Environmental Factors That Affect Parathyroid Hormone and Calcitonin Levels
Abstract
:1. Introduction
2. Involvement of PTH and Calcitonin in the Regulation of Calcium and Phosphate Levels
3. Environmental Factors That Affect PTH and Calcitonin Levels
3.1. Lifestyle Factors
3.1.1. Smoking
3.1.2. Body Mass Index
3.1.3. Diet
Micronutrients
3.1.4. Alcohol
3.1.5. Exercise
| Factor | Effect on Hormone Levels | Number of Participants | Participants | Reference | |
|---|---|---|---|---|---|
| Smoking | Smoking | ↓PTH | 170 (men) | Healthy adults | [113] |
| Smoking | ↓PTH | 376 | Healthy adults | [114] | |
| Smoking | ↓PTH | 510 | Healthy adults | [62] | |
| Smoking | ↔PTH | 535 | Healthy adults | [115] | |
| Smoking | ↔PTH | 1203 | Healthy adults | [116] | |
| Smoking | ↓iPTH | 177 | Healthy adults | [117] | |
| Smoking | ↓PTH (in mothers and their new-borns) | 61 | Mothers and their new-borns | [33] | |
| Smoking | ↓iPTH | 31 (men) | Healthy adults | [118] | |
| Smoking | ↔iPTH | 43 (women) | Healthy adults | [118] | |
| Smoking | ↓PTH | 7896 | Healthy adults | [30] | |
| Smoking | ↓PTH | 405 (women) | Healthy adults | [37] | |
| Smoking | ↓PTH | 958 (men) | Healthy adults | [119] | |
| Smoking | ↔PTH | 136 | Healthy adults | [92] | |
| Smoking | ↓PTH | 406 | Healthy adults | [38] | |
| Smoking | ↔PTH | 3212 | 2758 healthy adults + 454 participants with coronary heart disease | [120] | |
| Smoking | ↓iPTH | 347 | Healthy adults | [61] | |
| Smoking | ↔PTH | 1206 | Healthy adults | [121] | |
| Smoking | ↔PTH | 1068 | Healthy adults | [122] | |
| Smoking | ↓iPTH | 345 | 216 healthy adults + 129 men with earlier partial gastrectomy | [123] | |
| Smoking | ↓PTH | 7561 | Healthy adults | [31] | |
| Smoking | ↓iPTH | 3949 | Healthy adults | [124] | |
| Smoking | ↔PTH | 32 | Healthy adults | [125] | |
| Smoking | ↓PTH | 1288 | Healthy adults | [63] | |
| Smoking | ↓PTH | 7652 | Healthy adults | [32] | |
| Smoking | ↔PTH | 414 | Healthy adults | [126] | |
| Smoking | ↓PTH | 2810 | Healthy adults | [127] | |
| Smoking | ↔PTH | 1205 | Healthy adults | [128] | |
| ↔PTH | 719 (men) | ||||
| Smoking | ↑PTH | 128 (participants with low body weight (≤75 kg)) | Healthy adults | [129] | |
| Smoking | ↓PTH | 1067 (women) | Healthy adults | [130] | |
| Smoking | ↓PTH | 47 (women) | Healthy adults | [131] | |
| Smoking | ↔PTH | 489 (women) | Healthy adults | [91] | |
| Smoking | ↓PTH | 908 | Healthy adults | [132] | |
| Smoking | ↓PTH | 294 (women) | Healthy adults | [18] | |
| Smoking | ↔PTH | 58 | Healthy adults | [133] | |
| Alcohol consumption | Alcohol | ↔PTH | 535 | Healthy adults | [115] |
| Alcohol | ↔PTH | 510 | Healthy adults | [62] | |
| Alcohol | ↔PTH | 1203 | Healthy adults | [116] | |
| Alcohol | ↓PTH | 7896 | Healthy adults | [30] | |
| Alcohol | ↓PTH | 136 | Healthy adults | [92] | |
| Alcohol | ↔PTH | 1206 | Healthy adults | [121] | |
| Alcohol | ↓iPTH | 3949 | Healthy adults | [124] | |
| Alcohol | ↔PTH | 1288 | Healthy adults | [63] | |
| Alcohol | ↔PTH | 414 | Healthy adults | [126] | |
| Alcohol | ↔PTH | 1205 | Healthy adults | [128] | |
| Alcohol | ↔PTH | 7652 | Healthy adults | [32] | |
| Alcohol | ↔PTH | 27 (men) | Healthy adults, alcoholics | [134] | |
| Alcohol | ↔PTH | 21 (men) | Healthy adults, alcoholics | [135] | |
| Alcohol | ↓PTH | 6 | Healthy adults | [90] | |
| Alcohol | ↔PTH | 47 | Healthy adults, alcoholics | [95] | |
| Alcohol | ↔PTH | 26 | Healthy adults | [136] | |
| Alcohol | ↓PTH | 136 | Healthy adults | [92] | |
| Alcohol | ↓PTH (increase in PTH levels after alcohol withdrawal) | 26 | Healthy adults, alcoholics | [137] | |
| Alcohol | ↔iPTH | 36 (men) | Healthy adults, alcoholics | [138] | |
| Alcohol | ↓immunoreactive PTH | 104 (men) | Healthy adults | [139] | |
| Increased BMI | ↑BMI | ↔PTH | 535 | Healthy adults | [115] |
| ↑BMI | ↑PTH | 510 | Healthy adults | [62] | |
| ↑BMI | ↑PTH | 1203 | Healthy adults | [116] | |
| ↑BMI | ↑PTH | 7896 | Healthy adults | [30] | |
| ↑BMI | ↑PTH | 7561 | Healthy adults | [31] | |
| ↑BMI | ↑PTH | 3212 | 2758 healthy adults + 454 participants with coronary heart disease | [120] | |
| ↑BMI | ↑iPTH | 347 | Healthy adults | [61] | |
| ↑BMI | ↑PTH | 1206 | Healthy adults | [121] | |
| ↑BMI | ↑PTH | 2810 | Healthy adults | [127] | |
| ↑BMI | ↑PTH | 1205 | Healthy adults | [128] | |
| ↑BMI | ↑PTH | 7652 | Healthy adults | [32] | |
| ↑BMI | ↑PTH | 1288 | Healthy adults | [63] | |
| ↑BMI | ↑iPTH | 3949 | Healthy adults | [124] | |
| ↑BMI | ↑iPTH | 160 | Healthy adults | [140] | |
| ↑BMI | ↑PTH | 483 | Healthy adults | [141] | |
| ↑BMI | ↔PTH | 57 | Healthy adults | [79] | |
| ↑BMI | ↑PTH | 57 (men) | Healthy adults | [142] | |
| ↑BMI | ↑PTH | 1628 | Dialysis patients | [143] | |
| ↑BMI | ↑PTH | 419 | Children | [144] | |
| ↑BMI | ↑PTH | 82 (women) | Healthy adults | [145] | |
| ↑BMI | ↑PTH | 316 | Healthy adults | [146] | |
| ↑BMI | ↑iPTH | 332 | Healthy adults | [147] | |
| ↑BMI | ↑PTH | 40 | Bariatric surgery patients and healthy controls | [148] | |
| ↑BMI | ↑PTH | 316 | Patients who had attended the obesity clinics | [149] | |
| ↑BMI | ↑PTH | 42 | Patients undergoing sleeve gastrectomy | [150] | |
| ↑BMI | ↑PTH | 516 | Healthy adults | [151] | |
| ↑BMI | ↑PTH | 3248 (women) | Healthy adults | [152] | |
| ↑BMI | ↑PTH | 669 (men) | Healthy adults | [153] | |
| ↑BMI | ↑iPTH | 590 | Hemodialysis patients | [154] | |
| ↑BMI | ↑PTH | 2758 healthy adults + 454 participants with coronary heart disease | Healthy adults | [155] | |
| ↑BMI | ↑PTH | 250 | Healthy adults | [156] | |
| ↑BMI | ↑PTH | 608 | Healthy adults | [157] | |
| ↑BMI | ↑PTH | 496 (men) | Patients with chronic kidney disease | [158] | |
| ↑BMI | ↔PTH | 1436 | Healthy adults | [159] | |
| ↑BMI | ↑PTH | 304 (women) | Healthy adults | [160] | |
| ↑BMI | ↑PTH | 156 | Obese children | [161] | |
| ↑BMI | ↑PTH | 3002 | Healthy adults | [162] | |
| ↑BMI | ↑PTH | 810 (women) | Healthy adults | [163] | |
| ↑BMI | ↑PTH (PTH = 21.4–65.8 pg/ mL) | 131 | Healthy adults and subjects with primary hyperparathyroidism | [41] | |
| ↓PTH (PTH = 147–2511.7 pg/mL) | 132 | ||||
| ↑BMI | ↑PTH | 383 (women) | Healthy adults | [164] | |
| ↑BMI | ↑PTH | 2848 | Healthy adults | [165] | |
| ↑BMI | ↑PTH | 453 | Healthy adults | [166] | |
| ↑BMI | ↑PTH | 25 | Anorexia nervosa patients | [167] | |
| ↑BMI | ↑PTH | 98 | Healthy adults | [168] | |
| ↑BMI | ↑PTH | 625 | Healthy adults | [71] | |
| ↑BMI | ↑PTH | 294 | Healthy adults | [18] | |
| Diet | Different sorts of vegetables, sausages, salami, mushrooms, eggs, white bread | ↑PTH | 1180 | Healthy adults | [21] |
| Bran bread | ↓PTH | ||||
| Traditional Inuit diet (diet mainly of marine origin taken by Greenland inhabitants) | ↓PTH | 535 | Healthy adults | [115] | |
| ↑Total calorie intake | ↔iPTH | 3949 | Healthy adults | [124] | |
| Protein intake | ↔PTH | 7652 | Healthy adults | [32] | |
| Coronary Health Improvement Project (CHIP). CHIP intervention, which promotes a plant-based diet with little dairy intake and meat consumption | ↑PTH (after 6 weeks) | 119 (women) | Healthy adults | [57] | |
| High-phosphorus, low-calcium diets | ↑PTH | 16 | Healthy adults | [50] | |
| The traditional Brazilian diet (fruits, vegetables, and small amounts of meat) | ↓PTH | 111 | Severely obese adults | [169] | |
| Extra virgin olive oil supplementation | ↔PTH | 111 | Severely obese adults | [169] | |
| Moderate dietary protein restriction | ↑PTH | 18 | Patients with idiopathic hypercalciuria and calcium nephrolithiasis | [55] | |
| Vegans vs omnivores | ↑PTH in vegans | 155 | Healthy adults | [58] | |
| The “Dietary Approaches to Stop Hypertension” (DASH) diet, rich in fiber and low-fat dairy | ↔PTH | 334 | Healthy adults | [170] | |
| Vegans vs. omnivores | ↔PTH | 210 (women) | Healthy adults | [171] | |
| High protein and high dairy group | ↓PTH | 30 (women) | Healthy adults | [56] | |
| Adequate protein and medium dairy group | ↓PTH | 30 (women) | Healthy adults | [56] | |
| Adequate protein and low dairy | ↑PTH | 30 (women) | Healthy adults | [56] | |
| Diet with low calcium:phosphorus ratio | ↑PTH | 147 (women) | Healthy adults | [51] | |
| Low-protein diets (diets containing 0.7 and 0.8 g protein/kg) | ↑PTH | 8 (women) | Healthy adults | [54] | |
| Higher consumption of a proinflammatory diet | ↑PTH | 7679 | Adults with/without chronic kidney disease | [53] | |
| High fruit and vegetable intake (consuming more than 3 servings of fruit and vegetables) | ↓PTH | 56 | Children | [172] | |
| Dietary calorie, vitamin D, and magnesium intake | ↔PTH | 98 | Healthy adults | [168] | |
| Vegetarians vs. controls | ↑iPTH | 44 | Healthy adults | [59] | |
| Intake of dietary fiber | ↑iPTH | ||||
| Dietary calcium intake | ↓iPTH | ||||
| Coffee | ↓iPTH | 181 (men) | Healthy adults | [61] | |
| Coffee, tea | ↔PTH | 510 | Healthy adults | [62] | |
| Coffee | ↓PTH | 3427 (men) | Healthy adults | [30] | |
| Caffeine intake | ↔PTH | 7652 | Healthy adults | [32] | |
| Caffeine intake | ↔PTH | 1288 | Healthy adults | [63] | |
| Vitamin D supplements | ↔PTH | 510 | Healthy adults | [62] | |
| Vitamin D supplements | ↓PTH | 4469 (women) | Healthy adults | [30] | |
| Vitamin D supplements | ↓iPTH | 3949 | Healthy adults | [124] | |
| Vitamin D supplements | ↔PTH | 1288 | Healthy adults | [63] | |
| Vitamin D supplements | ↓PTH | 414 | Healthy adults | [126] | |
| Vitamin D intake | ↓PTH | 316 | Healthy adults | [146] | |
| Vitamin D supplementation | ↓PTH | 250 | Healthy adults | [156] | |
| Vitamin D intake | ↓PTH | 376 (women) | Healthy adults | [173] | |
| Vitamin D supplementation | ↓PTH | Meta-analysis | [70] | ||
| Vitamin D and calcium supplementation | ↓PTH | 77 | Healthy adults | [174] | |
| Vitamin D and calcium supplementation | ↓PTH | 247 (women) | Healthy adults | [175] | |
| Vitamin D and calcium supplementation | ↓PTH | 877 (women) | Healthy adults | [176] | |
| Vitamin D supplementation | ↓PTH | 270 (women) | Healthy adults | [75] | |
| Vitamin D and calcium supplementation | ↓PTH | 313 | Healthy adults | [177] | |
| Vitamin D and calcium supplementation | ↓PTH | 103 (women) | Elderly institutionalised women | [178] | |
| Vitamin D supplementation | ↔PTH | 128 (women) | Healthy adults | [179] | |
| Vitamin D and calcium supplementation | ↓PTH | 145 (women) | Healthy adults | [180] | |
| Vitamin D supplementation | ↓PTH | 60 (men) | Healthy adults | [181] | |
| Vitamin D and calcium supplementation | ↓PTH | 192 (women) | Healthy adults | [182] | |
| Vitamin D and calcium supplementation | ↓PTH | 191 (women) | Ambulatory elderly women | [183] | |
| Vitamin D supplementation | ↔PTH | 208 (women) | Healthy adults | [184] | |
| Vitamin D and calcium supplementation | ↓PTH | 314 | Healthy adults | [185] | |
| Vitamin D and calcium supplementation | ↓PTH | 1368 | Healthy adults | [127] | |
| Vitamin D supplementation | ↓PTH | 338 | Healthy adults | [186] | |
| Vitamin D and calcium supplementation | ↓PTH | 218 | Older patients | [187] | |
| Vitamin D supplementation | ↔PTH | 215 | Healthy adults | [188] | |
| Vitamin D and calcium supplementation | ↓PTH | 242 | Healthy adults | [189] | |
| Vitamin D supplementation | ↓PTH | 165 | Healthy overweight subjects | [190] | |
| Vitamin D and calcium supplementation | ↓PTH | 153 | Healthy adults | [191] | |
| Multiple micronutrient and calcium supplementation | ↓PTH | 153 (women) | Healthy adults | [191] | |
| Vitamin D and calcium supplementation | ↓PTH | 158 | Overweight subjects | [192] | |
| Vitamin D supplementation | ↓PTH | 202 | Healthy adults | [193] | |
| Vitamin D supplementation | ↓PTH | 94 | Healthy adults | [194] | |
| Vitamin D supplementation | ↔PTH | 90 | Coronary artery disease patients | [195] | |
| Vitamin D supplementation | ↔PTH | 151 | Healthy adults | [196] | |
| Vitamin D supplementation | ↓PTH | 89 | Obese with pre- or early diabetes | [197] | |
| Vitamin D supplementation | ↓PTH | 112 | Hypertensive patients | [198] | |
| Vitamin D supplementation | ↓PTH | 230 | Adults with depression | [199] | |
| Vitamin D supplementation | ↓PTH | 77 (women) | Healthy adults | [200] | |
| Vitamin D and calcium supplementation | ↓PTH | 173 (women) | Healthy adults | [201] | |
| Vitamin D supplementation | ↓PTH | 112 | Parkinson disease | [202] | |
| Vitamin D supplementation | ↔PTH | 82 | Healthy adults | [203] | |
| Vitamin A intake | ↔PTH | 606 | Healthy adults | [72] | |
| Total calcium and vitamin A intake | ↓PTH | 625 | Healthy adults | [71] | |
| Vitamin A intake | ↓PTH | 1288 | Healthy adults | [63] | |
| The dietary intake of minerals (calcium, phosphate, and magnesium) and vitamin D | ↔PTH | 127 | Healthy adults | [204] | |
| Calcium supplements | ↓PTH | 414 | Healthy adults | [126] | |
| Calcium supplements | ↓PTH | 51 | Toddlers | [205] | |
| Calcium intake | ↓PTH | 7896 | Healthy adults | [30] | |
| Dietary calcium intake | ↓PTH | 181 | Healthy adolescents | [206] | |
| Calcium intake | ↓PTH | 1203 | Healthy adults | [116] | |
| Calcium intake | ↓PTH | 3212 | 2758 healthy adults + 454 participants with coronary heart disease | [120] | |
| Calcium intake | ↔PTH | 1288 | Healthy adults | [63] | |
| Calcium intake | ↓iPTH | 3949 | Healthy adults | [124] | |
| Dietary calcium intake | ↓PTH | 7652 | Healthy adults | [32] | |
| Calcium intake | ↔PTH | 57 | Healthy adults | [79] | |
| Animal/total calcium intake | ↓PTH | 316 | Healthy adults | [146] | |
| Dietary calcium | ↔PTH | 155 (women) | Healthy adults | [207] | |
| Calcium supplements | ↓PTH | 566 | Healthy adults | [208] | |
| Intake of calcium | ↓PTH | 82 | Healthy adults | [203] | |
| Calcium intake derived from milk | ↓PTH | 245 (women) | Healthy adults | [173] | |
| Magnesium intake | ↔PTH | 57 | Healthy adults | [79] | |
| Magnesium intake | ↔PTH | 7652 | Healthy adults | [32] | |
| Magnesium supplementation | ↑PTH | 10 (patients with hypoparathyroidism) | Patients with osteoporosis | [78] | |
| ↓PTH | 10 (patients with vitamin D insufficiency) | ||||
| Magnesium supplementation | ↑iPTH | 23 | Children with diabetes | [77] | |
| Zinc infusion | ↔PTH | 38 | Patients of short stature, diabetes mellitus, and controls | [83] | |
| Phosphorus intake | ↔PTH | 7652 | Healthy adults | [32] | |
| Intervention group (exercise, vitamin D, calcium, protein supplementation) | ↓iPTH | 220 | Patients that were on bariatric surgery | [209] | |
| Exercise | Exercise | ↓PTH | 7561 | Healthy adults | [31] |
| Exercise | ↔PTH | 1288 | Healthy adults | [63] | |
| Exercise | ↓PTH | 3427 (men) | Healthy adults | [30] | |
| Exercise | ↔PTH | 414 | Healthy adults | [126] | |
| Exercise | ↔PTH | 1205 | Healthy adults | [128] | |
| ↑Sitting | ↑PTH | 566 | Healthy adults | [208] | |
| Exercise | ↓PTH | 625 | Healthy adults | [71] | |
| Exercise | ↑PTH | 12 (men) | Healthy adults | [210] | |
| Exercise | ↑PTH | 20 | Healthy adults | [211] | |
| Exercise | ↓PTH | 54 | Chronic kidney disease patients | [212] | |
| Exercise | ↑PTH | 29 | Boys and young men | [213] | |
| Exercise | ↑PTH | 11 (men) | Healthy adults | [214] | |
| Exercise | ↑PTH | 25 | Healthy adults | [215] | |
| Exercise | ↑PTH | 12 (men) | Healthy adults | [216] | |
| Exercise | ↔iPTH | 100 (women) | Healthy adults | [217] | |
| Exercise | ↑iPTH | 21 | Healthy adults | [218] | |
| Exercise | ↑iPTH | 7 (men) | Healthy adults | [219] | |
| Exercise | ↓PTH | 5 (women) | Healthy adults | [220] | |
| Exercise | ↑iPTH | 9 (men) | Healthy adults | [221] | |
| Exercise | ↑PTH (during the exercise with the highest intensity) | 10 (men) | Healthy adults | [222] | |
| Exercise | ↑PTH (during the exercise) ↔PTH (postexercise period) | 10 (men) | Healthy adults | [223] | |
| Exercise | ↑PTH | 10 (women) | Healthy adults | [104] | |
| Exercise | ↑PTH | 51 (men) | Healthy adults | [224] | |
| Exercise | ↓iPTH (moderate exercise) ↑iPTH (intensive exercise) | 21 (women) | Healthy adults | [225] | |
| Exercise | ↑PTH | 14 (women) | Healthy adults | [226] | |
| Exercise | ↓PTH (with the onset of exercise) ↑PTH (intensive exercise) | 10 (men) | Healthy adults | [227] | |
| Exercise | ↑PTH | 17 (men) | Healthy adults | [228] | |
| Exercise | ↑PTH | 100 (men) | Healthy adults | [229] | |
| Exercise | ↑PTH | 9 (men) | Healthy adults | [111] | |
| Exercise | ↑PTH | 26 (women) | Healthy adults | [230] | |
| Exercise | ↑PTH | 18 | Healthy adults | [112] | |
| Exercise | ↑iPTH | 8 (men) | Healthy adults | [231] | |
| Exercise | ↔PTH | 6 (men) | Healthy adults | [232] | |
| Exercise | ↑PTH | 6 (men) | Healthy adults | [109] | |
| Exercise | ↑PTH | 19 (men) | Healthy adults | [107] | |
| Exercise | ↔PTH | 13 (men) | Healthy adults | [110] | |
| Exercise | ↑PTH | 27 (men) | Healthy adults | [20] |
| Factor | Effect on Hormone Levels | Number of Participants | Participants | Reference | |
|---|---|---|---|---|---|
| Smoking | Smoking | ↔Calcitonin | 294 (women) | Healthy adults | [18] |
| Smoking | ↑Calcitonin | 9340 | People with type 2 diabetes | [48] | |
| Smoking | ↑Calcitonin | 142 (men) | Healthy adults | [233] | |
| Smoking | ↑Calcitonin | 58 | Healthy adults | [133] | |
| Smoking | ↑Calcitonin | 120 (men) | Healthy adults | [234] | |
| Smoking | ↑Calcitonin | 6341 (men) | Healthy adults | [17] | |
| Alcohol consumption | Alcohol | ↔Calcitonin | 26 | Healthy adults | [136] |
| Alcohol | ↔Calcitonin | 93 | Healthy adults | [96] | |
| Alcohol | ↓Calcitonin (in a heavy drinking group) | 47 | Alcoholics | [95] | |
| Alcohol | ↑Calcitonin | 50 | Alcoholics + controls | [94] | |
| Increased BMI | ↑BMI | ↔Calcitonin | 467 | Patients with Hashimoto’s thyroiditis | [235] |
| ↑BMI | ↓Calcitonin | 294 | Healthy adults | [18] | |
| ↑BMI | ↑Calcitonin | 9340 | People with type 2 diabetes | [48] | |
| ↑BMI | ↑Calcitonin | 287 | Healthy adults | [233] | |
| ↑BMI | ↔Calcitonin | 4638 | Healthy adults | [17] | |
| ↑BMI | ↑Calcitonin | 31 | Patients with chronic kidney disease on hemodialysis | [236] | |
| Vitamins and minerals | Vitamin D supplementation | ↔Calcitonin | 270 (women) | Healthy adults | [75] |
| Zinc infusion | ↓Calcitonin | 38 | Patients of short stature, diabetes mellitus, and controls | [83] | |
| High dietary zinc | ↓Calcitonin | 21 | Healthy adults | [86] | |
| High dietary copper | ↔Calcitonin | 21 | Healthy adults | [86] | |
| Exercise | Exercise | ↔Calcitonin | 9 (men) | Healthy adults | [111] |
| Exercise | ↔Calcitonin | 18 | Healthy adults | [112] | |
| Exercise | ↔Calcitonin | 6 (men) | Healthy adults | [109] | |
| Exercise | ↑Calcitonin | 19 (men) | Healthy adults | [107] | |
| Exercise | ↔Calcitonin | 13 (men) | Healthy adults | [110] | |
| Exercise | ↔Calcitonin | 27 (men) | Healthy adults | [20] | |
| Raloxifene combined with aerobic exercise | ↑Calcitonin | 70 | Patients with osteoporosis | [108] |
3.2. Pollutants
3.2.1. Heavy Metals
3.2.2. Chemicals
| Factor | Effect on Hormone Levels | Number of Participants | Participants | Reference | |
|---|---|---|---|---|---|
| Heavy metals | Arsenic | ↔PTH– | 196 | Healthy adults | [256] |
| Arsenic | ↔iPTH | 774 | Children and new-borns | [244] | |
| Cadmium | ↓PTH | 719 (women) | Healthy adults | [34] | |
| Cadmium | ↓PTH | 85 (women) | Healthy adults | [257] | |
| Cadmium | ↓PTH | 51 (men) | Participants exposed to cadmium | [258] | |
| Cadmium | ↔PTH | 46 | Participants exposed to cadmium for a long period (some suffering from decreased tubular function) | [240] | |
| Cadmium | ↔PTH | 41 (women) | Subjects with renal tubular dysfunction caused by exposure to cadmium | [259] | |
| Cadmium | ↓iPTH | 306 | Chronic peritoneal dialysis patients | [260] | |
| Cadmium in urine (maternal) | ↓PTH (in boys) ↑PTH (in girls) | 504 | 504 children in a mother–child cohort | [242] | |
| Cadmium in erythrocytes (maternal) | ↑PTH (in boys) ↓PTH (in girls) | 504 | |||
| Cadmium | ↔PTH | 60 | Patients with renal tubular damage caused by exposure to cadmium and healthy controls | [238] | |
| Cadmium | ↑PTH | 53 | Patients with renal tubular damage caused by exposure to cadmium and healthy controls | [241] | |
| Cadmium | ↓PTH (association lost after adjustment for smoking) | 908 (women) | Healthy adults | [132] | |
| Cadmium | ↓PTH, ↑Calcitonin | 294 (women) | Healthy adults | [18] | |
| Cadmium | ↔PTH | 146 | Healthy adults | [239] | |
| Lead | ↑PTH | 89 | Healthy adults | [245] | |
| Lead | ↔PTH | 719 (women) | Healthy adults | [34] | |
| Lead | ↔PTH | 51 | Dialysis patients | [261] | |
| Lead | ↑PTH | 146 (men) | Healthy adults | [262] | |
| Lead | ↑iPTH | 315 | Chronic peritoneal dialysis patients | [263] | |
| Lead | ↑PTH | 115 | Hemodialysis patients | [264] | |
| Lead | ↔PTH | 47 | Healthy adults | [265] | |
| Lead | ↑PTH | 73 (women) | Healthy adults | [266] | |
| Lead | ↑iPTH | 93 | Hemodialysis patients | [267] | |
| Uranium | ↔iPTH | 35 | Gulf War I veterans exposed to uranium | [268] | |
| Uranium | ↓iPTH | 35 | Gulf War I veterans exposed to uranium | [246] | |
| Chemicals | Persistent organochlorine compounds (CB-153) | ↔PTH | 908 (women) | Healthy adults | [132] |
| Persistent organochlorine compounds (p,p’-DDE) | ↔PTH | ||||
| PFAS | ↑PTH | 100 (men) | Healthy adults | [251] | |
| PCBs (exposed prenatally) | ↔PTH | 110 | Children in a mother–child cohort | [250] | |
| Fluoride | ↑PTH | 196 | Healthy adults | [256] | |
| Fluoride | ↑PTH | 84 | Patients with endemic fluorosis and healthy controls | [252] | |
| Fluoride | ↓PTH (in pregnant women) | 180 | Pregnant women and their new-borns | [269] | |
| ↔PTH (in new-borns) | |||||
| Lithium | ↔iPTH | 178 | Mother–child cohort | [270] | |
| Perchlorate | ↓PTH | 2207 (women) | Healthy adults | [19] | |
| Nitrate | ↓PTH | 4265 | Healthy adults | [19] | |
| Thiocyanate | ↓PTH | 4265 | Healthy adults | [19] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tebben, P.J.; Kumar, R. The hormonal regulation of calcium metabolism. In Seldin and Geibisch’s The Kidney; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 2, pp. 2249–2272. ISBN 9780123814623. [Google Scholar]
- Choi, N.W. Kidney and phosphate metabolism. Electrolytes Blood Press. 2008, 6, 77–85. [Google Scholar] [CrossRef]
- Gattineni, J.; Friedman, P.A. Regulation of hormone-sensitive renal phosphate transport. Vitam. Horm. 2015, 98, 249–306. [Google Scholar] [CrossRef] [PubMed]
- Talmage, R.V.; Vanderwiel, C.J.; Matthews, J.L. Calcitonin and phosphate. Mol. Cell. Endocrinol. 1981, 24, 235–251. [Google Scholar] [CrossRef]
- Jafari, N.; Abdollahpour, H.; Falahatkar, B. Stimulatory effects of short-term calcitonin administration on plasma calcium, magnesium, phosphate, and glucose in juvenile Siberian sturgeon Acipenser baerii. Fish Physiol. Biochem. 2020, 46, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Lofrese, J.J.; Basit, H.; Lappin, S.L. Physiology, Parathyroid; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Pearse, A.G. The cytochemistry of the thyroid C cells and their relationship to calcitonin. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1966, 164, 478–487. [Google Scholar] [CrossRef]
- Bae, Y.J.; Schaab, M.; Kratzsch, J. Calcitonin as biomarker for the medullary thyroid carcinoma. Recent Res. Cancer Res. 2015, 204, 117–137. [Google Scholar] [CrossRef]
- Hunter, D.; De Lange, M.; Snieder, H.; MacGregor, A.J.; Swaminathan, R.; Thakker, R.V.; Spector, T.D. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J. Bone Miner. Res. 2001, 16, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Cohen, C.; Lutsey, P.L.; Kleber, M.E.; Nielson, C.M.; Mitchell, B.D.; Bis, J.C.; Eny, K.M.; Portas, L.; Eriksson, J.; Lorentzon, M.; et al. Genetic variants associated with circulating parathyroid hormone. J. Am. Soc. Nephrol. 2017, 28, 1553–1565. [Google Scholar] [CrossRef] [Green Version]
- Matana, A.; Brdar, D.; Torlak, V.; Boutin, T.; Popović, M.; Gunjača, I.; Kolčić, I.; Boraska Perica, V.; Punda, A.; Polašek, O.; et al. Genome-wide meta-analysis identifies novel loci associated with parathyroid hormone level. Mol. Med. 2018, 24, 15. [Google Scholar] [CrossRef] [Green Version]
- Deftos, L.J.; Weisman, M.H.; Williams, G.W.; Karpf, D.B.; Frumar, A.M.; Davidson, B.J.; Parthemore, J.G.; Judd, H.L. Influence of age and sex on plasma calcitonin in human beings. N. Engl. J. Med. 1980, 302, 1351–1353. [Google Scholar] [CrossRef]
- Haden, S.T.; Brown, E.M.; Hurwitz, S.; Scott, J.; Fuleihan, G.E.H. The effects of age and gender on parathyroid hormone dynamics. Clin. Endocrinol. 2000, 52, 329–338. [Google Scholar] [CrossRef]
- Carrivick, S.J.; Walsh, J.P.; Brown, S.J.; Wardrop, R.; Hadlow, N.C. Brief report: Does PTH increase with age, independent of 25-hydroxyvitamin D, phosphate, renal function, and ionized calcium? J. Clin. Endocrinol. Metab. 2015, 100, 2131–2134. [Google Scholar] [CrossRef] [Green Version]
- Tiegs, R.D.; Body, J.J.; Barta, J.M.; Heath, H. Secretion and metabolism of monomeric human calcitonin: Effects of age, sex, and thyroid damage. J. Bone Miner. Res. 1986, 1, 339–349. [Google Scholar] [CrossRef]
- Mazeh, H.; Sippel, R.S.; Chen, H. The role of gender in primary hyperparathyroidism: Same disease, different presentation. Ann. Surg. Oncol. 2012, 19, 2958–2962. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Jeon, M.J.; Yoo, H.J.; Bae, S.J.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Kim, H.K.; Kim, W.G. Gender-dependent reference range of serum calcitonin levels in healthy Korean adults. Endocrinol. Metab. 2021, 36, 365–373. [Google Scholar] [CrossRef]
- Schutte, R.; Nawrot, T.S.; Richart, T.; Thijs, L.; Vanderschueren, D.; Kuznetsova, T.; Van Hecks, E.; Roels, H.A.; Staessen, J.A. Bone resorption and environmental exposure to cadmium in women: A population study. Environ. Health Perspect. 2008, 116, 777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, W.C.; Liu, C.L.; Lee, J.J.; Liu, T.P.; Yang, P.S.; Hsu, Y.C.; Cheng, S.P. Negative association between serum parathyroid hormone levels and urinary perchlorate, nitrate, and thiocyanate concentrations in U.S. adults: The national health and nutrition examination survey 2005–2006. PLoS ONE 2014, 9, e115245. [Google Scholar] [CrossRef] [Green Version]
- Soria, M.; Haro, C.G.; Ansón, M.A.; Iñigo, C.; Calvo, M.L.; Escanero, J.F. Variations in serum magnesium and hormonal levels during incremental exercise. Magnes. Res. 2014, 27, 155–164. [Google Scholar] [CrossRef]
- Popović, M.; Matana, A.; Torlak, V.; Brdar, D.; Gunjača, I.; Boraska Perica, V.; Barbalić, M.; Kolčić, I.; Punda, A.; Polašek, O.; et al. The effect of multiple nutrients on plasma parathyroid hormone level in healthy individuals. Int. J. Food Sci. Nutr. 2019, 70, 638–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penido, M.G.M.G.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [Green Version]
- Carter, P.; Schipani, E. The roles of parathyroid hormone and calcitonin in bone remodeling: Prospects for novel therapeutics. Endocr. Metab. Immune Disord. Drug Targets 2006, 6, 59–76. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and Vitamin, D. Compr. Physiol. 2016, 6, 561–601. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Levi, R. Regulation of PTH synthesis and secretion relevant to the management of secondary hyperparathyroidism in chronic kidney disease. Kidney Int. Suppl. 2005, 67, S8–S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Jüppner, H.; et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saki, F.; Kassaee, S.R.; Salehifar, A.; Omrani, G.H.R. Interaction between serum FGF-23 and PTH in renal phosphate excretion, a case-control study in hypoparathyroid patients. BMC Nephrol. 2020, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lanske, B.; Razzaque, M.S. Molecular interactions of FGF23 and PTH in phosphate regulation. Kidney Int. 2014, 86, 1072–1074. [Google Scholar] [CrossRef] [Green Version]
- Ben-Dov, I.Z.; Galitzer, H.; Lavi-Moshayoff, V.; Goetz, R.; Kuro-o, M.; Mohammadi, M.; Sirkis, R.; Naveh-Many, T.; Silver, J. The parathyroid is a target organ for FGF23 in rats. J. Clin. Investig. 2007, 117, 4003–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorde, R.; Saleh, F.; Figenschau, Y.; Kamycheva, E.; Haug, E.; Sundsfjord, J. Serum parathyroid hormone (PTH) levels in smokers and non-smokers. The fifth Tromsø study. Eur. J. Endocrinol. 2005, 152, 39–45. [Google Scholar] [CrossRef] [Green Version]
- He, J.L.; Scragg, R.K. Vitamin D, parathyroid hormone, and blood pressure in the National Health and Nutrition Examination Surveys. Am. J. Hypertens. 2011, 24, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Paik, J.M.; Farwell, W.R.; Taylor, E.N. Demographic, dietary, and serum factors and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos. Int. 2012, 23, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Gómez, N.M.; Mendoza, C.; González-González, N.L.; Barroso, F.; Jiménez-Sosa, A.; Domenech, E.; Clemente, I.; Barrios, Y.; Moya, M. Maternal smoking and the vitamin D-parathyroid hormone system during the perinatal period. J. Pediatr. 2007, 151, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Åkesson, A.; Bjellerup, P.; Lundh, T.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Skerfving, S.; Vahter, M. Cadmium-induced effects on bone in a population-based study of women. Environ. Health Perspect. 2006, 114, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.E.; Amini, H.; Heydarpour, P.; Amini Chermahini, F.; Godderis, L. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: Evidence and potential mechanisms. Environ. Int. 2019, 122, 67–90. [Google Scholar] [CrossRef] [PubMed]
- Al-Bashaireh, A.M.; Haddad, L.G.; Weaver, M.; Chengguo, X.; Kelly, D.L.; Yoon, S. The effect of tobacco smoking on bone mass: An overview of pathophysiologic mechanisms. J. Osteoporos. 2018, 2018, 1206235. [Google Scholar] [CrossRef] [Green Version]
- Need, A.G.; Kemp, A.; Giles, N.; Morris, H.A.; Horowitz, M.; Nordin, B.E.C. Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos. Int. 2002, 13, 83–88. [Google Scholar] [CrossRef]
- Jorde, R.; Stunes, A.K.; Kubiak, J.; Grimnes, G.; Thorsby, P.M.; Syversen, U. Smoking and other determinants of bone turnover. PLoS ONE 2019, 14, e0225539. [Google Scholar] [CrossRef]
- Babić Leko, M.; Gunjača, I.; Pleić, N.; Zemunik, T. Environmental factors affecting thyroid-stimulating hormone and thyroid hormone levels. Int. J. Mol. Sci. 2021, 22, 6521. [Google Scholar] [CrossRef]
- Tabassian, A.R.; Nylen, E.S.; Giron, A.E.; Snider, R.H.; Cassidy, M.M.; Becker, K.L. Evidence for cigarette smoke-induced calcitonin secretion from lungs of man and hamster. Life Sci. 1988, 42, 2323–2329. [Google Scholar] [CrossRef]
- Yuan, T.J.; Chen, L.P.; Pan, Y.L.; Lu, Y.; Sun, L.H.; Zhao, H.Y.; Wang, W.Q.; Tao, B.; Liu, J.M. An inverted U-shaped relationship between parathyroid hormone and body weight, body mass index, body fat. Endocrine 2021, 72, 844–851. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.B.; Ames, R.W.; Horne, A.M.; Gamble, G.D.; Reid, I.R. Fat mass is an important predictor of parathyroid hormone levels in postmenopausal women. Bone 2006, 38, 317–321. [Google Scholar] [CrossRef]
- Ni, Z.; Smogorzewski, M.; Massry, S.G. Effects of parathyroid hormone on cytosolic calcium of rat adipocytes. Endocrinology 1994, 135, 1837–1844. [Google Scholar] [CrossRef]
- McCarty, M.F.; Thomas, C.A. PTH excess may promote weight gain by impeding catecholamine-induced lipolysis-implications for the impact of calcium, vitamin D, and alcohol on body weight. Med. Hypotheses 2003, 61, 535–542. [Google Scholar] [CrossRef]
- Rickard, D.J.; Wang, F.L.; Rodriguez-Rojas, A.M.; Wu, Z.; Trice, W.J.; Hoffman, S.J.; Votta, B.; Stroup, G.B.; Kumar, S.; Nuttall, M.E. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone 2006, 39, 1361–1372. [Google Scholar] [CrossRef]
- He, Y.; Liu, R.X.; Zhu, M.T.; Shen, W.B.; Xie, J.; Zhang, Z.Y.; Chen, N.; Shan, C.; Guo, X.-z.; Tao, B.; et al. The browning of white adipose tissue and body weight loss in primary hyperparathyroidism. EBioMedicine 2019, 40, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Daniels, G.H.; Hegedüs, L.; Marso, S.P.; Nauck, M.A.; Zinman, B.; Bergenstal, R.M.; Mann, J.F.E.; Derving Karsbøl, J.; Moses, A.C.; Buse, J.B.; et al. LEADER 2: Baseline calcitonin in 9340 people with type 2 diabetes enrolled in the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial: Preliminary observations. Diabetes Obes. Metab. 2015, 17, 477–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathiesen, D.S.; Lund, A.; Vilsbøll, T.; Knop, F.K.; Bagger, J.I. Amylin and calcitonin: Potential therapeutic strategies to reduce body weight and liver fat. Front. Endocrinol. 2021, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Kumar, R.; Heath, H. Elevated secretion and action of serum parathyroid hormone in young adults consuming high phosphorus, low calcium diets assembled from common foods. J. Clin. Endocrinol. Metab. 1988, 66, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Kemi, V.E.; Kärkkäinen, M.U.M.; Rita, H.J.; Laaksonen, M.M.L.; Outila, T.A.; Lamberg-Allardt, C.J.E. Low calcium:phosphorus ratio in habitual diets affects serum parathyroid hormone concentration and calcium metabolism in healthy women with adequate calcium intake. Br. J. Nutr. 2010, 103, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Lederer, E. Regulation of serum phosphate. J. Physiol. 2014, 592, 3985–3995. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Yang, Q.; Liao, R.; Su, B. The association between dietary inflammatory index and parathyroid hormone in adults with/without chronic kidney disease. Front. Nutr. 2021, 8, 364. [Google Scholar] [CrossRef]
- Kerstetter, J.E.; Svastisalee, C.M.; Caseria, D.M.; Mitnick, M.A.E.; Insogna, K.L. A threshold for low-protein-diet-induced elevations in parathyroid hormone. Am. J. Clin. Nutr. 2000, 72, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Nobile, M.; Sartori, L.; Carbonare, L.D.; Ciuffreda, M.; Corrò, P.; D’Angelo, A.; Calò, L.; Crepaldi, G. Acute effects of moderate dietary protein restriction in patients with idiopathic hypercalciuria and calcium nephrolithiasis. Am. J. Clin. Nutr. 1999, 69, 267–271. [Google Scholar] [CrossRef]
- Josse, A.R.; Atkinson, S.A.; Tarnopolsky, M.A.; Phillips, S.M. Diets higher in dairy foods and dietary protein support bone health during diet- and exercise-induced weight loss in overweight and obese premenopausal women. J. Clin. Endocrinol. Metab. 2012, 97, 251–260. [Google Scholar] [CrossRef]
- Merrill, R.M.; Aldana, S.G. Consequences of a plant-based diet with low dairy consumption on intake of bone-relevant nutrients. J. Women’s Health 2009, 18, 691–698. [Google Scholar] [CrossRef]
- Hansen, T.H.; Madsen, M.T.B.; Jørgensen, N.R.; Cohen, A.S.; Hansen, T.; Vestergaard, H.; Pedersen, O.; Allin, K.H. Bone turnover, calcium homeostasis, and vitamin D status in Danish vegans. Eur. J. Clin. Nutr. 2018, 72, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Lamberg-Allardt, C.; Kärkkäinen, M.; Seppänen, R.; Biström, H. Low serum 25-hydroxyvitamin D concentrations and secondary hyperparathyroidism in middle-aged white strict vegetarians. Am. J. Clin. Nutr. 1993, 58, 684–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, R.; Huang, X.; Huang, J.; Li, Y.; Zhang, C.; Yin, Y.; Chen, Z.; Jin, Y.; Cai, J.; Cui, F. Long- and short-term health effects of pesticide exposure: A cohort study from China. PLoS ONE 2015, 10, e0128766. [Google Scholar] [CrossRef]
- Landin-Wilhelmsen, K.; Wilhelmsen, L.; Lappas, G.; Rosén, T.; Lindstedt, G.; Lundberg, P.A.; Wilske, J.; Bengtsson, B.Å. Serum intact parathyroid hormone in a random population sample of men and women: Relationship to anthropometry, life-style factors, blood pressure, and vitamin D. Calcif. Tissue Int. 1995, 56, 104–108. [Google Scholar] [CrossRef]
- Brot, C.; Jøorgensen, N.R.; Sørensen, O.H. The influence of smoking on vitamin D status and calcium metabolism. Eur. J. Clin. Nutr. 1999, 53, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Paik, J.M.; Curhan, G.C.; Forman, J.P.; Taylor, E.N. Determinants of plasma parathyroid hormone levels in young women. Calcif. Tissue Int. 2010, 87, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Parthemore, J.G.; Deftos, L.J. Calcitonin secretion in normal human subjects. J. Clin. Endocrinol. Metab. 1978, 47, 184–188. [Google Scholar] [CrossRef]
- Pedrazzoni, M.; Ciotti, G.; Davoli, L.; Pioli, G.; Girasole, G.; Palummeri, E.; Passeri, M. Meal-stimulated gastrin release and calcitonin secretion. J. Endocrinol. Investig. 1989, 12, 409–412. [Google Scholar] [CrossRef]
- Zayed, A.; Alzubaidi, M.; Atallah, S.; Momani, M.; Al-Delaimy, W. Should food intake and circadian rhythm be considered when measuring serum calcitonin level? Endocr. Pract. 2013, 19, 620–626. [Google Scholar] [CrossRef]
- Pointillart, A.; Garel, J.M.; Gueguen, L.; Colin, C. Plasma calcitonin and parathyroid hormone levels in growing pigs on different diets. I.—High phosphorus diet. Ann. Biol. Anim. Biochim. Biophys. 1978, 18, 699–709. [Google Scholar] [CrossRef]
- Deftos, L.J.; Miller, M.M.; Burton, D.W. A high-fat diet increases calcitonin secretion in the rat. Bone Miner. 1989, 5, 303–308. [Google Scholar] [CrossRef]
- Grundmann, M.; von Versen-Höynck, F. Vitamin D—Roles in women’s reproductive health? Reprod. Biol. Endocrinol. 2011, 9, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moslehi, N.; Shab-Bidar, S.; Mirmiran, P.; Hosseinpanah, F.; Azizi, F. Determinants of parathyroid hormone response to vitamin D supplementation: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 1360–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidya, A.; Curhan, G.C.; Paik, J.M.; Wang, M.; Taylor, E.N. Physical activity and the risk of primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 2016, 101, 1590–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engström, A.; Håkansson, H.; Skerfving, S.; Bjellerup, P.; Lidfeldt, J.; Lundh, T.; Samsioe, G.; Vahter, M.; Åkesson, A. Retinol may counteract the negative effect of cadmium on bone. J. Nutr. 2011, 141, 2198–2203. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ridefelt, P.; Akerström, G.; Hellman, P. Differentiation of human parathyroid cells in culture. J. Endocrinol. 2001, 168, 417–425. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, P.N.; Ritter, C.; Brown, A.J.; Slatopolsky, E. Retinoic acid suppresses parathyroid hormone (PTH) secretion and PreproPTH mRNA levels in bovine parathyroid cell culture. J. Clin. Investig. 1994, 93, 725–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooms, M.E.; Roos, J.C.; Bezemer, P.D.; van der Vijgh, W.J.F.; Bouter, L.M.; Lips, P. Prevention of bone loss by vitamin D supplementation in elderly women: A randomized double-blind trial. J. Clin. Endocrinol. Metab. 1995, 80, 1052–1058. [Google Scholar] [CrossRef] [Green Version]
- Naveh-Many, T.; Silver, J. Regulation of calcitonin gene transcription by vitamin D metabolites in vivo in the rat. J. Clin. Investig. 1988, 81, 270–273. [Google Scholar] [CrossRef]
- Saggese, G.; Federico, G.; Bertelloni, S.; Baroncelli, G.I.; Calisti, L. Hypomagnesemia and the parathyroid hormone-vitamin D endocrine system in children with insulin-dependent diabetes mellitus: Effects of magnesium administration. J. Pediatr. 1991, 118, 220–225. [Google Scholar] [CrossRef]
- Sahota, O.; Mundey, M.K.; San, P.; Godber, I.M.; Hosking, D.J. Vitamin D insufficiency and the blunted PTH response in established osteoporosis: The role of magnesium deficiency. Osteoporos. Int. 2006, 17, 1013–1021. [Google Scholar] [CrossRef]
- Cheung, M.M.; DeLuccia, R.; Ramadoss, R.K.; Aljahdali, A.; Volpe, S.L.; Shewokis, P.A.; Sukumar, D. Low dietary magnesium intake alters vitamin D-parathyroid hormone relationship in adults who are overweight or obese. Nutr. Res. 2019, 69, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.J.; Ritchie, G.; Kerstan, D.; Kang, H.S.; Cole, D.E.C.; Quamme, G.A. Magnesium transport in the renal distal convoluted tubule. Physiol. Rev. 2001, 81, 51–84. [Google Scholar] [CrossRef]
- McGonigle, R.; Weston, M.; Keenan, J.; Jackson, D.; Parsons, V. Effect of hypermagnesemia on circulating plasma parathyroid hormone in patients on regular hemodialysis therapy. Magnesium 1984, 3, 1–7. [Google Scholar] [PubMed]
- Ohya, M.; Negi, S.; Sakaguchi, T.; Koiwa, F.; Ando, R.; Komatsu, Y.; Shinoda, T.; Inaguma, D.; Joki, N.; Yamaka, T.; et al. Significance of serum magnesium as an independent correlative factor on the parathyroid hormone level in uremic patients. J. Clin. Endocrinol. Metab. 2014, 99, 3873–3878. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, S.; Nakamura, T.; Higashi, A.; Matsuda, I. Infusion of zinc inhibits serum calcitonin levels in patients with various zinc status. Calcif. Tissue Int. 1991, 49, 179–182. [Google Scholar] [CrossRef]
- Suzuki, T.; Kajita, Y.; Katsumata, S.I.; Matsuzaki, H.; Suzuki, K. Zinc deficiency increases serum concentrations of parathyroid hormone through a decrease in serum calcium and induces bone fragility in rats. J. Nutr. Sci. Vitaminol. 2015, 61, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Alkan Baylan, F.; Bankir, M.; Acıbucu, F.; Kılınç, M. Zinc copper levels in patients with primary hyperparathyroidism. Cumhur. Med. J. 2021, 43, 117–123. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Milne, D.B. A moderately high intake compared to a low intake of zinc depresses magnesium balance and alters indices of bone turnover in postmenopausal women. Eur. J. Clin. Nutr. 2004, 58, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Strause, L.; Saltman, P.; Smith, K.T.; Bracker, M.; Andon, M.B. Spinal bone loss in postmenopausal women supplemented with calcium and trace minerals. J. Nutr. 1994, 124, 1060–1064. [Google Scholar] [CrossRef] [Green Version]
- Eaton Evans, J.; Mcilrath, E.M.; Jackson, W.E.; Mccartney, H.; Strain, J.J. Copper supplementation and the maintenance of bone mineral density in middle-aged women. J. Trace Elem. Exp. Med. 1996, 9, 87–94. [Google Scholar] [CrossRef]
- Marrone, J.A.; Maddalozzo, G.F.; Branscum, A.J.; Hardin, K.; Cialdella-Kam, L.; Philbrick, K.A.; Breggia, A.C.; Rosen, C.J.; Turner, R.T.; Iwaniec, U.T. Moderate alcohol intake lowers biochemical markers of bone turnover in postmenopausal women. Menopause 2012, 19, 974–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laitinen, K.; Lamberg-Allardt, C.; Tunninen, R.; Karonen, S.-L.; Tähtelä, R.; Ylikahri, R.; Välimäki, M. Transient hypoparathyroidism during acute alcohol intoxication. N. Engl. J. Med. 1991, 324, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Rapuri, P.B.; Gallagher, J.C.; Balhorn, K.E.; Ryschon, K.L. Smoking and bone metabolism in elderly women. Bone 2000, 27, 429–436. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Brownbill, R.A.; Tamborini, L.; Crncevic-Orlic, Z. To drink or not to drink: How are alcohol, caffeine and past smoking related to bone mineral density in elderly women? J. Am. Coll. Nutr. 2002, 21, 536–544. [Google Scholar] [CrossRef]
- Rico, H. Alcohol and bone disease. Alcohol Alcohol. 1990, 25, 345–352. [Google Scholar] [PubMed]
- Vantyghem, M.C.; Danel, T.; Marcelli-Tourvieille, S.; Moriau, J.; Leclerc, L.; Cardot-Bauters, C.; Docao, C.; Carnaille, B.; Wemeau, J.L.; D’Herbomez, M. Calcitonin levels do not decrease with weaning in chronic alcoholism. Thyroid 2007, 17, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.; Koopmann, A.; Grosshans, M.; Reinhard, I.; Spanagel, R.; Kiefer, F. Association of plasma calcium concentrations with alcohol craving: New data on potential pathways. Eur. Neuropsychopharmacol. 2017, 27, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Ilias, I.; Paparrigopoulos, T.; Tzavellas, E.; Karaiskos, D.; Meristoudis, G.; Liappas, A.; Liappas, I. Inpatient alcohol detoxification and plasma calcitonin (with original findings). Hell. J. Nucl. Med. 2011, 14, 177–178. [Google Scholar]
- Kalafateli, A.L.; Vallöf, D.; Colombo, G.; Lorrai, I.; Maccioni, P.; Jerlhag, E. An amylin analogue attenuates alcohol-related behaviours in various animal models of alcohol use disorder. Neuropsychopharmacology 2019, 44, 1093–1102. [Google Scholar] [CrossRef]
- Kalafateli, A.L.; Satir, T.M.; Vallöf, D.; Zetterberg, H.; Jerlhag, E. An amylin and calcitonin receptor agonist modulates alcohol behaviors by acting on reward-related areas in the brain. Prog. Neurobiol. 2021, 200, 101969. [Google Scholar] [CrossRef]
- Ylli, D.; Wartofsky, L. Can we link thyroid status, energy expenditure, and body composition to management of subclinical thyroid dysfunction? J. Clin. Endocrinol. Metab. 2019, 104, 209–212. [Google Scholar] [CrossRef]
- Bouassida, A.; Latiri, I.; Bouassida, S.; Zalleg, D.; Zaouali, M.; Feki, Y.; Gharbi, N.; Zbidi, A.; Tabka, Z. Parathyroid hormone and physical exercise: A brief review. J. Sport. Sci. Med. 2006, 5, 367. [Google Scholar]
- Nichols, J.F.; Palmer, J.E.; Levy, S.S. Low bone mineral density in highly trained male master cyclists. Osteoporos. Int. 2003, 14, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Hind, K.; Truscott, J.G.; Evans, J.A. Low lumbar spine bone mineral density in both male and female endurance runners. Bone 2006, 39, 880–885. [Google Scholar] [CrossRef]
- Lombardi, G.; Ziemann, E.; Banfi, G.; Corbetta, S. Physical activity-dependent regulation of parathyroid hormone and calcium-phosphorous metabolism. Int. J. Mol. Sci. 2020, 21, 5388. [Google Scholar] [CrossRef]
- Shea, K.L.; Barry, D.W.; Sherk, V.D.; Hansen, K.C.; Wolfe, P.; Kohrt, W.M. Calcium supplementation and parathyroid hormone response to vigorous walking in postmenopausal women. Med. Sci. Sport. Exerc. 2014, 46, 2007–2013. [Google Scholar] [CrossRef] [Green Version]
- Blum, J.W.; Fischer, J.A.; Hunziker, W.H.; Binswanger, U.; Picotti, G.B.; Da Prada, M.; Guillebeau, A. Parathyroid hormone responses to catecholamines and to changes of extracellular calcium in cows. J. Clin. Investig. 1978, 61, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- López, I.; Aguilera-Tejero, E.; Estepa, J.C.; Rodríguez, M.; Felsenfeld, A.J. Role of acidosis-induced increases in calcium on PTH secretion in acute metabolic and respiratory acidosis in the dog. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E780–E785. [Google Scholar] [CrossRef]
- Lin, L.L.; Hsieh, S.S. Effects of strength and endurance exercise on calcium-regulating hormones between different levels of physical activity. J. Mech. Med. Biol. 2005, 5, 267–275. [Google Scholar] [CrossRef]
- Zhao, C.; Hou, H.; Chen, Y.; Lv, K. Effect of aerobic exercise and raloxifene combination therapy on senileosteoporosis. J. Phys. Ther. Sci. 2016, 28, 1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, S.A.; Graham, H.K.; Mollan, R.A.B.; Riddoch, C.; Sheridan, B.; Johnston, H. Calcium homeostasis and exercise. Int. Orthop. 1989, 13, 69–73. [Google Scholar] [CrossRef]
- O’Neill, M.E.; Wilkinson, M.; Robinson, B.G.; McDowall, D.B.; Cooper, K.A.; Mihailidou, A.S.; Frewin, D.B.; Clifton-Bligh, P.; Hunyor, S.N. The effect of exercise on circulating immunoreactive calcitonin in men. Horm. Metab. Res. 1990, 22, 546–550. [Google Scholar] [CrossRef]
- Klausen, T.; Breum, L.; Sørensen, H.A.; Schifter, S.; Sonne, B. Plasma levels of parathyroid hormone, vitamin D, calcitonin, and calcium in association with endurance exercise. Calcif. Tissue Int. 1993, 52, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Soria, M.; Anson, M.; Escanero, J.F. Correlation analysis of exercise-induced changes in plasma trace element and hormone levels during incremental exercise in well-trained athletes. Biol. Trace Elem. Res. 2016, 170, 55–64. [Google Scholar] [CrossRef]
- Çetin Kargin, N.; Marakoglu, K.; Unlu, A.; Kebapcilar, L.; Korucu, N. Comparison of bone turnover markers between male smoker and non-smoker. Acta Med. Mediterr. 2016, 32, 317–323. [Google Scholar] [CrossRef]
- Fujiyoshi, A.; Polgreen, L.E.; Gross, M.D.; Reis, J.P.; Sidney, S.; Jacobs, D.R. Smoking habits and parathyroid hormone concentrations in young adults: The CARDIA study. Bone Rep. 2016, 5, 104. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.; Noahsen, P.; Rex, K.F.; Fleischer, I.; Albertsen, N.; Jorgensen, M.E.; Schæbel, L.K.; Laursen, M.B. Serum 25-hydroxyvitamin D, calcium and parathyroid hormone levels in Native and European populations in Greenland. Br. J. Nutr. 2018, 119, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.R.; Sha, Y.; Chen, Y.D.; Shi, Y.; Yin, D.W.; Wang, H. Vitamin D, parathyroid hormone, and serum lipid profiles in a middle-aged and elderly Chinese population. Endocr. Pract. 2014, 20, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Cutillas-Marco, E.; Fuertes-Prosper, A.; Grant, W.B.; Morales-Suárez-Varela, M. Vitamin D deficiency in South Europe: Effect of smoking and aging. Photodermatol. Photoimmunol. Photomed. 2012, 28, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Supervía, A.; Nogués, X.; Enjuanes, A.; Vila, J.; Mellibovsky, L.; Serrano, S.; Aubía, J.; Díez-Pérez, A. Effect of smoking and smoking cessation on bone mass, bone remodeling, vitamin D, PTH and sex hormones. J. Musculoskelet. Neuronal Interact. 2006, 6, 234–241. [Google Scholar]
- Hagström, E.; Hellman, P.; Larsson, T.E.; Ingelsson, E.; Berglund, L.; Sundström, J.; Melhus, H.; Held, C.; Lind, L.; Michaëlsson, K.; et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation 2009, 119, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Kamycheva, E.; Sundsfjord, J.; Jorde, R. Serum parathyroid hormone levels predict coronary heart disease: The Tromsø Study. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 69–74. [Google Scholar] [CrossRef]
- Li, L.; Yin, X.; Yao, C.; Zhu, X.; Wu, X. Vitamin D, parathyroid hormone and their associations with hypertension in a Chinese population. PLoS ONE 2012, 7, e43344. [Google Scholar] [CrossRef] [Green Version]
- Lorentzon, M.; Mellström, D.; Haug, E.; Ohlsson, C. Smoking is associated with lower bone mineral density and reduced cortical thickness in young men. J. Clin. Endocrinol. Metab. 2007, 92, 497–503. [Google Scholar] [CrossRef]
- Mellstrom, D.; Johansson, C.; Johnell, O.; Lindstedt, G.; Lundberg, P.A.; Obrant, K.; Schoon, I.M.; Toss, G.; Ytterberg, B.O. Osteoporosis, metabolic aberrations, and increased risk for vertebral fractures after partial gastrectomy. Calcif. Tissue Int. 1993, 53, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Jones, T.M.; Hyre, A.D.; Melamed, M.L.; Alper, A.; Raggi, P.; Leonard, M.B. Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clin. J. Am. Soc. Nephrol. 2009, 4, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortego-Centeno, N.; Muñoz-Torres, M.; Jódar, E.; Hernández-Quero, J.; Jurado-Duce, A.; De La Higuera Torres-Puchol, J. Effect of tobacco consumption on bone mineral density in healthy young males. Calcif. Tissue Int. 1997, 60, 496–500. [Google Scholar] [CrossRef]
- Saquib, N.; Von Mühlen, D.; Garland, C.F.; Barrett-Connor, E. Serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in men: The Rancho Bernardo study. Osteoporos. Int. 2006, 17, 1734–1741. [Google Scholar] [CrossRef]
- Sneve, M.; Emaus, N.; Joakimsen, R.M.; Jorde, R. The association between serum parathyroid hormone and bone mineral density, and the impact of smoking: The Tromso Study. Eur. J. Endocrinol. 2008, 158, 401–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snijder, M.B.; Lips, P.; Seidell, J.C.; Visser, M.; Deeg, D.J.H.; Dekker, J.M.; Van Dam, R.M. Vitamin D status and parathyroid hormone levels in relation to blood pressure: A population-based study in older men and women. J. Intern. Med. 2007, 261, 558–565. [Google Scholar] [CrossRef]
- Szulc, P.; Garnero, P.; Claustrat, B.; Marchand, F.; Duboeuf, F.; Delmas, P.D. Increased bone resorption in moderate smokers with low body weight: The Minos study. J. Clin. Endocrinol. Metab. 2002, 87, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Alessi, A.; Girotti, G.; Zanforlini, B.M.; Bertocco, A.; Mazzochin, M.; Zoccarato, F.; Piovesan, F.; Dianin, M.; Giannini, S.; et al. The impact of smoking on bone metabolism, bone mineral density and vertebral fractures in postmenopausal women. J. Clin. Densitom. 2020, 23, 381–389. [Google Scholar] [CrossRef]
- Gudmundsson, J.A.; Ljunghall, S.; Bergquist, C.; Wide, L.; Nillius, S.J. Increased bone turnover during gonadotropin-releasing hormone superagonist-induced ovulation inhibition. J. Clin. Endocrinol. Metab. 1987, 65, 159–163. [Google Scholar] [CrossRef]
- Rignell-Hydbom, A.; Skerfving, S.; Lundh, T.; Lindh, C.H.; Elmståhl, S.; Bjellerup, P.; Jünsson, B.A.; Strümberg, U.; Akesson, A. Exposure to cadmium and persistent organochlorine pollutants and its association with bone mineral density and markers of bone metabolism on postmenopausal women. Environ. Res. 2009, 109, 991–996. [Google Scholar] [CrossRef]
- Eliasson, M.; Hagg, E.; Lundblad, D.; Karlsson, R.; Bucht, E. Influence of smoking and snuff use on electrolytes, adrenal and calcium regulating hormones. Acta Endocrinol. 1993, 128, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Stesin, A.; Halloran, B.; Steinbach, L.; Recker, R. Alcohol-induced bone disease: Relationship to age and parathyroid hormone levels. Alcohol. Clin. Exp. Res. 1993, 17, 690–695. [Google Scholar] [CrossRef]
- Johnell, O.; Kristensson, H.; Nilsson, B.E. Parathyroid activity in alcoholics. Br. J. Addict. 1982, 77, 93–95. [Google Scholar] [CrossRef]
- Sripanyakorn, S.; Jugdaohsingh, R.; Mander, A.; Davidson, S.L.; Thompson, R.P.H.; Powell, J.J. Moderate ingestion of alcohol is associated with acute ethanol-induced suppression of circulating CTX in a PTH-independent fashion. J. Bone Miner. Res. 2009, 24, 1380–1388. [Google Scholar] [CrossRef] [Green Version]
- Wilkens Knudsen, A.; Jensen, J.E.B.; Nordgaard-Lassen, I.; Almdal, T.; Kondrup, J.; Becker, U. Nutritional intake and status in persons with alcohol dependency: Data from an outpatient treatment programme. Eur. J. Nutr. 2014, 53, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Shim, M.S.; Kim, M.K.; Lee, Y.; Shin, Y.G.; Chung, C.H.; Kwon, S.O. Effect of chronic alcohol ingestion on bone mineral density in males without liver cirrhosis. Korean J. Intern. Med. 2003, 18, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.; Horowitz, M.; Fleming, S.; Kaiser, F.; Patrick, P.; Morley, J.; Cushman, W.; Bingham, S.; Perry, H. Effect of recent alcohol intake on parathyroid hormone and mineral metabolism in men. Alcohol. Clin. Exp. Res. 1998, 22, 1369–1375. [Google Scholar] [CrossRef]
- Al-Sultan, A.; Amin, T.; Abou-Seif, M.; Naboli, M. Al Vitamin D, parathyroid hormone levels and insulin sensitivity among obese young adult Saudis. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 135–147. [Google Scholar]
- Bischof, M.G.; Heinze, G.; Vierhapper, H. Vitamin D status and its relation to age and body mass index. Horm. Res. 2006, 66, 211–215. [Google Scholar] [CrossRef]
- Di Monaco, M.; Castiglioni, C.; Vallero, F.; Di Monaco, R.; Tappero, R. Parathyroid hormone is significantly associated with body fat compartment in men but not in women following a hip fracture. Aging Clin. Exp. Res. 2013, 25, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Grootendorst, D.C.; Boeschoten, E.W.; Krediet, R.T.; Wanner, C.; Dekker, F.W. Changes in parathyroid hormone, body mass index and the association with mortality in dialysis patients. Nephrol. Dial. Transplant. 2011, 26, 1340–1346. [Google Scholar] [CrossRef] [Green Version]
- Durá-Travé, T.; Gallinas-Victoriano, F.; Chueca-Guindulain, M.J.; Berrade-Zubiri, S.; Urretavizcaya-Martinez, M.; Ahmed-Mohamed, L. Assessment of vitamin D status and parathyroid hormone during a combined intervention for the treatment of childhood obesity. Nutr. Diabetes 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Abbasalizad Farhangi, M.; Ostadrahimi, A.; Mahboob, S. Serum calcium, magnesium, phosphorous and lipid profile in healthy Iranian premenopausal women. Biochem. Med. 2011, 21, 312–320. [Google Scholar] [CrossRef]
- Gannagé-Yared, M.H.; Chemali, R.; Sfeir, C.; Maalouf, G.; Halaby, G. Dietary calcium and vitamin D intake in an adult Middle Eastern population: Food sources and relation to lifestyle and PTH. Int. J. Vitam. Nutr. Res. 2005, 75, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.C.; Schuch, N.J.; Catania, A.S.; Gouvea Ferreira, S.R.; Martini, L.A. Parathyroid hormone has an important role in blood pressure regulation in vitamin D–insufficient individuals. Nutrition 2013, 29, 1147–1151. [Google Scholar] [CrossRef] [Green Version]
- Grethen, E.; Hill, K.M.; Jones, R.; Cacucci, B.M.; Gupta, C.E.; Acton, A.; Considine, R.V.; Peacock, M. Serum leptin, parathyroid hormone, 1,25-dihydroxyvitamin D, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in obesity. J. Clin. Endocrinol. Metab. 2012, 97, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Guasch, A.; Bulló, M.; Rabassa, A.; Bonada, A.; Del Castillo, D.; Sabench, F.; Salas-Salvadó, J. Plasma vitamin D and parathormone are associated with obesity and atherogenic dyslipidemia: A cross-sectional study. Cardiovasc. Diabetol. 2012, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guglielmi, V.; Bellia, A.; Gentileschi, P.; Lombardo, M.; D’Adamo, M.; Lauro, D.; Sbraccia, P. Parathyroid hormone in surgery-induced weight loss: No glucometabolic effects but potential adaptive response to skeletal loading. Endocrine 2018, 59, 288–295. [Google Scholar] [CrossRef]
- Gunnarsson, Ö.; Indridason, Ó.S.; Franzson, L.; Sigurdsson, G. Factors associated with elevated or blunted PTH response in vitamin D insufficient adults. J. Intern. Med. 2009, 265, 488–495. [Google Scholar] [CrossRef]
- Ha, J.; Jo, K.; Lim, D.J.; Lee, J.M.; Chang, S.A.; Kang, M.I.L.; Cha, B.Y.; Kim, M.H. Parathyroid hormone and vitamin D are associated with the risk of metabolic obesity in a middle-aged and older Korean population with preserved renal function: A cross-sectional study. PLoS ONE 2017, 12, e0175132. [Google Scholar] [CrossRef]
- Helal, O.; Kensara, O.; Azzeh, F.; Abdel Kafy, M. Effect of parathyroid hormone and body mass index on overall stability index in Saudi males with vitamin D deficiency. Life Sci. J. 2016, 13, 1–6. [Google Scholar]
- Ishimura, E.; Okuno, S.; Tsuboniwa, N.; Norimine, K.; Fukumoto, S.; Yamakawa, K.; Yamakawa, T.; Shoji, S.; Nishizawa, Y.; Inaba, M. Significant positive association between parathyroid hormone and fat mass and lean mass in chronic hemodialysis patients. J. Clin. Endocrinol. Metab. 2013, 98, 1264–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamycheva, E.; Sundsfjord, J.; Jorde, R. Serum parathyroid hormone level is associated with body mass index. The 5th Tromsø study. Eur. J. Endocrinol. 2004, 151, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Chandler, P.; Ng, K.; Manson, J.A.E.; Giovannucci, E. Obesity and efficacy of vitamin D 3 supplementation in healthy black adults. Cancer Causes Control 2020, 31, 303–307. [Google Scholar] [CrossRef]
- Kontogeorgos, G.; Trimpou, P.; Laine, C.M.; Oleröd, G.; Lindahl, A.; Landin-Wilhelmsen, K. Normocalcaemic, vitamin D-sufficient hyperparathyroidism—High prevalence and low morbidity in the general population: A long-term follow-up study, the WHO MONICA project, Gothenburg, Sweden. Clin. Endocrinol. 2015, 83, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Ahmadzadeh, S.; Anderson, J.E.; Kalantar-Zadeh, K. Obesity is associated with secondary hyperparathyroidism in men with moderate and severe chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 1024–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Lv, F.; Zhang, Z.; Deng, W.; Li, Y.; Deng, Z.; Jiang, Y.; Wang, O.; Xing, X.; Xu, L.; et al. Establishment of a normal reference value of parathyroid hormone in a large healthy Chinese population and evaluation of its relation to bone turnover and bone mineral density. Osteoporos. Int. 2016, 27, 1907–1916. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Garg, M.K.; Mahalle, N.; Bhadra, K.; Tandon, N. Role of parathyroid hormone in determination of fat mass in patients with Vitamin D deficiency. Indian J. Endocrinol. Metab. 2017, 21, 848. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; de Sousa, G.; Alexy, U.; Kersting, M.; Andler, W. Vitamin D status and parathyroid hormone in obese children before and after weight loss. Eur. J. Endocrinol. 2007, 157, 225–232. [Google Scholar] [CrossRef]
- Van Ballegooijen, A.J.; Kestenbaum, B.; Sachs, M.C.; De Boer, I.H.; Siscovick, D.S.; Hoofnagle, A.N.; Ix, J.H.; Visser, M.; Brouwer, I.A. Association of 25-hydroxyvitamin D and parathyroid hormone with incident hypertension: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2014, 63, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- You, L.; Chrn, L.; Pan, L.; Chen, J.; Peng, Y. Relation of body mass index to vitamin D, PTH, and bone turnover markers levels among women in Shanghai area. Chin. J. Endocrinol. Metab. 2013, 12, 566–569. [Google Scholar] [CrossRef]
- Shapses, S.A.; Lee, E.J.; Sukumar, D.; Durazo-Arvizu, R.; Schneider, S.H. The effect of obesity on the relationship between serum parathyroid hormone and 25-hydroxyvitamin D in women. J. Clin. Endocrinol. Metab. 2013, 98, E886–E890. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.-G.; Park, S.-K.; Kim, K.-M.; Kim, D.-H. Association of serum parathyroid hormone and vitamin D levels with cardiovascular risk factors. Korean J. Fam. Pract. 2017, 7, 55–59. [Google Scholar] [CrossRef]
- Snijder, M.B.; Van Dam, R.M.; Visser, M.; Deeg, D.J.H.; Dekker, J.M.; Bouter, L.M.; Seidell, J.C.; Lips, P. Adiposity in relation to vitamin D status and parathyroid hormone levels: A population-based study in older men and women. J. Clin. Endocrinol. Metab. 2005, 90, 4119–4123. [Google Scholar] [CrossRef] [Green Version]
- Svedlund, A.; Pettersson, C.; Tubic, B.; Magnusson, P.; Swolin-Eide, D. Vitamin D status in young Swedish women with anorexia nervosa during intensive weight gain therapy. Eur. J. Nutr. 2017, 56, 2061–2067. [Google Scholar] [CrossRef]
- Valiña-Tóth, A.L.B.; Lai, Z.; Yoo, W.; Abou-Samra, A.; Gadegbeku, C.A.; Flack, J.M. Relationship of vitamin D and parathyroid hormone to obesity and body composition in African Americans. Clin. Endocrinol. 2010, 72, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, C.K.d.S.; Santos, A.S.e.A.d.C.; Rosa, L.P.d.S.; Mendonça, C.R.; Vitorino, P.V.d.O.; Peixoto, M.D.R.G.; Silveira, É.A. Effect of extra virgin olive oil and traditional Brazilian diet on the bone health parameters of severely obese adults: A randomized controlled trial. Nutrients 2020, 12, 403. [Google Scholar] [CrossRef] [Green Version]
- Hassoon, A.; Michos, E.D.; Miller, E.R.; Crisp, Z.; Appel, L.J. Effects of different dietary interventions on calcitriol, parathyroid hormone, calcium, and phosphorus: Results from the DASH trial. Nutrients 2018, 10, 367. [Google Scholar] [CrossRef] [Green Version]
- Ho-Pham, L.T.; Vu, B.Q.; Lai, T.Q.; Nguyen, N.D.; Nguyen, T.V. Vegetarianism, bone loss, fracture and vitamin D: A longitudinal study in Asian vegans and non-vegans. Eur. J. Clin. Nutr. 2012, 66, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Tylavsky, F.A.; Holliday, K.; Danish, R.; Womack, C.; Norwood, J.; Carbone, L. Fruit and vegetable intakes are an independent predictor of bone size in early pubertal children. Am. J. Clin. Nutr. 2004, 79, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Kinyamu, H.K.; Gallagher, J.C.; Rafferty, K.A.; Balhorn, K.E. Dietary calcium and vitamin D intake in elderly women: Effect on serum parathyroid hormone and vitamin D metabolites. Am. J. Clin. Nutr. 1998, 67, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, M.C.; Chapuy, P.; Meunier, P.J. Calcium and vitamin D supplements: Effects on calcium metabolism in elderly people. Am. J. Clin. Nutr. 1987, 46, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Dallal, G.E.; Krall, E.A.; Harris, S.; Sokoll, L.J.; Falconer, G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann. Intern. Med. 1991, 115, 505–512. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992, 327, 1637–1642. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Harris, S.S.; Krall, E.A.; Dallal, G.E. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N. Engl. J. Med. 1997, 337, 670–676. [Google Scholar] [CrossRef]
- Krieg, M.A.; Jacquet, A.F.; Bremgartner, M.; Cuttelod, S.; Thiébaud, D.; Burckhardt, P. Effect of supplementation with vitamin D3 and calcium on quantitative ultrasound of bone in elderly institutionalized women: A longitudinal study. Osteoporos. Int. 1999, 9, 483–488. [Google Scholar] [CrossRef]
- Hunter, D.; Major, P.; Arden, N.; Swaminathan, R.; Andrew, T.; MacGregor, A.J.; Keen, R.; Snieder, H.; Spector, T.D. A randomized controlled trial of vitamin D supplementation on preventing postmenopausal bone loss and modifying bone metabolism using identical twin pairs. J. Bone Miner. Res. 2000, 15, 2276–2283. [Google Scholar] [CrossRef]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Nachtigall, D.; Hansen, C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J. Clin. Endocrinol. Metab. 2001, 86, 1633–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, A.M.; Biskup, B.; Robbins, B.; Marcella, G.; Burleson, J.A. Effects of vitamin D supplementation on strength, physical function, and health perception in older, community-dwelling men. J. Am. Geriatr. Soc. 2003, 51, 1762–1767. [Google Scholar] [CrossRef]
- Grados, F.; Brazier, M.; Kamel, S.; Mathieu, M.; Hurtebize, N.; Maamer, M.; Garabédian, M.; Sebert, J.L.; Fardellone, P. Prediction of bone mass density variation by bone remodeling markers in postmenopausal women with vitamin D insufficiency treated with calcium and vitamin D supplementation. J. Clin. Endocrinol. Metab. 2003, 88, 5175–5179. [Google Scholar] [CrossRef] [PubMed]
- Brazier, M.; Grados, F.; Kamel, S.; Mathieu, M.; Morel, A.; Maamer, M.; Sebert, J.L.; Fardellone, P. Clinical and laboratory safety of one year’s use of a combination calcium + vitamin D tablet in ambulatory elderly women with vitamin D insufficiency: Results of a multicenter, randomized, double-blind, placebo-controlled study. Clin. Ther. 2005, 27, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.A.; Aloia, J.F.; Pollack, S.; Yeh, J.K. Dose response to vitamin D supplementation among postmenopausal African American women. Am. J. Clin. Nutr. 2007, 86, 1657–1662. [Google Scholar] [CrossRef]
- Pittas, A.G.; Harris, S.S.; Stark, P.C.; Dawson-Hughes, B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007, 30, 980–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chel, V.; Wijnhoven, H.A.H.; Smit, J.H.; Ooms, M.; Lips, P. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos. Int. 2008, 19, 663–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björkman, M.; Sorva, A.; Risteli, J.; Tilvis, R. Vitamin D supplementation has minor effects on parathyroid hormone and bone turnover markers in vitamin D-deficient bedridden older patients. Age Ageing 2008, 37, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K.D.; Hill, T.R.; Lucey, A.J.; Taylor, N.; Seamans, K.M.; Muldowney, S.; FitzGerald, A.P.; Flynn, A.; Barnes, M.S.; Horigan, G.; et al. Estimation of the dietary requirement for vitamin D in healthy adults. Am. J. Clin. Nutr. 2008, 88, 1535–1542. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Suppan, K.; Fahrleitner-Pammer, A.; Dobnig, H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos. Int. 2009, 20, 315–322. [Google Scholar] [CrossRef]
- Zittermann, A.; Frisch, S.; Berthold, H.K.; Götting, C.; Kuhn, J.; Kleesiek, K.; Stehle, P.; Koertke, H.; Koerfer, R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am. J. Clin. Nutr. 2009, 89, 1321–1327. [Google Scholar] [CrossRef]
- Islam, M.Z.; Shamim, A.A.; Viljakainen, H.T.; Akhtaruzzaman, M.; Jehan, A.H.; Khan, H.U.; Al-Arif, F.A.; Lamberg-Allardt, C. Effect of vitamin D, calcium and multiple micronutrient supplementation on vitamin D and bone status in Bangladeshi premenopausal garment factory workers with hypovitaminosis D: A double-blinded, randomised, placebo-controlled 1-year intervention. Br. J. Nutr. 2010, 104, 241–247. [Google Scholar] [CrossRef]
- Jorde, R.; Sneve, M.; Torjesen, P.; Figenschau, Y.; Hansen, J.B. Parameters of the thrombogram are associated with serum 25-hydroxyvitamin D levels at baseline, but not affected during supplementation with vitamin D. Thromb. Res. 2010, 125, e210–e213. [Google Scholar] [CrossRef]
- Lips, P.; Binkley, N.; Pfeifer, M.; Recker, R.; Samanta, S.; Cohn, D.A.; Chandler, J.; Rosenberg, E.; Papanicolaou, D.A. Once-weekly dose of 8400 IU vitamin D(3) compared with placebo: Effects on neuromuscular function and tolerability in older adults with vitamin D insufficiency. Am. J. Clin. Nutr. 2010, 91, 985–991. [Google Scholar] [CrossRef] [Green Version]
- Grimnes, G.; Figenschau, Y.; Almås, B.; Jorde, R. Vitamin D, insulin secretion, sensitivity, and lipids: Results from a case-control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes 2011, 60, 2748–2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokol, S.I.; Srinivas, V.; Crandall, J.P.; Kim, M.; Tellides, G.; Lebastchi, A.; Yu, Y.; Gupta, A.K.; Alderman, M.H. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc. Med. 2012, 17, 394–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponda, M.P.; Dowd, K.; Finkielstein, D.; Holt, P.R.; Breslow, J.L. The short-term effects of vitamin D repletion on cholesterol: A randomized, placebo-controlled trial. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2510–2515. [Google Scholar] [CrossRef] [Green Version]
- Harris, S.S.; Pittas, A.G.; Palermo, N.J. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes. Metab. 2012, 14, 789–794. [Google Scholar] [CrossRef]
- Larsen, T.; Mose, F.H.; Bech, J.N.; Hansen, A.B.; Pedersen, E.B. Effect of cholecalciferol supplementation during winter months in patients with hypertension: A randomized, placebo-controlled trial. Am. J. Hypertens. 2012, 25, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Kjærgaard, M.; Waterloo, K.; Wang, C.E.A.; Almås, B.; Figenschau, Y.; Hutchinson, M.S.; Svartberg, J.; Jorde, R. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: Nested case-control study and randomised clinical trial. Br. J. Psychiatry 2012, 201, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Salehpour, A.; Hosseinpanah, F.; Shidfar, F.; Vafa, M.; Razaghi, M.; Dehghani, S.; Hoshiarrad, A.; Gohari, M. A 12-week double-blind randomized clinical trial of vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr. J. 2012, 11, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, R.; Vatsa, M.; Sreenivas, V.; Singh, U.; Gupta, N.; Lakshmy, R.; Aggarwal, S.; Ganapathy, A.; Joshi, P.; Bhatia, H. Skeletal muscle strength in young Asian Indian females after vitamin D and calcium supplementation: A double-blind randomized controlled clinical trial. J. Clin. Endocrinol. Metab. 2012, 97, 4709–4716. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Yoshioka, M.; Hashimoto, M.; Murakami, M.; Noya, M.; Takahashi, D.; Urashima, M. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am. J. Clin. Nutr. 2013, 97, 1004–1013. [Google Scholar] [CrossRef]
- Jorde, R.; Bønaa, K.H. Calcium from dairy products, vitamin D intake, and blood pressure: The Tromsø study. Am. J. Clin. Nutr. 2000, 71, 1530–1535. [Google Scholar] [CrossRef] [Green Version]
- Rudnicki, M.; Thode, J.; Jorgensen, T.; Heitmann, B.L.; Sorensen, O.H. Effects of age, sex, season and diet on serum ionized calcium, parathyroid hormone and vitamin D in a random population. J. Intern. Med. 1993, 234, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, A.; Mughal, M.Z.; Hanumante, N.; Sayyad, M.; Sanwalka, N.; Naik, S.; Fraser, W.D.; Joshi, A.; Khadilkar, V. Oral calcium supplementation reverses the biochemical pattern of parathyroid hormone resistance in underprivileged Indian toddlers. Arch. Dis. Child. 2009, 94, 932–937. [Google Scholar] [CrossRef]
- Patel, P.; Zulf Mughal, M.; Patel, P.; Yagnik, B.; Kajale, N.; Mandlik, R.; Khadilkar, V.; Chiplonkar, S.A.; Phanse, S.; Patwardhan, V.; et al. Dietary calcium intake influences the relationship between serum 25-hydroxyvitamin D3 (25OHD) concentration and parathyroid hormone (PTH) concentration. Arch. Dis. Child. 2016, 101, 316–319. [Google Scholar] [CrossRef]
- Gunther, C.W.; Legowski, P.A.; Lyle, R.M.; Weaver, C.M.; McCabe, L.D.; McCabe, G.P.; Peacock, M.; Teegarden, D. Parathyroid hormone is associated with decreased fat mass in young healthy women. Int. J. Obes. 2006, 30, 94–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, L.; Mason, R.S.; Mpofu, E.; Dibley, M.; Li, Y.; Brock, K.E. Vitamin D and parathyroid hormone status in a representative population living in Macau, China. J. Steroid Biochem. Mol. Biol. 2015, 148, 261–268. [Google Scholar] [CrossRef]
- Muschitz, C.; Kocijan, R.; Haschka, J.; Zendeli, A.; Pirker, T.; Geiger, C.; Müller, A.; Tschinder, B.; Kocijan, A.; Marterer, C.; et al. The impact of vitamin D, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: The BABS study. J. Bone Miner. Res. 2016, 31, 672–682. [Google Scholar] [CrossRef]
- Bouassida, A.; Zalleg, D.; Zaouali Ajina, M.; Gharbi, N.; Duclos, M.; Richalet, J.P.; Tabka, Z. Parathyroid hormone concentrations during and after two periods of high intensity exercise with and without an intervening recovery period. Eur. J. Appl. Physiol. 2003, 88, 339–344. [Google Scholar] [CrossRef]
- Brahm, H.; Piehl-Aulin, K.; Ljunghall, S. Bone metabolism during exercise and recovery: The influence of plasma volume and physical fitness. Calcif. Tissue Int. 1997, 61, 192–198. [Google Scholar] [CrossRef]
- Dashtidehkordi, A.; Shahgholian, N.; Sadeghian, J. The effect of exercise during hemodialysis on serum levels of albumin, calcium, phosphorus and parathyroid hormone: A randomized clinical trial. Res. Square 2021, 1–18. [Google Scholar] [CrossRef]
- Falk, B.; Haddad, F.; Klentrou, P.; Ward, W.; Kish, K.; Mezil, Y.; Radom-Aizik, S. Differential sclerostin and parathyroid hormone response to exercise in boys and men. Osteoporos. Int. 2016, 27, 1245–1249. [Google Scholar] [CrossRef] [Green Version]
- Kohrt, W.M.; Wherry, S.J.; Wolfe, P.; Sherk, V.D.; Wellington, T.; Swanson, C.M.; Weaver, C.M.; Boxer, R.S. Maintenance of serum ionized calcium during exercise attenuates parathyroid hormone and bone resorption responses. J. Bone Miner. Res. 2018, 33, 1326–1334. [Google Scholar] [CrossRef] [Green Version]
- Kohrt, W.M.; Wolfe, P.; Sherk, V.D.; Wherry, S.J.; Wellington, T.; Melanson, E.L.; Swanson, C.M.; Weaver, C.M.; Boxer, R.S. Dermal calcium loss is not the primary determinant of parathyroid hormone secretion during exercise. Med. Sci. Sport. Exerc. 2019, 51, 2117–2124. [Google Scholar] [CrossRef]
- Ljunghall, S.; Joborn, H.; Roxin, L.-E.; Rastad, J.; Wide, L.; Åkerström, G. Prolonged low-intensity exercise raises the serum parathyroid hormone levels. Clin. Endocrinol. 1986, 25, 535–542. [Google Scholar] [CrossRef]
- Moreira, L.D.F.; Fronza, F.C.A.O.; Dos Santos, R.N.; Zach, P.L.; Kunii, I.S.; Hayashi, L.F.; Teixeira, L.R.; Kruel, L.F.M.; Castro, M.L. The benefits of a high-intensity aquatic exercise program (HydrOS) for bone metabolism and bone mass of postmenopausal women. J. Bone Miner. Metab. 2014, 32, 411–419. [Google Scholar] [CrossRef]
- Maïmoun, L.; Simar, D.; Malatesta, D.; Caillaud, C.; Peruchon, E.; Couret, I.; Rossi, M.; Mariano-Goulart, D. Response of bone metabolism related hormones to a single session of strenuous exercise in active elderly subjects. Br. J. Sport. Med. 2005, 39, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Maïmoun, L.; Manetta, J.; Couret, I.; Dupuy, A.M.; Mariano-Goulart, D.; Micallef, J.P.; Peruchon, E.; Rossi, M. The intensity level of physical exercise and the bone metabolism response. Int. J. Sport. Med. 2006, 27, 105–111. [Google Scholar] [CrossRef]
- Mathis, S.L.; Pivovarova, A.I.; Hicks, S.M.; Alrefai, H.; MacGregor, G.G. Calcium loss in sweat does not stimulate PTH release: A study of Bikram hot yoga. Complement. Ther. Med. 2020, 51, 102417. [Google Scholar] [CrossRef]
- Salvesen, H.; Johansson, A.G.; Foxdal, P.; Wide, L.; Piehl-Aulin, K.; Ljunghall, S. Intact serum parathyroid hormone levels increase during running exercise in well-trained men. Calcif. Tissue Int. 1994, 54, 256–261. [Google Scholar] [CrossRef]
- Scott, J.P.R.; Sale, C.; Greeves, J.P.; Casey, A.; Dutton, J.; Fraser, W.D. The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise. J. Appl. Physiol. 2011, 110, 423–432. [Google Scholar] [CrossRef]
- Scott, J.P.R.; Sale, C.; Greeves, J.P.; Casey, A.; Dutton, J.; Fraser, W.D. Treadmill running reduces parathyroid hormone concentrations during recovery compared with a nonexercising control group. J. Clin. Endocrinol. Metab. 2014, 99, 1774–1782. [Google Scholar] [CrossRef] [Green Version]
- Sherk, V.D.; Wherry, S.J.; Barry, D.W.; Shea, K.L.; Wolfe, P.; Kohrt, W.M. Calcium supplementation attenuates disruptions in calcium homeostasis during exercise. Med. Sci. Sport. Exerc. 2017, 49, 1437. [Google Scholar] [CrossRef]
- Takada, H.; Washino, K.; Hanai, T.; Iwata, H. Response of parathyroid hormone to exercise and bone mineral density in adolescent female athletes. Environ. Health Prev. Med. 1998, 2, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Thorsen, K.; Kristoffersson, A.; Hultdin, J.; Lorentzon, R. Effects of moderate endurance exercise on calcium, parathyroid hormone, and markers of bone metabolism in young women. Calcif. Tissue Int. 1997, 60, 16–20. [Google Scholar] [CrossRef]
- Townsend, R.; Elliott-Sale, K.J.; Pinto, A.J.; Thomas, C.; Scott, J.P.R.; Currell, K.; Fraser, W.D.; Sale, C. Parathyroid hormone secretion is controlled by both ionized calcium and phosphate during exercise and recovery in men. J. Clin. Endocrinol. Metab. 2016, 101, 3231–3239. [Google Scholar] [CrossRef] [Green Version]
- Ljunghall, S.; Joborn, H.; Roxin, L.E.; Skarfors, E.T.; Wide, L.E.; Lithell, H.O. Increase in serum parathyroid hormone levels after prolonged physical exercise. Med. Sci. Sport. Exerc. 1988, 20, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Castro, J.; Mira-Rufino, P.J.; Moreno-Fernandez, J.; Chirosa, I.; Chirosa, J.L.; Guisado, R.; Ochoa, J.J. Ubiquinol supplementation modulates energy metabolism and bone turnover during high intensity exercise. Food Funct. 2020, 11, 7523–7531. [Google Scholar] [CrossRef]
- Malandish, A.; Tartibian, B.; Sheikhlou, Z.; Afsargharehbagh, R.; Rahmati, M. The effects of short-term moderate intensity aerobic exercise and long-term detraining on electrocardiogram indices and cardiac biomarkers in postmenopausal women. J. Electrocardiol. 2020, 60, 15–22. [Google Scholar] [CrossRef]
- Tsai, K.S.; Lin, J.C.; Chen, C.K.; Cheng, W.C.; Yang, C.H. Effect of exercise and exogenous glucocorticoid on serum level of intact parathyroid hormone. Int. J. Sport. Med. 1997, 18, 583–587. [Google Scholar] [CrossRef]
- Vora, N.M.; Kukreja, S.C.; York, P.A.J.; Bowser, E.N.; Hargis, G.K.; Williams, G.A. Effect of exercise on serum calcium and parathyroid hormone. J. Clin. Endocrinol. Metab. 1983, 57, 1067–1069. [Google Scholar] [CrossRef]
- D’Herbomez, M.; Caron, P.; Bauters, C.; Do Cao, C.; Schlienger, J.L.; Sapin, R.; Baldet, L.; Carnaille, B.; Wémeau, J.L. Reference range of serum calcitonin levels in humans: Influence of calcitonin assays, sex, age, and cigarette smoking. Eur. J. Endocrinol. 2007, 157, 749–755. [Google Scholar] [CrossRef] [Green Version]
- Gobba, N.A.E.K.; Hussein Ali, A.; El Sharawy, D.E.; Hussein, M.A. The potential hazardous effect of exposure to iron dust in Egyptian smoking and nonsmoking welders. Arch. Environ. Occup. Health 2017, 73, 189–202. [Google Scholar] [CrossRef]
- Cvek, M.; Punda, A.; Brekalo, M.; PLoSnić, M.; Barić, A.; Kaličanin, D.; Brčić, L.; Vuletić, M.; Gunjača, I.; Torlak Lovrić, V.; et al. Presence or severity of Hashimoto’s thyroiditis does not influence basal calcitonin levels: Observations from CROHT biobank. J. Endocrinol. Investig. 2021, 1–9. [Google Scholar] [CrossRef]
- Sabia, R.; Wagner, M.; Susa, K.; Lemke, J.; Rothermund, L.; Henne-Bruns, D.; Hillenbrand, A. Calcitonin concentrations in patients with chronic kidney disease on hemodialysis in reference to parathyroidectomy. BMC Res. Notes 2019, 12, 1–5. [Google Scholar] [CrossRef]
- Pilat-Marcinkiewicz, B.; Brzóska, M.; Moniuszko-Jakoniuk, J. Thyroid and parathyroid function and structure in male rats chronically exposed to cadmium. Pol. J. Environ. Stud. 2008, 17, 113–120. [Google Scholar]
- Nogawa, K.; Kobayashi, E.; Yamada, Y.; Honda, R.; Kido, T.; Tsuritani, I.; Ishizaki, M. Parathyroid hormone concentration in the serum of people with cadmium-induced renal damage. Int. Arch. Occup. Environ. Health 1984, 54, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Tsuritani, I.; Honda, R.; Lshizaki, M.; Yamada, Y.; Kido, T.; Nogawa, K. Impairment of vitamin D metabolism due to environmental cadmium exposure, and possible relevance to sex-related differences in vulnerability to the bone damage. J. Toxicol. Environ. Health 1992, 37, 519–533. [Google Scholar] [CrossRef]
- Jarup, L.; Persson, B.; Elinder, C.G. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup. Environ. Med. 1995, 52, 818. [Google Scholar] [CrossRef] [Green Version]
- Nogawa, K.; Tsuritani, I.; Kido, T.; Honda, R.; Yamada, Y.; Ishizaki, M. Mechanism for bone disease found in inhabitants environmentally exposed to cadmium: Decreased serum 1 alpha, 25-dihydroxyvitamin D level. Int. Arch. Occup. Environ. Health 1987, 59, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Malin Igra, A.; Vahter, M.; Raqib, R.; Kippler, M. Early-life cadmium exposure and bone-related biomarkers: A longitudinal study in children. Environ. Health Perspect. 2019, 127, 37003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Bone metabolism of male rats chronically exposed to cadmium. Toxicol. Appl. Pharmacol. 2005, 207, 195–211. [Google Scholar] [CrossRef]
- Ahmed, S.; Rekha, R.S.; Ahsan, K.B.; Doi, M.; Grandér, M.; Roy, A.K.; Ekström, E.C.; Wagatsuma, Y.; Vahter, M.; Raqib, R. Arsenic exposure affects plasma insulin-like growth factor 1 (IGF-1) in children in rural Bangladesh. PLoS ONE 2013, 8, e81530. [Google Scholar] [CrossRef]
- Mazumdar, I.; Goswami, K.; Ali, M.S. Status of serum calcium, vitamin D and parathyroid hormone and hematological indices among lead exposed jewelry workers in Dhaka, Bangladesh. Indian J. Clin. Biochem. 2017, 32, 110. [Google Scholar] [CrossRef] [Green Version]
- McDiarmid, M.A.; Engelhardt, S.M.; Dorsey, C.D.; Oliver, M.; Gucer, P.; Gaitens, J.M.; Kane, R.; Cernich, A.; Kaup, B.; Hoover, D.; et al. Longitudinal health surveillance in a cohort of Gulf War veterans 18 years after first exposure to depleted uranium. J. Toxicol. Environ. Health A 2011, 74, 678–691. [Google Scholar] [CrossRef]
- Yuan, G.; Lu, H.; Yin, Z.; Dai, S.; Jia, R.; Xu, J.; Song, X.; Li, L. Effects of mixed subchronic lead acetate and cadmium chloride on bone metabolism in rats. Int. J. Clin. Exp. Med. 2014, 7, 1378. [Google Scholar]
- Zhu, M.; Zhou, W.; Bai, L.; Li, H.; Wang, L.; Zou, X. Dietary cadmium chloride supplementation impairs renal function and bone metabolism of laying hens. Animals 2019, 9, 998. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Yamamoto, M.; Watanabe, K.; Kambegawa, A.; Hattori, A. Both mercury and cadmium directly influence calcium homeostasis resulting from the suppression of scale bone cells: The scale is a good model for the evaluation of heavy metals in bone metabolism. J. Bone Miner. Metab. 2004, 22, 439–446. [Google Scholar] [CrossRef]
- Guo, Y.L.; Lin, C.J.; Yao, W.J.; Ryan, J.J.; Hsu, C.C. Musculoskeletal changes in children prenatally exposed to polychlorinated biphenyls and related compounds (Yu-Cheng children). J. Toxicol. Environ. Health 1994, 41, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, A.; Rocca, M.S.; De Toni, L.; Sabovic, I.; Guidolin, D.; Dall’Acqua, S.; Acquasaliente, L.; De Filippis, V.; Plebani, M.; Foresta, C. Endocrine disruption of vitamin D activity by perfluoro-octanoic acid (PFOA). Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koroglu, B.K.; Ersoy, I.H.; Koroglu, M.; Balkarli, A.; Ersoy, S.; Varol, S.; Tamer, M.N. Serum parathyroid hormone levels in chronic endemic fluorosis. Biol. Trace Elem. Res. 2011, 143, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Skórka-Majewicz, M.; Goschorska, M.; Żwierełło, W.; Baranowska-Bosiacka, I.; Styburski, D.; Kapczuk, P.; Gutowska, I. Effect of fluoride on endocrine tissues and their secretory functions—Review. Chemosphere 2020, 260, 127565. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.A.; Elkaliny, H.H.; Abd-Elsalam, M.M. Lycopene ameliorates the effect of Aroclor 1254 on morphology, proliferation, and angiogenesis of the thyroid gland in rat. Toxicology 2021, 452, 152722. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015, 36, 1–150. [Google Scholar] [CrossRef]
- Zeng, Q.b.; Xu, Y.y.; Yu, X.; Yang, J.; Hong, F.; Zhang, A. hua Arsenic may be involved in fluoride-induced bone toxicity through PTH/PKA/AP1 signaling pathway. Environ. Toxicol. Pharmacol. 2014, 37, 228–233. [Google Scholar] [CrossRef]
- Engström, A.; Skerving, S.; Lidfeldt, J.; Burgaz, A.; Lundh, T.; Samsioe, G.; Vahter, M.; Åkesson, A. Cadmium-induced bone effect is not mediated via low serum 1,25-dihydroxy vitamin D. Environ. Res. 2009, 109, 188–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, K.S.; Beshir, S.; Shahy, E.M.; Shaheen, W.A. Effect of occupational cadmium exposure on parathyroid gland. Open Access Maced. J. Med. Sci. 2016, 4, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kido, T.; Honda, R.; Tsuritani, I.; Ishizaki, M.; Yamada, Y.; Nogawa, K.; Nakagawa, H.; Dohi, Y. Assessment of cadmium-induced osteopenia by measurement of serum bone Gla protein, parathyroid hormone, and 1 alpha,25-dihydroxyvitamin D. J. Appl. Toxicol. 1991, 11, 161–166. [Google Scholar] [CrossRef]
- Lee, C.C.; Weng, C.H.; Huang, W.H.; Yen, T.H.; Lin, J.L.; Lin, D.T.; Chen, K.H.; Hsu, C.W. Association between blood cadmium levels and mortality in peritoneal dialysis. Medicine 2016, 95, e3717. [Google Scholar] [CrossRef]
- Ghosh-Narang, J.; Jones, T.M.; Menke, A.; Todd, A.C.; Muntner, P.; Batuman, V. Parathyroid hormone status does not influence blood and bone lead levels in dialysis patients. Am. J. Med. Sci. 2007, 334, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Kristal-Boneh, E.; Froom, P.; Yerushalmi, N.; Harari, G.; Ribak, J. Calcitropic hormones and occupational lead exposure. Am. J. Epidemiol. 1998, 147, 458–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.L.; Lin-Tan, D.T.; Chen, K.H.; Hsu, C.W.; Yen, T.H.; Huang, W.H.; Huang, Y.L. Blood lead levels association with 18-month all-cause mortality in patients with chronic peritoneal dialysis. Nephrol. Dial. Transplant. 2010, 25, 1627–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colleoni, N.; Arrigo, G.; Gandini, E.; Corigliano, C.; D’Amico, G. Blood lead in hemodialysis patients. Am. J. Nephrol. 1993, 13, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, J.D. Observations on the effect of parathyroid hormone on environmental blood lead concentrations in humans. Environ. Res. 1991, 54, 8–16. [Google Scholar] [CrossRef]
- Potula, V.; Henderson, A.; Kaye, W. Calcitropic hormones, bone turnover, and lead exposure among female smelter workers. Arch. Environ. Occup. Health 2005, 60, 195–204. [Google Scholar] [CrossRef]
- Pouresmaeil, R.; Razeghi, E.; Ahmadi, F. Correlation of serum lead levels with inflammation, nutritional status, and clinical complications in hemodialysis patients. Ren. Fail. 2012, 34, 1114–1117. [Google Scholar] [CrossRef] [Green Version]
- McDiarmid, M.A.; Engelhardt, S.M.; Dorsey, C.D.; Oliver, M.; Gucer, P.; Wilson, P.D.; Kane, R.; Cernich, A.; Kaup, B.; Anderson, L.; et al. Surveillance results of depleted uranium-exposed Gulf War I veterans: Sixteen years of follow-up. J. Toxicol. Environ. Health A 2009, 72, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, H.M.; Devananda, D.; Nanditha Kumar, M.; Wormald, M.M.; Prashanth, S.N. The association of fluoride in drinking water with serum calcium, vitamin D and parathyroid hormone in pregnant women and newborn infants. Eur. J. Clin. Nutr. 2020, 75, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Harari, F.; Åkesson, A.; Casimiro, E.; Lu, Y.; Vahter, M. Exposure to lithium through drinking water and calcium homeostasis during pregnancy: A longitudinal study. Environ. Res. 2016, 147, 1–7. [Google Scholar] [CrossRef] [Green Version]
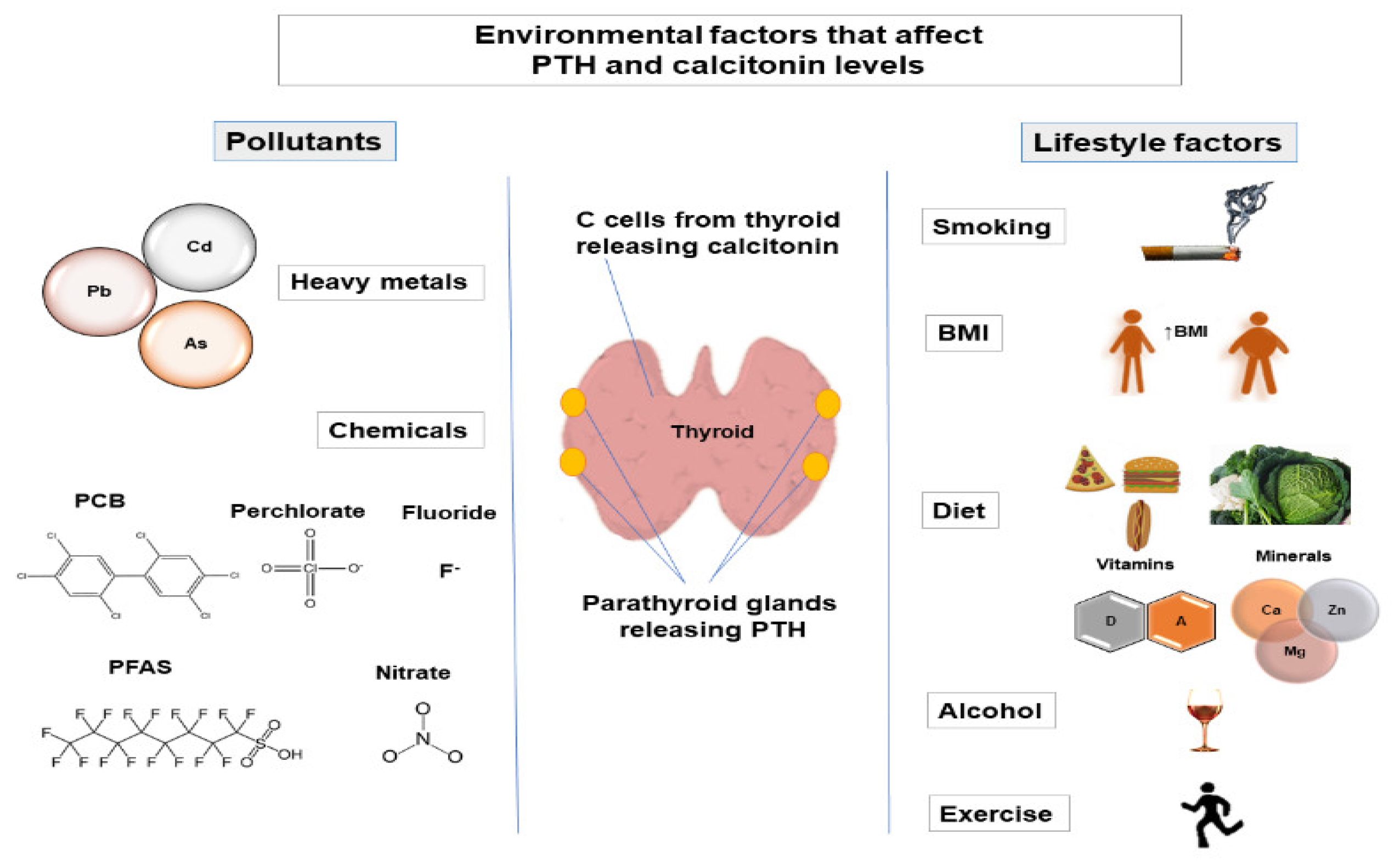
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babić Leko, M.; Pleić, N.; Gunjača, I.; Zemunik, T. Environmental Factors That Affect Parathyroid Hormone and Calcitonin Levels. Int. J. Mol. Sci. 2022, 23, 44. https://doi.org/10.3390/ijms23010044
Babić Leko M, Pleić N, Gunjača I, Zemunik T. Environmental Factors That Affect Parathyroid Hormone and Calcitonin Levels. International Journal of Molecular Sciences. 2022; 23(1):44. https://doi.org/10.3390/ijms23010044
Chicago/Turabian StyleBabić Leko, Mirjana, Nikolina Pleić, Ivana Gunjača, and Tatijana Zemunik. 2022. "Environmental Factors That Affect Parathyroid Hormone and Calcitonin Levels" International Journal of Molecular Sciences 23, no. 1: 44. https://doi.org/10.3390/ijms23010044
APA StyleBabić Leko, M., Pleić, N., Gunjača, I., & Zemunik, T. (2022). Environmental Factors That Affect Parathyroid Hormone and Calcitonin Levels. International Journal of Molecular Sciences, 23(1), 44. https://doi.org/10.3390/ijms23010044







