Pyranose Ring Puckering Thermodynamics for Glycan Monosaccharides Associated with Vertebrate Proteins
Abstract
1. Introduction
2. Results and Discussion
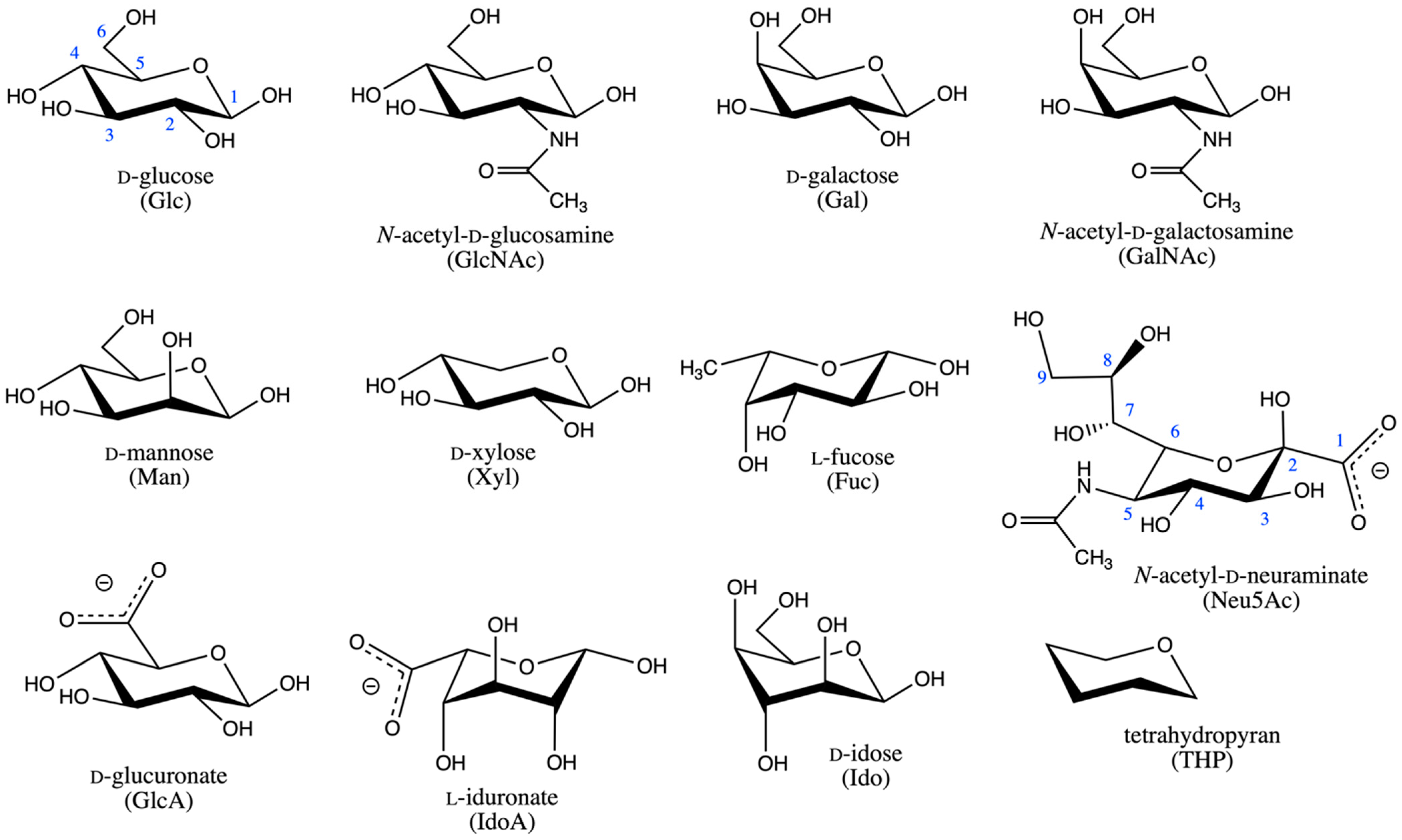
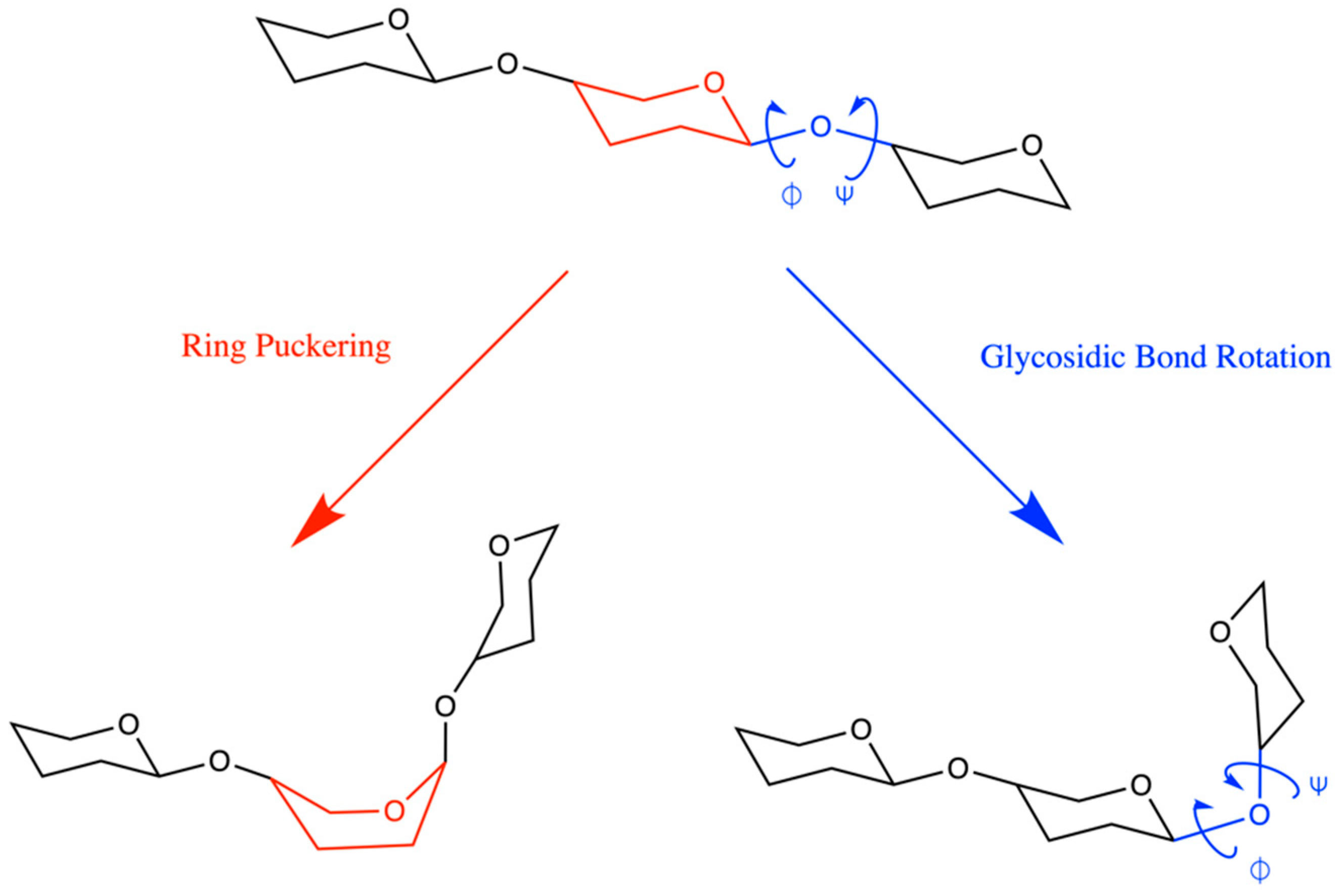
2.1. Reaction Coordinate and Sampling Approach
2.2. Extended System Adaptive Biasing Force (eABF) Sampling of the B-S (α1, α2) Reaction Coordinate
2.3. Using eABF-Computed ΔG(α1, α2) to Calculate Specific Ring Puckering Conformation Probabilities
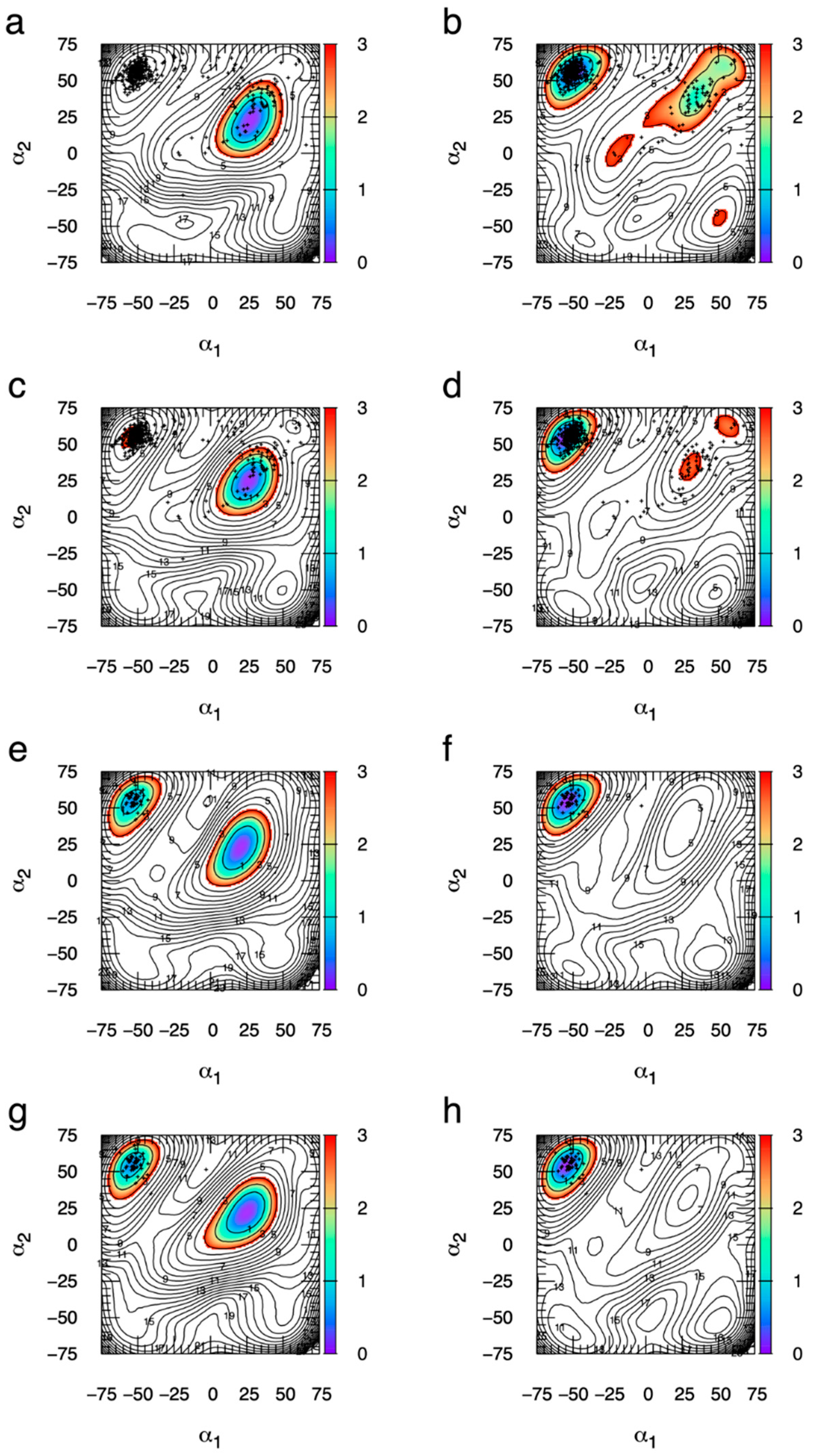
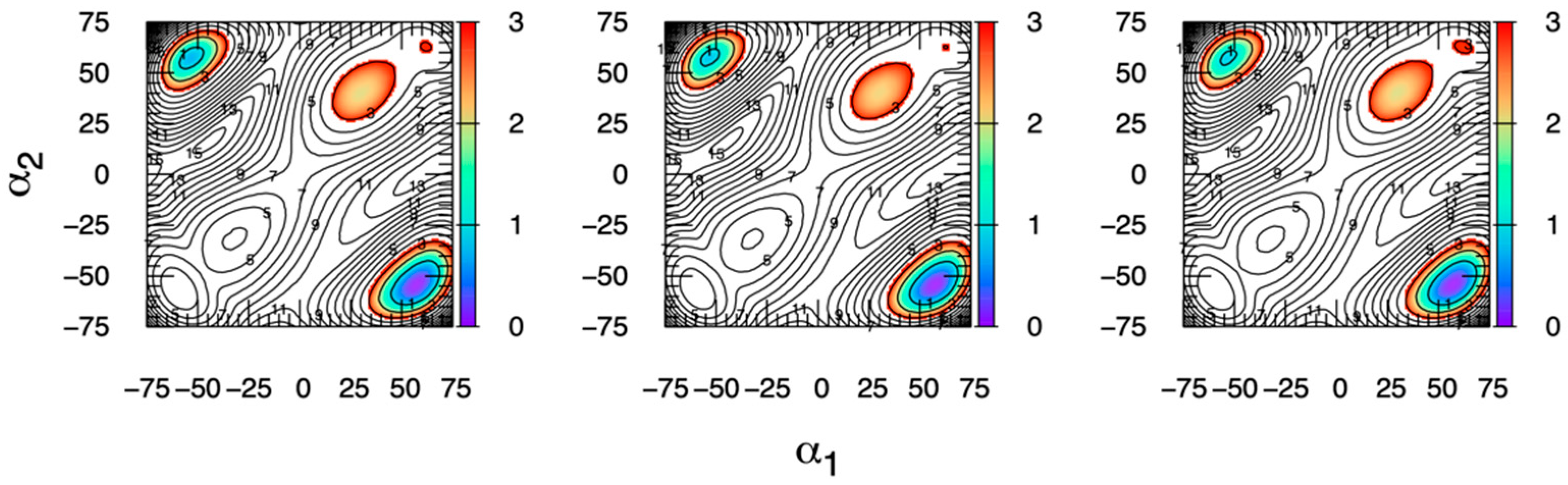
2.4. Ring Puckering Probabilities: Idose and Iduronate
2.5. Ring Puckering: ΔG(α1, α2) Minima for All Compounds
3. Materials and Methods
3.1. Force Field
3.2. System Construction
3.3. Molecular Dynamics Simulations
3.4. Molecular Dynamics Trajectory Analysis
3.5. Definition of 4C1, 1C4, 2SO, OS2, and Other Ring Puckering Conformations
- 4C1: 0° ≤ θ < 30°, 𝜙 = any
- Southern tropical: 30° ≤ θ < 60°, 𝜙 = any
- Equatorial: 60° ≤ θ < 120°, with specific conformations defined by,
- ○
- 3,OB: 0° ≤ 𝜙 < 15° or 345° ≤ 𝜙 < 360°
- ○
- 3S1: 15° ≤ 𝜙 < 45°
- ○
- B1,4: 45° ≤ 𝜙 < 75°
- ○
- 5S1: 75° ≤ 𝜙 < 105°
- ○
- 2,5B: 105° ≤ 𝜙 < 135°
- ○
- 2SO: 135° ≤ 𝜙 < 165°
- ○
- B3,O: 165° ≤ 𝜙 < 195°
- ○
- 1S3: 195° ≤ 𝜙 < 225°
- ○
- 1,4B: 225° ≤ 𝜙 < 255°
- ○
- 1S5: 255° ≤ 𝜙 < 285°
- ○
- B2,5: 285° ≤ 𝜙 < 315°
- ○
- OS2: 315° ≤ 𝜙 < 345°
- Northern tropical: 120° ≤ θ < 150°, 𝜙 = any
- 4C1: 150° ≤ θ ≤ 180°, 𝜙 = any
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| NC2D1 | CC3161 | CC3161 | CC3261 | 0.20 | 3 | 0.0 |
| CC312 | CC3163 | CC3161 | NC2D1 | 0.20 | 3 | 0.0 |
| OC3C61 | CC3163 | CC3161 | NC2D1 | 0.20 | 3 | 0.0 |
References
- Schnaar, R.L.; Kinoshita, T. Glycosphingolipids. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 125–135. [Google Scholar] [CrossRef]
- Seeberger, P.H. Monosaccharide Diversity. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017; pp. 19–30. [Google Scholar] [CrossRef]
- Stanley, P.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 99–111. [Google Scholar] [CrossRef]
- Brockhausen, I.; Stanley, P. O-GalNAc Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 113–123. [Google Scholar] [CrossRef]
- Lindahl, U.; Couchman, J.; Kimata, K.; Esko, J.D. Proteoglycans and Sulfated Glycosaminoglycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 207–221. [Google Scholar] [CrossRef]
- Varki, A.; Kornfeld, S. Historical Background and Overview. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 1–18. [Google Scholar] [CrossRef]
- Imberty, A.; Prestegard, J.H. Structural Biology of Glycan Recognition. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015; pp. 387–400. [Google Scholar] [CrossRef]
- Kumar, A.; Narayanan, V.; Sekhar, A. Characterizing Post-Translational Modifications and Their Effects on Protein Conformation Using NMR Spectroscopy. Biochemistry 2020, 59, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Radivojac, P. Post-translational modifications induce significant yet not extreme changes to protein structure. Bioinformatics 2012, 28, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Craveur, P.; Narwani, T.J.; Rebehmed, J.; de Brevern, A.G. Investigation of the impact of PTMs on the protein backbone conformation. Amino Acids 2019, 51, 1065–1079. [Google Scholar] [CrossRef]
- Kermani, A.A. A guide to membrane protein X-ray crystallography. FEBS J. 2021, 288, 5788–5804. [Google Scholar] [CrossRef]
- Woods, R.J. Predicting the Structures of Glycans, Glycoproteins, and Their Complexes. Chem. Rev. 2018, 118, 8005–8024. [Google Scholar] [CrossRef] [PubMed]
- Samsonov, S.A.; Pisabarro, M.T. Computational analysis of interactions in structurally available protein-glycosaminoglycan complexes. Glycobiology 2016, 26, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, E.K.; Vesenka, G.; Sihler, H.; Guvench, O. Efficient Construction of Atomic-Resolution Models of Non-Sulfated Chondroitin Glycosaminoglycan Using Molecular Dynamics Data. Biomolecules 2020, 10, 537. [Google Scholar] [CrossRef]
- Whitmore, E.K.; Martin, D.; Guvench, O. Constructing 3-Dimensional Atomic-Resolution Models of Nonsulfated Glycosaminoglycans with Arbitrary Lengths Using Conformations from Molecular Dynamics. Int. J. Mol. Sci. 2020, 21, 7699. [Google Scholar] [CrossRef]
- Bohne-Lang, A.; von der Lieth, C.-W. GlyProt: In silico glycosylation of proteins. Nucleic Acids Res. 2005, 33, W214–W219. [Google Scholar] [CrossRef]
- Singh, A.; Montgomery, D.; Xue, X.; Foley, B.L.; Woods, R.J. GAG Builder: A web-tool for modeling 3D structures of glycosaminoglycans. Glycobiology 2019, 29, 515–518. [Google Scholar] [CrossRef]
- Engelsen, S.B.; Hansen, P.I.; Perez, S. POLYS 2.0: An open source software package for building three-dimensional structures of polysaccharides. Biopolymers 2014, 101, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Kuttel, M.M.; Ståhle, J.; Widmalm, G. CarbBuilder: Software for building molecular models of complex oligo- and polysaccharide structures. J. Comput. Chem. 2016, 37, 2098–2105. [Google Scholar] [CrossRef]
- Clerc, O.; Deniaud, M.; Vallet, S.D.; Naba, A.; Rivet, A.; Perez, S.; Thierry-Mieg, N.; Ricard-Blum, S. MatrixDB: Integration of new data with a focus on glycosaminoglycan interactions. Nucleic Acids Res. 2019, 47, D376–D381. [Google Scholar] [CrossRef]
- Clerc, O.; Mariethoz, J.; Rivet, A.; Lisacek, F.; Pérez, S.; Ricard-Blum, S. A pipeline to translate glycosaminoglycan sequences into 3D models. Application to the exploration of glycosaminoglycan conformational space. Glycobiology 2019, 29, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Lee, J.; Qi, Y.; Kern, N.R.; Lee, H.S.; Jo, S.; Joung, I.; Joo, K.; Lee, J.; Im, W. CHARMM-GUI Glycan Modeler for modeling and simulation of carbohydrates and glycoconjugates. Glycobiology 2019, 29, 320–331. [Google Scholar] [CrossRef]
- Almond, A. Multiscale modeling of glycosaminoglycan structure and dynamics: Current methods and challenges. Curr. Opin. Struct. Biol. 2018, 50, 58–64. [Google Scholar] [CrossRef]
- Sattelle, B.M.; Shakeri, J.; Cliff, M.J.; Almond, A. Proteoglycans and their heterogeneous glycosaminoglycans at the atomic scale. Biomacromolecules 2015, 16, 951–961. [Google Scholar] [CrossRef]
- Frank, M. Conformational analysis of oligosaccharides and polysaccharides using molecular dynamics simulations. Methods Mol. Biol. 2015, 1273, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Widmalm, G. A perspective on the primary and three-dimensional structures of carbohydrates. Carbohydr. Res. 2013, 378, 123–132. [Google Scholar] [CrossRef]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; González-Outeiriño, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar] [CrossRef]
- Singh, A.; Tessier, M.B.; Pederson, K.; Wang, X.; Venot, A.P.; Boons, G.-J.; Prestegard, J.H.; Woods, R.J. Extension and validation of the GLYCAM force field parameters for modeling glycosaminoglycans. Can. J. Chem. 2016, 94, 927–935. [Google Scholar] [CrossRef]
- Pol-Fachin, L.; Rusu, V.H.; Verli, H.; Lins, R.D. GROMOS 53A6GLYC, an Improved GROMOS Force Field for Hexopyranose-Based Carbohydrates. J. Chem. Theory Comput. 2012, 8, 4681–4690. [Google Scholar] [CrossRef] [PubMed]
- Pol-Fachin, L.; Verli, H.; Lins, R.D. Extension and validation of the GROMOS 53A6GLYC parameter set for glycoproteins. J. Comput. Chem. 2014, 35, 2087–2095. [Google Scholar] [CrossRef]
- Hansen, H.S.; Hünenberger, P.H. A reoptimized GROMOS force field for hexopyranose-based carbohydrates accounting for the relative free energies of ring conformers, anomers, epimers, hydroxymethyl rotamers, and glycosidic linkage conformers. J. Comput. Chem. 2011, 32, 998–1032. [Google Scholar] [CrossRef]
- Plazinski, W.; Lonardi, A.; Hünenberger, P.H. Revision of the GROMOS 56A6CARBO force field: Improving the description of ring-conformational equilibria in hexopyranose-based carbohydrates chains. J. Comput. Chem. 2016, 37, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Panczyk, K.; Gaweda, K.; Drach, M.; Plazinski, W. Extension of the GROMOS 56a6CARBO/CARBO_R Force Field for Charged, Protonated, and Esterified Uronates. J. Phys. Chem. B 2018, 122, 3696–3710. [Google Scholar] [CrossRef]
- Damm, W.; Frontera, A.; Tirado-Rives, J.; Jorgensen, W.L. OPLS all-atom force field for carbohydrates. J. Comput. Chem. 1997, 18, 1955–1970. [Google Scholar] [CrossRef]
- Kony, D.; Damm, W.; Stoll, S.; van Gunsteren, W.F. An improved OPLS-AA force field for carbohydrates. J. Comput. Chem. 2002, 23, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Guvench, O.; Greene, S.N.; Kamath, G.; Brady, J.W.; Venable, R.M.; Pastor, R.W.; Mackerell, A.D., Jr. Additive empirical force field for hexopyranose monosaccharides. J. Comput. Chem. 2008, 29, 2543–2564. [Google Scholar] [CrossRef]
- Guvench, O.; Hatcher, E.R.; Venable, R.M.; Pastor, R.W.; MacKerell, A.D., Jr. CHARMM Additive All-Atom Force Field for Glycosidic Linkages between Hexopyranoses. J. Chem. Theory Comput. 2009, 5, 2353–2370. [Google Scholar] [CrossRef]
- Guvench, O.; Mallajosyula, S.S.; Raman, E.P.; Hatcher, E.; Vanommeslaeghe, K.; Foster, T.J.; Jamison, F.W., II; Mackerell, A.D., Jr. CHARMM additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate-protein modeling. J. Chem. Theory Comput. 2011, 7, 3162–3180. [Google Scholar] [CrossRef] [PubMed]
- Mallajosyula, S.S.; Guvench, O.; Hatcher, E.; MacKerell, A.D., Jr. CHARMM Additive All-Atom Force Field for Phosphate and Sulfate Linked to Carbohydrates. J. Chem. Theory Comput. 2012, 8, 759–776. [Google Scholar] [CrossRef]
- Sattelle, B.M.; Shakeri, J.; Almond, A. Does Microsecond Sugar Ring Flexing Encode 3D-Shape and Bioactivity in the Heparanome? Biomacromolecules 2013, 14, 1149–1159. [Google Scholar] [CrossRef]
- Sattelle, B.M.; Hansen, S.U.; Gardiner, J.; Almond, A. Free energy landscapes of iduronic acid and related monosaccharides. J. Am. Chem. Soc. 2010, 132, 13132–13134. [Google Scholar] [CrossRef] [PubMed]
- Sattelle, B.M.; Bose-Basu, B.; Tessier, M.; Woods, R.J.; Serianni, A.S.; Almond, A. Dependence of pyranose ring puckering on anomeric configuration: Methyl idopyranosides. J. Phys. Chem. B 2012, 116, 6380–6386. [Google Scholar] [CrossRef]
- Lesage, A.; Lelièvre, T.; Stoltz, G.; Hénin, J. Smoothed Biasing Forces Yield Unbiased Free Energies with the Extended-System Adaptive Biasing Force Method. J. Phys. Chem. B 2017, 121, 3676–3685. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Shao, X.; Chipot, C.; Cai, W. Extended Adaptive Biasing Force Algorithm. An On-the-Fly Implementation for Accurate Free-Energy Calculations. J. Chem. Theory Comput. 2016, 12, 3506–3513. [Google Scholar] [CrossRef]
- Plazinski, W.; Plazinska, A. Molecular dynamics simulations of hexopyranose ring distortion in different force fields. Pure Appl. Chemistry. Chim. Pure Appl. 2017, 89, 1283–1294. [Google Scholar] [CrossRef]
- Bose-Basu, B.; Zhang, W.; Kennedy, J.L.W.; Hadad, M.J.; Carmichael, I.; Serianni, A.S. 13C-Labeled Idohexopyranosyl Rings: Effects of Methyl Glycosidation and C6 Oxidation on Ring Conformational Equilibria. J. Org. Chem. 2017, 82, 1356–1370. [Google Scholar] [CrossRef]
- Lins, R.D.; Hünenberger, P.H. A new GROMOS force field for hexopyranose-based carbohydrates. J. Comput. Chem. 2005, 26, 1400–1412. [Google Scholar] [CrossRef]
- Panczyk, K.; Plazinski, W. Pyranose ring puckering in aldopentoses, ketohexoses and deoxyaldohexoses. A molecular dynamics study. Carbohydr. Res. 2018, 455, 62–70. [Google Scholar] [CrossRef]
- Guvench, O.; MacKerell, A.D., Jr. Automated conformational energy fitting for force-field development. J. Mol. Model. 2008, 14, 667–679. [Google Scholar] [CrossRef][Green Version]
- MacKerell, A.D., Jr.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef]
- MacKerell, A.D., Jr.; Feig, M.; Brooks, C.L., III. Improved treatment of the protein backbone in empirical force fields. J. Am. Chem. Soc. 2004, 126, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, P.E.; Mittal, J.; Feig, M.; Mackerell, A.D., Jr. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi(1) and chi(2) dihedral angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. [Google Scholar] [CrossRef]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef] [PubMed]
- Klauda, J.B.; Monje, V.; Kim, T.; Im, W. Improving the CHARMM Force Field for Polyunsaturated Fatty Acid Chains. J. Phys. Chem. B 2012, 116, 9424–9431. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J.A. General definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Dowd, M.K.; French, A.D.; Reilly, P.J. Modeling of aldopyranosyl ring puckering with MM3 (92). Carbohydr. Res. 1994, 264, 1–19. [Google Scholar] [CrossRef]
- Barducci, A.; Bussi, G.; Parrinello, M. Well-Tempered Metadynamics: A Smoothly Converging and Tunable Free-Energy Method. Phys. Rev. Lett. 2008, 100, 020603. [Google Scholar] [CrossRef]
- Barducci, A.; Bonomi, M.; Parrinello, M. Metadynamics. WIREs Comput. Mol. Sci. 2011, 1, 826–843. [Google Scholar] [CrossRef]
- Autieri, E.; Sega, M.; Pederiva, F.; Guella, G. Puckering free energy of pyranoses: A NMR and metadynamics-umbrella sampling investigation. J. Chem. Phys. 2010, 133, 095104. [Google Scholar] [CrossRef]
- Pickett, H.M.; Strauss, H.L. Conformational structure, energy, and inversion rates of cyclohexane and some related oxanes. J. Am. Chem. Soc. 1970, 92, 7281–7290. [Google Scholar] [CrossRef]
- Hansen, H.S.; Hünenberger, P.H. Using the local elevation method to construct optimized umbrella sampling potentials: Calculation of the relative free energies and interconversion barriers of glucopyranose ring conformers in water. J. Comput. Chem. 2010, 31, 1–23. [Google Scholar] [CrossRef]
- Boeyens, J.C.A.; Evans, D.G. Group theory of ring pucker. Acta Crystallogr. Sect. B 1989, 45, 577–581. [Google Scholar] [CrossRef]
- Sega, M.; Autieri, E.; Pederiva, F. Pickett angles and Cremer–Pople coordinates as collective variables for the enhanced sampling of six-membered ring conformations. Mol. Phys. 2011, 109, 141–148. [Google Scholar] [CrossRef]
- Babin, V.; Sagui, C. Conformational free energies of methyl-alpha-L-iduronic and methyl-beta-D-glucuronic acids in water. J. Chem. Phys. 2010, 132, 104108. [Google Scholar] [CrossRef]
- Alibay, I.; Bryce, R.A. Ring Puckering Landscapes of Glycosaminoglycan-Related Monosaccharides from Molecular Dynamics Simulations. J. Chem. Inf. Model. 2019, 59, 4729–4741. [Google Scholar] [CrossRef] [PubMed]
- Darve, E.; Rodríguez-Gómez, D.; Pohorille, A. Adaptive biasing force method for scalar and vector free energy calculations. J. Chem. Phys. 2008, 128, 144120. [Google Scholar] [CrossRef]
- Hénin, J.; Chipot, C. Overcoming free energy barriers using unconstrained molecular dynamics simulations. J. Chem. Phys. 2004, 121, 2904–2914. [Google Scholar] [CrossRef]
- Hénin, J.; Fiorin, G.; Chipot, C.; Klein, M.L. Exploring Multidimensional Free Energy Landscapes Using Time-Dependent Biases on Collective Variables. J. Chem. Theory Comput. 2010, 6, 35–47. [Google Scholar] [CrossRef]
- MacKerell, A.D., Jr.; Feig, M.; Brooks, C.L., III. Extending the treatment of backbone energetics in protein force fields: Limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem. 2004, 25, 1400–1415. [Google Scholar] [CrossRef] [PubMed]
- Angyal, S.J. The Composition and Conformation of Sugars in Solution. Angew. Chem. Int. Ed. Engl. 1969, 8, 157–166. [Google Scholar] [CrossRef]
- Spiwok, V.; Tvaroška, I. Conformational free energy surface of alpha-N-acetylneuraminic acid: An interplay between hydrogen bonding and solvation. J. Phys. Chem. B 2009, 113, 9589–9594. [Google Scholar] [CrossRef]
- Sattelle, B.M.; Almond, A. Is N-acetyl-D-glucosamine a rigid 4C1 chair? Glycobiology 2011, 21, 1651–1662. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Durell, S.R.; Brooks, B.R.; Ben-Naim, A. Solvent-induced forces between two hydrophilic groups. J. Phys. Chem. 1994, 98, 2198–2202. [Google Scholar] [CrossRef]
- Beglov, D.; Roux, B. Finite representation of an infinite bulk system: Solvent boundary potential for computer simulations. J. Chem. Phys. 1994, 100, 9050–9063. [Google Scholar] [CrossRef]
- Venable, R.M.; Luo, Y.; Gawrisch, K.; Roux, B.; Pastor, R.W. Simulations of anionic lipid membranes: Development of interaction-specific ion parameters and validation using NMR data. J. Phys. Chem. B 2013, 117, 10183–10192. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Brooks, C.L., III; MacKerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Steinbach, P.J.; Brooks, B.R. New spherical-cutoff methods for long-range forces in macromolecular simulation. J. Comput. Chem. 1994, 15, 667–683. [Google Scholar] [CrossRef]
- Shirts, M.R.; Mobley, D.L.; Chodera, J.D.; Pande, V.S. Accurate and efficient corrections for missing dispersion interactions in molecular simulations. J. Phys. Chem. B 2007, 111, 13052–13063. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of Cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kollman, P.A. Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Kubo, R.; Toda, M.; Hashitume, N. Statistical Physics II: Nonequilibrium Statistical Mechanics, 2nd ed.; Springer: New York, NY, USA, 1991. [Google Scholar]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Feller, S.E.; Zhang, Y.H.; Pastor, R.W.; Brooks, B.R. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Hénin, J. Fast and accurate multidimensional free energy integration. J. Chem. Theory Comput. 2021, 17, 6789–6798. [Google Scholar] [CrossRef] [PubMed]
- Fiorin, G.; Klein, M.L.; Hénin, J. Using collective variables to drive molecular dynamics simulations. Mol. Phys. 2013, 111, 3345–3362. [Google Scholar] [CrossRef]
- Eastman, P.; Swails, J.; Chodera, J.D.; McGibbon, R.T.; Zhao, Y.; Beauchamp, K.A.; Wang, L.P.; Simmonett, A.C.; Harrigan, M.P.; Stern, C.D.; et al. OpenMM 7: Rapid development of high performance algorithms for molecular dynamics. PLoS Comput. Biol. 2017, 13, e1005659. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Mallajosyula, S.S.; MacKerell, A.D. Influence of Solvent and Intramolecular Hydrogen Bonding on the Conformational Properties of O-Linked Glycopeptides. J. Phys. Chem. B 2011, 115, 11215–11229. [Google Scholar] [CrossRef]
- Faller, C.E.; Guvench, O. Sulfation and cation effects on the conformational properties of the glycan backbone of chondroitin sulfate disaccharides. J. Phys. Chem. B 2015, 119, 6063–6073. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Angles d’Ortoli, T.; Säwén, E.; Jana, M.; Widmalm, G.; MacKerell, A.D. Delineating the conformational flexibility of trisaccharides from NMR spectroscopy experiments and computer simulations. Phys. Chem. Chem. Phys. 2016, 18, 18776–18794. [Google Scholar] [CrossRef]
- Ng, C.; Premnath, P.N.; Guvench, O. Rigidity and flexibility in the tetrasaccharide linker of proteoglycans from atomic-resolution molecular simulation. J. Comput. Chem. 2017, 38, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Rönnols, J.; Engström, O.; Schnupf, U.; Säwén, E.; Brady, J.W.; Widmalm, G. Inter-residual Hydrogen Bonding in Carbohydrates Unraveled by NMR Spectroscopy and Molecular Dynamics Simulations. Chem. Bio. Chem. 2019, 20, 2519–2528. [Google Scholar] [CrossRef]
- Lutsyk, V.; Plazinski, W. Conformational Properties of Glycosaminoglycan Disaccharides: A Molecular Dynamics Study. J. Phys. Chem. B 2021, 125, 10900–10916. [Google Scholar] [CrossRef]
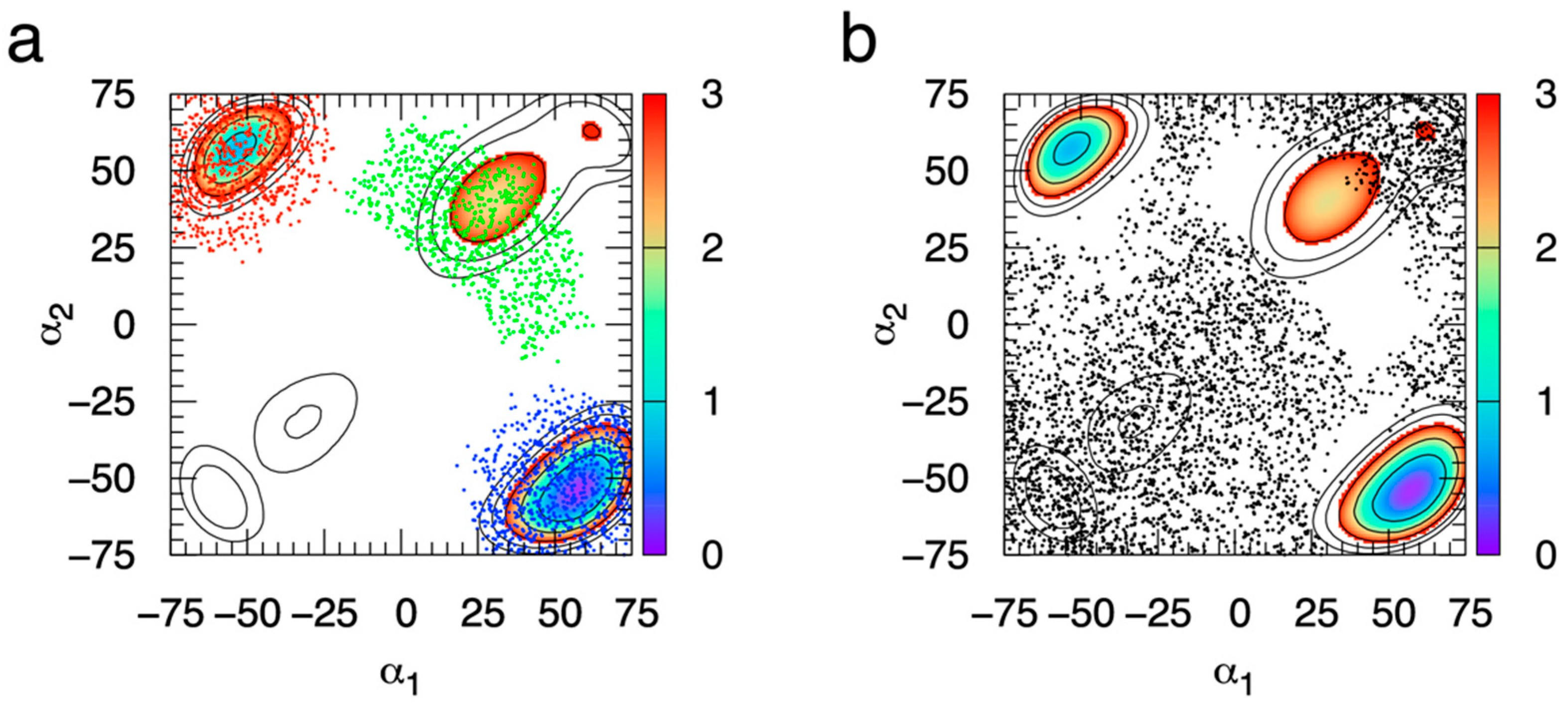

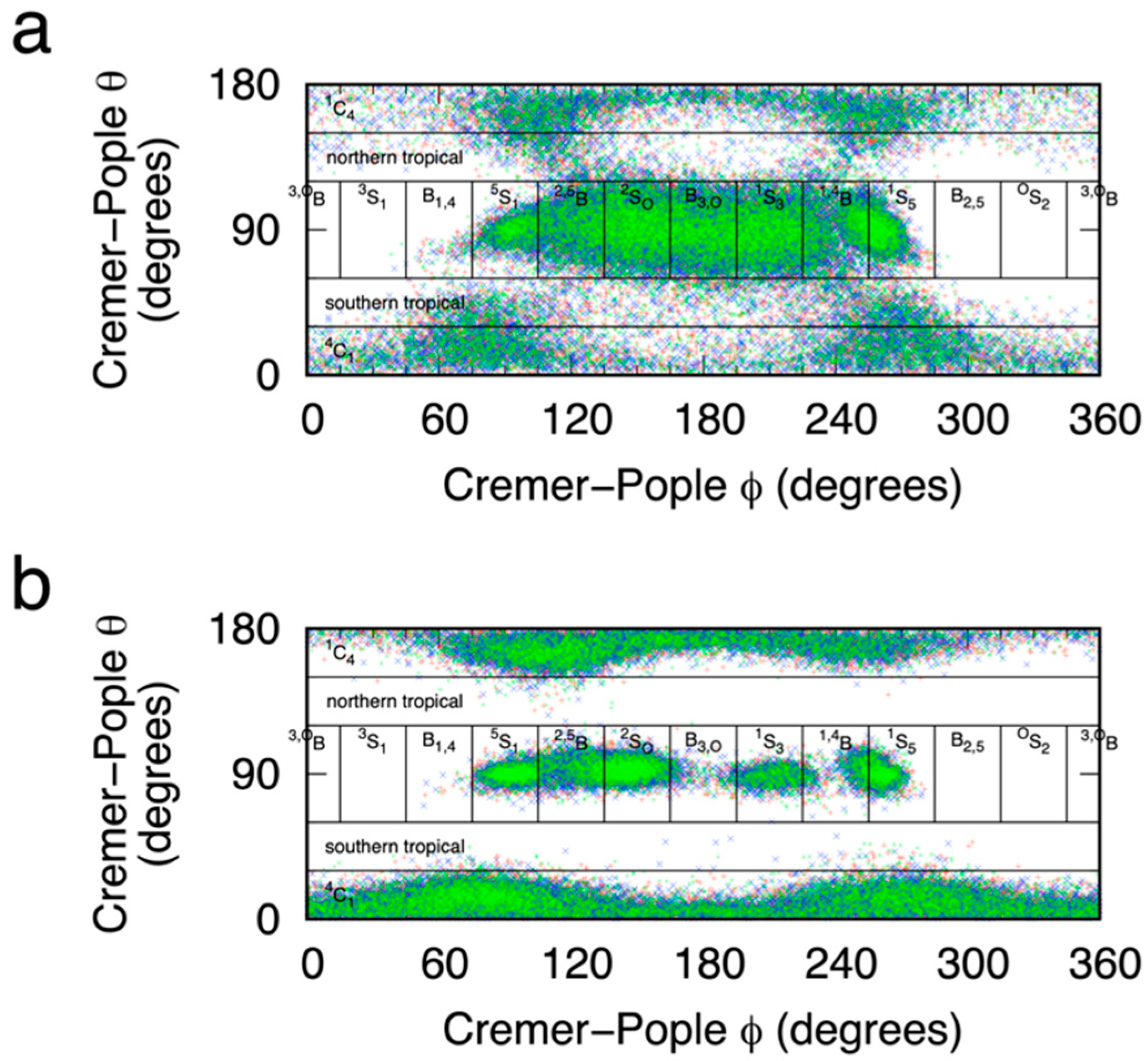

| Compound | eABF Simulations 1 | CMAP-Biased Simulations 1 | Experimental [46] |
|---|---|---|---|
| αIdo | 17.6:82.4 (1.8) | 15.1:84.9 (1.9) | 18:82 |
| MeαIdo | 16.1:83.9 (0.7) | 18.1:81.9 (2.1) | 42:58 |
| βIdo | 97.1:2.9 (0.7) | 90.7:9.3 (1.7) | 82:18 |
| MeβIdo | 82.8:17.2 (2.2) | 76.6:23.4 (1.5) | 74:26 |
| MeαIdoA | 82.9:17.1 (1.4) | 77.2:22.8 (1.0) | 61:39 |
| Compound | ΔG+,− 1 | ΔG−,+ 1 | ΔG−,− 1 | ΔG+,+ 1 | Major Pucker Conformation(s) 2 |
|---|---|---|---|---|---|
| αGlc | 0 | 5.47 (0.04) | 6.05 (0.04) | 8.46 (0.05) | 4C1 |
| MeαGlc | 0 | 6.83 (0.06) | 7.06 (0.05) | 9.39 (0.06) | 4C1 |
| βGlc | 0 | 8.43 (0.19) | 5.44 (0.04) | 7.03 (0.01) | 4C1 |
| MeβGlc | 0 | 8.27 (0.08) | 5.38 (0.03) | 6.91 (0.02) | 4C1 |
| αGlcNAc | 0 | 5.01 (0.05) | 6.11 (0.07) | 7.19 (0.05) | 4C1 |
| MeαGlcNAc | 0 | 6.19 (0.11) | 7.08 (0.04) | 8.20 (0.04) | 4C1 |
| βGlcNAc | 0 | 4.95 (0.09) | 4.01 (0.02) | 6.60 (0.05) | 4C1 |
| MeβGlcNAc | 0 | 4.73 (0.06) | 2.83 (0.09) | 6.85 (0.04) | 4C1 > 1S5 |
| αGal | 0 | 4.25 (0.06) | 6.11 (0.04) | 8.73 (0.06) | 4C1 |
| MeαGal | 0 | 5.62 (0.01) | 7.35 (0.04) | 8.74 (0.03) | 4C1 |
| βGal | 0 | 6.56 (0.09) | 5.80 (0.01) | 8.35 (0.04) | 4C1 |
| MeβGal | 0 | 7.10 (0.06) | 6.63 (0.04) | 8.29 (0.04) | 4C1 |
| αGalNAc | 0 | 3.09 (0.09) | 7.00 (0.07) | 7.72 (0.09) | 4C1 |
| MeαGalNAc | 0 | 4.33 (0.06) | 8.21 (0.05) | 7.77 (0.06) | 4C1 |
| βGalNAc | 0 | 2.47 (0.05) | 3.66 (0.07) | 7.03 (0.04) | 4C1 > 1C4 |
| MeβGalNAc | 0 | 2.90 (0.04) | 3.58 (0.01) | 6.87 (0.05) | 4C1 > 1C4 |
| αMan | 0 | 5.26 (0.6) | 6.83 (0.02) | 9.99 (0.06) | 4C1 |
| MeαMan | 0 | 5.82 (0.03) | 7.54 (0.06) | 10.74 (0.03) | 4C1 |
| βMan | 0 | 6.89 (0.05) | 7.27 (0.03) | 8.98 (0.04) | 4C1 |
| MeβMan | 0 | 6.20 (0.05) | 6.24 (0.05) | 8.14 (0.01) | 4C1 |
| αXyl | 0 | 2.17 (0.01) | 6.03 (0.02) | 6.00 (0.05) | 4C1 > 1C4 |
| MeαXyl | 0 | 3.60 (0.02) | 6.95 (0.02) | 6.73 (0.02) | 4C1 |
| βXyl | 0 | 3.77 (0.05) | 5.25 (0.01) | 3.24 0.01) | 4C1 |
| MeβXyl | 0 | 4.19 (0.00) | 4.91 (0.02) | 3.51 (0.01) | 4C1 |
| αFuc | 3.87 (0.06) | 0 | 8.11 (0.06) | 5.97 (0.02) | 1C4 |
| MeαFuc | 5.14 (0.03) | 0 | 8.15 (0.01) | 7.10 (0.02) | 1C4 |
| βFuc | 6.48 (0.03) | 0 | 8.10 (0.03) | 5.73 (0.01) | 1C4 |
| MeβFuc | 7.01 (0.04) | 0 | 7.86 (0.02) | 6.54 (0.04) | 1C4 |
| αNeu5Ac | 2.71 (0.09) | 0 | 2.79 (0.02) | 1.42 (0.07) | 2C5 > 3SO > 5C2 ≅ 4,OB |
| MeαNeu5Ac | 4.89 (0.29) | 0 | 6.37 (0.10) | 2.71 (0.24) | 2C5 > 3SO |
| βNeu5Ac | 6.79 (0.09) | 0 | 7.18 (0.10) | 4.01 (0.03) | 1C4 |
| MeβNeu5Ac | 8.76 (0.08) | 0 | 8.88 (0.10) | 5.93 (0.11) | 1C4 |
| αGlcA | 0 | 4.53 (0.12) | 5.64 (0.04) | 6.28 (0.05) | 4C1 |
| MeαGlcA | 0 | 5.80 (0.04) | 6.75 (0.03) | 7.20 (0.03) | 4C1 |
| βGlcA | 0 | 5.96 (0.11) | 5.69 (0.01) | 4.31 (0.06) | 4C1 |
| MeβGlcA | 0 | 8.30 (0.09) | 6.49 (0.02) | 6.22 (0.04) | 4C1 |
| αIdoA | 0 | 0.31 (0.09) | 3.84 (0.04) | 1.74 (0.02) | 4C1 ≅ 1C4 > 2SO |
| MeαIdoA | 0 | 0.77 (0.05) | 3.23 (0.01) | 2.04 (0.02) | 4C1 > 1C4 > 2SO |
| βIdoA | 2.29 (0.03) | 0 | 4.47 (0.08) | 3.81 (0.03) | 1C4 > 4C1 |
| MeβIdoA | 3.29 (0.06) | 0 | 4.31 (0.05) | 3.53 (0.06) | 1C4 |
| αIdo | 0.73 (0.08) | 0 | 0.88 (0.08) | 3.20 (0.06) | 1C4 > 4C1 ≅ OS2 |
| MeαIdo | 0.82 (0.04) | 0 | 1.00 (0.02) | 2.81 (0.03) | 1C4 > 4C1 ≅ OS2 > 3S1 |
| βIdo | 0 | 2.15 (0.10) | 4.17 (0.08) | 5.30 (0.09) | 4C1 > 1C4 |
| MeβIdo | 0 | 1.12 (0.09) | 3.49 (0.04) | 5.00 (0.09) | 4C1 > 1C4 |
| THP | 0 | 0.03 (0.01) | 5.14 (0.01) | 5.13 (0.00) | 4C1 = 1C4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guvench, O.; Martin, D.; Greene, M. Pyranose Ring Puckering Thermodynamics for Glycan Monosaccharides Associated with Vertebrate Proteins. Int. J. Mol. Sci. 2022, 23, 473. https://doi.org/10.3390/ijms23010473
Guvench O, Martin D, Greene M. Pyranose Ring Puckering Thermodynamics for Glycan Monosaccharides Associated with Vertebrate Proteins. International Journal of Molecular Sciences. 2022; 23(1):473. https://doi.org/10.3390/ijms23010473
Chicago/Turabian StyleGuvench, Olgun, Devon Martin, and Megan Greene. 2022. "Pyranose Ring Puckering Thermodynamics for Glycan Monosaccharides Associated with Vertebrate Proteins" International Journal of Molecular Sciences 23, no. 1: 473. https://doi.org/10.3390/ijms23010473
APA StyleGuvench, O., Martin, D., & Greene, M. (2022). Pyranose Ring Puckering Thermodynamics for Glycan Monosaccharides Associated with Vertebrate Proteins. International Journal of Molecular Sciences, 23(1), 473. https://doi.org/10.3390/ijms23010473





