Transport and Toxicity of Methylmercury-Cysteine in Cultured BeWo Cells
Abstract
:1. Introduction
2. Results
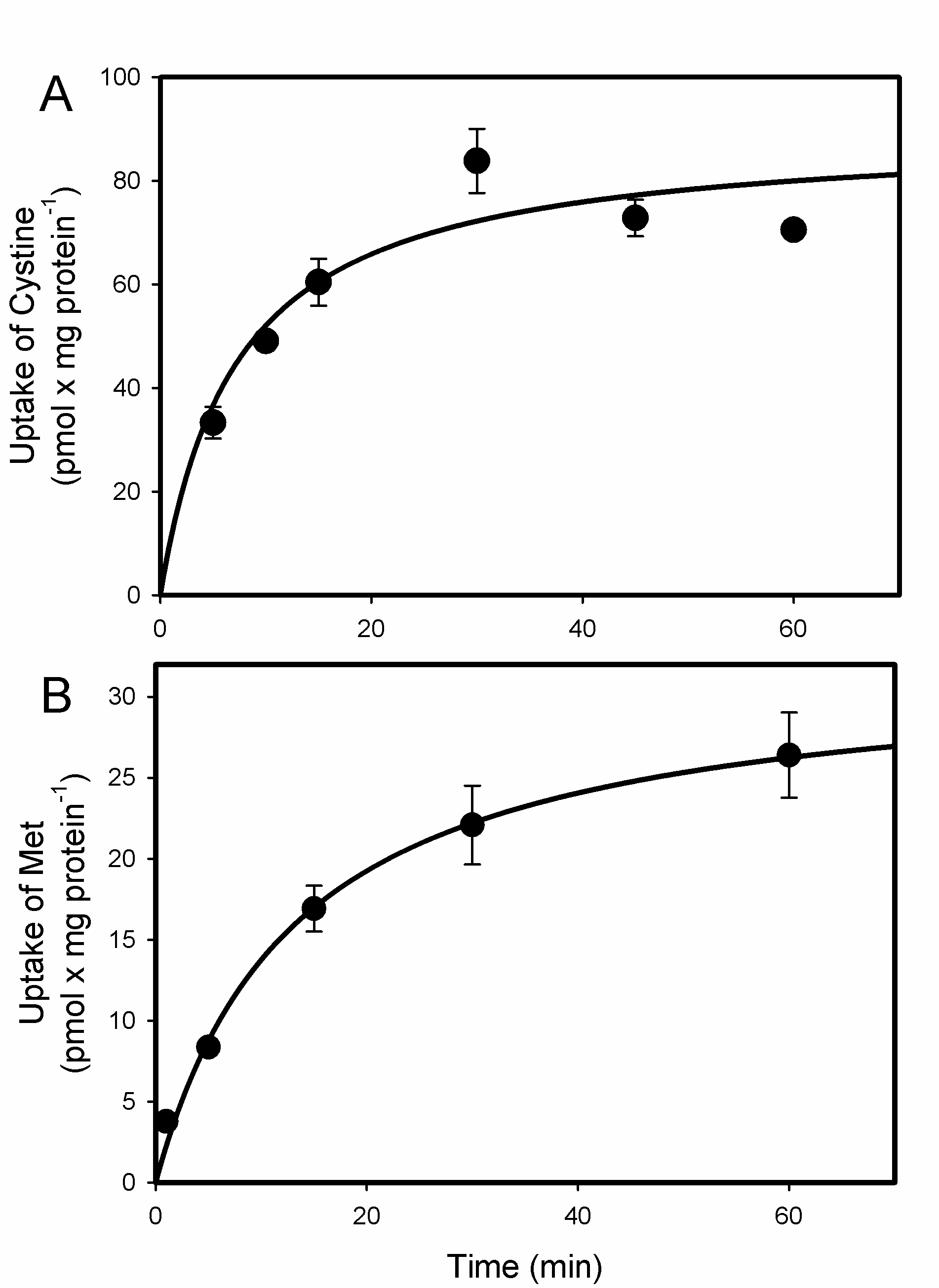
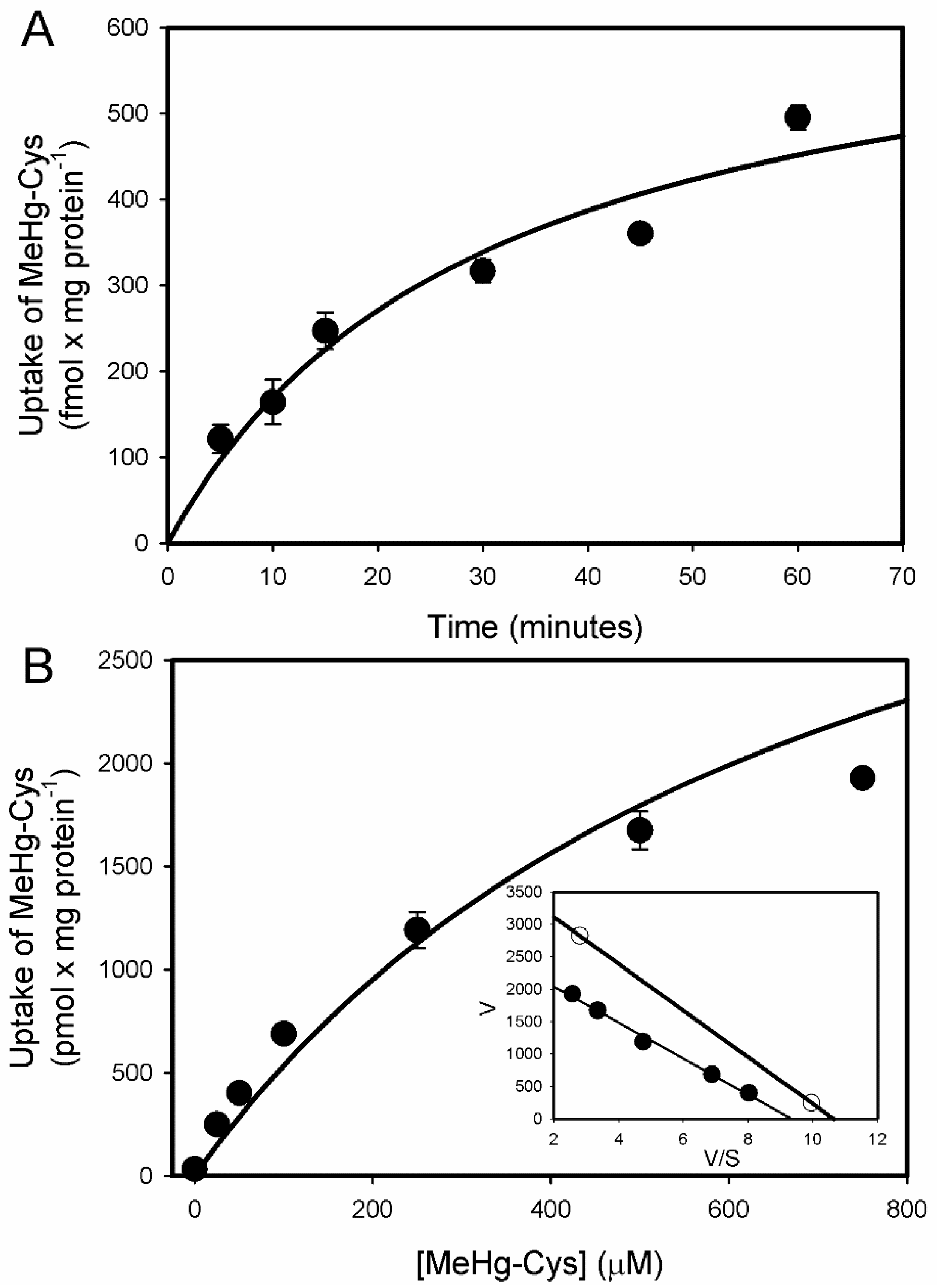
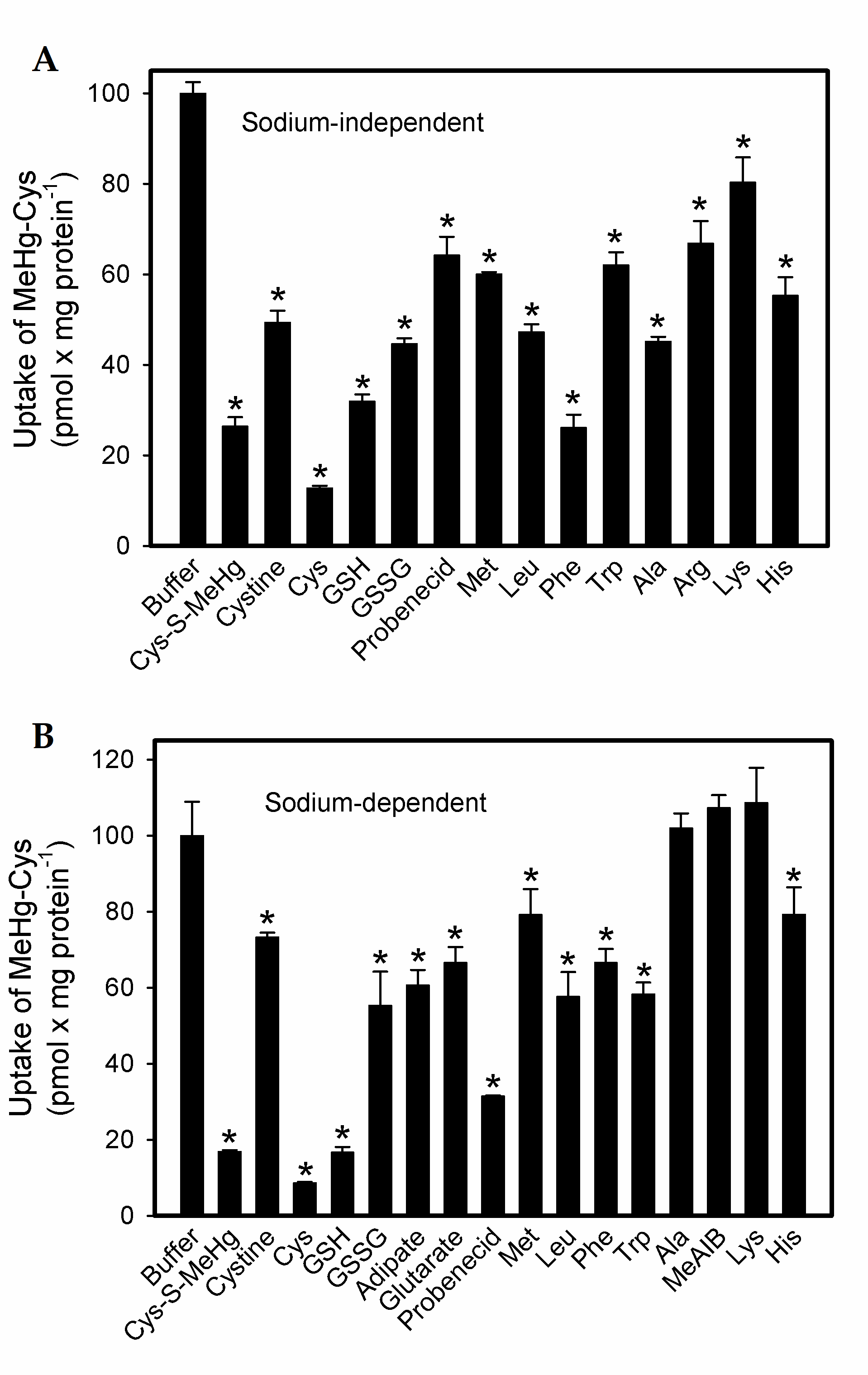
2.1. Uptake of Cystine and MeHg-Cys in BeWo Cells
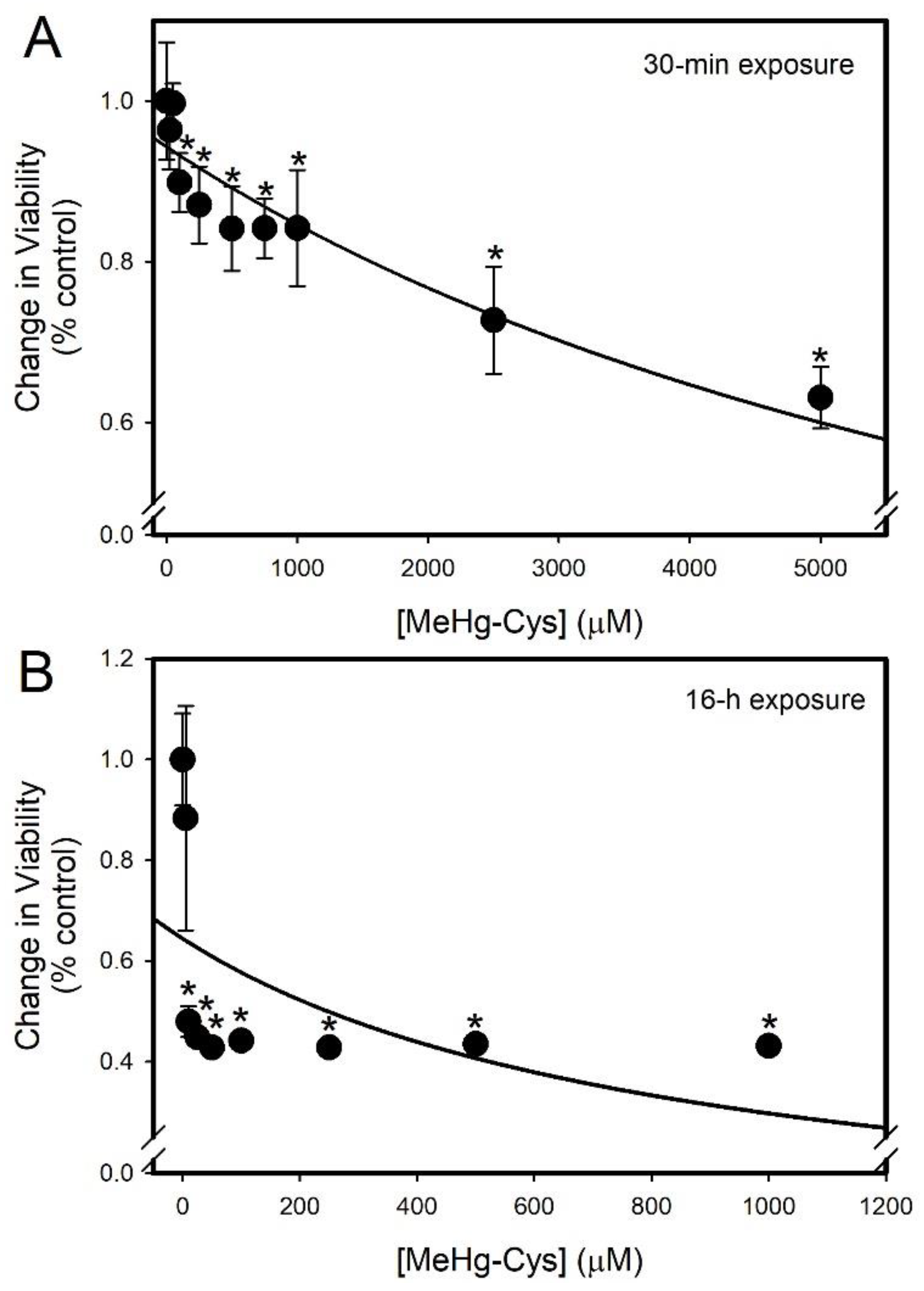
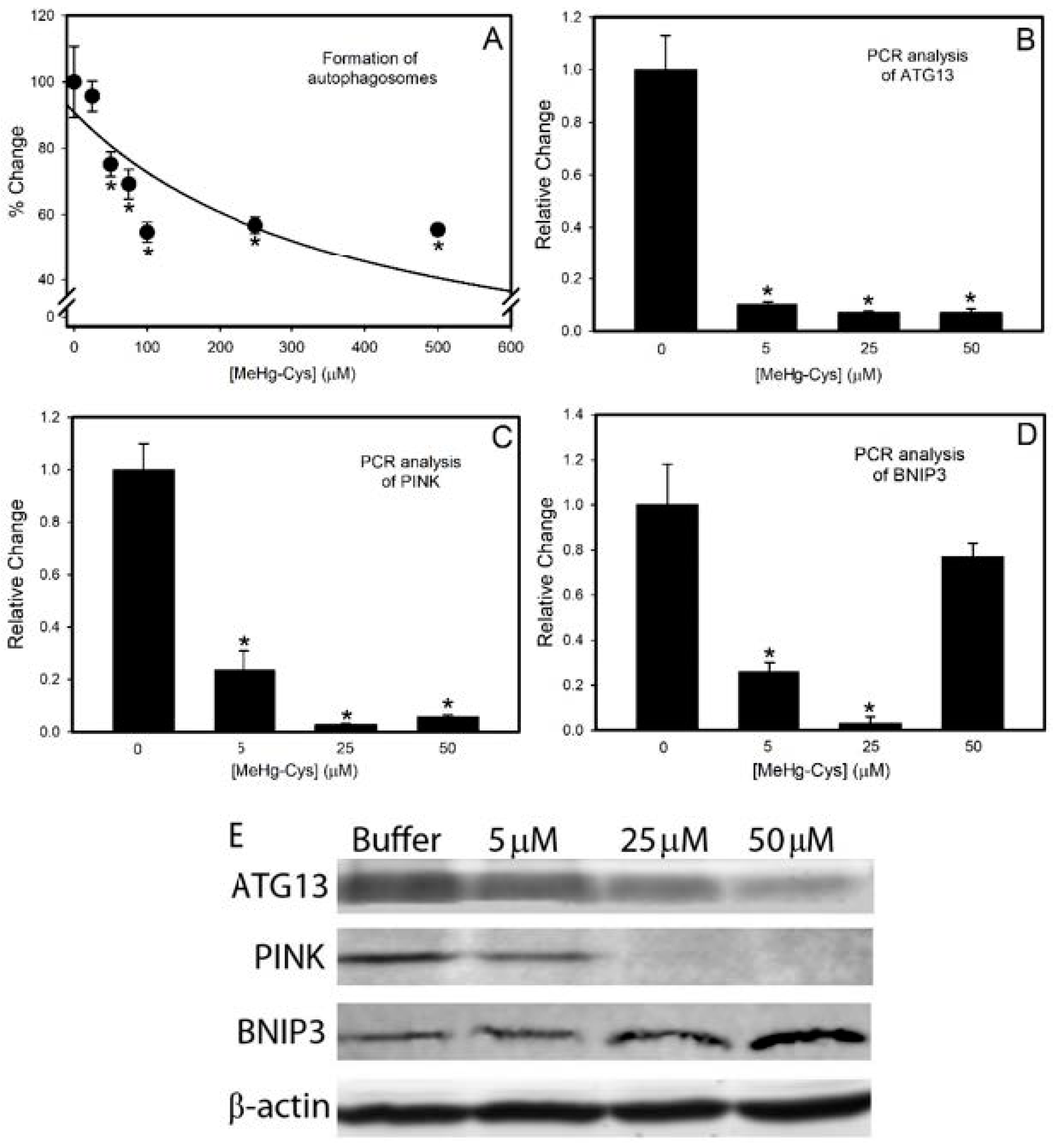
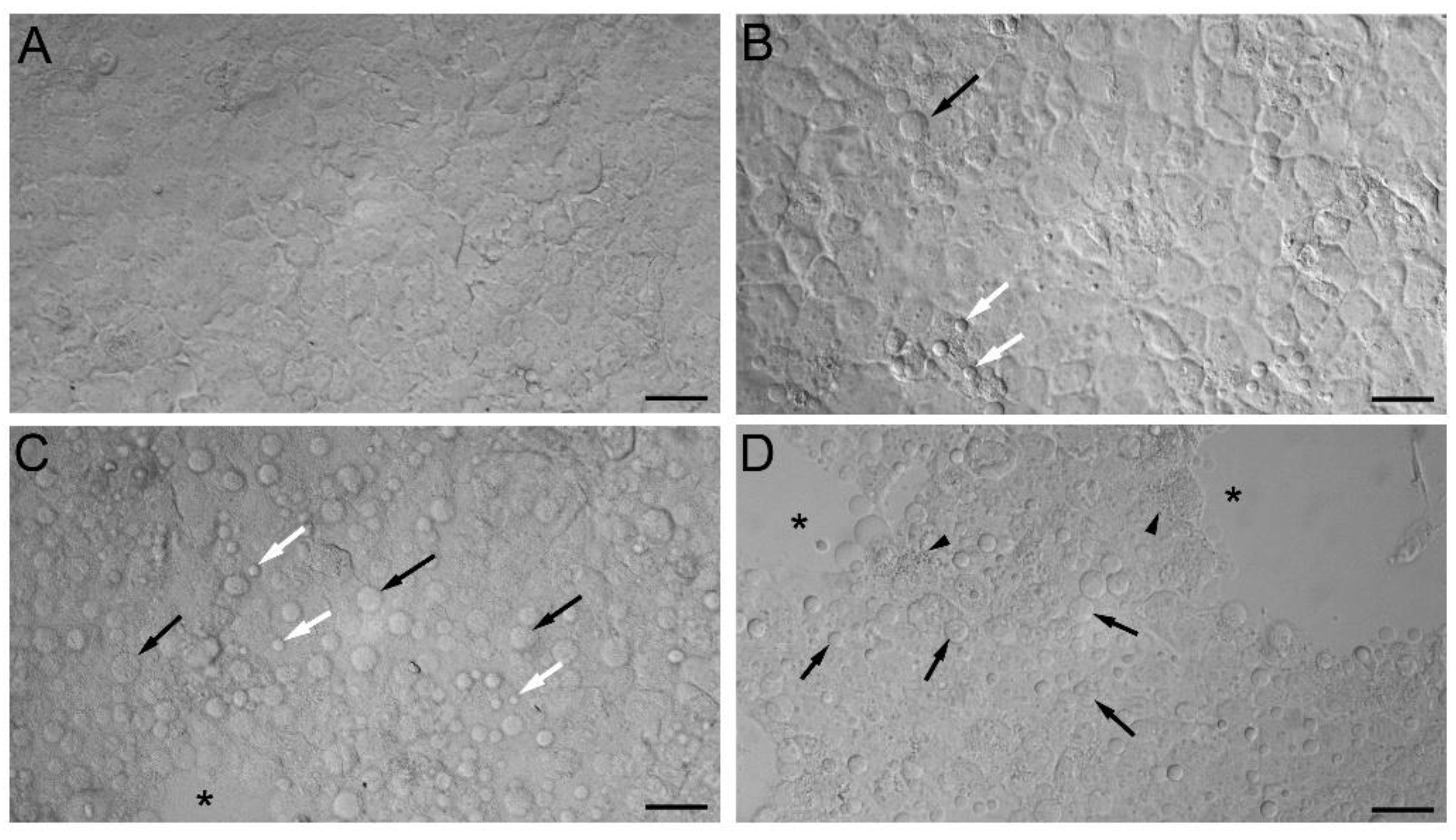
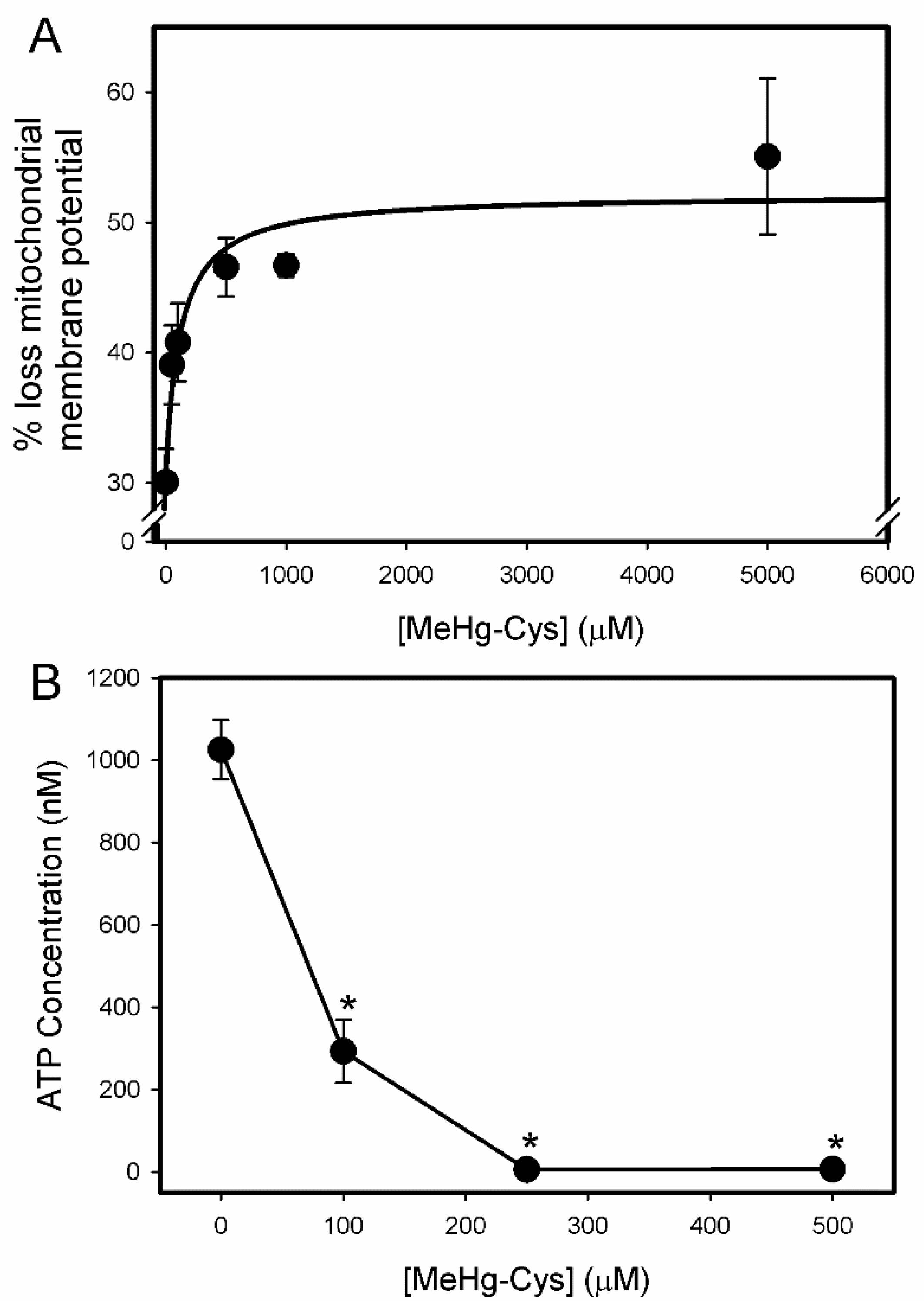
2.2. Cell Viability and Function Assays
2.3. Markers of Intracellular Injury and Oxidative Stress
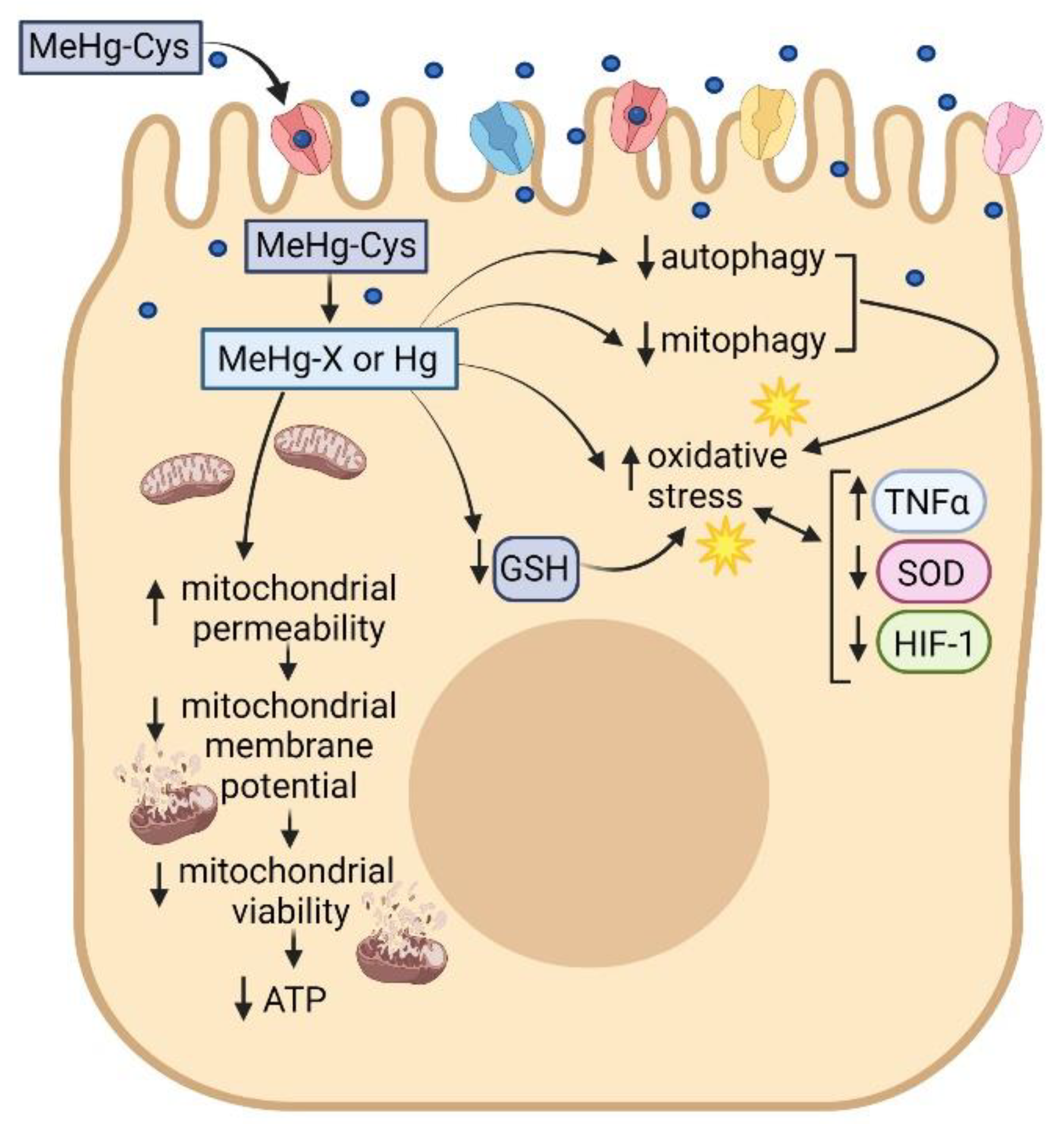
3. Discussion
4. Materials and Methods
4.1. Tissue Culture
4.2. Uptake Assays
4.3. Cell Viability Analyses
4.4. Autophagy Assay
4.5. ATP Assay
4.6. TBARS (Thiobarbituric Acid Reactive Substances) Assay
4.7. Quantitative PCR
4.8. GSH/GSSG Assay
4.9. Western Blotting
4.10. Statistical Analyses
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- ATSDR, Agency for Toxic Substances and Disease Registry, Toxicological Profile for Mercury. U.S. Department of Health and Human Services; Public Health Service, Centers for Disease Control: Atlanta, GA, USA, 2008. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf (accessed on 21 July 2021).
- Donohue, A.; Wagner, C.L.; Burch, J.B.; Rothenberg, S.E. Blood total mercury and methylmercury among pregnant mothers in Charleston, South Carolina, USA. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Wickliffe, J.K.; Lichtveld, M.Y.; Zijlmans, C.W.; MacDonald-Ottevanger, S.; Shafer, M.; Dahman, C.; Harville, E.W.; Drury, S.; Landburg, G.; Ouboter, P. Exposure to total and methylmercury among pregnant women in Suriname: Sources and public health implications. J. Expo. Sci. Environ. Epidemiol. 2020, 31, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Llorente Ballesteros, M.T.; Garcia Barrado, B.; Navarro Serrano, I.; Izquierdo Alvarez, S.; Del Pueyo Garcia Anaya, M.; Gonzalez Munoz, M.J. Evaluation of blood mercury and serum selenium levels in the pregnant population of the Community of Madrid, Spain. J. Trace Elements Med. Biol. 2020, 57, 60–67. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. Mechanisms involved in the transport of mercuric ions in target tissues. Arch. Toxicol. 2017, 91, 63–81. [Google Scholar] [CrossRef] [Green Version]
- Ask, K.; Akesson, A.; Berglund, M.; Vahter, M. Inorganic mercury and methylmercury in placentas of Swedish women. Environ. Health Perspect. 2002, 110, 523–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inouye, M.; Kajiwara, Y. Developmental disturbances of the fetal brain in guinea-pigs caused by methylmercury. Arch. Toxicol. 1988, 62, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Inouye, M.; Murao, K.; Kajiwara, Y. Behavioral and neuropathological effects of prenatal methylmercury exposure in mice. Neurobehav. Toxicol. Teratol. 1985, 7, 227–232. [Google Scholar] [PubMed]
- Moss, R.F.; George, H.S.; Nijhara, S.; Orr, S.E.; Joshee, L.; Barkin, J.L.; Bridges, C.C. Chronic kidney disease in pregnant mothers affects maternal and fetal disposition of mercury. Reprod. Toxicol. 2020, 93, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Spyker, D.A.; Spyker, J.M. Response model analysis for cross-fostering studies: Prenatal versus postnatal effects on offspring exposed to methylmercury dicyandiamide. Toxicol. Appl. Pharmacol. 1977, 40, 511–527. [Google Scholar] [CrossRef]
- Su, M.Q.; Okita, G.T. Embryocidal and teratogenic effects of methylmercury in mice. Toxicol. Appl. Pharmacol. 1976, 38, 207–216. [Google Scholar] [CrossRef]
- Murakami, U. Embryo-fetotoxic effect of some organic mercury compounds. Annu. Rep. Res. Inst. Environ. Med. Nagoya Univ. 1970, 18, 33–43. [Google Scholar]
- Sakamoto, M.; Chan, H.M.; Domingo, J.L.; Koriyama, C.; Murata, K. Placental transfer and levels of mercury, selenium, vitamin E, and docosahexaenoic acid in maternal and umbilical cord blood. Environ. Int. 2018, 111, 309–315. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Yasutake, A.; Adachi, T.; Hirayama, K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch. Toxicol. 1996, 70, 310–314. [Google Scholar] [CrossRef]
- Simmons-Willis, T.A.; Koh, A.S.; Clarkson, T.W.; Ballatori, N. Transport of a neurotoxicant by molecular mimicry: The methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem. J. 2002, 367 Pt 1, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Balthasar, C.; Stangl, H.; Widhalm, R.; Granitzer, S.; Hengstschlager, M.; Gundacker, C. Methylmercury Uptake into BeWo Cells Depends on LAT2-4F2hc, a System L Amino Acid Transporter. Int. J. Mol. Sci. 2017, 18, 1730. [Google Scholar] [CrossRef] [Green Version]
- Straka, E.; Ellinger, I.; Balthasar, C.; Scheinast, M.; Schatz, J.; Szattler, T.; Bleichert, S.; Saleh, L.; Knofler, M.; Zeisler, H.; et al. Mercury toxicokinetics of the healthy human term placenta involve amino acid transporters and ABC transporters. Toxicology 2016, 340, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Grillo, M.A.; Lanza, A.; Colombatto, S. Transport of amino acids through the placenta and their role. Amino Acids 2008, 34, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Peng, S.; He, L.; Wang, Y.; Li, Y.; Ma, D.; Sun, L.; Zheng, H.; Yang, W.; Dai, F.; et al. Methylmercury cytotoxicity and possible mechanisms in human trophoblastic HTR-8/SVneo cells. Ecotoxicol. Environ. Saf. 2021, 207, 111520. [Google Scholar] [CrossRef] [PubMed]
- Brigham, M.P.; Stein, W.H.; Moore, S. The Concentrations of Cysteine and Cystine in Human Blood Plasma. J. Clin. Investig. 1960, 39, 1633–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popelka, H.; Klionsky, D.J. The molecular mechanism of Atg13 function in autophagy induction: What is hidden behind the data? Autophagy 2017, 13, 449–451. [Google Scholar] [CrossRef] [Green Version]
- Bingol, B.; Sheng, M. Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radic. Biol. Med. 2016, 100, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Jiang, J.; Xie, F.; Chen, L. Bnip3 in mitophagy: Novel insights and potential therapeutic target for diseases of secondary mitochondrial dysfunction. Clin. Chim. Acta 2020, 506, 72–83. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. 2005, 204, 274–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaccioli, F.; Aye, I.L.; Roos, S.; Lager, S.; Ramirez, V.I.; Kanai, Y.; Powell, T.L.; Jansson, T. Expression and functional characterisation of System L amino acid transporters in the human term placenta. Reprod. Biol. Endocrinol. RBE 2015, 13, 57. [Google Scholar] [CrossRef] [Green Version]
- Dallmann, A.; Liu, X.I.; Burckart, G.J.; van den Anker, J. Drug Transporters Expressed in the Human Placenta and Models for Studying Maternal-Fetal Drug Transfer. J. Clin. Pharmacol. 2019, 59 (Suppl. 1), S70–S81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleal, J.K.; Lofthouse, E.M.; Sengers, B.G.; Lewis, R.M. A systems perspective on placental amino acid transport. J. Physiol. 2018, 596, 5511–5522. [Google Scholar] [CrossRef] [Green Version]
- Chairoungdua, A.; Kanai, Y.; Matsuo, H.; Inatomi, J.; Kim, D.K.; Endou, H. Identification and characterization of a novel member of the heterodimeric amino acid transporter family presumed to be associated with an unknown heavy chain. J. Biol. Chem. 2001, 276, 49390–49399. [Google Scholar] [CrossRef] [Green Version]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OATs and OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012, 165, 1260–1287. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Sugawara, J.; Sato, T.; Mizutamari, H.; Suzuki, T.; Ito, A.; Mikkaichi, T.; Onogawa, T.; Tanemoto, M.; Unno, M.; et al. Expression of organic anion transporting polypeptide E (OATP-E) in human placenta. Placenta 2003, 24, 144–148. [Google Scholar] [CrossRef]
- Moe, A.J.; Furesz, T.C.; Smith, C.H. Functional characterization of L-alanine transport in a placental choriocarcinoma cell line (BeWo). Placenta 1994, 15, 797–802. [Google Scholar] [CrossRef]
- Ma, L.; Bi, K.D.; Fan, Y.M.; Jiang, Z.Y.; Zhang, X.Y.; Zhang, J.W.; Zhao, J.; Jiang, F.L.; Dong, J.X. In vitro modulation of mercury-induced rat liver mitochondria dysfunction. Toxicol. Res. 2018, 7, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Orr, S.E.; Barnes, M.C.; Joshee, L.; Uchakina, O.; McKallip, R.J.; Bridges, C.C. Potential mechanisms of cellular injury following exposure to a physiologically relevant species of inorganic mercury. Toxicol. Lett. 2019, 304, 13–20. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Safiulina, D.; Veksler, V.; Zharkovsky, A.; Kaasik, A. Loss of mitochondrial membrane potential is associated with increase in mitochondrial volume: Physiological role in neurones. J. Cell Physiol. 2006, 206, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.A.; Korotkov, S.M.; Saris, N.E. In vitro modulation of heavy metal-induced rat liver mitochondria dysfunction: A comparison of copper and mercury with cadmium. J. Trace Elem. Med. Biol. 2011, 25 (Suppl. 1), S63–S73. [Google Scholar] [CrossRef]
- Gawel, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Alhusaini, A.; Alghilani, S.; Alhuqbani, W.; Hasan, I.H. Vitamin E and Lactobacillus Provide Protective Effects against Liver Injury Induced by HgCl2: Role of CHOP, GPR87, and mTOR Proteins. Dose-Response A Publ. Int. Hormesis Soc. 2021, 19, 15593258211011360. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, Z.; Gao, J.; Sun, L.; Wang, Y.; Liu, Y.; Gooneratne, R. Comparative Study of Cytotoxicity, DNA Damage and Oxidative Stress Induced by Heavy Metals Cd(II), Hg(II) and Cr(III) in Yeast. Curr. Microbiol. 2021, 78, 1856–1863. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twig, G.; Shirihai, O.S. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Tang, X.; Ye, Z.; Wang, S.; Xiao, X. Methylmercury disrupts autophagic flux by inhibiting autophagosome-lysosome fusion in mouse germ cells. Ecotoxicol. Environ. Saf. 2020, 198, 110667. [Google Scholar] [CrossRef]
- El Asar, H.M.; Mohammed, E.A.; Aboulhoda, B.E.; Emam, H.Y.; Imam, A.A. Selenium protection against mercury neurotoxicity: Modulation of apoptosis and autophagy in the anterior pituitary. Life Sci. 2019, 231, 116578. [Google Scholar] [CrossRef]
- Lin, T.; Ruan, S.; Huang, D.; Meng, X.; Li, W.; Wang, B.; Zou, F. MeHg-induced autophagy via JNK/Vps34 complex pathway promotes autophagosome accumulation and neuronal cell death. Cell Death Dis. 2019, 10, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.L.; Shi, X.T.; Xu, X.F.; Zhou, G.X.; Xiong, Y.W.; Yi, S.J.; Liu, W.B.; Dai, L.M.; Cao, X.L.; Xu, D.X.; et al. Melatonin protects against environmental stress-induced fetal growth restriction via suppressing ROS-mediated GCN2/ATF4/BNIP3-dependent mitophagy in placental trophoblasts. Redox. Biol. 2021, 40, 101854. [Google Scholar] [CrossRef]
- De Sousa Abreu, R.; Penalva, L.O.; Marcotte, E.M.; Vogel, C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 2009, 5, 1512–1526. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridges, C.C.; Bauch, C.; Verrey, F.; Zalups, R.K. Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0,+): Implications of molecular mimicry. J. Am. Soc. Nephrol. 2004, 15, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.C.; Zalups, R.K. Homocysteine, system b0, + and the renal epithelial transport and toxicity of inorganic mercury. Am. J. Pathol. 2004, 165, 1385–1394. [Google Scholar] [CrossRef] [Green Version]
- Zalups, R.K.; Bridges, C.C. Relationships between the renal handling of DMPS and DMSA and the renal handling of mercury. Chem. Res. Toxicol. 2012, 25, 1825–1838. [Google Scholar] [CrossRef] [Green Version]


| Treatment | GSH (μM) | GSSG (μM) | GSH:GSSG |
|---|---|---|---|
| Buffer | 1.8 ± 0.48 | 0.21 ± 0.05 | 9:1 |
| 5 μM MeHg-Cys | 1.38 ± 0.36 | 0.31 ± 0.08 | 5:1 * |
| 25 μM MeHg-Cys | 2.49 ± 0.65 | 0.49 ± 0.13 | 5:1 * |
| 50 μM MeHg-Cys | 0.39 ± 0.10 | 0.19 ± 0.05 | 2:1 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganapathy, S.; Farrell, E.R.; Vaghela, S.; Joshee, L.; Ford, E.G., IV; Uchakina, O.; McKallip, R.J.; Barkin, J.L.; Bridges, C.C. Transport and Toxicity of Methylmercury-Cysteine in Cultured BeWo Cells. Int. J. Mol. Sci. 2022, 23, 394. https://doi.org/10.3390/ijms23010394
Ganapathy S, Farrell ER, Vaghela S, Joshee L, Ford EG IV, Uchakina O, McKallip RJ, Barkin JL, Bridges CC. Transport and Toxicity of Methylmercury-Cysteine in Cultured BeWo Cells. International Journal of Molecular Sciences. 2022; 23(1):394. https://doi.org/10.3390/ijms23010394
Chicago/Turabian StyleGanapathy, Srividya, Elisa R. Farrell, Simran Vaghela, Lucy Joshee, Earl G. Ford, IV, Olga Uchakina, Robert J. McKallip, Jennifer L. Barkin, and Christy C. Bridges. 2022. "Transport and Toxicity of Methylmercury-Cysteine in Cultured BeWo Cells" International Journal of Molecular Sciences 23, no. 1: 394. https://doi.org/10.3390/ijms23010394






