Polyurethane Foam Rafts Supported In Vitro Cultures of Rindera graeca Roots for Enhanced Production of Rinderol, Potent Proapoptotic Naphthoquinone Compound
Abstract
1. Introduction
2. Results and Discussion
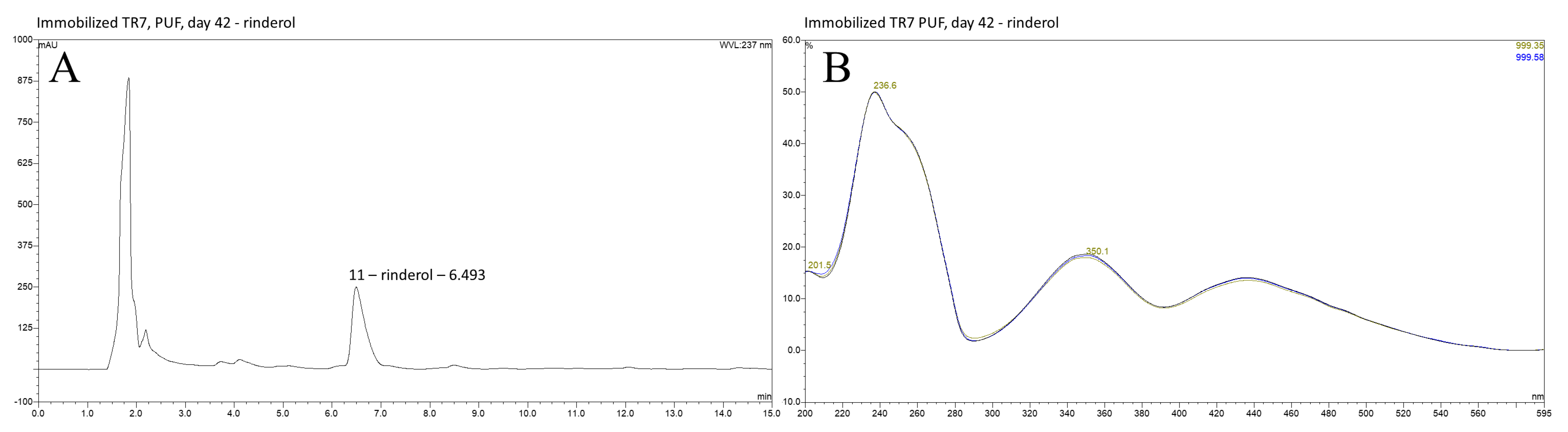
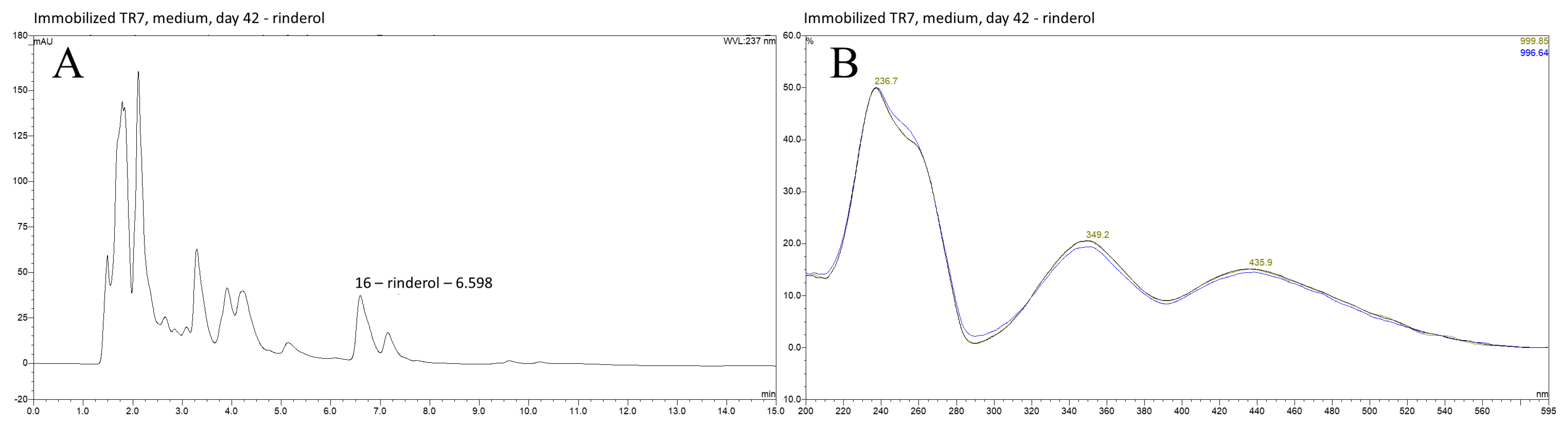
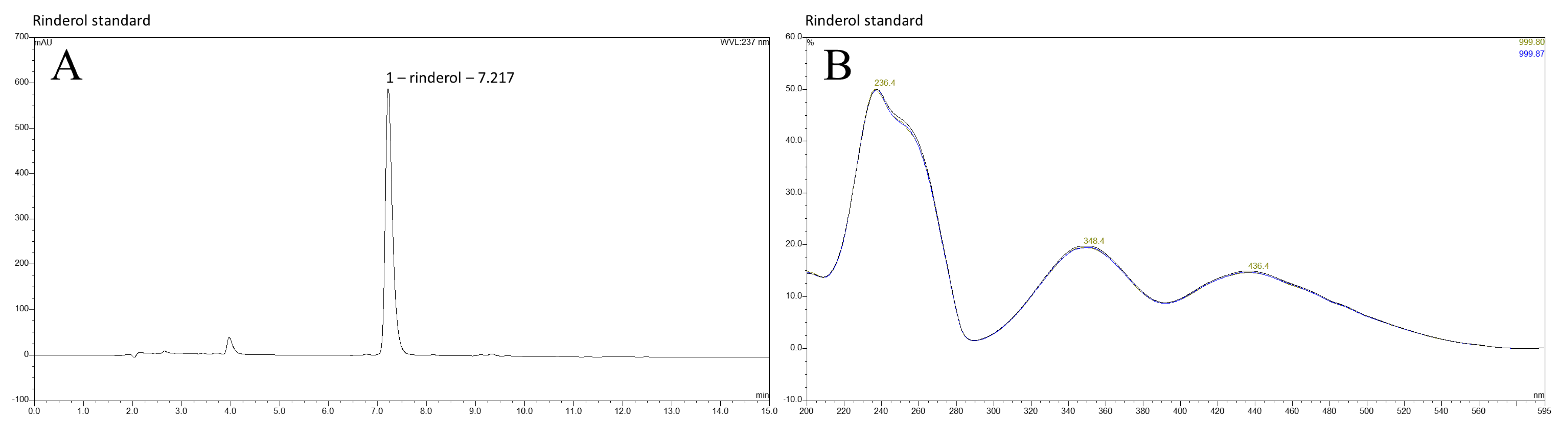
2.1. HPLC DAD Analysis of R. graeca In Vitro Root Cultures
2.2. Effect of Root Immobilization on Biomass Growth
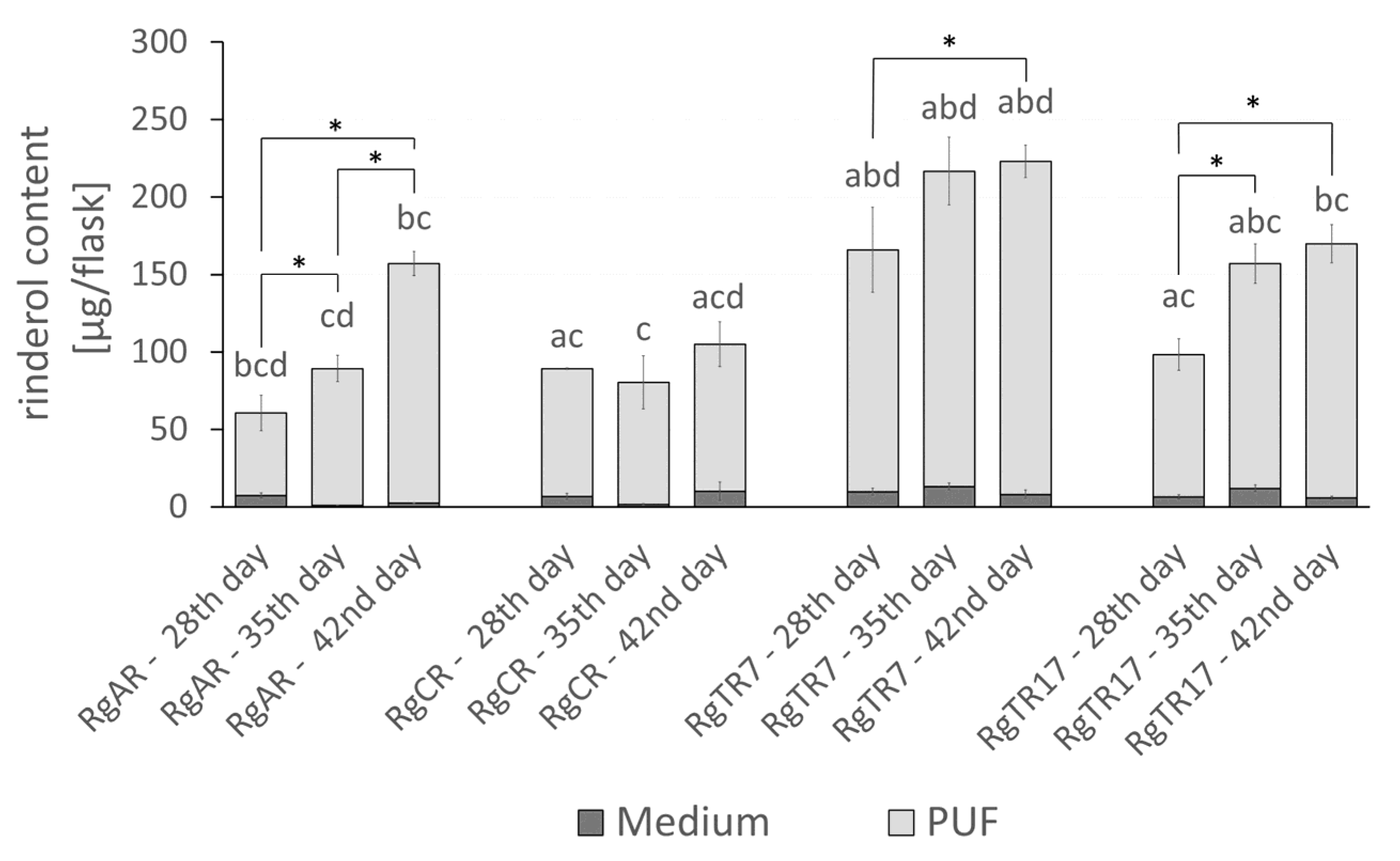
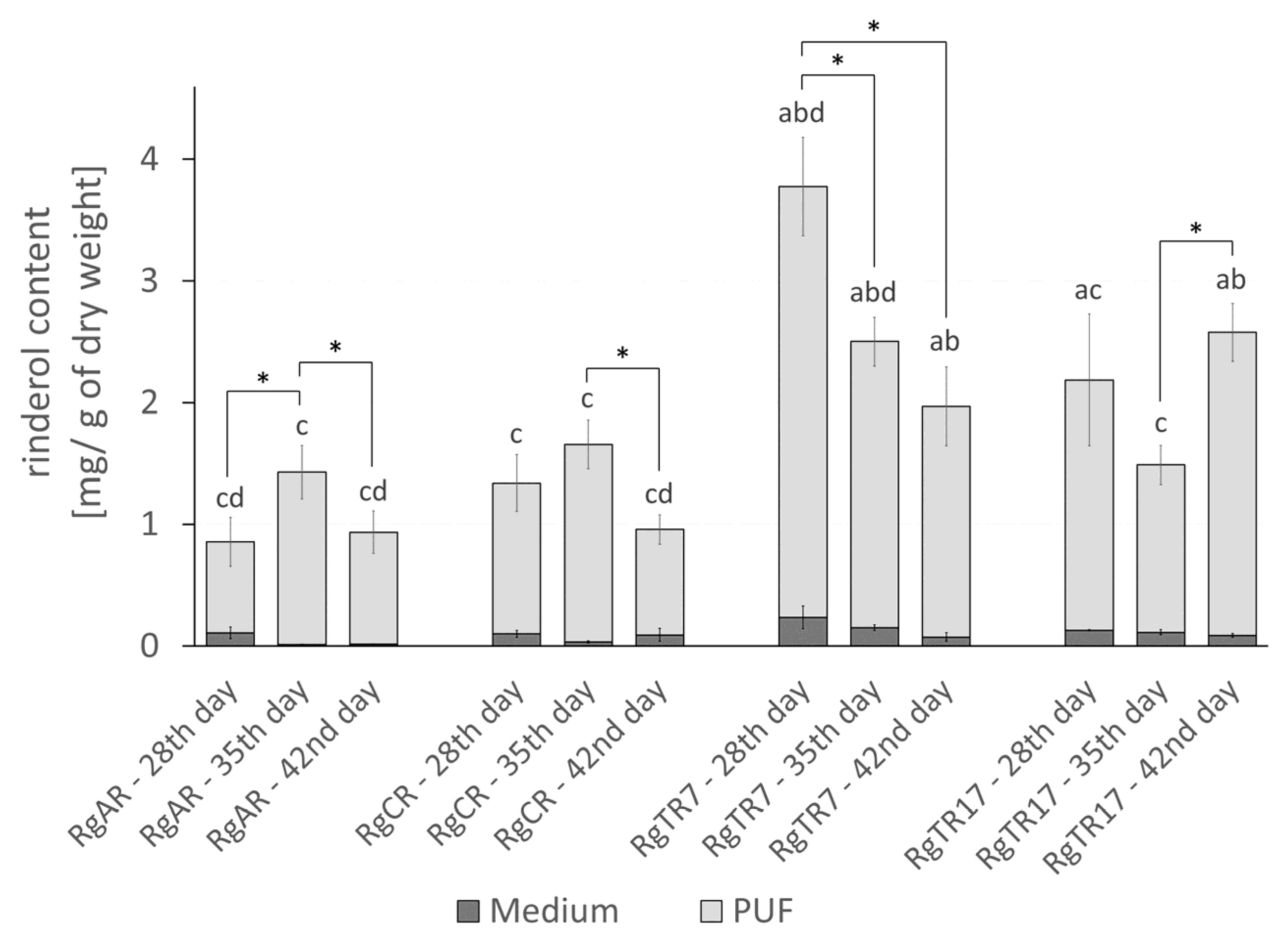
2.3. Effect of Root Immobilization on Rinderol Production
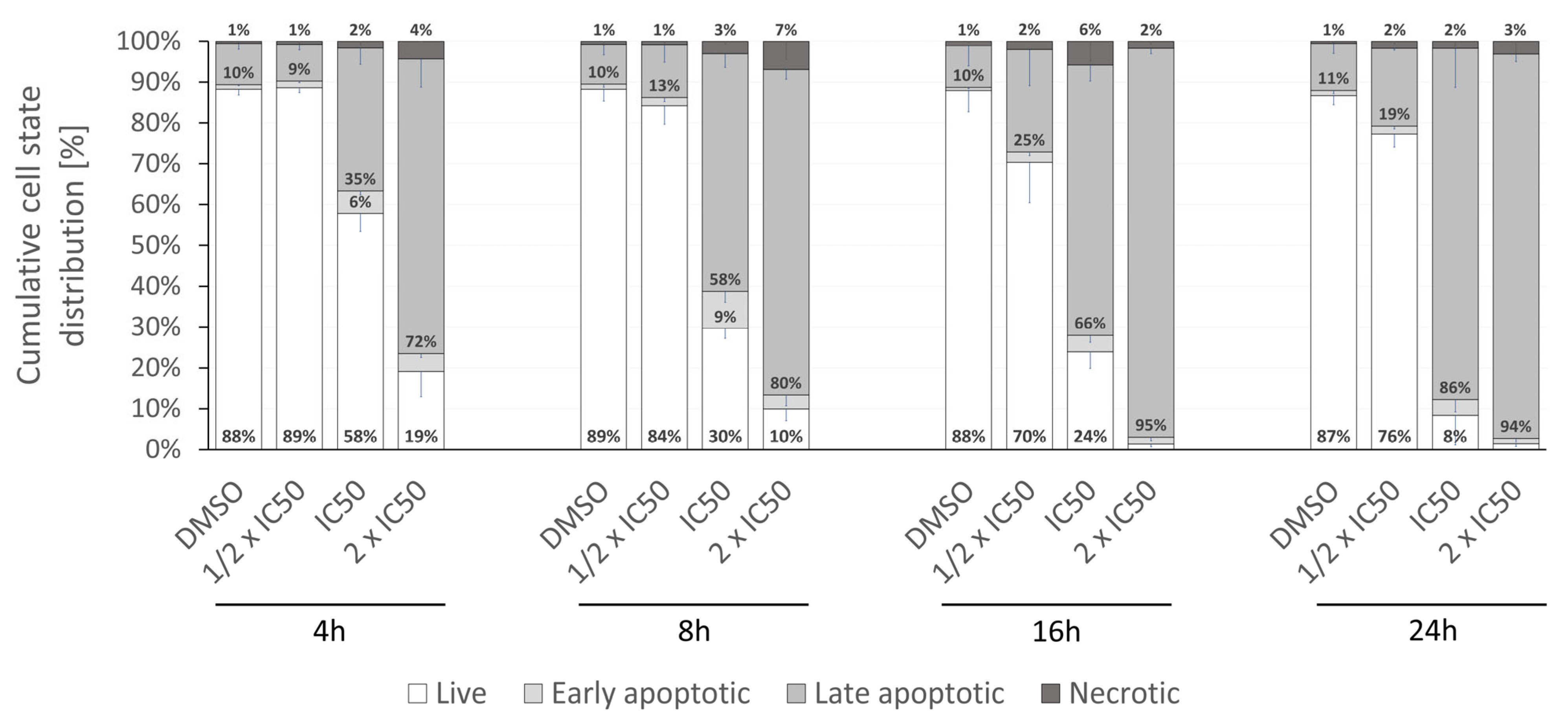
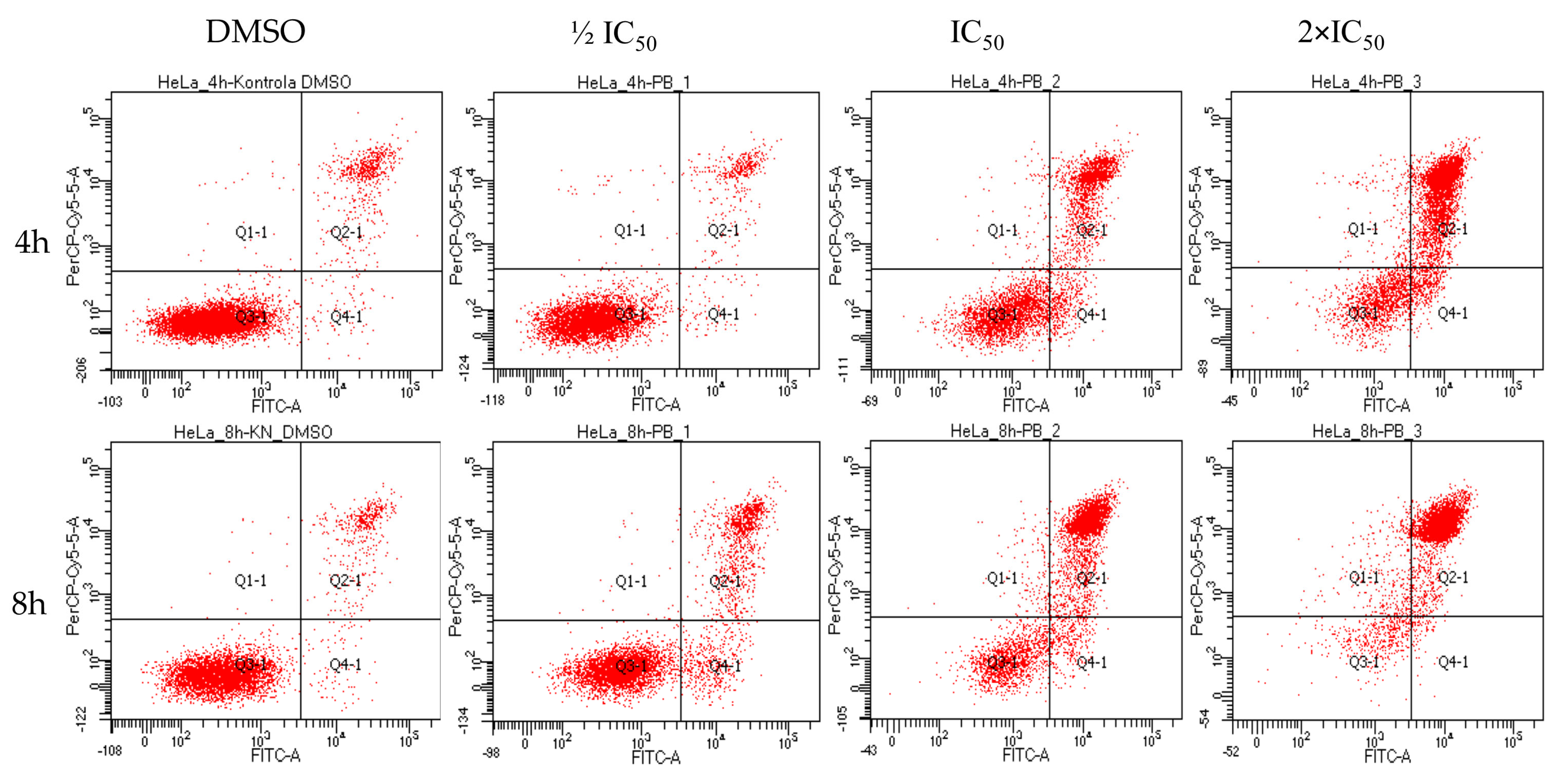
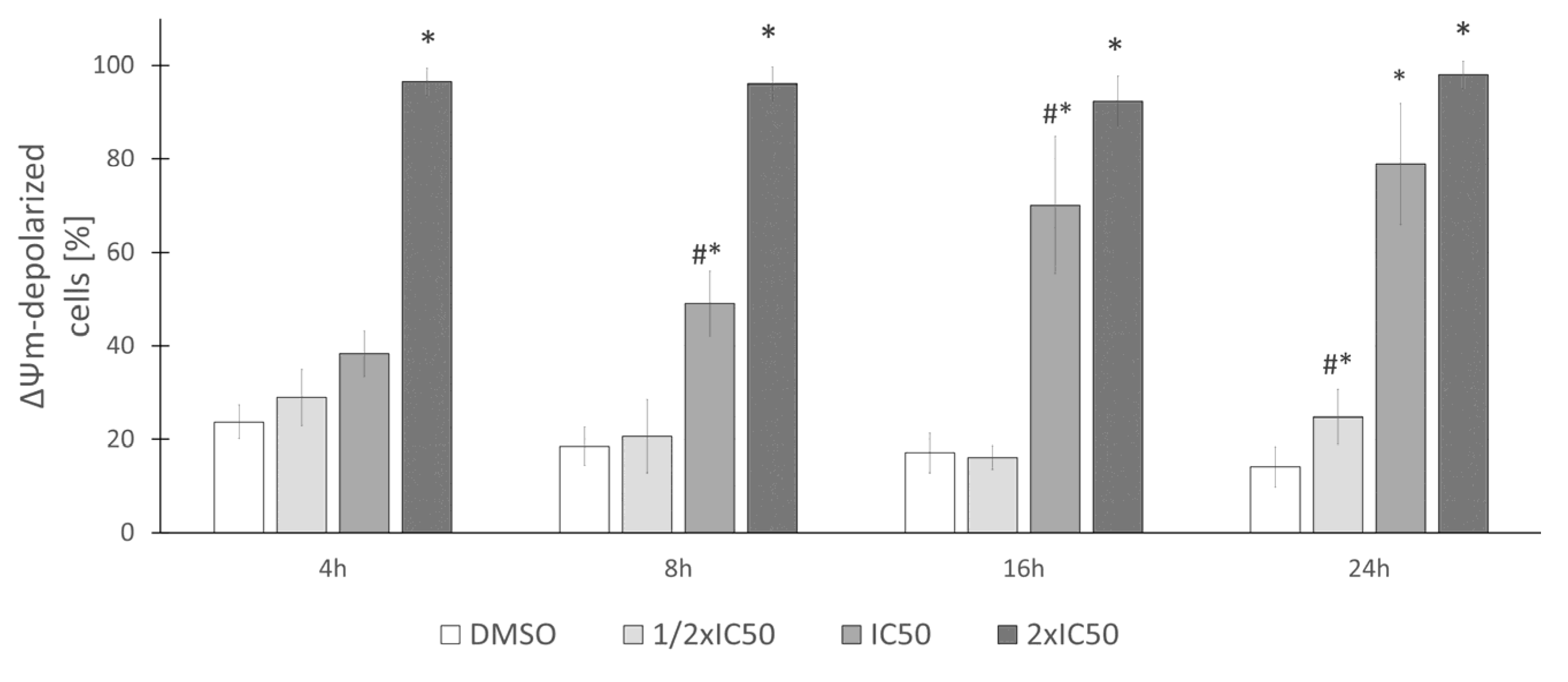
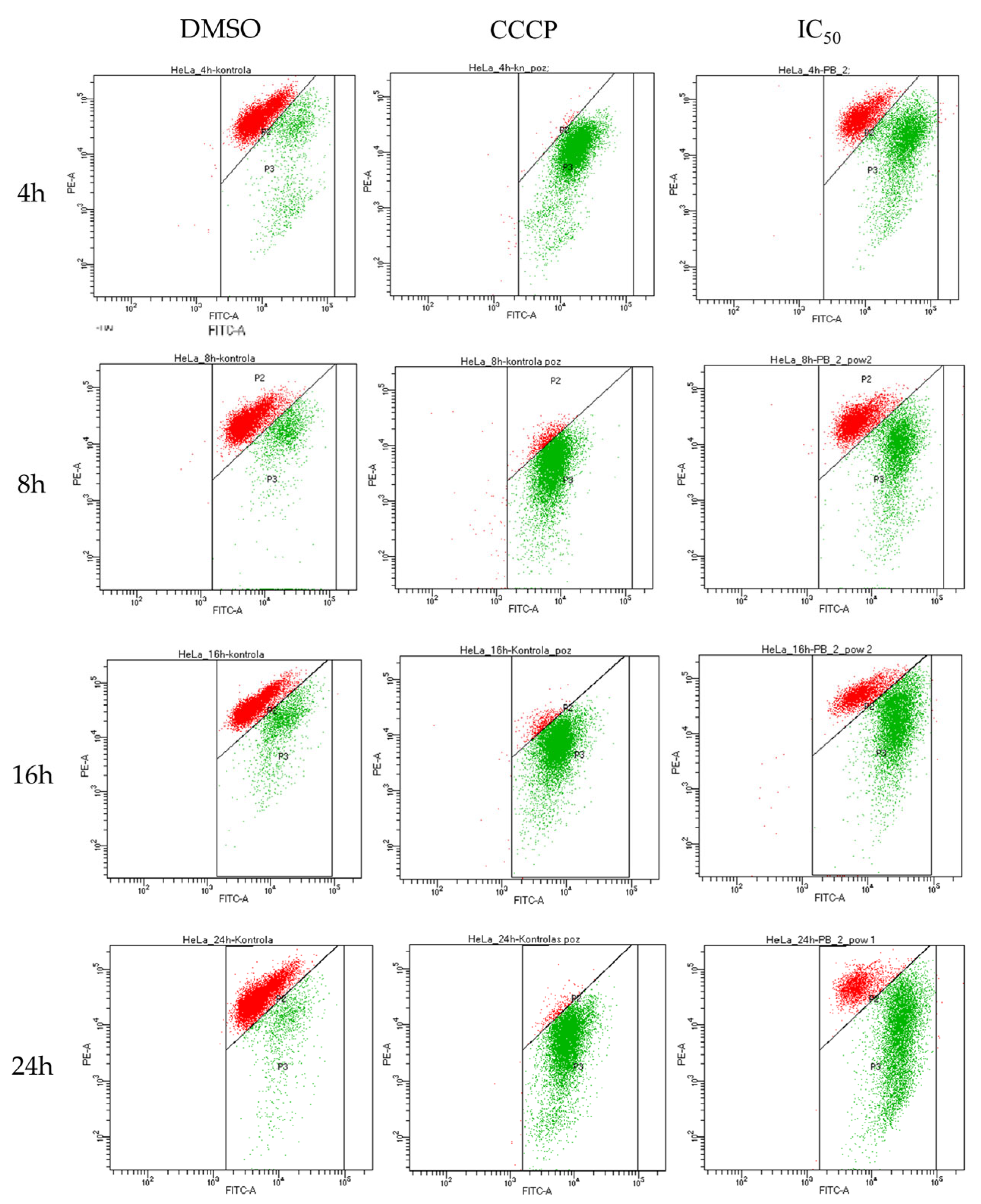
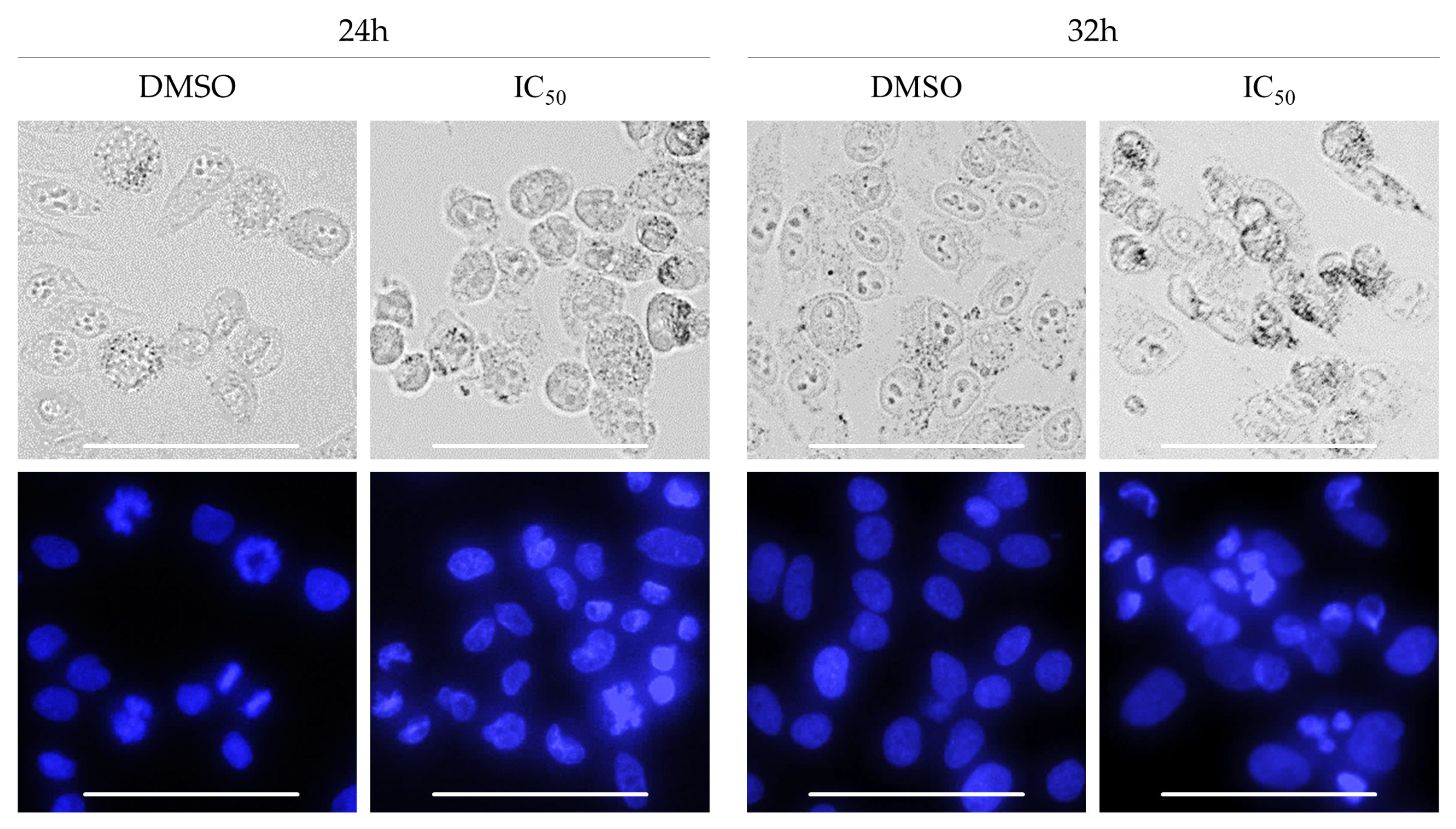
2.4. Biological Activity of Rinderol
3. Materials and Methods
3.1. Plant Material
3.2. Root Immobilization
3.3. Extraction Procedure
3.4. Isolation of Rinderol and HPLC-DAD-UV-Vis Analysis
3.5. Cancer Cell Line Culture
3.6. Cytotoxicity Assay
3.7. Flow Cytometry
3.8. Apoptosis Assay by Annexin V/Propidium Iodide (PI)
3.9. Mitochondrial Membrane Potential (ΔΨm) Assay
3.10. Morphological Analysis of Cells Nuclei
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.W.-H.; Vederas, J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of Plant Secondary Metabolites: A Historical Perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Karuppusamy, S. A Review on Trends in Production of Secondary Metabolites from Higher Plants by in Vitro Tissue, Organ and Cell Cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Plant Cell Culture Strategies for the Production of Natural Products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Smetanska, I. Production of Secondary Metabolites Using Plant Cell Cultures. Adv. Biochem. Eng. Biotechnol. 2008, 111, 187–228. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Qiu, W.; Tiwari, S.; Bhargava, R.; Gao, W.; Xing, B. Consumer-Grade Polyurethane Foam Functions as a Large and Selective Absorption Sink for Bisphenol A in Aqueous Media. J. Mater. Chem. A 2015, 3, 8870–8881. [Google Scholar] [CrossRef]
- Han, J.; Cao, Z.; Qiu, W.; Gao, W.; Hu, J.; Xing, B. Probing the Specificity of Polyurethane Foam as a ‘Solid-Phase Extractant’: Extractability-Governing Molecular Attributes of Lipophilic Phenolic Compounds. Talanta 2017, 172, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Jeziorek, M.; Damianakos, H.; Kawiak, A.; Laudy, A.E.; Zakrzewska, K.; Sykłowska-Baranek, K.; Chinou, I.; Pietrosiuk, A. Bioactive Rinderol and Cynoglosol Isolated from Cynoglossum Columnae Ten. in Vitro Root Culture. Ind. Crops Prod. 2019, 137, 446–452. [Google Scholar] [CrossRef]
- Graikou, K.; Damianakos, H.; Ganos, C.; Sykłowska-Baranek, K.; Jeziorek, M.; Pietrosiuk, A.; Roussakis, C.; Chinou, I. Chemical Profile and Screening of Bioactive Metabolites of Rindera graeca (A. DC.) Bois. & Heldr. (Boraginaceae) In Vitro Cultures. Plants 2021, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Liu, D.; Li, Y.-H.; Chen, X.-Q.; Luo, K.; Zhang, Y.-M.; Li, R.-T. Naphthoquinones from Onosma Paniculatum with Potential Anti-Inflammatory Activity. Planta Med. 2017, 83, 631–635. [Google Scholar] [CrossRef]
- Bentebibel, S.; Moyano, E.; Palazón, J.; Cusidó, R.M.; Bonfill, M.; Eibl, R.; Piñol, M.T. Effects of Immobilization by Entrapment in Alginate and Scale-up on Paclitaxel and Baccatin III Production in Cell Suspension Cultures of Taxus Baccata. Biotechnol. Bioeng. 2005, 89, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, M.J.C.; Smith, J.I.; Robins, R.J. Factors Affecting the Immobilisation of Plant Cells on Reticulated Polyurethane Foam Particles. Appl. Microbiol. Biotechnol. 1987, 26, 28–35. [Google Scholar] [CrossRef]
- Yuan, Q.; Xu, H.; Hu, Z. Two-Phase Culture for Enhanced Alkaloid Synthesis and Release in a New Airlift Reactor by Catharanthus Roseus. Biotechnol. Tech. 1999, 13, 107–109. [Google Scholar] [CrossRef]
- Taya, M.; Yoyama, A.; Kondo, O.; Kobayashi, T.; Matsui, C. Growth Characteristics of Plant Hairy Roots and Their Cultures in Bioreactors. J. Chem. Eng. Jpn. 1989, 22, 84–89. [Google Scholar] [CrossRef]
- Thakore, D.; Srivastava, A.K.; Sinha, A.K. Mass Production of Ajmalicine by Bioreactor Cultivation of Hairy Roots of Catharanthus Roseus. Biochem. Eng. J. 2017, 119, 84–91. [Google Scholar] [CrossRef]
- Kawka, M.; Pilarek, M.; Sykłowska-Baranek, K.; Pietrosiuk, A. In Situ Extraction Approach for Enhancement of Secondary Metabolites Production in Rindera graeca Hairy Root Cultures. In Proceedings of the 13th Warsaw International Medical Congress, Warsaw, Poland, 11–14 May 2017. [Google Scholar]
- Leva, A.R.; Petruccelli, R.; Rinaldi, L.M.R. Somaclonal Variation in Tissue Culture: A Case Study with Olive. In Recent Advances in Plant In Vitro Culture; BoD: Norderstedt, Germany, 2012. [Google Scholar] [CrossRef]
- Brodelius, P. The Potential Role of Immobilization in Plant Cell Biotechnology. Trends Biotechnol. 1985, 3, 280–285. [Google Scholar] [CrossRef]
- Valdiani, A.; Hansen, O.K.; Nielsen, U.B.; Johannsen, V.K.; Shariat, M.; Georgiev, M.I.; Omidvar, V.; Ebrahimi, M.; Tavakoli Dinanai, E.; Abiri, R. Bioreactor-Based Advances in Plant Tissue and Cell Culture: Challenges and Prospects. Crit. Rev. Biotechnol. 2018, 1–15. [Google Scholar] [CrossRef]
- Vaněk, T.; Valterová, I.; Pospíšilová, R.; Vaisar, T. The Effect of Immobilization on the Course of Biotransformation Reactions by Plant Cells. Biotechnol. Tech. 1994, 8, 289–294. [Google Scholar] [CrossRef]
- Taticek, R.A.; Moo-Young, M.; Legge, R.L. The Scale-up of Plant Cell Culture: Engineering Considerations. Plant Cell Tissue Organ Cult. 1991, 24, 139–158. [Google Scholar] [CrossRef]
- Asada, M.; Shuler, M.L. Stimulation of Ajmalicine Production and Excretion from Catharanthus Roseus: Effects of Adsorption in Situ, Elicitors and Alginate Immobilization. Appl. Microbiol. Biotechnol. 1989, 30, 475–481. [Google Scholar] [CrossRef]
- Gilleta, F.; Roisin, C.; Fliniaux, M.; Jacquin–Dubreuil, A.; Barbotin, J.; Nava–Saucedo, J. Immobilization of Nicotiana tabacum plant cell suspensions within calcium alginate gel beads for the production of enhanced amounts of scopolin. Enzym. Microb. Technol. 2000, 26, 229–234. [Google Scholar] [CrossRef]
- Kim, D.J.; Chang, H.N. Enhanced Shikonin Production from Lithospermum Erythrorhizon by in Situ Extraction and Calcium Alginate Immobilization. Biotechnol. Bioeng. 1990, 36, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Komaraiah, P.; Ramakrishna, S.V.; Reddanna, P.; Kavi Kishor, P.B. Enhanced Production of Plumbagin in Immobilized Cells of Plumbago Rosea by Elicitation and in Situ Adsorption. J. Biotechnol. 2003, 101, 181–187. [Google Scholar] [CrossRef]
- Cai, Z.; Kastell, A.; Knorr, D.; Smetanska, I. Exudation: An Expanding Technique for Continuous Production and Release of Secondary Metabolites from Plant Cell Suspension and Hairy Root Cultures. Plant Cell Rep. 2012, 31, 461–477. [Google Scholar] [CrossRef]
- Woodley, J.M.; Bisschops, M.; Straathof, A.J.J.; Ottens, M. Future Directions for In-Situ Product Removal (ISPR). J. Chem. Technol. Biotechnol. 2008, 83, 121–123. [Google Scholar] [CrossRef]
- Pan, J.; Hirai, K.I.; Simamura, E.; Koyama, J.; Shimada, H.; Kuwabara, S. Mitochondrial Damage by a New Antitumour Agent Furanonaphthoquinone Derivative in Human Cervical Cancer HeLa Cells. Microscopy 1997, 46, 181–187. [Google Scholar] [CrossRef]
- Kretschmer, N.; Deutsch, A.; Durchschein, C.; Rinner, B.; Stallinger, A.; Higareda-Almaraz, J.C.; Scheideler, M.; Lohberger, B.; Bauer, R. Comparative Gene Expression Analysis in WM164 Melanoma Cells Revealed That β-β-Dimethylacrylshikonin Leads to ROS Generation, Loss of Mitochondrial Membrane Potential, and Autophagy Induction. Molecules 2018, 23, 2823. [Google Scholar] [CrossRef]
- Mao, X.; Rong Yu, C.; Hua Li, W.; Xin Li, W. Induction of Apoptosis by Shikonin through a ROS/JNK-Mediated Process in Bcr/Abl-Positive Chronic Myelogenous Leukemia (CML) Cells. Cell Res. 2008, 18, 879–888. [Google Scholar] [CrossRef]
- Wiench, B.; Eichhorn, T.; Paulsen, M.; Efferth, T. Shikonin Directly Targets Mitochondria and Causes Mitochondrial Dysfunction in Cancer Cells. Available online: https://www.hindawi.com/journals/ecam/2012/726025/ (accessed on 13 April 2020).
- Yang, J.-T.; Li, Z.-L.; Wu, J.-Y.; Lu, F.-J.; Chen, C.-H. An Oxidative Stress Mechanism of Shikonin in Human Glioma Cells. PLoS ONE 2014, 9, e94180. [Google Scholar] [CrossRef]
- Liu, B.; Jin, J.; Zhang, Z.; Zuo, L.; Jiang, M.; Xie, C. Shikonin Exerts Antitumor Activity by Causing Mitochondrial Dysfunction in Hepatocellular Carcinoma through PKM2-AMPK-PGC1α Signaling Pathway. Biochem. Cell Biol. Biochim. Biol. Cell. 2019, 97, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Stallinger, A.; Kretschmer, N.; Kleinegger, F.; Brvar, L.; Liegl-Atzwanger, B.; Prokesch, A.; Durchschein, C.; Bauer, R.; Deutsch, A.; Rinner, B. β,β-Dimethylacrylshikonin Induces Apoptosis in Melanoma Cell Lines by NOXA Upregulation. J. Nat. Prod. 2020, 83, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, L.; Li, L.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. P53-Mediated Cell Cycle Arrest and Apoptosis Induced by Shikonin via a Caspase-9-Dependent Mechanism in Human Malignant Melanoma A375-S2 Cells. J. Pharmacol. Sci. 2004, 94, 166–176. [Google Scholar] [CrossRef]
- Froeling, F.E.M.; Swamynathan, M.M.; Deschênes, A.; Chio, I.I.C.; Brosnan, E.; Yao, M.A.; Alagesan, P.; Lucito, M.; Li, J.; Chang, A.-Y.; et al. Bioactivation of Napabucasin Triggers Reactive Oxygen Species-Mediated Cancer Cell Death. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 7162–7174. [Google Scholar] [CrossRef]
- Silvers, M.A.; Deja, S.; Singh, N.; Egnatchik, R.A.; Sudderth, J.; Luo, X.; Beg, M.S.; Burgess, S.C.; DeBerardinis, R.J.; Boothman, D.A.; et al. The NQO1 Bioactivatable Drug, β-Lapachone, Alters the Redox State of NQO1+ Pancreatic Cancer Cells, Causing Perturbation in Central Carbon Metabolism. J. Biol. Chem. 2017, 292, 18203–18216. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Jin, A.; Sun, J.; Cui, X.; Yang, Y.; Chen, L.; Lin, Z. Clinicopathological Implications of NQO1 Overexpression in the Prognosis of Pancreatic Adenocarcinoma. Oncol. Lett. 2017, 13, 2996–3002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oh, E.-T.; Kim, J.; Kim, J.M.; Kim, S.J.; Lee, J.-S.; Hong, S.-S.; Goodwin, J.; Ruthenborg, R.J.; Jung, M.G.; Lee, H.-J.; et al. NQO1 Inhibits Proteasome-Mediated Degradation of HIF-1α. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Oh, E.-T.; Park, H.J. Implications of NQO1 in Cancer Therapy. BMB Rep. 2015, 48, 609–617. [Google Scholar] [CrossRef]
- Löcken, H.; Clamor, C.; Müller, K. Napabucasin and Related Heterocycle-Fused Naphthoquinones as STAT3 Inhibitors with Antiproliferative Activity against Cancer Cells. J. Nat. Prod. 2018, 81, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Syklowska, K.; Graikou, K.; Damianakos, H.; Jeziorek, M.; Chinou, I. Phenolic Compounds from in Vitro Cultures of Rindera graeca Boiss. & Feldr. Planta Med. 2013, 79. [Google Scholar] [CrossRef]
- Syklowska, K.; Kuźma, Ł.; Chinou, I.; Kongel, M.; Jeziorek, M. Establishment of Rindera graeca Transgenic Root Culture as a Source of Shikonin Derivatives. Planta Med. 2008, 74. [Google Scholar] [CrossRef]
- Gupta, P.K.; Durzan, D.J. Shoot Multiplication from Mature Trees of Douglas-Fir (Pseudotsuga Menziesii) and Sugar Pine (Pinus Lambertiana). Plant Cell Rep. 1985, 4, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid Colorimetric Assay for Cell Growth and Survival. Modifications to the Tetrazolium Dye Procedure Giving Improved Sensitivity and Reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a Tetrazolium-Based Semiautomated Colorimetric Assay: Assessment of Chemosensitivity Testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Méry, B.; Guy, J.-B.; Vallard, A.; Espenel, S.; Ardail, D.; Rodriguez-Lafrasse, C.; Rancoule, C.; Magné, N. In Vitro Cell Death Determination for Drug Discovery: A Landscape Review of Real Issues. J. Cell Death 2017, 10. [Google Scholar] [CrossRef]
- Fallahi-Sichani, M.; Honarnejad, S.; Heiser, L.M.; Gray, J.W.; Sorger, P.K. Metrics Other than Potency Reveal Systematic Variation in Responses to Cancer Drugs. Nat. Chem. Biol. 2013, 9, 708–714. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawka, M.; Bubko, I.; Koronkiewicz, M.; Gruber-Bzura, B.; Graikou, K.; Chinou, I.; Jeziorek, M.; Pietrosiuk, A.; Sykłowska-Baranek, K. Polyurethane Foam Rafts Supported In Vitro Cultures of Rindera graeca Roots for Enhanced Production of Rinderol, Potent Proapoptotic Naphthoquinone Compound. Int. J. Mol. Sci. 2022, 23, 56. https://doi.org/10.3390/ijms23010056
Kawka M, Bubko I, Koronkiewicz M, Gruber-Bzura B, Graikou K, Chinou I, Jeziorek M, Pietrosiuk A, Sykłowska-Baranek K. Polyurethane Foam Rafts Supported In Vitro Cultures of Rindera graeca Roots for Enhanced Production of Rinderol, Potent Proapoptotic Naphthoquinone Compound. International Journal of Molecular Sciences. 2022; 23(1):56. https://doi.org/10.3390/ijms23010056
Chicago/Turabian StyleKawka, Mateusz, Irena Bubko, Mirosława Koronkiewicz, Beata Gruber-Bzura, Konstantia Graikou, Ioanna Chinou, Małgorzata Jeziorek, Agnieszka Pietrosiuk, and Katarzyna Sykłowska-Baranek. 2022. "Polyurethane Foam Rafts Supported In Vitro Cultures of Rindera graeca Roots for Enhanced Production of Rinderol, Potent Proapoptotic Naphthoquinone Compound" International Journal of Molecular Sciences 23, no. 1: 56. https://doi.org/10.3390/ijms23010056
APA StyleKawka, M., Bubko, I., Koronkiewicz, M., Gruber-Bzura, B., Graikou, K., Chinou, I., Jeziorek, M., Pietrosiuk, A., & Sykłowska-Baranek, K. (2022). Polyurethane Foam Rafts Supported In Vitro Cultures of Rindera graeca Roots for Enhanced Production of Rinderol, Potent Proapoptotic Naphthoquinone Compound. International Journal of Molecular Sciences, 23(1), 56. https://doi.org/10.3390/ijms23010056






