Lipidomic Profile and Enzymes Activity in Hepatic Microsomes of Rats in Physiological and Pathological Conditions
Abstract
:1. Introduction
2. Results
2.1. FA Profile in Hepatic Microsomes
2.2. CFA Profile in Hepatic Microsomes
2.3. Chemometric Analysis
2.4. Peroxidability Index, LA Isomerization Index and Desaturases Indices
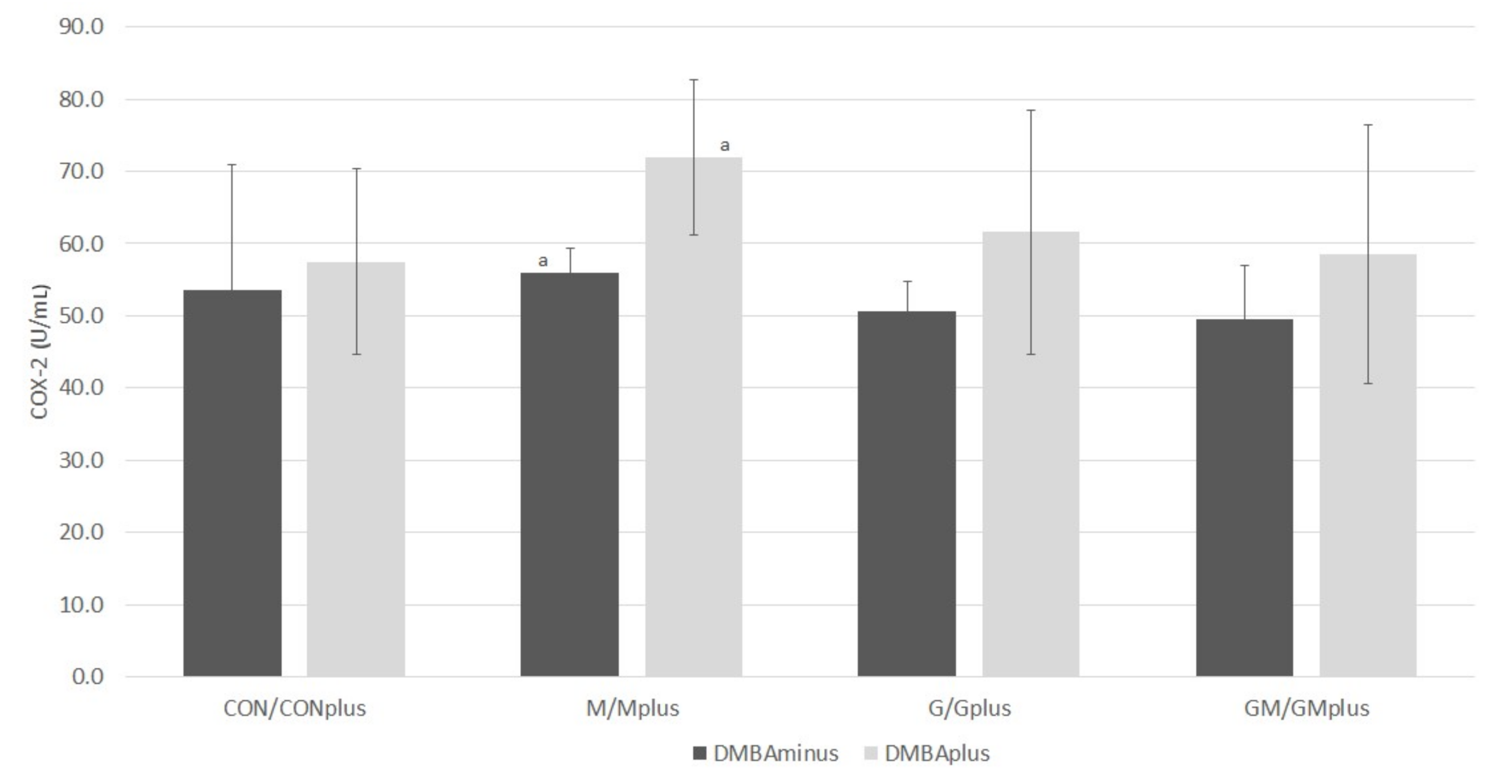
2.5. COX-2 Activity
2.6. CYP1B1 Content
3. Discussion
4. Materials and Methods
4.1. Dietary Supplements: PSO and BME
4.2. Animals
- −
- CON and CONplus—control groups without diet supplementation, fed a standard diet and water ad libitum,
- −
- M and Mplus—animals fed a standard diet supplemented with 1% aqueous extract of bitter melon dried fruits (BM) ad libitum,
- −
- G and Gplus—animals were fed the standard diet and water ad libitum and were given 0.15 mL/d PSO via intragastric gavage,
- −
- GM and GMplus—animals were fed the standard diet and were supplemented with both 0.15 mL/d PSO via intragastric gavage and 1% BM ad libitum.
4.3. Preparation of Hepatic Microsomes
4.4. Determination of Fatty Acids in Hepatic Microsomes
4.5. Determination of Conjugated Fatty Acid (CFA) Isomers in Hepatic Microsomes
4.6. Determination of Peroxidability Index, LA Isomerization Index and Indices of Desaturases Activity in Hepatic Microsomes
4.7. Determination of COX-2 Activity in Hepatic Microsomes
4.8. Determination of CYP2B1 Isoform Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marmot, M.; Atinmo, T.; Byers, T.; Chen, J.; Hirohata, T.; Jackson, A.; James, W.; Kolonel, L.; Kumanyika, S.; Leitzmann, C.; et al. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; AICR: Washington, DC, USA, 2007. [Google Scholar]
- Krawinkel, M.B.; Keding, G.B. Bitter Gourd (Momordica charantia): A Dietary Approach to Hyperglycemia. Nutr. Rev. 2006, 64, 331–337. [Google Scholar] [CrossRef]
- Virdi, J.; Sivakami, S.; Shahani, S.; Suthar, A.C.; Banavalikar, M.M.; Biyani, M.K. Antihyperglycemic effects of three extracts from Momordica charantia. J. Ethnopharmacol. 2003, 88, 107–111. [Google Scholar] [CrossRef]
- Białek, A.; Teryks, M.; Tokarz, A. Sprzężone trieny kwasu linolenowego (conjugated linolenic acid—CLnA, super CLA)—źródła i działanie biologiczne. Postepy Hig. Med. Dosw. 2014, 68, 1238–1250. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Le Bloc’H, J.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Al-Jahdari, W.S.; Yamamoto, K.; Hiraoka, H.; Nakamura, K.; Goto, F.; Horiuchi, R. Prediction of total propofol clearance based on enzyme activities in microsomes from human kidney and liver. Eur. J. Clin. Pharmacol. 2006, 62, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Lepionka, T.; Białek, M.; Czauderna, M.; Białek, A. Pomegranate seed oil and bitter melon extract supplemented in diet influence the lipid profile and intensity of peroxidation in livers of SPRD rats exposed to a chemical carcinogen. Prostaglandins Lipid Mediat. 2021, 152, 106495. [Google Scholar] [CrossRef]
- Białek, A.; Jelińska, M.; Białek, M.; Lepionka, T.; Czerwonka, M.; Czauderna, M. The Effect of Diet Supplementation with Pomegranate and Bitter Melon on Lipidomic Profile of Serum and Cancerous Tissues of Rats with Mammary Tumours. Antioxidants 2020, 9, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Białek, A.; Białek, M.; Lepionka, T.; Pachniewicz, P.; Czauderna, M. Oxysterols and lipidomic profile of myocardium of rats supplemented with pomegranate seed oil and/or bitter melon aqueous extract—Cardio-oncological animal model research. Chem. Phys. Lipids 2021, 235, 105057. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, Y.; Cambon-Gros, C.; Deltour, P.; Muntané, J.; Canal, M.T.; Mitjavila, S. Dietary polyunsaturated fatty acid deficiency: Consequences for Ca2+ transport by hepatic microsomal membranes in relation with their physicochemical state. Food Addit. Contam. 1990, 7, S158–S161. [Google Scholar] [CrossRef]
- Andrianaivo-Rafehivola, A.A.; Siess, M.H.; Gaydou, E.M. Modifications of hepatic drug metabolizing enzyme activities in rats fed baobab seed oil containing cyclopropenoid fatty acids. Food Chem. Toxicol. 1995, 33, 377–382. [Google Scholar] [CrossRef]
- Rustan, A.C.; Christiansen, E.N.; Drevon, C.A. Serum lipids, hepatic glycerolipid metabolism and peroxisomal fatty acid oxidation in rats fed ω-3 and ω-6 fatty acids. Biochem. J. 1992, 283, 333–339. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, X.; Cheng, C.; Li, N.; Liu, Y.; Cao, Y. The implications of signaling lipids in cancer metastasis. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Du, G. Dysregulated lipid metabolism in cancer. World J. Biol. Chem. 2012, 3, 167–174. [Google Scholar] [CrossRef]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. 2018, 38, 27. [Google Scholar] [CrossRef] [PubMed]
- Lepionka, T.; Białek, A.; Białek, M.; Czauderna, M.; Stawarska, A.; Wrzesień, R.; Bielecki, W.; Paśko, P.; Galanty, A.; Bobrowska-Korczak, B. Mammary cancer risk and serum lipid profile of rats supplemented with pomegranate seed oil and bitter melon extract. Prostaglandins Lipid Mediat. 2019, 142, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Dower, W.V. Metabolic pathways of 7,12 dimethylbenz[a]anthracene in hepatic microsomes. Proc. Natl. Acad. Sci. USA 1975, 72, 2601–2605. [Google Scholar] [CrossRef] [Green Version]
- Badal, S.; Shields, M.; Delgo, R. Cytochrome P450 Enzyme Inhibitors from Nature. Enzym. Inhib. Bioapplications 2012, 40–56. [Google Scholar]
- Semiz, A.; Sen, A. Antioxidant and chemopreventive properties of Momordica charantia L. (butter melon) fruit extract. Afr. J. Biotechnol. 2007, 6, 273–277. [Google Scholar]
- Kasimsetty, S.G.; Bialonska, D.; Reddy, M.K.; Thornton, C.; Willett, K.L.; Ferreira, D. Effects of pomegranate chemical constituents/intestinal microbial metabolites on CYP1B1 in 22Rv1 prostate cancer cells. J. Agric. Food Chem. 2009, 57, 10636–10644. [Google Scholar] [CrossRef]
- Faria, A.; Monteiro, R.; Azevedo, I.; Calhau, C. Pomegranate juice effects on cytochrome p450s expression: In vivo studies. J. Med. Food 2007, 10, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Mallhi, T.H.; Sarriff, A.; Adnan, A.S.; Khan, Y.H.; Qadir, M.I.; Hamzah, A.A.; Khan, A.H. Effect of fruit/vegetable-drug interactions on CYP450, OATP and p-glycoprotein: A systematic review. Trop. J. Pharm. Res. 2015, 14, 1927–1935. [Google Scholar] [CrossRef]
- Białek, M.; Białek, A.; Czauderna, M. Conjugated linoleic acid isomers affect profile of lipid compounds and intensity of their oxidation in heart of rats with chemically induced mammary tumors—Preliminary study. Nutrients 2019, 11, 2032. [Google Scholar] [CrossRef] [Green Version]
- Białek, M.; Białek, A.; Czauderna, M. Maternal and Early Postnatal Diet Supplemented with Conjugated Linoleic Acid Isomers Affect Lipid Profile in Hearts of Offspring Rats with Mammary Tumors. Animals 2020, 10, 464. [Google Scholar] [CrossRef] [Green Version]
- Białek, A.; Białek, M.; Lepionka, T.; Tober, E.; Czauderna, M. The Quality Determination of Selected Commercial Online Purchased Edible Pomegranate Seed Oils With New Argentometric Liquid Chromatography Method. J. Diet. Suppl. 2020, 18, 351–371. [Google Scholar] [CrossRef]
- Kohno, H.; Suzuki, R.; Yasui, Y.; Hosokawa, M.; Miyashita, K.; Tanaka, T. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004, 95, 481–486. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Kawakami, Y.; Abe, R.; Nakagawa, K.; Koba, K.; Imamura, J.; Iwata, T.; Ikeda, I.; Miyazawa, T. Conjugated Linolenic Acid Is Slowly Absorbed in Rat Intestine, but Quickly Converted to Conjugated Linoleic Acid. J. Nutr. 2006, 163, 2153–2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, G.; Sun, H.; Sinclair, A.J.; Li, D. Effects of conjugated linolenic acid and conjugated linoleic acid on lipid metabolism in mice. Eur. J. Lipid Sci. Technol. 2009, 111, 537–545. [Google Scholar] [CrossRef]
- Go, R.E.; Hwang, K.A.; Choi, K.C. Cytochrome P450 1 family and cancers. J. Steroid Biochem. Mol. Biol. 2015, 147, 24–30. [Google Scholar] [CrossRef]
- Manzanares, M.A.; Solanas, M.; Moral, R.; Escrich, R.; Vela, E.; Costa, I.; Escrich, E. Dietary extra-virgin olive oil and corn oil differentially modulate the mRNA expression of xenobiotic-metabolizing enzymes in the liver and in the mammary gland in a rat chemically induced breast cancer model. Eur. J. Cancer Prev. 2015, 24, 215–222. [Google Scholar] [CrossRef]
- Burdan, F.; Chałas, A.; Szumiło, J. Cyklooksygenaza i prostanoidy—Znaczenie biologiczne. Postepy Hig. Med. Dosw. 2006, 60, 129–141. [Google Scholar]
- Soslow, R.A.; Dannenberg, A.J.; Rush, D.; Woerner, B.M.; Nasir Khan, K.; Masferrer, J.; Koki, A.T. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 2000, 89, 2637–2645. [Google Scholar] [CrossRef]
- Chan, G.; Boyle, J.O.; Yang, E.K.; Zhang, F.; Sacks, P.G.; Shah, J.P.; Edelstein, D.; Soslow, R.A.; Koki, A.T.; Woerner, B.M.; et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999, 59, 991–994. [Google Scholar]
- Lii, C.K.; Chen, H.W.; Yun, W.T.; Liu, K.L. Suppressive effects of wild bitter gourd (Momordica charantia Linn. var. abbreviata ser.) fruit extracts on inflammatory responses in RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 122, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Sung, P.J.; Wang, W.H.; Kuo, Y.H. Anti-inflammatory effect of momordica charantia in sepsis mice. Molecules 2014, 19, 12777–12788. [Google Scholar] [CrossRef] [Green Version]
- Białek, A.; Stawarska, A.; Bodecka, J.; Białek, M.; Tokarz, A. Pomegranate seed oil influences the fatty acids profile and reduces the activity of desaturases in livers of Sprague-Dawley rats. Prostaglandins Lipid Mediat. 2017, 131, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Stawarska, A.; Białek, A.; Stanimirova, I.; Stawarski, T.; Tokarz, A. The effect of Conjugated Linoleic Acids (CLA) supplementation on the activity of enzymes participating in the formation of arachidonic acid in liver microsomes of rats—Probable mechanism of CLA anticancer activity. Nutr. Cancer 2015, 67, 145–155. [Google Scholar] [CrossRef]
- Liu, K.L.; Belury, M.A. Conjugated linoleic acid reduces arachidonic acid content and PGE2 synthesis in murine keratinocytes. Cancer Lett. 1998, 127, 15–22. [Google Scholar] [CrossRef]
- Kucharski, M.; Kaczor, U. Desaturaza stearylo-CoA—Regulatormetabolizmu lipidów. Postepy Hig. Med. Dosw. 2014, 68, 334–342. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, H.; Park, S.S.; Chang, C.; Kim, E. Stearoyl CoA desaturase (SCD) facilitates proliferation of prostate cancer cells through enhancement of androgen receptor transactivation. Mol. Cells 2011, 31, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Białek, M.; Białek, A.; Lepionka, T.; Paśko, P.; Galanty, A.; Tokarz, A.; Czauderna, M. Punica granatum (Pomegranate) Seed Oil and Momordica charantia (Bitter Melon) Extract Affect the Lipid’s Profile and Oxidative Stability of Femoral Muscles of Rats. Eur. J. Lipid Sci. Technol. 2019, 121, 1800429. [Google Scholar] [CrossRef]
- Stawarska, A.; Białek, A.; Tokarz, A. Heating of vegetable oils influences the activity of enzymes participating in arachidonic acid formation in Wistar rats. Nutr. Res. 2015, 35, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method forthe isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Marounek, M.; Mlchalski, J.P.; Rozbicka-Wieczorek, A.J.; Krajewska, K.A. A new internal standard for HPLC assay of conjugated linoleic acid in animal tissues and milk. Czech J. Anim. Sci. 2011, 56, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Białek, M.; Czauderna, M.; Białek, A. Partial replacement of rapeseed oil with fish oil, and dietary antioxidants supplementation affects concentrations of biohydrogenation products and conjugated fatty acids in rumen and selected lamb tissues. Anim. Feed Sci. Technol. 2018, 241, 63–74. [Google Scholar] [CrossRef]
- Yun, J.; Surh, J. Fatty Acid Composition as a Predictor for the Oxidation Stability of Korean Vegetable Oils with or without Induced Oxidative Stress. Prev. Nutr. Food Sci. 2012, 17, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Fatty Acid | Group | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | M | G | GM | CONplus | Mplus | Gplus | GMplus | ||
| C12:0 | 3.22 ± 2.03 | 4.11 ± 2.60 | 4.26 ± 2.45 | 4.94 ± 1.63 | 4.77 ± 2.65 | 4.34 ± 1.49 | 5.22 ± 3.16 | 4.49 ± 1.66 | ns |
| C14:0 | 13.5 ± 10.8 a,b,c | 17.7 ± 12.4 d,e | 19.6 ± 5.96 f | 18.3 ± 5.83 g | 28.8 ± 6.61 a | 22.9 ± 9.19 | 40.1 ± 23.2 b,d | 38.9 ± 9.02 c,e,f,g | <0.0001 |
| C15:0 | 13.4 ± 9.36 a,b | 13.5 ± 8.96 c,d | 11.1 ± 1.74 e,f | 13.7 ± 1.74 g | 12.9 ± 4.15 h,i | 17.4 ± 6.90 | 29.1 ± 9.46 a,c,e,h | 33.7 ± 11.3 b,d,f,g,i | <0.0001 |
| C16:0 (mg/g) | 1.22 ± 0.67 | 1.40 ± 0.90 | 1.12 ± 0.11 | 1.38 ± 0.23 | 1.10 ± 0.34 | 1.78 ± 0.67 | 1.75 ± 0.64 | 1.49 ± 0.43 | ns |
| C17:0 | 57.9 ± 37.6 a | 64.4 ± 48.5 b | 45.3 ± 8.22 c,d,e | 56.9 ± 9.25 | 64.8 ± 23.5 | 102 ± 37.7 c | 95.9 ± 29.5 d | 107 ± 35.2 a,b,e | <0.0001 |
| C18:0 (mg/g) | 1.73 ± 0.90 | 2.06 ± 1.51 | 1.60 ± 0.24 a | 1.92 ± 0.27 | 1.76 ± 0.54 | 2.71 ± 0.93 a | 1.89 ± 0.55 | 2.02 ± 0.54 | 0.0209 |
| C20:0 | 4.26 ± 2.97 | 6.64 ± 4.50 | 4.24 ± 1.50 | 4.91 ± 1.46 | 2.86 ± 1.12 a | 3.64 ± 1.23 | 4.29 ± 2.30 | 6.04 ± 2.88 a | 0.0198 |
| C21:0 | 3.55 ± 2.67 | 3.42 ± 2.11 | 2.87 ± 1.65 | 3.51 ± 2.17 | 2.46 ± 1.12 | 2.02 ± 0.15 | 5.52 ± 3.47 | 4.47 ± 2.00 | ns |
| C22:0 | 4.56 ± 1.96 | 1.64 ± 0.81 | 2.32 ± 0.20 | 2.75 ± 2.12 | nd | nd | 5.10 ± 2.76 | 3.85 ± 2.63 | ns |
| Sum of SFA (mg/g) | 3.04 ± 1.63 | 3.57 ± 2.47 | 2.80 ± 0.34 a | 3.40 ± 0.49 | 2.97 ± 0.90 | 4.63 ± 1.58 a | 3.82 ± 1.09 | 3.57 ± 0.86 | 0.0051 |
| c7C16:1 | 11.4 ± 7.87 a | 13.8 ±12.1 b | 11.0 ± 5.27 c | 12.1 ± 4.82 | 15.9 ± 5.10 | 12.0 ± 4.47 | 23.8 ± 9.62 a,b,c | 15.6 ± 4.20 | 0.0031 |
| c9C16:1 | 42.9 ± 29.5 a,b | 59.1 ± 46.6 | 45.0 ± 16.5 c,d | 50.7 ± 19.2 | 76.9 ± 39.2 | 77.4 ± 35.8 | 110 ± 54.7 a,c | 101 ± 40.0 b,d | 0.0001 |
| c9C17:1 | 7.17 ± 5.14 a,b | 6.67 ± 4.44 c,d | 5.39 ± 2.55 e,f | 6.71 ± 2.77 g,h | 10.0 ± 5.75 | 10.6 ± 4.98 | 23.5 ± 11.7 a,c,e,g | 19.8 ± 3.75 b,d,f,h | <0.0001 |
| t9C18:1 | 3.77 ± 3.32 | 4.87 ± 3.12 | 4.27 ± 2.25 | 5.33 ± 1.72 | 3.24 ± 0.97 | 5.39 ± 3.33 | 5.10 ± 3.59 | 6.44 ± 2.09 | ns |
| c6C18:1 | 6.61 ± 5.99 | 5.81 ± 4.94 | 3.78 ± 1.23 | 6.43 ± 2.50 a | 2.31 ± 1.11 a,b | 5.53 ± 2.67 b | 4.59 ± 2.50 | 3.63 ± 2.22 | 0.0031 |
| c9C18:1 | 529 ± 306 | 646 ± 413 | 436 ± 134 | 512 ± 130 | 477 ± 135 | 597 ± 184 | 749 ± 371 | 566 ± 172 | ns |
| c11C18:1 | 92.2 ± 57.8 a | 104 ± 68.9 b | 71.8 ± 14.2 c,d,e,f | 89.7 ± 13.8 g | 142 ± 55.8 c | 228 ± 85.6 a,b,d,g | 179 ± 71.9 e | 163 ± 40.3 f | <0.0001 |
| c14C18:1 | nd | nd | nd | nd | 1.66 ± 0.00 | 3.16 ± 1.55 | 4.12 ± 2.07 | 7.07 ± 3.89 | ns |
| c11C20:1 | 3.78 ± 2.61 | 4.78 ± 4.06 | 4.73 ± 2.11 | 4.48 ± 2.20 | 5.84 ± 2.09 | 5.40 ± 2.32 | 8.11 ± 4.35 | 7.30 ± 2.38 | 0.0281 |
| c5C24:1 | 11.3 ± 6.41 | 11.3 ± 7.03 | 7.76 ± 3.31 | 8.62 ± 5.42 | 14.9 ± 13.2 | nd | 18.7 ± 2.07 | 12.6 ± 4.74 | ns |
| Sum of MUFA (mg/g) | 0.70 ± 0.42 | 0.85 ± 0.55 | 0.58 ± 0.15a | 0.69 ± 0.17 | 0.74 ± 0.23 | 0.90 ± 0.32 | 1.10 ± 0.45a | 0.83 ± 0.31 | 0.0192 |
| c9c12C18:2 (mg/g) | 1.08 ± 0.62 | 1.21 ± 0.77 | 1.03 ± 0.13 | 1.10 ± 0.17 a | 0.61 ± 0.24 a,b | 1.27 ± 0.58 b | 0.96 ± 0.42 | 0.96 ± 0.45 | 0.0135 |
| c6c9c12C18:3 | 8.25 ± 3.97 a,b | 12.9 ± 9.35 | 19.8 ± 6.13 a,c | 13.2 ± 4.90 | 9.77 ± 5.91 c,d | 25.3 ± 12.7 b,d | 11.9 ± 8.63 | 10.4 ± 3.73 | <0.0001 |
| c11c14C20:2 | 5.19 ± 3.10 a,b | 6.93 ± 4.77 c,d | 5.32 ± 2.65 e,f | 7.00 ± 1.87 g | 8.15 ± 3.35 | 9.62 ± 4.29 | 13.8 ± 4.56 a,c,e | 14.9 ± 4.47 b,d,f,g | <0.0001 |
| c8c11c14C20:3 | 23.3 ± 12.2 | 31.6 ± 27.6 | 21.2 ± 6.12 a,b | 30.8 ± 4.42 | 24.4 ± 9.40 | 29.5 ± 12.0 | 50.7 ± 30.6 a | 46.9 ± 22.5 b | 0.0003 |
| c5c8c11c14C20:4 (mg/g) | 1.20 ± 0.66 a | 1.59 ± 1.24 b | 1.37 ± 0.22 c | 1.53 ± 0.23 | 1.53 ± 0.54 | 2.73 ± 1.06 a,b,c | 1.52 ± 0.47 | 1.68 ± 0.54 | 0.0013 |
| c9c12c15C18:3 | 70.0 ± 50.3 a,b | 71.3 ± 41.5 c,d | 70.4 ± 21.4 e,f,g,h | 73.5 ± 16.9 i,j,k,l | 10.4 ± 4.27 a,c,e,i | 13.6 ± 6.00 b,d,f,j | 18.8 ± 7.61 g,k | 21.5 ± 9.22 h,l | <0.0001 |
| c5c8c11c14c17C20:5 | 37.2 ± 17.6 a,b | 51.2 ± 37.5 c,d | 44.8 ± 16.8 e,f | 46.6 ± 9.4 g,h | 12.2 ± 8.9 a,c,e,g | 10.6 ± 4.5 b,d,f,h | 29.1 ± 19.1 | 23.8 ± 10.2 | <0.0001 |
| c7c10c13c16c19C22:5 | 77.4 ± 40.8 | 83.8 ± 59.2 | 60.8 ± 9.31 | 86.1 ± 9.68 a | 37.0 ± 17.4 a,b,c | 71.0 ± 39.8 | 96.5 ± 53.7 b | 105.1 ± 56.6 c | 0.0002 |
| c4c7c10c13c16c19C22:6 | 518 ± 300 a | 579 ± 405 b | 469 ± 56.1c | 591 ± 113 | 608 ± 236 | 1072 ± 413 a,b,c | 601 ± 156 | 664 ± 211 | 0.0008 |
| c9t11C18:2 CLA | nd | nd | 15.7 ± 3.36 | 23.1 ± 7.76 a,b | nd | nd | 7.05 ± 3.63 a | 5.35 ± 4.58 b | <0.0001 |
| Sum of PUFA (mg/g) | 3.01 ± 1.69 a | 3.63 ± 2.57 b | 3.09 ± 0.39 | 3.40 ± 0.43 | 2.85 ± 1.03 | 5.23 ± 2.01 a,b | 3.30 ± 0.97 | 3.53 ± 1.28 | 0.0124 |
| Sum of n3 PUFA | 703 ± 401 | 785 ± 537 a | 638 ± 71.5 b | 791 ± 113 | 668 ± 257 | 1166 ± 455 a,b | 744 ± 208 | 815 ± 270 | 0.0083 |
| Sum of n6 PUFA | 2311 ± 1290 a | 2842 ± 2045 b | 2440 ± 330 | 2589 ± 382 | 2184 ± 777 c | 4060 ± 1561 a,b,c | 2549 ± 775 | 2711 ± 1007 | 0.0142 |
| n6/n3 | 3.31 ± 0.31 a | 3.62 ± 0.56 | 3.82 ± 0.30 a,b,c | 3.43 ± 0.27 | 3.32 ± 0.23 b | 3.49 ± 0.23 | 3.43 ± 0.41 | 3.29 ± 0.27 c | 0.0095 |
| Group | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | M | G | GM | CONplus | Mplus | Gplus | GMplus | ||
| CFA | 42.0 ± 25.0 a,b,c | 41.1 ± 15.8 d,e,f | 343 ± 136 a,d,g,h | 371 ± 173 b,e,i,j | 29.8 ± 7.48 g,i,k,l | 36.0 ± 11.6 h,j,m,n | 264 ± 229 k,m | 690 ± 469 c,f,l,n | <0.0001 |
| CD | 31.8 ± 19.3 a,b,c,d | 34.6 ± 18.1 e,f,g,h | 304 ± 118 a,e,i,j | 325 ± 149 b,f,k,l | 28.7 ± 6.66 i,k,m,n | 38.4 ± 15.6 j,l,o | 247 ± 209 c,g,m | 443 ± 194 d,h,n,o | <0.0001 |
| tt | 18.8 ± 10.0 a,b,c,d | 23.1 ± 13.9 e,f,g | 82.1 ± 35.6 a,e,h,i | 85.8 ± 36.4 b,f,j,k | 16.9 ± 4.34 h,j,l,m | 22.7 ± 8.50 i,k,n | 93.7 ± 77.0 c,l | 210 ± 136 d,g,m,n | <0.0001 |
| ct | 7.77 ± 6.23 a,b,c | 10.1 ± 6.63 d,e,f | 216 ± 83.3 a,d,g,h | 232 ± 112 b,e,i,j | 5.59 ± 1.79 g,i,k,l | 10.5 ± 5.82 h,j,m | 94.7 ± 78.4 k | 308 ± 283 c,f,l,m | <0.0001 |
| c9t11CLA | 7.57 ± 5.27 a,b,c | 8.02 ± 6.40 d,e,f | 199 ± 78.1 a,d,g,h | 217 ± 108 b,e,i,j | 3.68 ± 1.16 g,i,k,l | 8.87 ± 6.26 h,j,m | 62.5 (18.4–212) k | 247 ± 238 c,f,m | <0.0001 |
| cc | 3.22 ± 2.67 a,b | 3.43 ± 1.95c | 6.24 ± 2.24 d | 7.17 ± 4.12 e | 6.83 ± 2.31 | 5.19 ± 2.11 f | 12.1 (5.27–27.6) a | 49.5 ± 28.8 b,c,d,e,f | <0.0001 |
| CT | 3.31 (1.34–8.15) a,b | 2.97 (1.07–8.26) c,d | 38.8 ± 24.3 a,c,e,f | 45.4 ± 28.6 b,d,g,h | 0.59 ± 0.57 e,g,i | 0.98 ± 0.67 f,h,j | 4.68 (1.10–19.9) | 20.9 (5.53–78.8) i,j | <0.0001 |
| ttt | 5.20 (0.77–35.2) a | 6.42 (0.89–46.5) | 33.71 ± 24.56 | 40.67 ± 22.75 a | nd | nd | 23.8 (0.88–645) | 157.75 ± 0.00 | ns |
| ttc/ctt | 1.71 ± 1.12 | 2.28 ± 1.55 | 3.82 ± 2.75 a | 1.33 (0.60–2.98) b | 0.39 ± 0.27 a,c,d | 0.81 ± 0.67 e | 7.78 ± 5.64 c | 15.6 ± 12.6 b,d,e | <0.0001 |
| cct | 1.74 ± 1.50 a | 2.00 (0.64–6.31) | 3.85 ± 1.97 | 6.89 ± 3.60 b | 1.91 ± 0.00 | 0.50 ± 0.27 b,c | 3.74 (0.53–26.2) | 158 (36.1–699) a,c | 0.0007 |
| Group | p Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CON | M | G | GM | CONplus | Mplus | Gplus | GMplus | |||
| CD (%CFA) | 80.7 ± 23.2 a,b | 85.1 ± 18.6 | 89.2 ± 3.7 c,d | 88.6 ± 4.3 e,f | 96.6 ± 4.6 a,c,e | 97.8 ± 2.0 b,d,f | 94.9 ± 5.2 | 87.3 ± 26.7 | <0.0001 | |
| tt (%CD) | 63.3 ± 16.2 a,b | 64.7 ± 11.3 c,d | 26.7 ± 3.5 a,c,e,f | 27.1 ± 4.3 b,d,g,h | 59.2 ± 9.2 e,g | 59.9 ± 4.1 f,h | 41.9 ± 15.5 | 41.2 ± 11.6 | <0.0001 | |
| ct (%CD) | 27.5 ± 14.3 a,b | 31.5 ± 12.9 c,d | 71.1 ± 3.5 a,c,e,f | 70.6 ± 4.1 b,d,g,h | 20.1 ± 6.4 e,g,i | 26.4 ± 4.3 f,h | 40.0 ± 22.2 | 52.0 ± 14.8 i | <0.0001 | |
| cc (%CD) | 9.3 ± 5.2 | 9.2 ± 2.2 | 2.2 ± 0.8 a,b,c | 2.3 ± 1.0 d,e | 22.8 ± 5.7 a,d | 13.8 ± 3.2 b,e | 10.2 ± 5.9 c | 9.7 ± 4.3 | <0.0001 | |
| c9t11CLA (%CFA) | 23.0 ± 9.6 a,b | 20.3 ± 12.1 c,d | 58.4 ± 4.9 a,c,e,f | 58.2 ± 4.0 b,d,g,h | 12.9 ± 4.6 e,g,i | 20.2 ± 4.5 f,h | 38.3 ± 15.4 i | 37.4 ± 16.7 | <0.0001 | |
| c9t11CLA (%CD) | 26.5 ± 10.7 a,b | 25.2 ± 12.5 c,d | 65.4 ± 3.9 a,c,e,f | 65.7 ± 3.9 b,d,g,h | 13.4 ± 4.8 e,g,I,j | 21.9 ± 6.1 f,h | 40.8 ± 17.2 i | 42.4 ± 15.8 j | <0.0001 | |
| CT (%CFA) | 13.4 ± 11.0 a,b | 7.0 (2.5–25.1) | 10.8 ± 3.7 c,d | 11.4 ± 4.3 e,f | 2.2 ± 2.2 a,c,e | 2.6 ± 1.9 b,d,f | 4.6 ± 4.2 | 6.7 ± 6.0 | <0.0001 | |
| ttt (%CT) | 49.2 ± 24.8 | 36.7 ± 3.7 | 80.4 ± 19.0 | 75.8 ± 26.8 | - | - | - | - | n.s. | |
| ttc/ctt (%CT) | 51.7 ± 35.4 | 61.1 ± 30.5 a | 11.7 ± 8.5 b,c | 11.8 ± 29.3 a,d,e,f | 90.7 ± 29.5 b,d | 61.6 ± 30.1 | 77.7 ± 35.6 c,e | 72.5 ± 42.8 f | <0.0001 | |
| cct (%CT) | 35.9 ± 21.2 | 53.7 ± 22.7 | 21.3 ± 17.3 | 17.0 ± 13.0 a | 93.3 ± 2.5 | 66.3 ± 34.3 a | 49.0 ± 38.1 | 82.6 ± 8.7 | 0.0084 | |
| Coefficients of Canonical Variables | ||
|---|---|---|
| Variable (Discriminatory Power) | DF1 (88.5%) | DF2 (11.5%) |
| c9c11C18:2 | 2.80842 | −0.30746 |
| c14C18:1 | −2.18297 | 1.43115 |
| c7C15:1 | 0.10547 | −0.95926 |
| c15C24:1 | −0.89805 | −0.15973 |
| ttc/ctt | −1.25729 | −0.25606 |
| CT | 1.30678 | 0.53155 |
| c6C18:1 | 0.23964 | 0.56732 |
| ct | 0.38138 | 0.77035 |
| c6c9c12C18:3 | 0.48747 | −0.34854 |
| cc | −0.06846 | −0.54010 |
| C15:0 | −0.41474 | 0.71331 |
| c9C17:1 | 0.62505 | −0.03485 |
| c11C20:1 | −0.38061 | −0.02071 |
| t9C18:1 | −0.35859 | −0.27043 |
| c7c10c13c16c19C22:5 | −0.33071 | −0.46821 |
| C20:0 | 0.09110 | 0.28954 |
| c11c14C20:2 | 0.43479 | 0.08553 |
| Average Value of Canonical Variables | ||
| Sk1 | 6.80004 | −0.12432 |
| Sk2 | −1.94173 | 2.35397 |
| Sk3 | −2.42916 | −1.11482 |
| Actual Cluster | Correct Classification (%) | Predicted Cluster Classification | ||
|---|---|---|---|---|
| Sk1 | Sk2 | Sk3 | ||
| Sk1 | 100.0 | 22 | 0 | 0 |
| Sk2 | 81.8 | 0 | 18 | 4 |
| Sk3 | 97.7 | 1 | 0 | 43 |
| All | 94.3 | 23 | 18 | 47 |
| Group | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | M | G | GM | CONplus | Mplus | Gplus | GMplus | ||
| PI | 117 ± 4.63 a | 118 ± 11.7 b | 120 ± 21.2 | 125 ± 6.41 | 128 ± 10.9 c | 141 ± 7.47 a,b,d,e | 113 ± 11.8 c,d | 125 ± 18.0 e | <0.0001 |
| D4D | 6.74 ± 1.06 a,b | 6.91 ± 0.80 c,d | 7.80 ± 0.97 | 6.84 ± 0.70 e,f | 17.2 ± 3.86 a,c,e | 16.6 ± 3.66 b,d,f | 7.38 ± 2.98 | 7.33 ± 2.83 | <0.0001 |
| D5D | 52.7 ± 10.4 a | 52.3 ± 11.9 b | 68.6 ± 18.8 c,d | 50.7 ± 11.7 e | 64.9 ± 18.7 f | 95.3 ± 18.5 a,b,e,g,h | 36.1 ± 13.9 c,f,g | 39.8 ± 12.1 d,h | <0.0001 |
| D9D_C16 | 0.03 ± 0.01 a,b,c | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.01 d,e,f | 0.07 ± 0.04 a,d | 0.04 ± 0.01 | 0.07 ± 0.03 b,e | 0.07 ± 0.02 c,f | <0.0001 |
| D9D_C18 | 0.31 ± 0.06 a | 0.35 ± 0.19 | 0.25 ± 0.05 | 0.27 ± 0.07 | 0.29 ± 0.09 | 0.21 ± 0.08 a,b | 0.42 ± 0.24 a,b | 0.29 ± 0.06 | 0.0010 |
| D9D_total | 0.19 ± 0.03 | 0.22 ± 0.10 | 0.17 ± 0.03 | 0.17 ± 0.04 | 0.20 ± 0.06 | 0.14 ± 0.04 a | 0.23 ± 0.07 a | 0.18 ± 0.06 | 0.0390 |
| Iso-LA | - | - | 0.02 ± 0.00 | 0.02 ± 0.01 a | - | - | 0.01 ± 0.00 | 0.01 ± 0.01 a | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepionka, T.; Białek, M.; Czauderna, M.; Szlis, M.; Białek, A. Lipidomic Profile and Enzymes Activity in Hepatic Microsomes of Rats in Physiological and Pathological Conditions. Int. J. Mol. Sci. 2022, 23, 442. https://doi.org/10.3390/ijms23010442
Lepionka T, Białek M, Czauderna M, Szlis M, Białek A. Lipidomic Profile and Enzymes Activity in Hepatic Microsomes of Rats in Physiological and Pathological Conditions. International Journal of Molecular Sciences. 2022; 23(1):442. https://doi.org/10.3390/ijms23010442
Chicago/Turabian StyleLepionka, Tomasz, Małgorzata Białek, Marian Czauderna, Michał Szlis, and Agnieszka Białek. 2022. "Lipidomic Profile and Enzymes Activity in Hepatic Microsomes of Rats in Physiological and Pathological Conditions" International Journal of Molecular Sciences 23, no. 1: 442. https://doi.org/10.3390/ijms23010442
APA StyleLepionka, T., Białek, M., Czauderna, M., Szlis, M., & Białek, A. (2022). Lipidomic Profile and Enzymes Activity in Hepatic Microsomes of Rats in Physiological and Pathological Conditions. International Journal of Molecular Sciences, 23(1), 442. https://doi.org/10.3390/ijms23010442









