Potential Therapeutic Application of Regulatory T Cells in Diabetes Mellitus Type 1
Abstract
:1. Introduction
- Stage 1: Multiple islet antibodies, normal blood glucose, pre-symptomatic;
- Stage 2: Multiple islet antibodies, raised blood glucose, pre-symptomatic;
- Stage 3: Islet autoimmunity, raised blood glucose, symptomatic;
- Stage 4: Long-standing type 1 diabetes [3].
2. Formation and Selection of Tregs
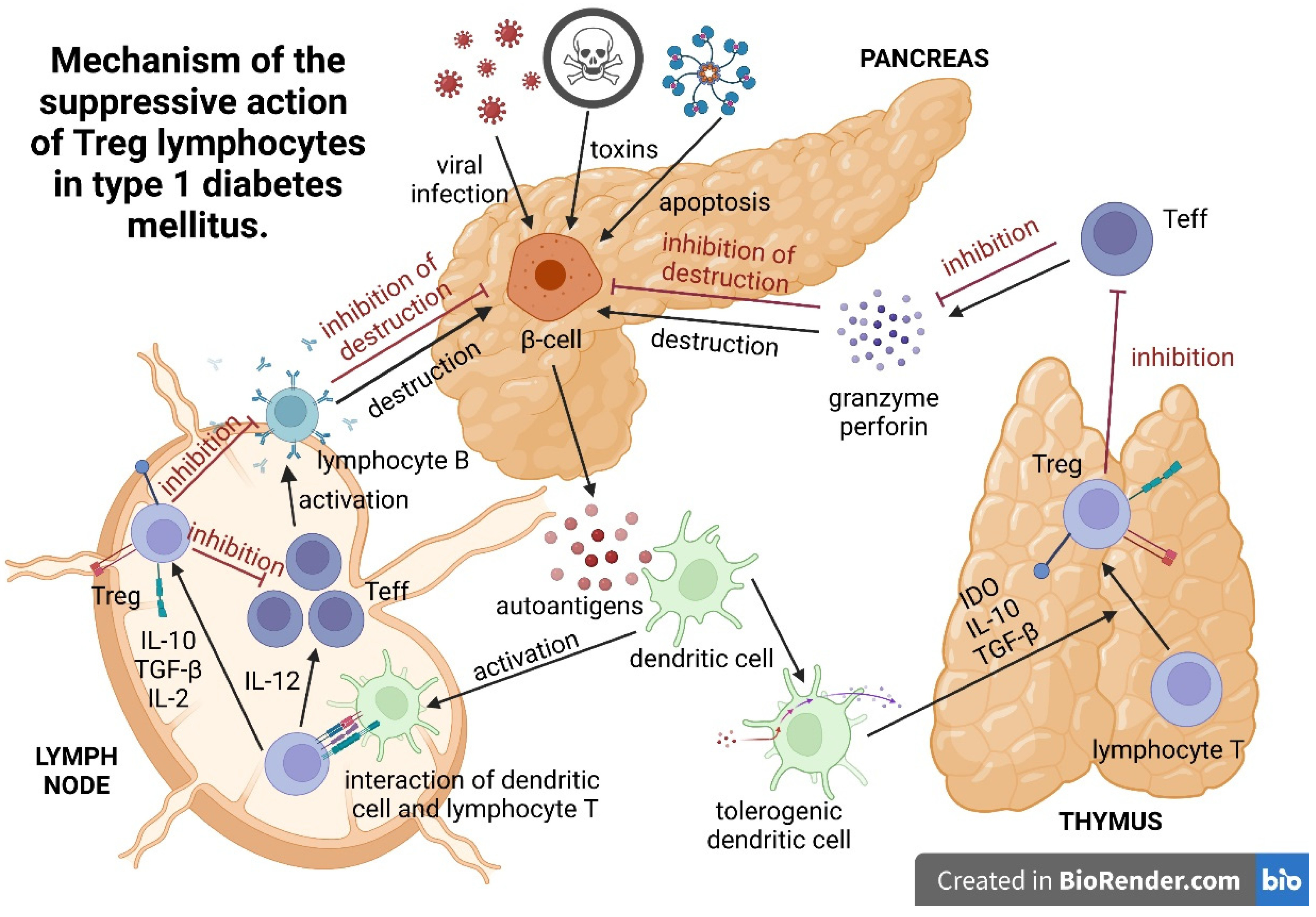
3. Mechanism of the Suppressive Action of Treg Lymphocytes
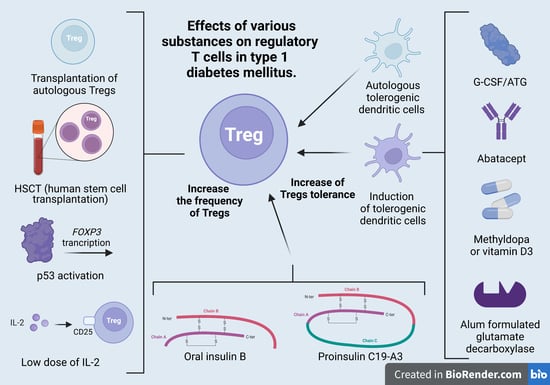
4. Treg Cells in the Treatment of T1DM
4.1. Treg-Cell Transplantation
4.2. Induction of Tregs
4.3. Pancreatic Islet Transplantation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desai, S.; Deshmukh, A. Mapping of Type 1 Diabetes Mellitus. Curr. Diabetes Rev. 2020, 16, 438–441. [Google Scholar] [CrossRef]
- Norris, J.M.; Johnson, R.K.; Stene, L.C. Type 1 diabetes—Early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020, 8, 226–238. [Google Scholar] [CrossRef]
- Watkins, R.A.; Evans-Molina, C.; Blum, J.S.; DiMeglio, L.A. Established and emerging biomarkers for the prediction of type 1 diabetes: A systematic review. Transl. Res. 2014, 164, 110–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalska, M.; Wąż, P.; Zorena, K.; Bartoszewicz, M.; Korzeniowska, K.; Krawczyk, S.; Beń-Skowronek, I.; Myśliwiec, M. Potential effects of microbial air quality on the number of new cases of diabetes type 1 in children in two regions of Poland: A pilot study. Infect. Drug Resist. 2019, 12, 2323–2334. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [PubMed]
- Tabarkiewicz, J.; Piekarski, R.; Wasiak, M.; Wdowiak, P.; Roliński, J.; Szewczyk, L. Evaluation of circulating dendritic cells and T regulatory cells in children with newly diagnosed type 1 diabetes mellitus. Pediatric. Endocrinol. 2009, 8, 27–34. [Google Scholar]
- Putnam, A.L.; Vendrame, F.; Dotta, F.; Gottlieb, P.A. CD4+CD25high regulatory T cells in human autoimmune diabetes. J. Autoimmun. 2005, 24, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Mhanna, V.; Fourcade, G.; Barennes, P.; Quiniou, V.; Pham, H.P.; Ritvo, P.-G.; Brimaud, F.; Gouritin, B.; Churlaud, G.; Six, A.; et al. Impaired Activated/Memory Regulatory T Cell Clonal Expansion Instigates Diabetes in NOD Mice. Diabetes 2021, 70, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Caridade, M.; Graca, L.; Ribeiro, R. Mechanisms Underlying CD4+ Treg Immune Regulation in the Adult: From Experiments to Models. Front. Immunol. 2013, 4, 378. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Xiang, Y.; Chang, C.; Zhou, Z. Alteration of regulatory T cells in type 1 diabetes mellitus: A comprehensive review. Clin. Rev. Allergy Immunol. 2014, 47, 234–243. [Google Scholar] [CrossRef]
- Oling, V.; Marttila, J.; Knip, M.; Simell, O.; Ilonen, J. Circulating CD4+CD25high regulatory T cells and natural killer T cells in children with newly diagnosed type 1 diabetes or with diabetes-associated autoantibodies. Ann. N. Y. Acad. Sci. 2007, 1107, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Glisic-Milosavljevic, S.; Wang, T.; Koppen, M.; Kramer, J.; Ehlenbach, S.; Waukau, J.; Jailwala, P.; Jana, S.; Alemzadeh, R.; Ghosh, S. Dynamic changes in CD4+ CD25+ high T cell apoptosis after the diagnosis of type 1 diabetes. Clin. Exp. Immunol. 2007, 150, 75–82. [Google Scholar] [CrossRef]
- Viisanen, T.; Gazali, A.M.; Ihantola, E.-L.; Ekman, I.; Näntö-Salonen, K.; Veijola, R.; Toppari, J.; Knip, M.; Ilonen, J.; Kinnunen, T. FOXP3+ Regulatory T Cell Compartment Is Altered in Children With Newly Diagnosed Type 1 Diabetes but Not in Autoantibody-Positive at-Risk Children. Front. Immunol. 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łuczyński, W.; Stasiak-Barmuta, A.; Wawrusiewicz-Kurylonek, N.; Kowalczuk, O.; Iłendo, E.; Głowińska-Olszewska, B.; Urban, R.; Szczepański, W.; Urban, M.; Kretowski, A.; et al. Disturbances in some gene expression in T regulatory cells separated from children with metabolic syndrome. Scand. J. Immunol. 2010, 71, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Kindelan, C.; Mackey, S.; Sands, A.; Rodriguez, J.; Vazquez, C.; Pugliese, A.; Bayer, A.L. Immunomodulation Followed by Antigen-Specific Treg Infusion Controls Islet Autoimmunity. Diabetes 2020, 69, 215–227. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Viglietta, V.; Hafler, D.A. Human CD4+CD25+ regulatory T cells. Semin. Immunol. 2004, 16, 89–98. [Google Scholar] [CrossRef]
- Hope, C.M.; Welch, J.; Mohandas, A.; Pederson, S.; Hill, D.; Gundsambuu, B.; Eastaff-Leung, N.; Grosse, R.; Bresatz, S.; Ang, G.; et al. Peptidase inhibitor 16 identifies a human regulatory T-cell subset with reduced FOXP3 expression over the first year of recent onset type 1 diabetes. Eur. J. Immunol. 2019, 49, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Ovcinnikovs, V.; Walker, L.S.K. Regulatory T Cells in Autoimmune Diabetes: Mechanisms of Action and Translational Potential. Prog. Mol. Biol. Transl. Sci. 2015, 136, 245–277. [Google Scholar] [CrossRef]
- Bacchetta, R.; Weinberg, K. Thymic origins of autoimmunity-lessons from inborn errors of immunity. Semin. Immunopathol. 2021, 43, 65–83. [Google Scholar] [CrossRef]
- Pacholczyk, R.; Kern, J. The T-cell receptor repertoire of regulatory T cells. Immunology 2008, 125, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Marx, A.; Yamada, Y.; Simon-Keller, K.; Schalke, B.; Willcox, N.; Ströbel, P.; Weis, C.-A. Thymus and autoimmunity. Semin. Immunopathol. 2021, 43, 45–64. [Google Scholar] [CrossRef]
- Volfson-Sedletsky, V.; Jones, A.; Hernandez-Escalante, J.; Dooms, H. Emerging Therapeutic Strategies to Restore Regulatory T Cell Control of Islet Autoimmunity in Type 1 Diabetes. Front. Immunol. 2021, 12, 635767. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.A.; Klawon, D.E.J.; Miller, C.H. Regulatory T Cell Development. Annu. Rev. Immunol. 2020, 38, 421–453. [Google Scholar] [CrossRef] [Green Version]
- Marfil-Garza, B.A.; Hefler, J.; Bermudez De Leon, M.; Pawlick, R.; Dadheech, N.; Shapiro, A.M.J. Progress in Translational Regulatory T Cell Therapies for Type 1 Diabetes and Islet Transplantation. Endocr. Rev. 2021, 42, 198–218. [Google Scholar] [CrossRef]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat. Immunol. 2005, 6, 1071–1072. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Christie, J.; Ramsdell, F.; Brunkow, M.E.; Ferguson, P.J.; Whitesell, L.; Kelly, T.E.; Saulsbury, F.T.; Chance, P.F.; Ochs, H.D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001, 27, 20–21. [Google Scholar] [CrossRef]
- Serr, I.; Drost, F.; Schubert, B.; Daniel, C. Antigen-Specific Treg Therapy in Type 1 Diabetes—Challenges and Opportunities. Front. Immunol. 2021, 12, 712870. [Google Scholar] [CrossRef] [PubMed]
- Pesenacker, A.M.; Cook, L.; Levings, M.K. The role of FOXP3 in autoimmunity. Curr. Opin. Immunol. 2016, 43, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.H.; Zelenay, S.; Bergman, M.-L.; Martins, A.C.; Demengeot, J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur. J. Immunol. 2009, 39, 948–955. [Google Scholar] [CrossRef]
- Aboelnazar, S.; Ghoneim, H.; Moaaz, M.; Bahgat, E.T.; Shalaby, T. Low-dose interleukin-2-loaded nanoparticle effect on NK and T-reg cell expression in experimentally induced type 1 diabetes mellitus. Prz. Gastroenterol. 2021, 16, 67–82. [Google Scholar] [CrossRef]
- Salomon, B.; Bluestone, J.A. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 2001, 19, 225–252. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007, 450, 566–569. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.-F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Cai, S.F.; Fehniger, T.A.; Song, J.; Collins, L.I.; Piwnica-Worms, D.R.; Ley, T.J. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 2007, 27, 635–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, S.; Gagliani, N.; Esplugues, E.; O’Connor, W.; Huber, F.J.; Chaudhry, A.; Kamanaka, M.; Kobayashi, Y.; Booth, C.J.; Rudensky, A.Y.; et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 2011, 34, 554–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apetoh, L.; Quintana, F.J.; Pot, C.; Joller, N.; Xiao, S.; Kumar, D.; Burns, E.J.; Sherr, D.H.; Weiner, H.L.; Kuchroo, V.K. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010, 11, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Hull, C.M.; Peakman, M.; Tree, T.I.M. Regulatory T cell dysfunction in type 1 diabetes: What’s broken and how can we fix it? Diabetologia 2017, 60, 1839–1850. [Google Scholar] [CrossRef] [Green Version]
- Chien, C.-H.; Chiang, B.-L. Regulatory T cells induced by B cells: A novel subpopulation of regulatory T cells. J. Biomed. Sci. 2017, 24, 86. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Lee, W.-H.; Yun, P.; Snow, P.; Liu, C.-P. Induction of autoantigen-specific Th2 and Tr1 regulatory T cells and modulation of autoimmune diabetes. J. Immunol. 2003, 171, 733–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Gagliani, N.; Ishigame, H.; Huber, S.; Zhu, S.; Esplugues, E.; Herold, K.C.; Wen, L.; Flavell, R.A. Intestinal type 1 regulatory T cells migrate to periphery to suppress diabetogenic T cells and prevent diabetes development. Proc. Natl. Acad. Sci. USA 2017, 114, 10443–10448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chujo, D.; Nguyen, T.-S.; Foucat, E.; Blankenship, D.; Banchereau, J.; Nepom, G.T.; Chaussabel, D.; Ueno, H. Adult-onset type 1 diabetes patients display decreased IGRP-specific Tr1 cells in blood. Clin. Immunol. 2015, 161, 270–277. [Google Scholar] [CrossRef]
- Petrich de Marquesini, L.G.; Fu, J.; Connor, K.J.; Bishop, A.J.; McLintock, N.E.; Pope, C.; Wong, F.S.; Dayan, C.M. IFN-gamma and IL-10 islet-antigen-specific T cell responses in autoantibody-negative first-degree relatives of patients with type 1 diabetes. Diabetologia 2010, 53, 1451–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arif, S.; Tree, T.I.; Astill, T.P.; Tremble, J.M.; Bishop, A.J.; Dayan, C.M.; Roep, B.O.; Peakman, M. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J. Clin. Investig. 2004, 113, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Herold, K.C.; Gitelman, S.E.; Ehlers, M.R.; Gottlieb, P.A.; Greenbaum, C.J.; Hagopian, W.; Boyle, K.D.; Keyes-Elstein, L.; Aggarwal, S.; Phippard, D.; et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: Metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 2013, 62, 3766–3774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; He, X.; Cai, P.; Li, T.; Peng, R.; Dang, J.; Li, Y.; Li, H.; Huang, F.; Shi, G.; et al. Induced regulatory T cells suppress Tc1 cells through TGF-β signaling to ameliorate STZ-induced type 1 diabetes mellitus. Cell. Mol. Immunol. 2021, 18, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Kroger, C.J.; Ke, Q.; Tisch, R.M. The Role of T Cell Receptor Signaling in the Development of Type 1 Diabetes. Front. Immunol. 2020, 11, 615371. [Google Scholar] [CrossRef]
- Battaglia, M.; Stabilini, A.; Draghici, E.; Migliavacca, B.; Gregori, S.; Bonifacio, E.; Roncarolo, M.-G. Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes 2006, 55, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Jonuleit, H.; Schmitt, E.; Kakirman, H.; Stassen, M.; Knop, J.; Enk, A.H. Infectious tolerance: Human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J. Exp. Med. 2002, 196, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Baecher-Allan, C.; Viglietta, V.; Hafler, D.A. Inhibition of human CD4+CD25+high regulatory T cell function. J. Immunol. 2002, 169, 6210–6217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, T.; Takasawa, K.; Kamiya, T.; Kirino, S.; Gau, M.; Inoue, K.; Hoshino, A.; Kashimada, K.; Kanegane, H.; Morio, T. Hematopoietic stem cell transplantation recovers insulin deficiency in type 1 diabetes mellitus associated with IPEX syndrome. Pediatr. Diabetes 2019, 20, 1035–1040. [Google Scholar] [CrossRef]
- Marek, N.; Bieniaszewska, M.; Krzystyniak, A.; Juścińska, J.; Myśliwska, J.; Witkowski, P.; Hellmann, A.; Trzonkowski, P. The time is crucial for ex vivo expansion of T regulatory cells for therapy. Cell Transpl. 2011, 20, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-S.; Wang, J.; Shen, D.-F.; Yuan, X.-L.; Dong, P.; Li, M.-X.; Xue, J.; Zhang, F.-M.; Ge, H.-L.; Xu, D. CD4+CD25+CD127low/− regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin. Immunol. 2009, 131, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.I.; Reneer, M.C.; Vélez-Ortega, A.C.; McCool, A.; Martí, F. Generation of induced regulatory T cells from primary human naïve and memory T cells. J. Vis. Exp. 2012, 62, e3738. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, C.; Weiner, H.L. Therapeutic anti-CD3 monoclonal antibodies: From bench to bedside. Immunotherapy 2016, 8, 889–906. [Google Scholar] [CrossRef] [Green Version]
- Song, J. Development of Auto Antigen-specific Regulatory T Cells for Diabetes Immunotherapy. Immune Netw. 2016, 16, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, C.; Ortiz, A.Z.; Presa, M.; Malo, S.; Montoya, A.; Garabatos, N.; Mora, C.; Verdaguer, J.; Stratmann, T. Treatment of T1D via optimized expansion of antigen-specific Tregs induced by IL-2/anti-IL-2 monoclonal antibody complexes and peptide/MHC tetramers. Sci. Rep. 2018, 8, 8106. [Google Scholar] [CrossRef]
- Brusko, T.; Bluestone, J. Clinical application of regulatory T cells for treatment of type 1 diabetes and transplantation. Eur. J. Immunol. 2008, 38, 931–934. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Buckner, J.H.; Fitch, M.; Gitelman, S.E.; Gupta, S.; Hellerstein, M.K.; Herold, K.C.; Lares, A.; Lee, M.R.; Li, K.; et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015, 7, 315ra189. [Google Scholar] [CrossRef] [Green Version]
- Yeh, W.-I.; Seay, H.R.; Newby, B.; Posgai, A.L.; Moniz, F.B.; Michels, A.; Mathews, C.E.; Bluestone, J.A.; Brusko, T.M. Avidity and Bystander Suppressive Capacity of Human Regulatory T Cells Expressing De Novo Autoreactive T-Cell Receptors in Type 1 Diabetes. Front. Immunol. 2017, 8, 1313. [Google Scholar] [CrossRef] [PubMed]
- Green, E.; Choi, Y.; Flavell, R.A. Pancreatic Lymph Node-Derived CD4+CD25+ Treg Cells. Immunity 2002, 16, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Henriksen, K.J.; Bi, M.; Finger, E.B.; Szot, G.; Ye, J.; Masteller, E.L.; McDevitt, H.; Bonyhadi, M.; Bluestone, J.A. In Vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004, 199, 1455–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.C.; Dash, P.; McCullers, J.A.; Doherty, P.C.; Thomas, P.G. T cell receptor αβ diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Sci. Transl. Med. 2012, 4, 128ra42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marek-Trzonkowska, N.; Mysliwiec, M.; Dobyszuk, A.; Grabowska, M.; Techmanska, I.; Juscinska, J.; Wujtewicz, M.A.; Witkowski, P.; Mlynarski, W.; Balcerska, A.; et al. Administration of CD4+CD25highCD127− regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care 2012, 35, 1817–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marek-Trzonkowska, N.; Myśliwiec, M.; Dobyszuk, A.; Grabowska, M.; Derkowska, I.; Juścińska, J.; Owczuk, R.; Szadkowska, A.; Witkowski, P.; Młynarski, W.; et al. Therapy of type 1 diabetes with CD4+CD25highCD127− regulatory T cells prolongs survival of pancreatic islets-results of one year follow-up. Clin. Immunol. 2014, 153, 23–30. [Google Scholar] [CrossRef]
- Marek-Trzonkowska, N.; Myśliwiec, M.; Iwaszkiewicz-Grześ, D.; Gliwiński, M.; Derkowska, I.; Żalińska, M.; Zieliński, M.; Grabowska, M.; Zielińska, H.; Piekarska, K.; et al. Factors affecting long-term efficacy of T regulatory cell-based therapy in type 1 diabetes. J. Transl. Med. 2016, 14, 332. [Google Scholar] [CrossRef] [Green Version]
- Gliwiński, M.; Iwaszkiewicz-Grześ, D.; Wołoszyn-Durkiewicz, A.; Tarnowska, M.; Żalińska, M.; Hennig, M.; Zielińska, H.; Dukat-Mazurek, A.; Zielkowska-Dębska, J.; Zieliński, M.; et al. Proinsulin-specific T regulatory cells may control immune responses in type 1 diabetes: Implications for adoptive therapy. BMJ Open Diabetes Res. Care 2020, 8, e000873. [Google Scholar] [CrossRef] [Green Version]
- Sodré, F.M.C.; Bissenova, S.; Bruggeman, Y.; Tilvawala, R.; Cook, D.P.; Berthault, C.; Mondal, S.; Callebaut, A.; You, S.; Scharfmann, R.; et al. Peptidylarginine Deiminase Inhibition Prevents Diabetes Development in NOD Mice. Diabetes 2021, 70, 516–528. [Google Scholar] [CrossRef]
- Hartemann, A.; Bensimon, G.; Payan, C.A.; Jacqueminet, S.; Bourron, O.; Nicolas, N.; Fonfrede, M.; Rosenzwajg, M.; Bernard, C.; Klatzmann, D. Low-dose interleukin 2 in patients with type 1 diabetes: A phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013, 1, 295–305. [Google Scholar] [CrossRef]
- Todd, J.A.; Evangelou, M.; Cutler, A.J.; Pekalski, M.L.; Walker, N.M.; Stevens, H.E.; Porter, L.; Smyth, D.J.; Rainbow, D.B.; Ferreira, R.C.; et al. Regulatory T Cell Responses in Participants with Type 1 Diabetes after a Single Dose of Interleukin-2: A Non-Randomised, Open Label, Adaptive Dose-Finding Trial. PLoS Med. 2016, 13, e1002139. [Google Scholar] [CrossRef]
- Rosenzwajg, M.; Salet, R.; Lorenzon, R.; Tchitchek, N.; Roux, A.; Bernard, C.; Carel, J.-C.; Storey, C.; Polak, M.; Beltrand, J.; et al. Low-dose IL-2 in children with recently diagnosed type 1 diabetes: A Phase I/II randomised, double-blind, placebo-controlled, dose-finding study. Diabetologia 2020, 63, 1808–1821. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Paiva, R.; Flavell, R.A. Harnessing the power of regulatory T-cells to control autoimmune diabetes: Overview and perspective. Immunology 2018, 153, 161–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Michels, A.W.; Rosenthal, S.M.; Shuster, J.J.; Zou, B.; Brusko, T.M.; Hulme, M.A.; Wasserfall, C.H.; et al. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J. Clin. Investig. 2015, 125, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Haller, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Michels, A.W.; Perry, D.J.; Schultz, A.R.; Hulme, M.A.; Shuster, J.J.; Zou, B.; Wasserfall, C.H.; et al. Antithymocyte Globulin Plus G-CSF Combination Therapy Leads to Sustained Immunomodulatory and Metabolic Effects in a Subset of Responders With Established Type 1 Diabetes. Diabetes 2016, 65, 3765–3775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannoukakis, N.; Phillips, B.; Finegold, D.; Harnaha, J.; Trucco, M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 2011, 34, 2026–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, D.; Wegmann, D.R. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9-23). Proc. Natl. Acad. Sci. USA 1996, 93, 956–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orban, T.; Farkas, K.; Jalahej, H.; Kis, J.; Treszl, A.; Falk, B.; Reijonen, H.; Wolfsdorf, J.; Ricker, A.; Matthews, J.B.; et al. Autoantigen-specific regulatory T cells induced in patients with type 1 diabetes mellitus by insulin B-chain immunotherapy. J. Autoimmun. 2010, 34, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Alhadj Ali, M.; Liu, Y.-F.; Arif, S.; Tatovic, D.; Shariff, H.; Gibson, V.B.; Yusuf, N.; Baptista, R.; Eichmann, M.; Petrov, N.; et al. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci. Transl. Med. 2017, 9, eaaf7779. [Google Scholar] [CrossRef] [Green Version]
- Gibson, V.B.; Nikolic, T.; Pearce, V.Q.; Demengeot, J.; Roep, B.O.; Peakman, M. Proinsulin multi-peptide immunotherapy induces antigen-specific regulatory T cells and limits autoimmunity in a humanized model. Clin. Exp. Immunol. 2015, 182, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Martinez, N.R.; Augstein, P.; Moustakas, A.K.; Papadopoulos, G.K.; Gregori, S.; Adorini, L.; Jackson, D.C.; Harrison, L.C. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J. Clin. Investig. 2003, 111, 1365–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, F.; Citro, A.; Squeri, G.; Sanvito, F.; Monti, P.; Gregori, S.; Roncarolo, M.G.; Annoni, A. InsB9-23 Gene Transfer to Hepatocyte-Based Combined Therapy Abrogates Recurrence of Type 1 Diabetes After Islet Transplantation. Diabetes 2021, 70, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Assfalg, R.; Knoop, J.; Hoffman, K.L.; Pfirrmann, M.; Zapardiel-Gonzalo, J.M.; Hofelich, A.; Eugster, A.; Weigelt, M.; Matzke, C.; Reinhardt, J.; et al. Oral insulin immunotherapy in children at risk for type 1 diabetes in a randomised controlled trial. Diabetologia 2021, 64, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shi, J.; Li, J.; Wu, M.; Li, Y.; Jia, S.; Ma, C.; Wang, X.; Li, Z.; Hu, N.; et al. The Effect of Immunosuppressive Adjuvant Kynurenine on Type 1 Diabetes Vaccine. Front. Immunol. 2021, 12, 681328. [Google Scholar] [CrossRef]
- Brusko, T.M.; Russ, H.A.; Stabler, C.L. Strategies for durable β cell replacement in type 1 diabetes. Science 2021, 373, 516–522. [Google Scholar] [CrossRef]
- Koprivica, I.; Gajic, D.; Saksida, T.; Cavalli, E.; Auci, D.; Despotovic, S.; Pejnovic, N.; Stosic-Grujicic, S.; Nicoletti, F.; Stojanovic, I. Orally delivered all-trans-retinoic acid- and transforming growth factor-β-loaded microparticles ameliorate type 1 diabetes in mice. Eur. J. Pharmacol. 2019, 864, 172721. [Google Scholar] [CrossRef]
- Knip, M.; Siljander, H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, I.; Saksida, T.; Miljković, Đ.; Pejnović, N. Modulation of Intestinal ILC3 for the Treatment of Type 1 Diabetes. Front. Immunol. 2021, 12, 653560. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, H.; Abdolmaleki, M.; Ebrahimzadeh, M.A.; Mojtabavi, N.; Mousavi, T.; Izad, M. Methanolic Extract of Sambucus ebulus Ameliorates Clinical Symptoms in Experimental Type 1 Diabetes through Anti-Inflammatory and Immunomodulatory Actions. Cell J. 2021, 23, 465–473. [Google Scholar] [CrossRef]
- Duan, W.; Ding, Y.; Yu, X.; Ma, D.; Yang, B.; Li, Y.; Huang, L.; Chen, Z.; Zheng, J.; Yang, C. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. Am. J. Transl. Res. 2019, 11, 2393–2402. [Google Scholar]
- Spangler, J.B.; Tomala, J.; Luca, V.C.; Jude, K.M.; Dong, S.; Ring, A.M.; Votavova, P.; Pepper, M.; Kovar, M.; Garcia, K.C. Antibodies to Interleukin-2 Elicit Selective T Cell Subset Potentiation through Distinct Conformational Mechanisms. Immunity 2015, 42, 815–825. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-R.; Huang, S.-H.; Wu, C.-H.; Chen, Y.-W.; Hong, Z.-J.; Cheng, C.-P.; Sytwu, H.-K.; Lin, G.-J. Valproic Acid Suppresses Autoimmune Recurrence and Allograft Rejection in Islet Transplantation through Induction of the Differentiation of Regulatory T Cells and Can Be Used in Cell Therapy for Type 1 Diabetes. Pharmaceuticals 2021, 14, 475. [Google Scholar] [CrossRef]
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S.; et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N. Engl. J. Med. 2019, 381, 603–613. [Google Scholar] [CrossRef] [Green Version]

| Regulatory T Cells | Foxp3+Tregs | Tregs of B Cells (B-Cell-Induced Tregs) | Th3 | Tr1 | CD8+ Tregs | nTregs | iTregs |
|---|---|---|---|---|---|---|---|
| Selection place | Naïve Tregs—thymus. Peripheral Tregs—peripheral tissues and MALT (mucosa-associated lymphoid tissue). Induced Tregs—in vitro-induced Tregs | Spleen MALT | Gut MALT | Spleen Lymph node | Spleen Lymph node | Thymus | In vitro/ Peripheral tissues |
| Phenotype | CD4+, CD25+, Foxp3+, CTLA-4+ | CD4+CD25+Foxp3- LAG 3+ ICOS+PD1+GITR+ OX40+ | CD4+CD25-Foxp3- LAP+ | CD4+CD25− Foxp3+, CD49b+, LAG3+, CD226+ | CD25+, Foxp3+ CD28- | CD4+, CD25+, Foxp3+ | CD25+, Foxp3+ |
| Mechanism of suppressive action | Direct cell–cell contact Granzyme-B-dependent formation of TGF beta | IL-10 | TGF beta IL-10 (strong) | IL-10 (strong) TGF beta CTLA4 CD226 | IL-4, IL-5, IL-10 | TGF beta IL-2 CD28 | TGF beta |
| Role in T1DM development | Reduction in the functional capacity of Foxp3+Treg populations contributes to disease development in type 1 diabetes | Immunomodulatory effect? | Th3 cells probably originate from naïve T cells as a result of stimulation with immature dendritic cells (iDCs), presenting various antigens, including autoantigens. Such stimulation results in the in vivo and in vitro formation of anergic cells with suppressive properties. | It has been shown that newly diagnosed T1DM patients and their first-degree relatives have fewer IL-10-secreting cells than healthy controls; this deficit in Treg function is amplified by an increased Teff function, which is reflected by increased antigen-specific IFN-c secretion. | The main triggers of β-cell autoimmunity | Inhibiting the immune response of effector T cells and maintaining immune tolerance | Inhibition of Th1 cells by a FasL-mediated cytotoxic effect Decreased Tc1-cell proliferation, increased Tc1-cell apoptosis, decreased Tc1-cell infiltration and/or inhibition of Tc1-cell differentiation |
| Methods of Therapy | Authors | Number of Patients | Results |
|---|---|---|---|
| Transplantation of autologous Tregs | Bluestone 2015 (42) Marek-Trzonkowska et al., 2012, 2014, 2016, 2020 (43–45) Brusko 2008 (58) | 14 12 | The therapy resulted in beta-cell regeneration, insulin production, and a strong decrease in therapeutic insulin intake. T1DM development was associated with changing proportions of naïve and memory Tregs and slowly increasing proinflammatory activity, which was only partially controlled by the administered Tregs. The authors suggest that the therapy should be administered early to protect the highest possible mass of islets and to utilize the preserved content of Tregs in the earlier phases of T1DM. The therapy extended remission of the T1MD (honeymoon) and decreased the doses of insulin necessary for treatment. |
| Low dose of IL-2 | Hartemann et al., 2013 (47) Todd et al., 2016 (48) Rosenzwaig 2015, 2020 (49, 50) | 24 40 24 | The authors defined a well-tolerated and immunologically effective dose range of IL2 for application to type 1 diabetes therapy and prevention. Early intervention with IL2 could help to re-establish a proper immune milieu and slow down or even reverse the pathological processes in T1DM. This therapy may improve maintenance of induced C-peptide production at 1 year. The adverse effects were influenza-like syndrome and injection-site reactions. |
| Induction of Tregs by tolerogenic DCs | |||
| Anti-thymocyte globulin and G-CSF (ATG/GCSF) | Haller et al., 2015, 2017, 2019 (52,53,64) | 17 | Immune responses returned to normal upon withdrawal of therapy. |
| CTLA-4-Ig (abatacept) | Orban et al., 2013 (65) | 112 | Co-stimulation modulation with abatacept slowed the decline of beta-cell function and improved HbA1c in recent-onset T1DM. The beneficial effect was sustained for at least one year after cessation of abatacept infusions or three years from T1DM diagnosis. |
| Methyldopa | Ostrov et al., 2018 (66) | 20 | Methyldopa specifically blocked DQ8 in patients with recent-onset T1D, highlighting the relevance of blocking disease-specific MHC class II antigen presentation to treat autoimmunity. |
| Alum formulated glutamate decarboxylase | Elding Larsson 2018 (67) | 50 | The subcutaneous prime and boost administration of GAD-Alum was safe but did not affect progression of T1DM. |
| Vitamin D3 | Piekarski 2012 (68) | 90 | Alfadiol, an analogue of vitamin D3, increased or maintained the value of C-peptide during the annual monitoring as compared with baseline values. This therapy extended the honeymoon in children with newly diagnosed T1DM. |
| Autologous tolerogenic dendritic cells | Giannoukakis 2011 (54) | 10 | The autologous tolerogenic dendritic cells in patients between 1 and 5 years of T1DM upregulated the frequency of B220+CD11c-B-cells, but the average insulin dose in patients remained unchanged. |
| Induction of Treg by tolerogenic peptides | |||
| Oral insulin B | Orban et al., 2010 (56) Krischer 2017 (69) | 12 560 | Oral insulin (7.5 mg/day) did not delay or prevent the development of type 1 diabetes. A higher dose (67.5 mg/day) was reported to produce protective insulin-responsive regulatory T-cell responses in genetically at-risk young relatives. |
| Proinsulin C19-A3 | Alhadj et al., 2017 (5) | 27 | Proinsulin peptide therapy restored immune tolerance in preclinical phase of T1DM but did not accelerate the decrease in beta-cell function. |
| Induction of Tregs by p53 activation | Pellegrino (70) | 10 | Due to the Teff dysregulation upon p53 activation, molecules promoting p53 cannot be part of the therapy for T1DM. |
| HSCT (human stem-cell transplantation) | Li 2012 (71) Zhang 2012 (72) Gu 2012 (73) D’Addio 2014 (74) Carlsson 2014 (75) | 13 9 28 65 20 1 | Human stem-cell transplantation is a safe and promising method for T1DM treatment and leads to an increase in C-peptide and insulin secretion. This method is more effective in the early stages of T1DM. Patients diagnosed in ketoacidosis at diagnosis have minimal chance for beta-cell recovery. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Skowronek, I.; Sieniawska, J.; Pach, E.; Wrobel, W.; Skowronek, A.; Tomczyk, Z.; Rosolowska, I. Potential Therapeutic Application of Regulatory T Cells in Diabetes Mellitus Type 1. Int. J. Mol. Sci. 2022, 23, 390. https://doi.org/10.3390/ijms23010390
Ben-Skowronek I, Sieniawska J, Pach E, Wrobel W, Skowronek A, Tomczyk Z, Rosolowska I. Potential Therapeutic Application of Regulatory T Cells in Diabetes Mellitus Type 1. International Journal of Molecular Sciences. 2022; 23(1):390. https://doi.org/10.3390/ijms23010390
Chicago/Turabian StyleBen-Skowronek, Iwona, Joanna Sieniawska, Emilia Pach, Wiktoria Wrobel, Anna Skowronek, Zaklina Tomczyk, and Iga Rosolowska. 2022. "Potential Therapeutic Application of Regulatory T Cells in Diabetes Mellitus Type 1" International Journal of Molecular Sciences 23, no. 1: 390. https://doi.org/10.3390/ijms23010390
APA StyleBen-Skowronek, I., Sieniawska, J., Pach, E., Wrobel, W., Skowronek, A., Tomczyk, Z., & Rosolowska, I. (2022). Potential Therapeutic Application of Regulatory T Cells in Diabetes Mellitus Type 1. International Journal of Molecular Sciences, 23(1), 390. https://doi.org/10.3390/ijms23010390