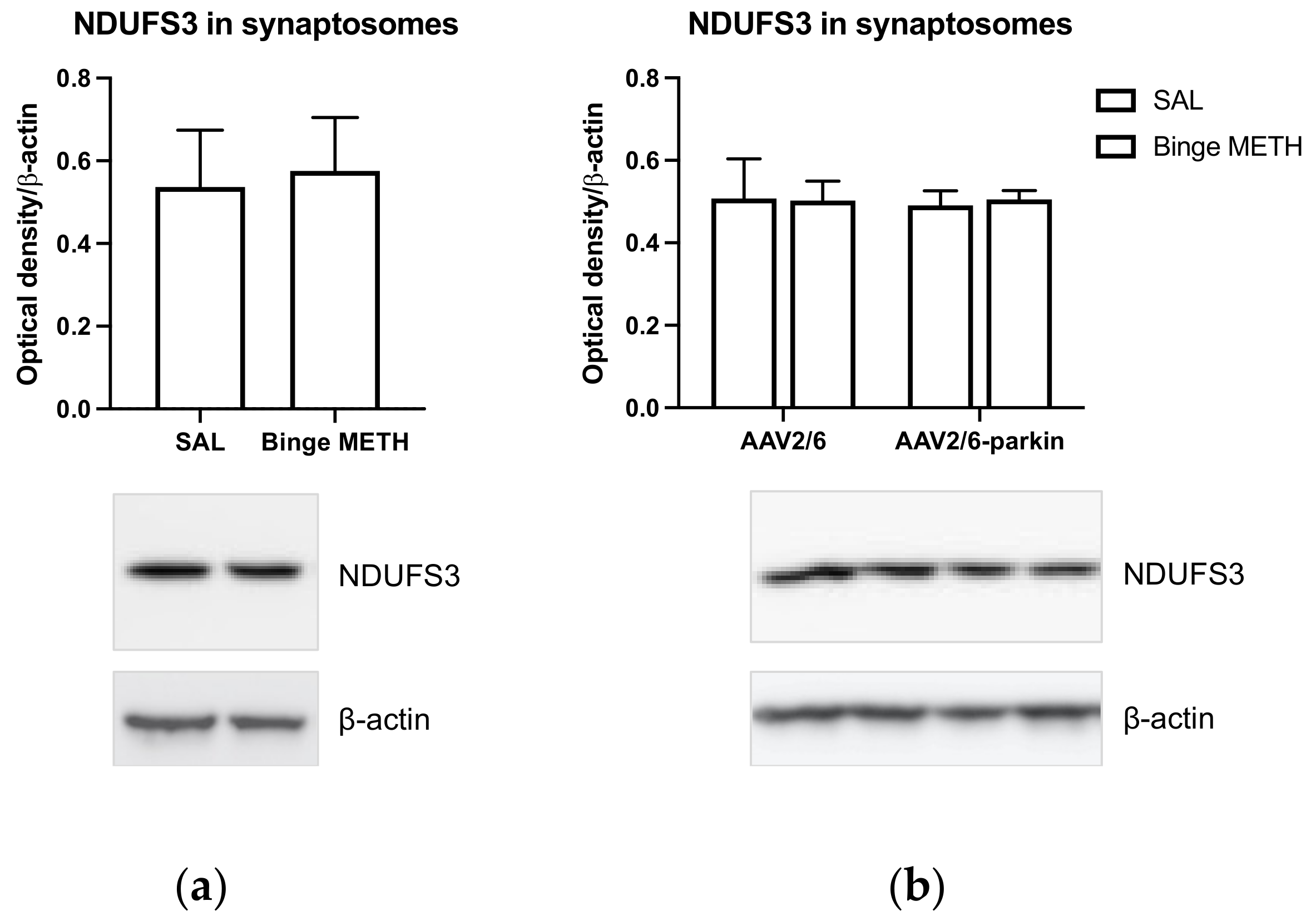
2.6. Effects of Dopamine and Methamphetamine on Levels of the Protein Parkin
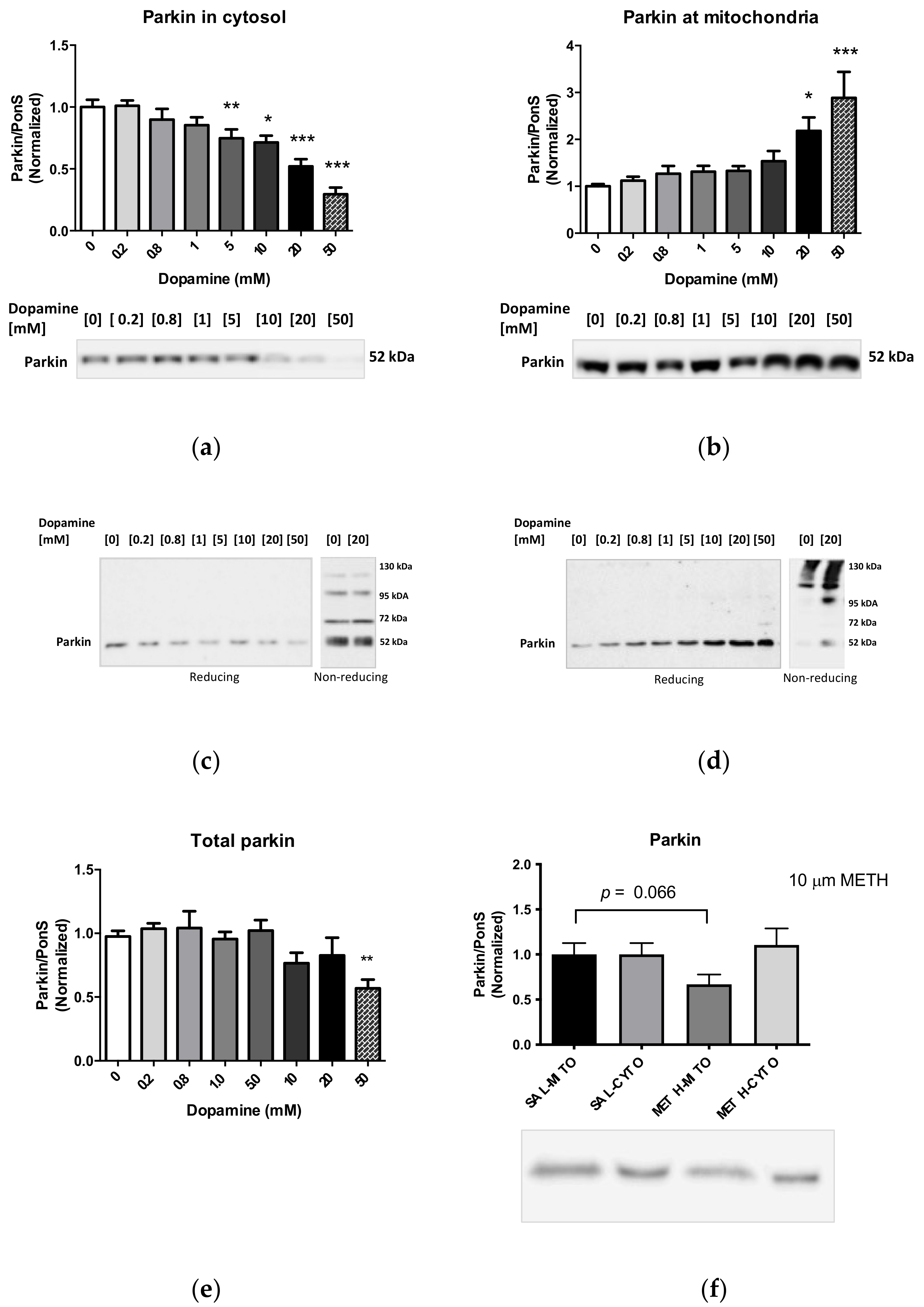

2.7. Effects of Parkin Overexpression and Binge Methamphetamine on Levels of NDUFS3 Subunit
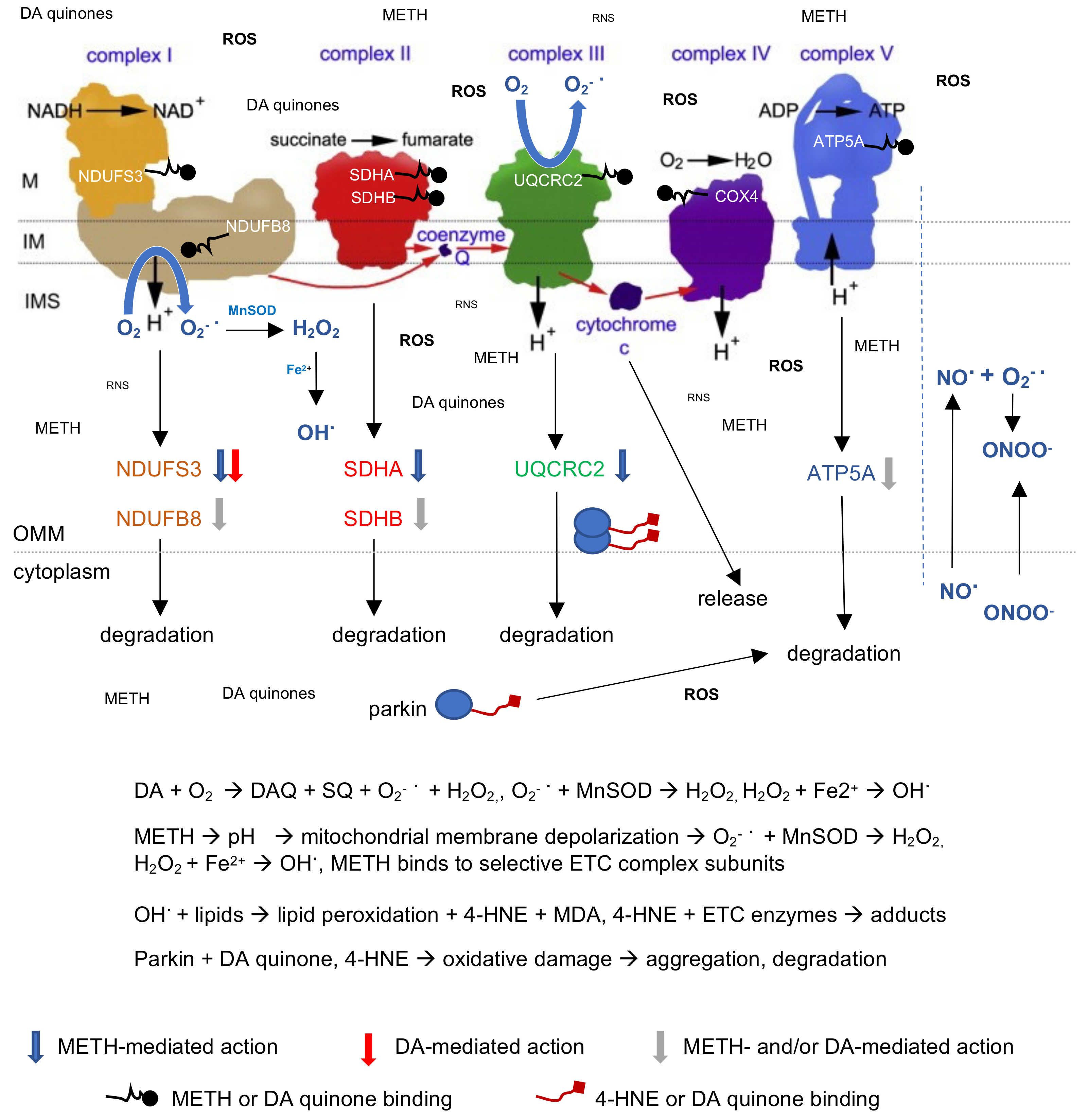
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of Mitochondrial Suspensions
4.3. Preparation of Synaptosomal Suspensions
4.4. Dopamine and Methamphetamine Treatment
4.5. Electrophoresis and Western Blotting
4.6. Overexpression of Parkin In Vivo
4.7. Binge Methamphetamine Treatment In Vivo
4.8. Statistical Analyses
5. Conclusions
- METH itself is a factor promoting dysfunction of striatal mitochondria.
- DA and METH decrease activities of the ETC complexes via oxidative damage to their subunits.
- Parkin does not regulate NDUFS3 turnover in rat striatum.
- Synaptosomal mitochondria may be somewhat “resistant” to DA/METH-induced disruption in mitochondrial ETC complexes than perikaryal mitochondria.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Integrity of Isolated Mitochondria
Appendix A.1.1. Incubation at 37 °C

Appendix A.1.2. Dopamine Treatment
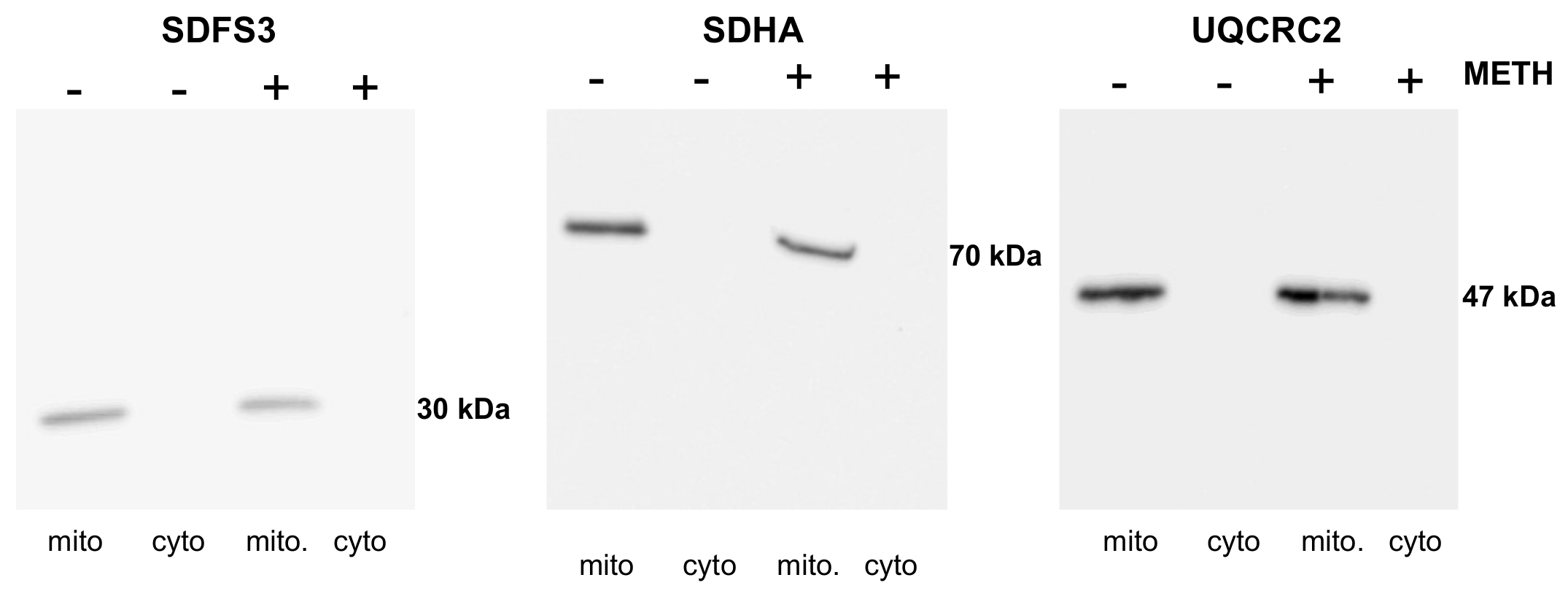
Appendix A.2. NDUFS3, SDHA, and UQCRC2 Deficits Are Not Caused by a Leakage or Aggregation
Appendix A.2.1. Supplementary Data
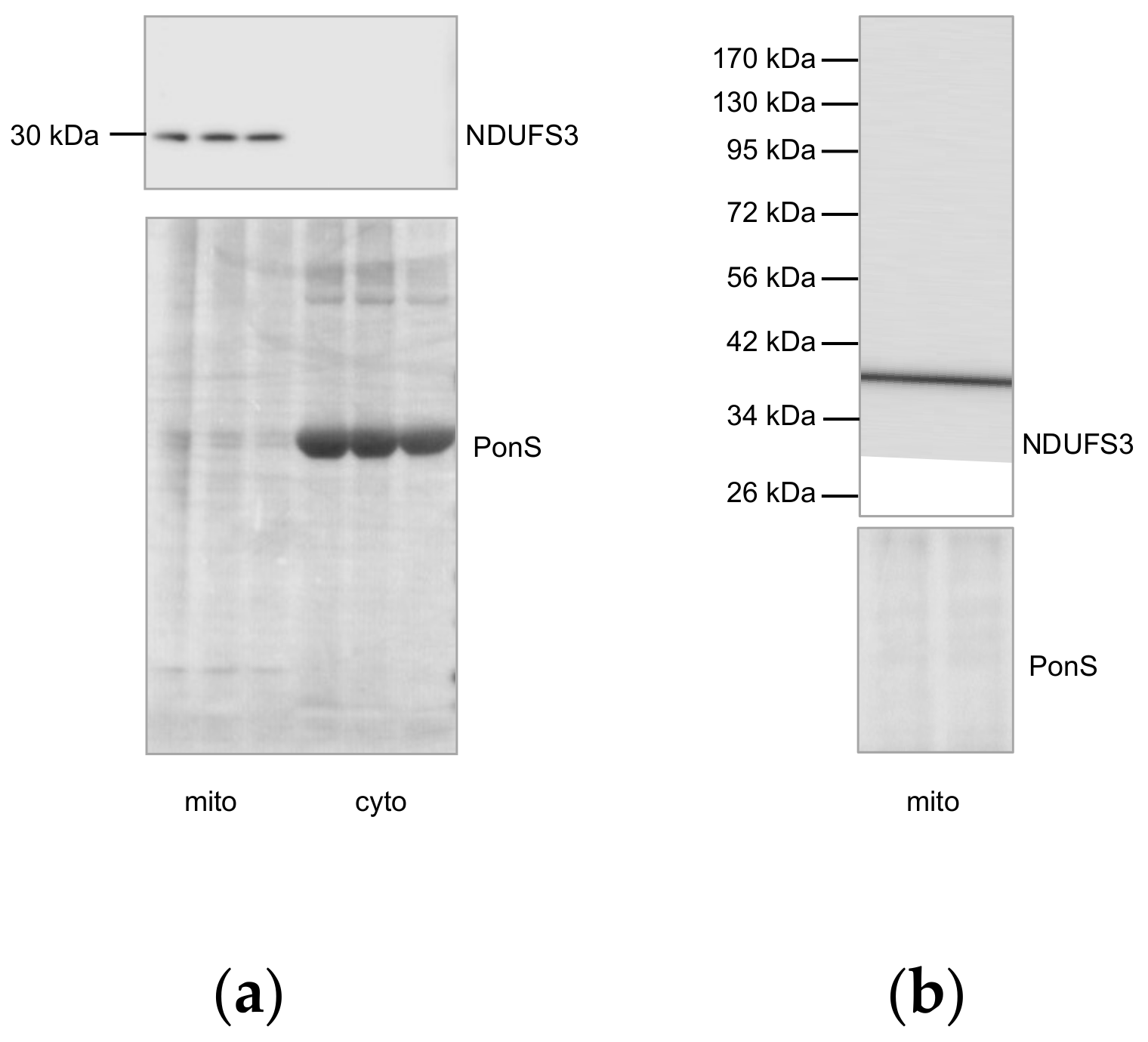
Appendix A.2.2. Supplementary Data
References
- SAMHSA. Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health; SAMHSA: Rockville, MD, USA, 2020.
- Courtney, K.E.; Ray, L.A. Methamphetamine: An update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014, 143, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Rusyniak, D.E. Neurologic manifestations of chronic methamphetamine abuse. Neurol. Clin. 2011, 29, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Garwood, E.R.; Bekele, W.; McCulloch, C.E.; Christine, C.W. Amphetamine exposure is elevated in Parkinson’s disease. Neurotoxicology 2006, 27, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, R.C.; Cunningham, J.K.; Sykes, J.; Kish, S.J. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012, 120, 35–40. [Google Scholar] [CrossRef]
- Curtin, K.; Fleckenstein, A.E.; Robison, R.J.; Crookston, M.J.; Smith, K.R.; Hanson, G.R. Methamphetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: A population-based assessment. Drug Alcohol Depend. 2015, 146, 30–38. [Google Scholar] [CrossRef]
- Davidson, C.; Gow, A.J.; Lee, T.H.; Ellinwood, E.H. Methamphetamine neurotoxicity: Necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res. Brain Res. Rev. 2001, 36, 1–22. [Google Scholar] [CrossRef]
- Ruan, Q.T.; Yazdani, N.; Blum, B.C.; Beierle, J.A.; Lin, W.; Coelho, M.A.; Fultz, E.K.; Healy, A.F.; Shahin, J.R.; Kandola, A.K.; et al. A Mutation in Hnrnph1 That Decreases Methamphetamine-Induced Reinforcement, Reward, and Dopamine Release and Increases Synaptosomal hnRNP H and Mitochondrial Proteins. J. Neurosci. 2020, 40, 107–130. [Google Scholar] [CrossRef]
- Sharma, A.; Bazylianska, V.; Moszczynska, A. Parkin-deficient rats are resistant to neurotoxicity of chronic high-dose methamphetamine. Exp. Neurol. 2021, 345, 113811. [Google Scholar] [CrossRef]
- Greenamyre, J.T.; MacKenzie, G.; Peng, T.I.; Stephans, S.E. Mitochondrial dysfunction in Parkinson’s disease. Biochem. Soc. Sympos. 1999, 66, 85–97. [Google Scholar]
- Bosch, P.J.; Peng, L.; Kivell, B.M. Proteomics Analysis of Dorsal Striatum Reveals Changes in Synaptosomal Proteins following Methamphetamine Self-Administration in Rats. PLoS ONE 2015, 10, e0139829. [Google Scholar] [CrossRef][Green Version]
- Killinger, B.; Shah, M.; Moszczynska, A. Co-administration of betulinic acid and methamphetamine causes toxicity to dopaminergic and serotonergic nerve terminals in the striatum of late adolescent rats. J. Neurochem. 2014, 128, 764–775. [Google Scholar] [CrossRef]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Li, Q.; Zhong, Y.; Chen, L.; Du, Y.; He, J.; Liao, L.; Xiong, K.; Yi, C.X.; et al. The Main Molecular Mechanisms Underlying Methamphetamine- Induced Neurotoxicity and Implications for Pharmacological Treatment. Front. Mol. Neurosci. 2018, 11, 186. [Google Scholar] [CrossRef]
- Sulzer, D.; Chen, T.K.; Lau, Y.Y.; Kristensen, H.; Rayport, S.; Ewing, A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J. Neurosci. 1995, 15 Pt 2, 4102–4108. [Google Scholar] [CrossRef]
- Jayanthi, S.; Deng, X.; Noailles, P.A.; Ladenheim, B.; Cadet, J.L. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004, 18, 238–251. [Google Scholar] [CrossRef]
- Beauvais, G.; Atwell, K.; Jayanthi, S.; Ladenheim, B.; Cadet, J.L. Involvement of dopamine receptors in binge methamphetamine-induced activation of endoplasmic reticulum and mitochondrial stress pathways. PLoS ONE 2011, 6, e28946. [Google Scholar] [CrossRef]
- Tanaka, A. Parkin-mediated selective mitochondrial autophagy, mitophagy: Parkin purges damaged organelles from the vital mitochondrial network. FEBS Lett. 2010, 584, 1386–1392. [Google Scholar] [CrossRef]
- Gehrke, S.; Wu, Z.; Klinkenberg, M.; Sun, Y.; Auburger, G.; Guo, S.; Lu, B. PINK1 and Parkin control localized translation of respiratory chain component mRNAs on mitochondria outer membrane. Cell Metabol. 2015, 21, 95–108. [Google Scholar] [CrossRef]
- Vincow, E.S.; Merrihew, G.; Thomas, R.E.; Shulman, N.J.; Beyer, R.P.; MacCoss, M.J.; Pallanck, L.J. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 6400–6405. [Google Scholar] [CrossRef]
- Mortiboys, H.; Thomas, K.J.; Koopman, W.J.; Klaffke, S.; Abou-Sleiman, P.; Olpin, S.; Wood, N.W.; Willems, P.H.; Smeitink, J.A.; Cookson, M.R.; et al. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann. Neurol. 2008, 64, 555–565. [Google Scholar] [CrossRef]
- Shimura, H.; Hattori, N.; Kubo, S.; Yoshikawa, M.; Kitada, T.; Matsumine, H.; Asakawa, S.; Minoshima, S.; Yamamura, Y.; Shimizu, N.; et al. Immunohistochemical and subcellular localization of Parkin protein: Absence of protein in autosomal recessive juvenile parkinsonism patients. Ann. Neurol. 1999, 45, 668–672. [Google Scholar] [CrossRef]
- Zhu, J.; Chu, C.T. Mitochondrial dysfunction in Parkinson’s disease. J. Alzheimer’s Dis. 2010, 20 (Suppl. 2), S325–S334. [Google Scholar] [CrossRef] [PubMed]
- Moszczynska, A.; Yamamoto, B.K. Methamphetamine oxidatively damages parkin and decreases the activity of 26S proteasome in vivo. J. Neurochem. 2011, 116, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Traini, R.; Killinger, B.; Schneider, B.; Moszczynska, A. Overexpression of parkin in the rat nigrostriatal dopamine system protects against methamphetamine neurotoxicity. Exp. Neurol. 2013, 247, 359–372. [Google Scholar] [CrossRef]
- Akude, E.; Zherebitskaya, E.; Chowdhury, S.K.; Smith, D.R.; Dobrowsky, R.T.; Fernyhough, P. Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats. Diabetes 2011, 60, 288–297. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Hu, Y.; Huang, P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by beta-phenethyl isothiocyanate: Mechanisms for anti-leukemia activity. Antiox. Redox Signal. 2011, 15, 2911–2921. [Google Scholar] [CrossRef]
- Dudkina, N.V.; Kouril, R.; Peters, K.; Braun, H.P.; Boekema, E.J. Structure and function of mitochondrial supercomplexes. Biochim. Biophys. Acta 2010, 1797, 664–670. [Google Scholar] [CrossRef]
- Junn, E.; Mouradian, M.M. Apoptotic signaling in dopamine-induced cell death: The role of oxidative stress, p38 mitogen-activated protein kinase, cytochrome c and caspases. J. Neurochem. 2001, 78, 374–383. [Google Scholar] [CrossRef]
- Jana, S.; Sinha, M.; Chanda, D.; Roy, T.; Banerjee, K.; Munshi, S.; Patro, B.S.; Chakrabarti, S. Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: Implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochim. Biophys. Acta 2011, 1812, 663–673. [Google Scholar] [CrossRef]
- Rustin, P.; Rotig, A. Inborn errors of complex II-unusual human mitochondrial diseases. Biochim. Biophys. Acta 2002, 1553, 117–122. [Google Scholar] [CrossRef]
- Neutzner, A.; Youle, R.J.; Karbowski, M. Outer mitochondrial membrane protein degradation by the proteasome. Novartis Found. Symp. 2007, 287, 4–14, discussion 14–20. [Google Scholar]
- LaVoie, M.J.; Ostaszewski, B.L.; Weihofen, A.; Schlossmacher, M.G.; Selkoe, D.J. Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 2005, 11, 1214–1221. [Google Scholar] [CrossRef]
- Herst, P.M.; Rowe, M.R.; Carson, G.M.; Berridge, M.V. Functional Mitochondria in Health and Disease. Front. Endocrinol 2017, 8, 296. [Google Scholar] [CrossRef]
- Ow, Y.P.; Green, D.R.; Hao, Z.; Mak, T.W. Cytochrome c: Functions beyond respiration. Nat. Rev. Mol. Cell Biol. 2008, 9, 532–542. [Google Scholar] [CrossRef]
- Gunter, T.E.; Pfeiffer, D.R. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 1990, 258 Pt 1, C755–C786. [Google Scholar] [CrossRef]
- Rugarli, E.I.; Langer, T. Mitochondrial quality control: A matter of life and death for neurons. EMBO J. 2012, 31, 1336–1349. [Google Scholar] [CrossRef]
- Ferraro, E.; Pulicati, A.; Cencioni, M.T.; Cozzolino, M.; Navoni, F.; di Martino, S.; Nardacci, R.; Carri, M.T.; Cecconi, F. Apoptosome-deficient cells lose cytochrome c through proteasomal degradation but survive by autophagy-dependent glycolysis. Mol. Biol. Cell 2008, 19, 3576–3588. [Google Scholar] [CrossRef]
- Mashayekhi, V.; Eskandari, M.R.; Kobarfard, F.; Khajeamiri, A.; Hosseini, M.J. Induction of mitochondrial permeability transition (MPT) pore opening and ROS formation as a mechanism for methamphetamine-induced mitochondrial toxicity. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 47–58. [Google Scholar] [CrossRef]
- Czerniczyniec, A.; Bustamante, J.; Lores-Arnaiz, S. Dopamine enhances mtNOS activity: Implications in mitochondrial function. Biochim. Biophys. Acta 2007, 1767, 1118–1125. [Google Scholar] [CrossRef]
- Brenner-Lavie, H.; Klein, E.; Zuk, R.; Gazawi, H.; Ljubuncic, P.; Ben-Shachar, D. Dopamine modulates mitochondrial function in viable SH-SY5Y cells possibly via its interaction with complex I: Relevance to dopamine pathology in schizophrenia. Biochim. Biophys. Acta 2008, 1777, 173–185. [Google Scholar] [CrossRef]
- Gluck, M.; Ehrhart, J.; Jayatilleke, E.; Zeevalk, G.D. Inhibition of brain mitochondrial respiration by dopamine: Involvement of H(2)O(2) and hydroxyl radicals but not glutathione-protein-mixed disulfides. J. Neurochem. 2002, 82, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.H.; Sen, T.; Maiti, A.K.; Jana, S.; Chatterjee, U.; Chakrabarti, S. Inhibition of rat brain mitochondrial electron transport chain activity by dopamine oxidation products during extended in vitro incubation: Implications for Parkinson’s disease. Biochim. Biophys. Acta 2005, 1741, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Molec. Pharmacol. 1978, 14, 633–643. [Google Scholar]
- Bombicino, S.S.; Iglesias, D.E.; Zaobornyj, T.; Boveris, A.; Valdez, L.B. Mitochondrial nitric oxide production supported by reverse electron transfer. Arch. Biochem Biophys 2016, 607, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Ghafourifar, P.; Richter, C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997, 418, 291–296. [Google Scholar] [CrossRef]
- Giulivi, C.; Poderoso, J.J.; Boveris, A. Production of nitric oxide by mitochondria. J. Biol. Chem. 1998, 273, 11038–11043. [Google Scholar] [CrossRef] [PubMed]
- Lacza, Z.; Pankotai, E.; Busija, D.W. Mitochondrial nitric oxide synthase: Current concepts and controversies. Front. Biosci. 2015, 14, 4436–4443. [Google Scholar] [CrossRef]
- Radi, R.; Cassina, A.; Hodara, R.; Quijano, C.; Castro, L. Peroxynitrite reactions and formation in mitochondria. Free Rad. Biol. Med. 2002, 33, 1451–1464. [Google Scholar] [CrossRef]
- Picklo, M.J.; Amarnath, V.; McIntyre, J.O.; Graham, D.G.; Montine, T.J. 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J. NeuroChem. 1999, 72, 1617–1624. [Google Scholar] [CrossRef]
- Wu, J.; Luo, X.; Yan, L.J. Two dimensional blue native/SDS-PAGE to identify mitochondrial complex I subunits modified by 4-hydroxynonenal (HNE). Front. Physiol. 2015, 6, 98. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Potula, R.; Hawkins, B.J.; Cenna, J.M.; Fan, S.; Dykstra, H.; Ramirez, S.H.; Morsey, B.; Brodie, M.R.; Persidsky, Y. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J. Immunol. 2010, 185, 2867–2876. [Google Scholar] [CrossRef]
- Hastings, T.G. The role of dopamine oxidation in mitochondrial dysfunction: Implications for Parkinson’s disease. J. Bioenerget. Biomembran. 2009, 41, 469–472. [Google Scholar] [CrossRef]
- Van Laar, V.S.; Dukes, A.A.; Cascio, M.; Hastings, T.G. Proteomic analysis of rat brain mitochondria following exposure to dopamine quinone: Implications for Parkinson disease. NeuroBiol. Dis. 2008, 29, 477–489. [Google Scholar] [CrossRef]
- Van Laar, V.S.; Mishizen, A.J.; Cascio, M.; Hastings, T.G. Proteomic identification of dopamine-conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. NeuroBiol. Dis. 2009, 34, 487–500. [Google Scholar] [CrossRef]
- Clarke, K.J.; Adams, A.E.; Manzke, L.H.; Pearson, T.W.; Borchers, C.H.; Porter, R.K. A role for ubiquitinylation and the cytosolic proteasome in turnover of mitochondrial uncoupling protein 1 (UCP1). Biochim. Biophys. Acta 2012, 1817, 1759–1767. [Google Scholar] [CrossRef]
- Koppen, M.; Langer, T. Protein degradation within mitochondria: Versatile activities of AAA proteases and other peptidases. Crit. Rev. BioChem. Mol. Biol. 2007, 42, 221–242. [Google Scholar] [CrossRef]
- Burrows, K.B.; Gudelsky, G.; Yamamoto, B.K. Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. Eur. J. Pharmacol. 2000, 398, 11–18. [Google Scholar] [CrossRef]
- Bachmann, R.F.; Wang, Y.; Yuan, P.; Zhou, R.; Li, X.; Alesci, S.; Du, J.; Manji, H.K. Common effects of lithium and valproate on mitochondrial functions: Protection against methamphetamine-induced mitochondrial damage. Int. J. Neuropsychopharmacol. 2009, 12, 805–822. [Google Scholar] [CrossRef]
- Thrash, B.; Karuppagounder, S.S.; Uthayathas, S.; Suppiramaniam, V.; Dhanasekaran, M. Neurotoxic effects of methamphetamine. NeuroChem. Res. 2010, 35, 171–179. [Google Scholar] [CrossRef]
- Heales, S.J.; Bolanos, J.P.; Stewart, V.C.; Brookes, P.S.; Land, J.M.; Clark, J.B. Nitric oxide, mitochondria and neurological disease. Biochim. Biophys. Acta 1999, 1410, 215–228. [Google Scholar] [CrossRef]
- Musatov, A.; Robinson, N.C. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Rad. Res. 2012, 46, 1313–1326. [Google Scholar] [CrossRef]
- Boveris, A.; Costa, L.E.; Poderoso, J.J.; Carreras, M.C.; Cadenas, E. Regulation of mitochondrial respiration by oxygen and nitric oxide. Ann. N. Y. Acad. Sci. 2000, 899, 121–135. [Google Scholar] [CrossRef]
- Chin, M.H.; Qian, W.J.; Wang, H.; Petyuk, V.A.; Bloom, J.S.; Sforza, D.M.; Lacan, G.; Liu, D.; Khan, A.H.; Cantor, R.M.; et al. Mitochondrial dysfunction, oxidative stress, and apoptosis revealed by proteomic and transcriptomic analyses of the striata in two mouse models of Parkinson’s disease. J. Prot. Res. 2008, 7, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Schroter, J.; Schott, K.J.; Purtill, M.A.; Neuhoff, V. Lysosomal protein degradation in experimental hyperphenylalaninaemia. J. Inherit. Metab. Dis. 1986, 9, 273–282. [Google Scholar] [CrossRef]
- Brown, J.M.; Quinton, M.S.; Yamamoto, B.K. Methamphetamine-induced inhibition of mitochondrial complex II: Roles of glutamate and peroxynitrite. J. NeuroChem. 2005, 95, 429–436. [Google Scholar] [CrossRef]
- Thrash-Williams, B.; Ahuja, M.; Karuppagounder, S.S.; Uthayathas, S.; Suppiramaniam, V.; Dhanasekaran, M. Assessment of therapeutic potential of amantadine in methamphetamine induced neurotoxicity. NeuroChem. Res. 2013, 38, 2084–2094. [Google Scholar] [CrossRef]
- Feier, G.; Valvassori, S.S.; Varela, R.B.; Resende, W.R.; Bavaresco, D.V.; Morais, M.O.; Scaini, G.; Andersen, M.L.; Streck, E.L.; Quevedo, J. Lithium and valproate modulate energy metabolism in an animal model of mania induced by methamphetamine. Pharmacol. BioChem. Behav. 2013, 103, 589–596. [Google Scholar] [CrossRef]
- Klongpanichapak, S.; Govitrapong, P.; Sharma, S.K.; Ebadi, M. Attenuation of cocaine and methamphetamine neurotoxicity by coenzyme Q10. NeuroChem. Res. 2006, 31, 303–311. [Google Scholar] [CrossRef]
- Czerniczyniec, A.; Bustamante, J.; Lores-Arnaiz, S. Improvement of mouse brain mitochondrial function after deprenyl treatment. Neuroscience 2007, 144, 685–693. [Google Scholar] [CrossRef]
- Gluck, M.R.; Zeevalk, G.D. Inhibition of brain mitochondrial respiration by dopamine and its metabolites: Implications for Parkinson’s disease and catecholamine-associated diseases. J. NeuroChem. 2004, 91, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.H.; Zeevalk, G.D. Characterization of reduced and oxidized dopamine and 3,4-dihydrophenylacetic acid, on brain mitochondrial electron transport chain activities. Biochim. Biophys. Acta 2011, 1807, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Sullivan, P.G.; Geddes, J.W. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J. Biol. Chem. 2006, 281, 11658–11668. [Google Scholar] [CrossRef]
- Yu, W.H.; Wolfgang, W.; Forte, M. Subcellular localization of human voltage-dependent anion channel isoforms. J. Biol. Chem. 1995, 270, 13998–14006. [Google Scholar] [CrossRef]
- Hobson, B.D.; Sims, P.A. Critical Analysis of Particle Detection Artifacts in Synaptosome Flow Cytometry. eNeuro 2019, 6, 3. [Google Scholar] [CrossRef]
- Kim, S.; Westphalen, R.; Callahan, B.; Hatzidimitriou, G.; Yuan, J.; Ricaurte, G.A. Toward development of an in vitro model of methamphetamine-induced dopamine nerve terminal toxicity. J. Pharmacol. Exp. Ther. 2000, 293, 625–633. [Google Scholar]
- Choi, S.W.; Gerencser, A.A.; Lee, D.W.; Rajagopalan, S.; Nicholls, D.G.; Andersen, J.K.; Brand, M.D. Intrinsic bioenergetic properties and stress sensitivity of dopaminergic synaptosomes. J. Neurosci. 2011, 31, 4524–4534. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef]
- LaVoie, M.J.; Cortese, G.P.; Ostaszewski, B.L.; Schlossmacher, M.G. The effects of oxidative stress on parkin and other E3 ligases. J. NeuroChem. 2007, 103, 2354–2368. [Google Scholar] [CrossRef]
- Choi, P.; Ostrerova-Golts, N.; Sparkman, D.; Cochran, E.; Lee, J.M.; Wolozin, B. Parkin is metabolized by the ubiquitin/proteosome system. Neuroreport 2000, 11, 2635–2638. [Google Scholar] [CrossRef]
- Cherra, S.J., 3rd; Dagda, R.K.; Tandon, A.; Chu, C.T. Mitochondrial autophagy as a compensatory response to PINK1 deficiency. Autophagy 2009, 5, 1213–1214. [Google Scholar] [CrossRef]
- Kuroda, Y.; Sako, W.; Goto, S.; Sawada, T.; Uchida, D.; Izumi, Y.; Takahashi, T.; Kagawa, N.; Matsumoto, M.; Matsumoto, M.; et al. Parkin interacts with Klokin1 for mitochondrial import and maintenance of membrane potential. Human Mol. Genet. 2012, 21, 991–1003. [Google Scholar] [CrossRef]
- Corti, O.; Brice, A. Mitochondrial quality control turns out to be the principal suspect in parkin and PINK1-related autosomal recessive Parkinson’s disease. Curr. Opin. Neurobiol. 2013, 23, 100–108. [Google Scholar] [CrossRef]
- Palacino, J.J.; Sagi, D.; Goldberg, M.S.; Krauss, S.; Motz, C.; Wacker, M.; Klose, J.; Shen, J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 2004, 279, 18614–18622. [Google Scholar] [CrossRef]
- Radi, R.; Cassina, A.; Hodara, R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol. Chem. 2002, 383, 401–409. [Google Scholar] [CrossRef]
- Yamamoto, B.K.; Moszczynska, A.; Gudelsky, G.A. Amphetamine toxicities: Classical and emerging mechanisms. Ann. N. Y. Acad. Sci. 2010, 1187, 101–121. [Google Scholar] [CrossRef]
- Matuszewich, L.; Yamamoto, B.K. Chronic stress augments the long-term and acute effects of methamphetamine. Neuroscience 2004, 124, 637–646. [Google Scholar] [CrossRef]
- Jones, S.R.; Gainetdinov, R.R.; Wightman, R.M.; Caron, M.G. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J. Neurosci. 1998, 18, 1979–1986. [Google Scholar] [CrossRef]
- Nash, J.F.; Yamamoto, B.K. Methamphetamine neurotoxicity and striatal glutamate release: Comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992, 581, 237–243. [Google Scholar] [CrossRef]
- Nash, J.F.; Yamamoto, B.K. Effect of D-amphetamine on the extracellular concentrations of glutamate and dopamine in iprindole-treated rats. Brain Res. 1993, 627, 1–8. [Google Scholar] [CrossRef]
- Lavin, A.; Nogueira, L.; Lapish, C.C.; Wightman, R.M.; Phillips, P.E.; Seamans, J.K. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J. Neurosci. 2005, 25, 5013–5023. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Brit. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Michel, B.; Bosshard, H.R. Spectroscopic analysis of the interaction between cytochrome c and cytochrome c oxidase. J. Biol. Chem. 1984, 259, 10085–10091. [Google Scholar] [CrossRef]
- Sampson, V.; Alleyne, T. Cytochrome c/cytochrome c oxidase interaction. Direct structural evidence for conformational changes during enzyme turnover. Eur. J. Biochem. 2001, 268, 6534–6544. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazylianska, V.; Sharma, A.; Chauhan, H.; Schneider, B.; Moszczynska, A. Dopamine and Methamphetamine Differentially Affect Electron Transport Chain Complexes and Parkin in Rat Striatum: New Insight into Methamphetamine Neurotoxicity. Int. J. Mol. Sci. 2022, 23, 363. https://doi.org/10.3390/ijms23010363
Bazylianska V, Sharma A, Chauhan H, Schneider B, Moszczynska A. Dopamine and Methamphetamine Differentially Affect Electron Transport Chain Complexes and Parkin in Rat Striatum: New Insight into Methamphetamine Neurotoxicity. International Journal of Molecular Sciences. 2022; 23(1):363. https://doi.org/10.3390/ijms23010363
Chicago/Turabian StyleBazylianska, Viktoriia, Akhil Sharma, Heli Chauhan, Bernard Schneider, and Anna Moszczynska. 2022. "Dopamine and Methamphetamine Differentially Affect Electron Transport Chain Complexes and Parkin in Rat Striatum: New Insight into Methamphetamine Neurotoxicity" International Journal of Molecular Sciences 23, no. 1: 363. https://doi.org/10.3390/ijms23010363
APA StyleBazylianska, V., Sharma, A., Chauhan, H., Schneider, B., & Moszczynska, A. (2022). Dopamine and Methamphetamine Differentially Affect Electron Transport Chain Complexes and Parkin in Rat Striatum: New Insight into Methamphetamine Neurotoxicity. International Journal of Molecular Sciences, 23(1), 363. https://doi.org/10.3390/ijms23010363






