The Mechanisms of Thin Filament Assembly and Length Regulation in Muscles
Abstract
1. Introduction
2. Thin Filament Structure
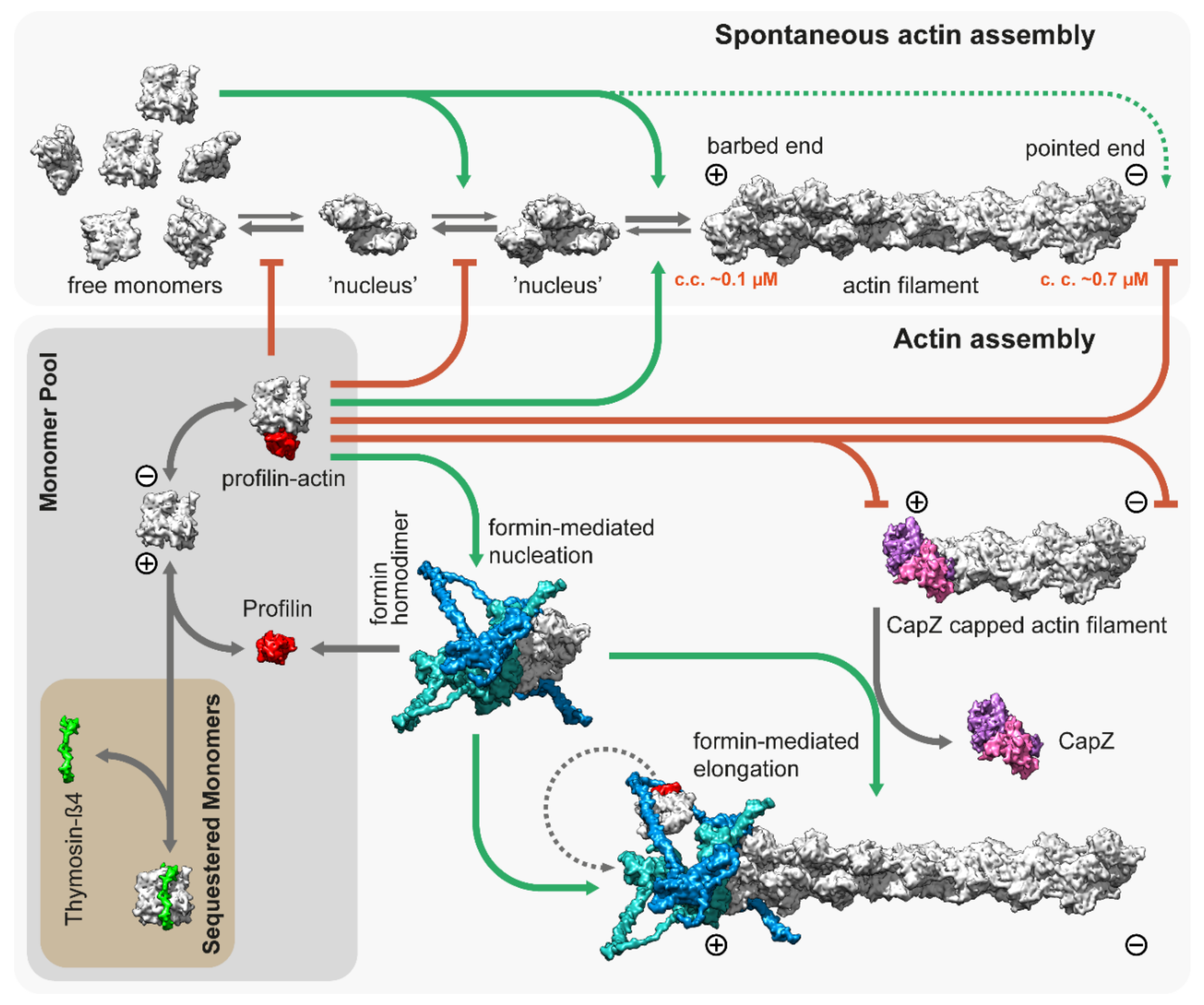
3. Thin Filament Assembly
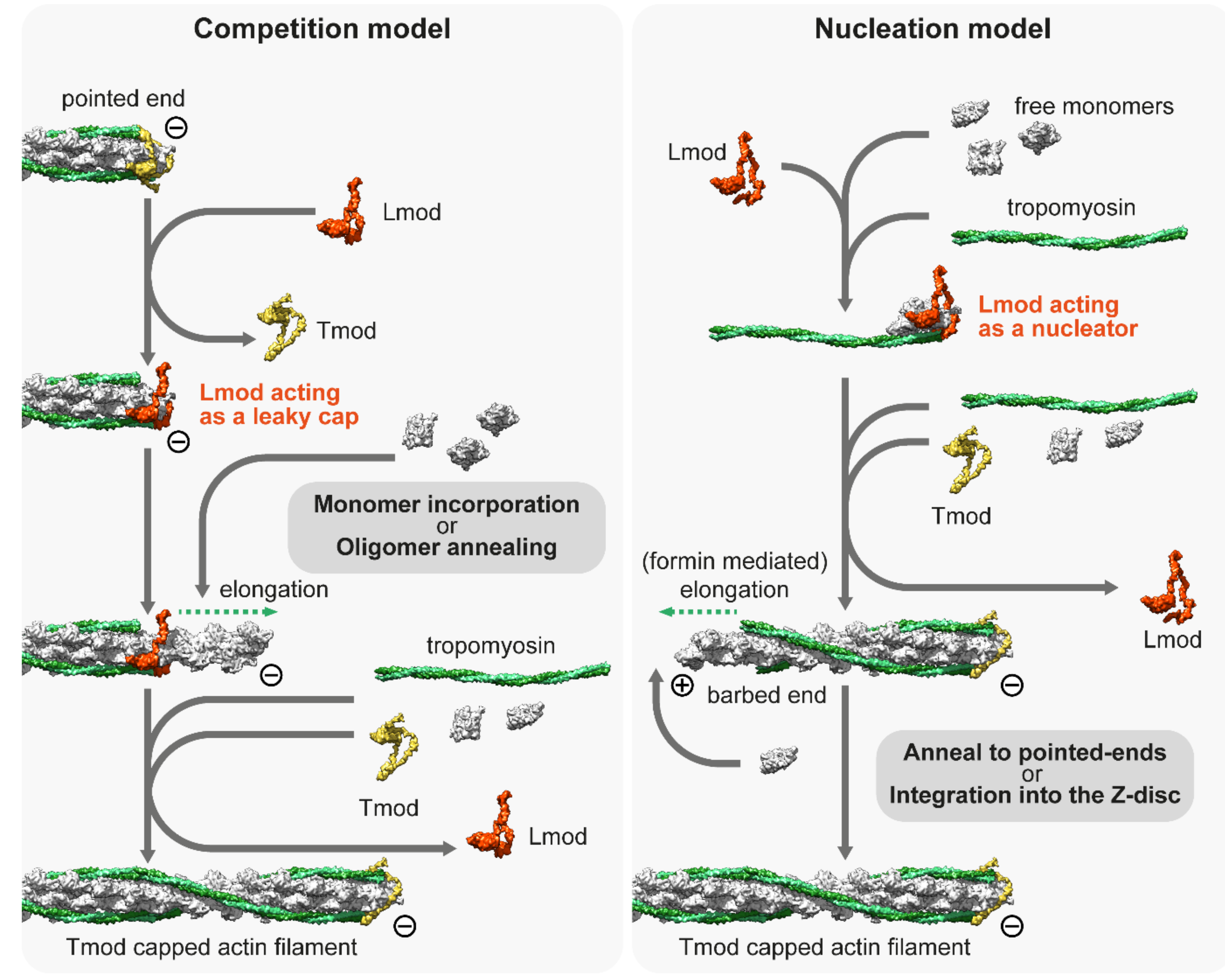
4. Thin Filament Elongation
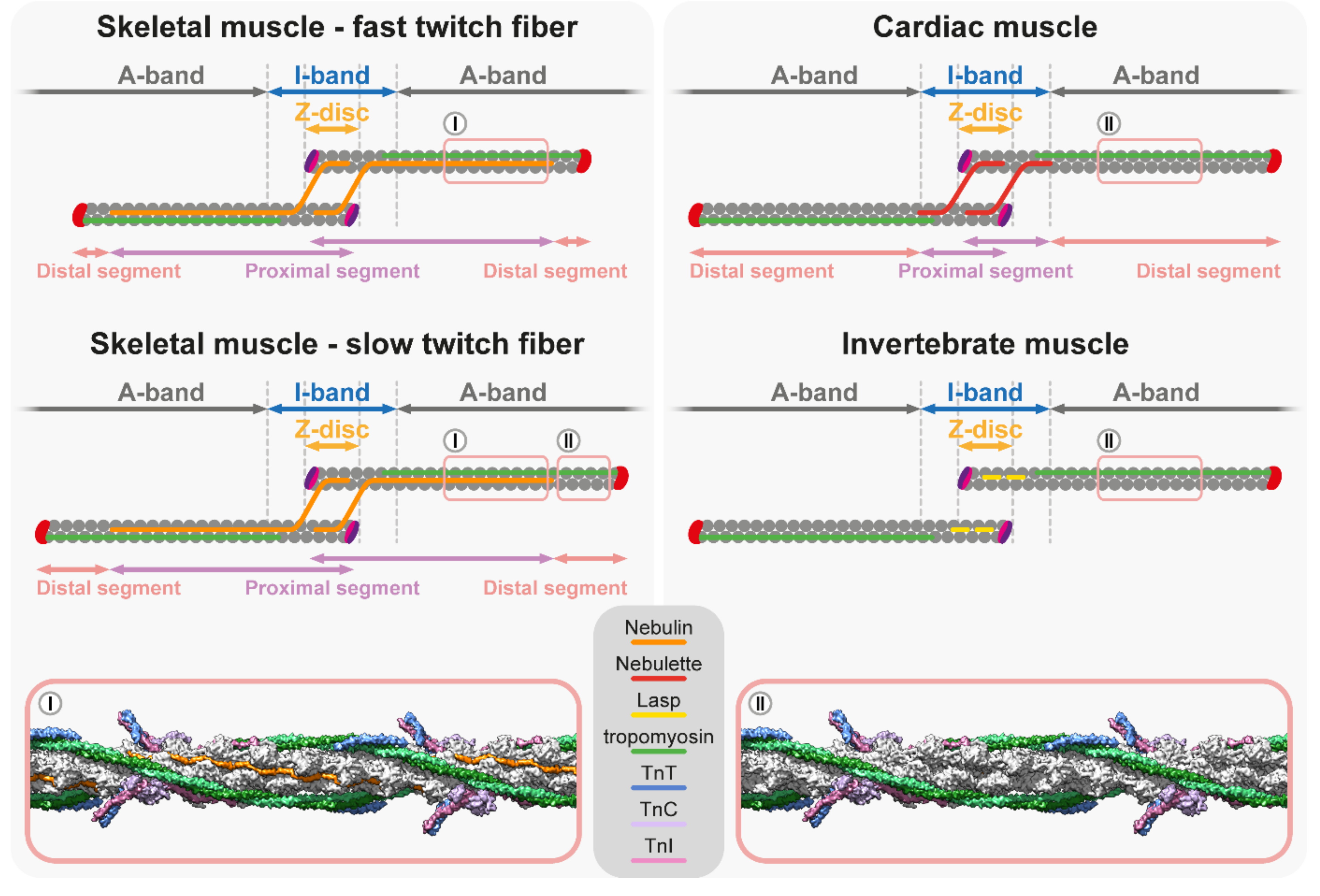
5. Thin Filament Length Determination
5.1. Nebulin Ruler Hypothesis
5.2. Titin/Myosin Scaffold Model
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Huxley, A.F.; Niedergerke, R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature 1954, 173, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Edman, K.A. The relation between sarcomere length and active tension in isolated semitendinosus fibres of the frog. J. Physiol. 1966, 183, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Huxley, A.F.; Julian, F.J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 1966, 184, 170–192. [Google Scholar] [CrossRef]
- Castillo, A.; Nowak, R.; Littlefield, K.P.; Fowler, V.M.; Littlefield, R.S. A nebulin ruler does not dictate thin filament lengths. Biophys. J. 2009, 96, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Gokhin, D.S.; Kim, N.E.; Lewis, S.A.; Hoenecke, H.R.; D’Lima, D.D.; Fowler, V.M. Thin-filament length correlates with fiber type in human skeletal muscle. Am. J. Physiol. Cell Physiol. 2011, 302, C555–C565. [Google Scholar] [CrossRef][Green Version]
- Gokhin, D.S.; Lewis, R.A.; McKeown, C.R.; Nowak, R.B.; Kim, N.E.; Littlefield, R.S.; Lieber, R.L.; Fowler, V.M. Tropomodulin isoforms regulate thin filament pointed-end capping and skeletal muscle physiology. J. Cell Biol. 2010, 189, 95–109. [Google Scholar] [CrossRef]
- Granzier, H.L.; Akster, H.A.; Ter Keurs, H.E. Effect of thin filament length on the force-sarcomere length relation of skeletal muscle. Am. J. Physiol. Cell Physiol. 1991, 260, C1060–C1070. [Google Scholar] [CrossRef]
- Ono, S. Regulation of structure and function of sarcomeric actin filaments in striated muscle of the nematode Caenorhabditis elegans. Anat. Rec. 2014, 297, 1548–1559. [Google Scholar] [CrossRef]
- Gieseler, K.; Qadota, H.; Benian, G.M. Development, Structure, and Maintenance of C. Elegans Body Wall Muscle. WormBook Online Rev. C. Elegans Biol. 2017, 2017, 1–59. [Google Scholar] [CrossRef]
- Prill, K.; Dawson, J.F. Assembly and Maintenance of Sarcomere Thin Filaments and Associated Diseases. Int. J. Mol. Sci. 2020, 21, 542. [Google Scholar] [CrossRef]
- De Winter, J.M.; Ottenheijm, C.A.C. Sarcomere Dysfunction in Nemaline Myopathy. J. Neuromuscul. Dis. 2017, 4, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Laitila, J.; Wallgren-Pettersson, C. Recent advances in nemaline myopathy. Neuromuscul. Disord. NMD 2021, 31, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Whitby, F.G.; Phillips, G.N., Jr. Crystal structure of tropomyosin at 7 Angstroms resolution. Proteins 2000, 38, 49–59. [Google Scholar] [CrossRef]
- Takeda, S.; Yamashita, A.; Maeda, K.; Maéda, Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature 2003, 424, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, M.V.; Stone, D.B.; Malanina, G.G.; Karatzaferi, C.; Cooke, R.; Mendelson, R.A.; Fletterick, R.J. Ca(2+)-regulated structural changes in troponin. Proc. Natl. Acad. Sci. USA 2005, 102, 5038–5043. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Iwane, A.H.; Yanagida, T.; Namba, K. Direct visualization of secondary structures of F-actin by electron cryomicroscopy. Nature 2010, 467, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Lehman, W.; Craig, R.; Vibert, P. Ca2+-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature 1994, 368, 65–67. [Google Scholar] [CrossRef]
- Xu, C.; Craig, R.; Tobacman, L.; Horowitz, R.; Lehman, W. Tropomyosin Positions in Regulated Thin Filaments Revealed by Cryoelectron Microscopy. Biophys. J. 1999, 77, 985–992. [Google Scholar] [CrossRef]
- Narita, A.; Yasunaga, T.; Ishikawa, T.; Mayanagi, K.; Wakabayashi, T. Ca(2+)-induced switching of troponin and tropomyosin on actin filaments as revealed by electron cryo-microscopy. J. Mol. Biol. 2001, 308, 241–261. [Google Scholar] [CrossRef]
- Oda, T.; Iwasa, M.; Aihara, T.; Maéda, Y.; Narita, A. The nature of the globular- to fibrous-actin transition. Nature 2009, 457, 441–445. [Google Scholar] [CrossRef]
- Paul, D.M.; Squire, J.M.; Morris, E.P. A novel approach to the structural analysis of partially decorated actin based filaments. J. Struct. Biol. 2010, 170, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Barbu-Tudoran, L.; Orzechowski, M.; Craig, R.; Trinick, J.; White, H.; Lehman, W. Three-dimensional organization of troponin on cardiac muscle thin filaments in the relaxed state. Biophys. J. 2014, 106, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.M.; Squire, J.M.; Morris, E.P. Relaxed and active thin filament structures; a new structural basis for the regulatory mechanism. J. Struct. Biol. 2017, 197, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Namba, K.; Fujii, T. Cardiac muscle thin filament structures reveal calcium regulatory mechanism. Nat. Commun. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Risi, C.M.; Pepper, I.; Belknap, B.; Landim-Vieira, M.; White, H.D.; Dryden, K.; Pinto, J.R.; Chase, P.B.; Galkin, V.E. The structure of the native cardiac thin filament at systolic Ca(2+) levels. Proc. Natl. Acad. Sci. USA 2021, 118, e2024288118. [Google Scholar] [CrossRef]
- Miller, A.; Tregear, E.T. Structure of insect fibrillar flight muscle in the presence and absence of ATP. J. Mol. Biol. 1972, 70, 85–104. [Google Scholar] [CrossRef]
- Reedy, M.K.; Reedy, M.C. Rigor crossbridge structure in tilted single filament layers and flared-X formations from insect flight muscle. J. Mol. Biol. 1985, 185, 145–176. [Google Scholar] [CrossRef]
- Schmitz, H.; Lucaveche, C.; Reedy, M.K.; Taylor, K.A. Oblique section 3-D reconstruction of relaxed insect flight muscle reveals the cross-bridge lattice in helical registration. Biophys. J. 1994, 67, 1620–1633. [Google Scholar] [CrossRef]
- Cooper, J.A. Actin Dynamics: Tropomyosin Provides Stability. Curr. Biol. 2002, 12, R523–R525. [Google Scholar] [CrossRef]
- Orzechowski, M.; Li, X.E.; Fischer, S.; Lehman, W. An atomic model of the tropomyosin cable on F-actin. Biophys. J. 2014, 107, 694–699. [Google Scholar] [CrossRef]
- Marston, S.; Zamora, J.E. Troponin structure and function: A view of recent progress. J. Muscle Res. Cell Motil. 2020, 41, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Von der Ecken, J.; Heissler, S.M.; Pathan-Chhatbar, S.; Manstein, D.J.; Raunser, S. Cryo-EM structure of a human cytoplasmic actomyosin complex at near-atomic resolution. Nature 2016, 534, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.N.; Madasu, Y.; Dominguez, R. Mechanism of actin filament pointed-end capping by tropomodulin. Science 2014, 345, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.; Merino, F.; Schaks, M.; Rottner, K.; Raunser, S.; Bieling, P. A barbed end interference mechanism reveals how capping protein promotes nucleation in branched actin networks. Nat. Commun. 2021, 12, 5329. [Google Scholar] [CrossRef]
- Murakami, K.; Stewart, M.; Nozawa, K.; Tomii, K.; Kudou, N.; Igarashi, N.; Shirakihara, Y.; Wakatsuki, S.; Yasunaga, T.; Wakabayashi, T. Structural basis for tropomyosin overlap in thin (actin) filaments and the generation of a molecular swivel by troponin-T. Proc. Natl. Acad. Sci. USA 2008, 105, 7200–7205. [Google Scholar] [CrossRef]
- Hanson, J. Axial Period of Actin Filaments. Nature 1967, 213, 353–356. [Google Scholar] [CrossRef]
- Egelman, E.H.; Francis, N.; DeRosier, D.J. F-actin is a helix with a random variable twist. Nature 1982, 298, 131–135. [Google Scholar] [CrossRef]
- Narita, A.; Oda, T.; Maéda, Y. Structural basis for the slow dynamics of the actin filament pointed end. EMBO J. 2011, 30, 1230–1237. [Google Scholar] [CrossRef]
- Zsolnay, V.; Katkar, H.H.; Chou, S.Z.; Pollard, T.D.; Voth, G.A. Structural basis for polarized elongation of actin filaments. Proc. Natl. Acad. Sci. USA 2020, 117, 30458–30464. [Google Scholar] [CrossRef]
- Bugyi, B.; Papp, G.; Hild, G.; Lõrinczy, D.; Nevalainen, E.M.; Lappalainen, P.; Somogyi, B.; Nyitrai, M. Formins regulate actin filament flexibility through long range allosteric interactions. J. Biol. Chem. 2006, 281, 10727–10736. [Google Scholar] [CrossRef]
- Papp, G.; Bugyi, B.; Ujfalusi, Z.; Barkó, S.; Hild, G.; Somogyi, B.; Nyitrai, M. Conformational changes in actin filaments induced by formin binding to the barbed end. Biophys. J. 2006, 91, 2564–2572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McGough, A.; Pope, B.; Chiu, W.; Weeds, A. Cofilin changes the twist of F-actin: Implications for actin filament dynamics and cellular function. J. Cell Biol. 1997, 138, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Galkin, V.E.; Orlova, A.; Lukoyanova, N.; Wriggers, W.; Egelman, E.H. Actin depolymerizing factor stabilizes an existing state of F-actin and can change the tilt of F-actin subunits. J. Cell Biol. 2001, 153, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Grintsevich, E.E.; Phillips, M.L.; Reisler, E.; Gimzewski, J.K. Atomic force microscopy reveals drebrin induced remodeling of f-actin with subnanometer resolution. Nano Lett. 2011, 11, 825–827. [Google Scholar] [CrossRef]
- Burbaum, L.; Schneider, J.; Scholze, S.; Böttcher, R.T.; Baumeister, W.; Schwille, P.; Plitzko, J.M.; Jasnin, M. Molecular-scale visualization of sarcomere contraction within native cardiomyocytes. Nat. Commun. 2021, 12, 4086. [Google Scholar] [CrossRef]
- Wang, Z.; Grange, M.; Wagner, T.; Kho, A.L.; Gautel, M.; Raunser, S. The molecular basis for sarcomere organization in vertebrate skeletal muscle. Cell 2021, 184, 2135–2150.e2113. [Google Scholar] [CrossRef]
- Szikora, S.; Gajdos, T.; Novák, T.; Farkas, D.; Földi, I.; Lenart, P.; Erdélyi, M.; Mihály, J. Nanoscopy reveals the layered organization of the sarcomeric H-zone and I-band complexes. J. Cell Biol. 2020, 219, e201907026. [Google Scholar] [CrossRef]
- Schueder, F.; Mangeol, P.; Chan, E.H.; Rees, R.; Schünemann, J.; Jungmann, R.; Görlich, D.; Schnorrer, F. Nanobodies combined with DNA-PAINT super-resolution reveal a staggered titin nano-architecture in flight muscles. bioRxiv 2022. [Google Scholar] [CrossRef]
- Sigal, Y.M.; Zhou, R.; Zhuang, X. Visualizing and discovering cellular structures with super-resolution microscopy. Science 2018, 361, 880–887. [Google Scholar] [CrossRef]
- Szikora, S.; Görög, P.; Kozma, C.; Mihály, J. Drosophila Models Rediscovered with Super-Resolution Microscopy. Cells 2021, 10, 1924. [Google Scholar] [CrossRef]
- Carlier, M.F.; Pantaloni, D. Control of actin assembly dynamics in cell motility. J. Biol. Chem. 2007, 282, 23005–23009. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Schutt, C.E.; Myslik, J.C.; Rozycki, M.D.; Goonesekere, N.C.W.; Lindberg, U. The structure of crystalline profilin–β-actin. Nature 1993, 365, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Yamashita, M.; Higashi, T.; Suetsugu, S.; Sato, Y.; Ikeda, T.; Shirakawa, R.; Kita, T.; Takenawa, T.; Horiuchi, H.; Fukai, S.; et al. Crystal structure of human DAAM1 formin homology 2 domain. Genes Cells 2007, 12, 1255–1265. [Google Scholar] [CrossRef]
- Kursula, P.; Kursula, I.; Massimi, M.; Song, Y.H.; Downer, J.; Stanley, W.A.; Witke, W.; Wilmanns, M. High-resolution structural analysis of mammalian profilin 2a complex formation with two physiological ligands: The formin homology 1 domain of mDia1 and the proline-rich domain of VASP. J. Mol. Biol. 2008, 375, 270–290. [Google Scholar] [CrossRef]
- Otomo, T.; Tomchick, D.R.; Otomo, C.; Panchal, S.C.; Machius, M.; Rosen, M.K. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature 2005, 433, 488–494. [Google Scholar] [CrossRef]
- Pollard, T.D.; Blanchoin, L.; Mullins, R.D. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 545–576. [Google Scholar] [CrossRef]
- Funk, J.; Merino, F.; Venkova, L.; Heydenreich, L.; Kierfeld, J.; Vargas, P.; Raunser, S.; Piel, M.; Bieling, P. Profilin and formin constitute a pacemaker system for robust actin filament growth. eLife 2019, 8, e50963. [Google Scholar] [CrossRef]
- Shimizu, N.; Obinata, T. Presence of Three Actin Types in Skeletal Muscle of Chick Embryos. Dev. Growth Differ. 1980, 22, 789–796. [Google Scholar] [CrossRef]
- Shimizu, N.; Obinata, T. Actin Concentration and Monomer-Polymer Ratio in Developing Chicken Skeletal Muscle 1. J. Biochem. 1986, 99, 751–759. [Google Scholar] [CrossRef]
- Safer, D.; Nachmias, V.T. Beta thymosins as actin binding peptides. BioEssays News Rev. Mol. Cell. Dev. Biol. 1994, 16, 473–479. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, E.M.; Ostap, E.M.; Brundage, R.A.; Reddy, K.S.; Sweeney, H.L.; Safer, D. Thymosin-β4 Changes the Conformation and Dynamics of Actin Monomers. Biophys. J. 2000, 78, 2516–2527. [Google Scholar] [CrossRef][Green Version]
- Babcock, G.; Rubenstein, P.A. Control of profilin and actin expression in muscle and nonmuscle cells. Cell Motil. Cytoskelet. 1993, 24, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, R.; Minami, N.; Hayakawa, K.; Abe, H.; Obinata, T. Quantitative analysis of low molecular weight G-actin-binding proteins, cofilin, ADF and profilin, expressed in developing and degenerating chicken skeletal muscles. J. Muscle Res. Cell Motil. 1996, 17, 463–473. [Google Scholar] [CrossRef]
- Kooij, V.; Viswanathan, M.C.; Lee, D.I.; Rainer, P.P.; Schmidt, W.; Kronert, W.A.; Harding, S.E.; Kass, D.A.; Bernstein, S.I.; Van Eyk, J.E.; et al. Profilin modulates sarcomeric organization and mediates cardiomyocyte hypertrophy. Cardiovasc. Res. 2016, 110, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R. The WH2 Domain and Actin Nucleation: Necessary but Insufficient. Trends Biochem. Sci. 2016, 41, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Higgs, H.N.; Peterson, K.J. Phylogenetic analysis of the formin homology 2 domain. Mol. Biol. Cell 2005, 16, 1–13. [Google Scholar] [CrossRef]
- Pring, M.; Evangelista, M.; Boone, C.; Yang, C.; Zigmond, S.H. Mechanism of formin-induced nucleation of actin filaments. Biochemistry 2003, 42, 486–496. [Google Scholar] [CrossRef]
- Goode, B.L.; Eck, M.J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 2007, 76, 593–627. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.S.; Pollard, T.D. Review of the mechanism of processive actin filament elongation by formins. Cell Motil. Cytoskelet. 2009, 66, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Kovar, D.R.; Kuhn, J.R.; Tichy, A.L.; Pollard, T.D. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 2003, 161, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, S.H.; Evangelista, M.; Boone, C.; Yang, C.; Dar, A.C.; Sicheri, F.; Forkey, J.; Pring, M. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr. Biol. CB 2003, 13, 1820–1823. [Google Scholar] [CrossRef]
- Moseley, J.B.; Sagot, I.; Manning, A.L.; Xu, Y.; Eck, M.J.; Pellman, D.; Goode, B.L. A Conserved Mechanism for Bni1- and mDia1-induced Actin Assembly and Dual Regulation of Bni1 by Bud6 and Profilin. Mol. Biol. Cell 2003, 15, 896–907. [Google Scholar] [CrossRef]
- Gutsche-Perelroizen, I.; Lepault, J.; Ott, A.; Carlier, M.F. Filament assembly from profilin-actin. J. Biol. Chem. 1999, 274, 6234–6243. [Google Scholar] [CrossRef]
- Kinosian, H.; Selden, L.; Gershman, L.; Estes, J. Actin Filament Barbed End Elongation with Nonmuscle MgATP−Actin and MgADP−Actin in the Presence of Profilin. Biochemistry 2002, 41, 6734–6743. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Quantitative analysis of the effect of Acanthamoeba profilin on actin filament nucleation and elongation. Biochemistry 1984, 23, 6631–6641. [Google Scholar] [CrossRef]
- Pring, M.; Weber, A.; Bubb, M.R. Profilin-actin complexes directly elongate actin filaments at the barbed end. Biochemistry 1992, 31, 1827–1836. [Google Scholar] [CrossRef]
- Bartolini, F.; Gundersen, G.G. Formins and microtubules. Biochim. Biophys. Acta 2010, 1803, 164–173. [Google Scholar] [CrossRef]
- Bartolini, F.; Moseley, J.B.; Schmoranzer, J.; Cassimeris, L.; Goode, B.L.; Gundersen, G.G. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J. Cell Biol. 2008, 181, 523–536. [Google Scholar] [CrossRef]
- Gaillard, J.; Ramabhadran, V.; Neumanne, E.; Gurel, P.; Blanchoin, L.; Vantard, M.; Higgs, H.N. Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules. Mol. Biol. Cell 2011, 22, 4575–4587. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.S.; Higgs, H.N. Biochemical Analysis of Mammalian Formin Effects on Actin Dynamics. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2006; Volume 406, pp. 190–214. [Google Scholar]
- Wen, Y.; Eng, C.H.; Schmoranzer, J.; Cabrera-Poch, N.; Morris, E.J.; Chen, M.; Wallar, B.J.; Alberts, A.S.; Gundersen, G.G. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 2004, 6, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Vig, A.T.; Földi, I.; Szikora, S.; Migh, E.; Gombos, R.; Tóth, M.; Huber, T.; Pintér, R.; Talián, G.C.; Mihály, J.; et al. The activities of the C-terminal regions of the formin protein disheveled-associated activator of morphogenesis (DAAM) in actin dynamics. J. Biol. Chem. 2017, 292, 13566–13583. [Google Scholar] [CrossRef] [PubMed]
- Szikora, S.; Földi, I.; Tóth, K.; Migh, E.; Vig, A.; Bugyi, B.; Maléth, J.; Hegyi, P.; Kaltenecker, P.; Sanchez-Soriano, N.; et al. The formin DAAM is required for coordination of the actin and microtubule cytoskeleton in axonal growth cones. J. Cell Sci. 2017, 130, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Foldi, I.; Szikora, S.; Mihály, J. Formin’ bridges between microtubules and actin filaments in axonal growth cones. Neural Regen. Res. 2017, 12, 1971–1973. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.; Barber, C.F.; Berciu, C.; Feldman, S.; Birren, S.J.; Nicastro, D.; Goode, B.L. Critical roles for multiple formins during cardiac myofibril development and repair. Mol. Biol. Cell 2014, 25, 811–827. [Google Scholar] [CrossRef]
- Spletter, M.L.; Barz, C.; Yeroslaviz, A.; Zhang, X.; Lemke, S.B.; Bonnard, A.; Brunner, E.; Cardone, G.; Basler, K.; Habermann, B.H.; et al. A transcriptomics resource reveals a transcriptional transition during ordered sarcomere morphogenesis in flight muscle. eLife 2018, 7, e34058. [Google Scholar] [CrossRef]
- Al Haj, A.; Mazur, A.J.; Radaszkiewicz, K.; Radaszkiewicz, T.; Makowiecka, A.; Stopschinski, B.E.; Schönichen, A.; Geyer, M.; Mannherz, H.G. Distribution of formins in cardiac muscle: FHOD1 is a component of intercalated discs and costameres. Eur. J. Cell Biol. 2015, 94, 101–113. [Google Scholar] [CrossRef]
- Dwyer, J.; Pluess, M.; Iskratsch, T.; Dos Remedios, C.G.; Ehler, E. The formin FHOD1 in cardiomyocytes. Anat. Rec. 2014, 297, 1560–1570. [Google Scholar] [CrossRef]
- Kanaya, H.; Takeya, R.; Takeuchi, K.; Watanabe, N.; Jing, N.; Sumimoto, H. Fhos2, a novel formin-related actin-organizing protein, probably associates with the nestin intermediate filament. Genes Cells 2005, 10, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Takeya, R.; Suetsugu, S.; Kan, O.M.; Narusawa, M.; Shiose, A.; Tominaga, R.; Sumimoto, H. Mammalian formin fhod3 regulates actin assembly and sarcomere organization in striated muscles. J. Biol. Chem. 2009, 284, 29873–29881. [Google Scholar] [CrossRef] [PubMed]
- Iskratsch, T.; Lange, S.; Dwyer, J.; Kho, A.L.; dos Remedios, C.; Ehler, E. Formin follows function: A muscle-specific isoform of FHOD3 is regulated by CK2 phosphorylation and promotes myofibril maintenance. J. Cell Biol. 2010, 191, 1159–1172. [Google Scholar] [CrossRef]
- Kan-o, M.; Takeya, R.; Taniguchi, K.; Tanoue, Y.; Tominaga, R.; Sumimoto, H. Expression and Subcellular Localization of Mammalian Formin Fhod3 in the Embryonic and Adult Heart. PLoS ONE 2012, 7, e34765. [Google Scholar] [CrossRef] [PubMed]
- Arimura, T.; Takeya, R.; Ishikawa, T.; Yamano, T.; Matsuo, A.; Tatsumi, T.; Nomura, T.; Sumimoto, H.; Kimura, A. Dilated cardiomyopathy-associated FHOD3 variant impairs the ability to induce activation of transcription factor serum response factor. Circ. J. 2013, 77, 2990–2996. [Google Scholar] [CrossRef] [PubMed]
- Wooten, E.C.; Hebl, V.B.; Wolf, M.J.; Greytak, S.R.; Orr, N.M.; Draper, I.; Calvino, J.E.; Kapur, N.K.; Maron, M.S.; Kullo, I.J.; et al. Formin homology 2 domain containing 3 variants associated with hypertrophic cardiomyopathy. Circulation. Cardiovasc. Genet. 2013, 6, 10–18. [Google Scholar] [CrossRef]
- Ochoa, J.P.; Sabater-Molina, M.; García-Pinilla, J.M.; Mogensen, J.; Restrepo-Córdoba, A.; Palomino-Doza, J.; Villacorta, E.; Martinez-Moreno, M.; Ramos-Maqueda, J.; Zorio, E.; et al. Formin Homology 2 Domain Containing 3 (FHOD3) Is a Genetic Basis for Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2018, 72, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Dos Remedios, C.G.; Li, A.; Lal, S. Non-sarcomeric causes of heart failure: A Sydney Heart Bank perspective. Biophys. Rev. 2018, 10, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, T.; Fujimoto, N.; Matsuyama, S.; Kan, O.M.; Kiyonari, H.; Shioi, G.; Kage, Y.; Yamasaki, S.; Takeya, R.; Sumimoto, H. The actin-organizing formin protein Fhod3 is required for postnatal development and functional maintenance of the adult heart in mice. J. Biol. Chem. 2018, 293, 148–162. [Google Scholar] [CrossRef]
- Shwartz, A.; Dhanyasi, N.; Schejter, E.D.; Shilo, B.-Z. The Drosophila formin Fhos is a primary mediator of sarcomeric thin-filament array assembly. eLife 2016, 5, e16540. [Google Scholar] [CrossRef]
- Schönichen, A.; Mannherz, H.G.; Behrmann, E.; Mazur, A.J.; Kühn, S.; Silván, U.; Schoenenberger, C.-A.; Fackler, O.T.; Raunser, S.; Dehmelt, L.; et al. FHOD1 is a combined actin filament capping and bundling factor that selectively associates with actin arcs and stress fibers. J. Cell Sci. 2013, 126, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Oztug Durer, Z.A.; van Loon, A.P.; Bremer, K.V.; Quinlan, M.E. Drosophila and human FHOD family formin proteins nucleate actin filaments. J. Biol. Chem. 2018, 293, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Kan, O.M.; Ushijima, T.; Kage, Y.; Tominaga, R.; Sumimoto, H.; Takeya, R. Transgenic Expression of the Formin Protein Fhod3 Selectively in the Embryonic Heart: Role of Actin-Binding Activity of Fhod3 and Its Sarcomeric Localization during Myofibrillogenesis. PLoS ONE 2016, 11, e0148472. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Kage, Y.; Fujimoto, N.; Ushijima, T.; Tsuruda, T.; Kitamura, K.; Shiose, A.; Asada, Y.; Sumimoto, H.; Takeya, R. Interaction between cardiac myosin-binding protein C and formin Fhod3. Proc. Natl. Acad. Sci. USA 2018, 115, E4386–E4395. [Google Scholar] [CrossRef]
- Li, D.; Hallett, M.A.; Zhu, W.; Rubart, M.; Liu, Y.; Yang, Z.; Chen, H.; Haneline, L.S.; Chan, R.J.; Schwartz, R.J.; et al. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development 2011, 138, 303–315. [Google Scholar] [CrossRef]
- Bao, B.; Zhang, L.; Hu, H.; Yin, S.; Liang, Z. Deletion of a single-copy DAAM1 gene in congenital heart defect: A case report. BMC Med. Genet. 2012, 13, 63. [Google Scholar] [CrossRef]
- Ajima, R.; Bisson, J.A.; Helt, J.-C.; Nakaya, M.-A.; Habas, R.; Tessarollo, L.; He, X.; Morrisey, E.E.; Yamaguchi, T.P.; Cohen, E.D. DAAM1 and DAAM2 are co-required for myocardial maturation and sarcomere assembly. Dev. Biol. 2015, 408, 126–139. [Google Scholar] [CrossRef]
- Molnár, I.; Migh, E.; Szikora, S.; Kalmár, T.; Végh, A.G.; Deák, F.; Barkó, S.; Bugyi, B.; Orfanos, Z.; Kovács, J.; et al. DAAM Is Required for Thin Filament Formation and Sarcomerogenesis during Muscle Development in Drosophila. PLoS Genet. 2014, 10, e1004166. [Google Scholar] [CrossRef]
- Higashi, T.; Ikeda, T.; Shirakawa, R.; Kondo, H.; Kawato, M.; Horiguchi, M.; Okuda, T.; Okawa, K.; Fukai, S.; Nureki, O.; et al. Biochemical characterization of the Rho GTPase-regulated actin assembly by diaphanous-related formins, mDia1 and Daam1, in platelets. J. Biol. Chem. 2008, 283, 8746–8755. [Google Scholar] [CrossRef]
- Barkó, S.; Bugyi, B.; Carlier, M.-F.; Gombos, R.; Matusek, T.; Mihály, J.; Nyitrai, M. Characterization of the Biochemical Properties and Biological Function of the Formin Homology Domains of Drosophila DAAM*. J. Biol. Chem. 2010, 285, 13154–13169. [Google Scholar] [CrossRef]
- Deng, S.; Silimon, R.L.; Balakrishnan, M.; Bothe, I.; Juros, D.; Soffar, D.B.; Baylies, M.K. The actin polymerization factor Diaphanous and the actin severing protein Flightless I collaborate to regulate sarcomere size. Dev. Biol. 2021, 469, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Bothe, I.; Baylies, M.K. The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 Regulation. PLoS Genet. 2015, 11, e1005381. [Google Scholar] [CrossRef] [PubMed]
- Mi-Mi, L.; Votra, S.; Kemphues, K.; Bretscher, A.; Pruyne, D. Z-line formins promote contractile lattice growth and maintenance in striated muscles of C. elegans. J. Cell Biol. 2012, 198, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Pappas, C.T.; Mayfield, R.M.; Henderson, C.; Jamilpour, N.; Cover, C.; Hernandez, Z.; Hutchinson, K.R.; Chu, M.; Nam, K.H.; Valdez, J.M.; et al. Knockout of Lmod2 results in shorter thin filaments followed by dilated cardiomyopathy and juvenile lethality. Proc. Natl. Acad. Sci. USA 2015, 112, 13573–13578. [Google Scholar] [CrossRef]
- Kolb, J.; Li, F.; Methawasin, M.; Adler, M.; Escobar, Y.-N.; Nedrud, J.; Pappas, C.T.; Harris, S.P.; Granzier, H. Thin filament length in the cardiac sarcomere varies with sarcomere length but is independent of titin and nebulin. J. Mol. Cell. Cardiol. 2016, 97, 286–294. [Google Scholar] [CrossRef]
- Littlefield, R.; Almenar-Queralt, A.; Fowler, V.M. Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat. Cell Biol. 2001, 3, 544–551. [Google Scholar] [CrossRef]
- Mardahl-Dumesnil, M.; Fowler, V.M. Thin filaments elongate from their pointed ends during myofibril assembly in Drosophila indirect flight muscle. J. Cell Biol. 2001, 155, 1043–1053. [Google Scholar] [CrossRef]
- Michele, D.E.; Albayya, F.P.; Metzger, J.M. Thin Filament Protein Dynamics in Fully Differentiated Adult Cardiac Myocytes: Toward A Model of Sarcomere Maintenance. J. Cell Biol. 1999, 145, 1483–1495. [Google Scholar] [CrossRef]
- Schafer, D.A.; Hug, C.; Cooper, J.A. Inhibition of CapZ during myofibrillogenesis alters assembly of actin filaments. J. Cell Biol. 1995, 128, 61–70. [Google Scholar] [CrossRef]
- Weber, A.; Pennise, C.R.; Babcock, G.G.; Fowler, V.M. Tropomodulin caps the pointed ends of actin filaments. J. Cell Biol. 1994, 127, 1627–1635. [Google Scholar] [CrossRef]
- Yamashiro, S.; Gokhin, D.S.; Kimura, S.; Nowak, R.B.; Fowler, V.M. Tropomodulins: Pointed-end capping proteins that regulate actin filament architecture in diverse cell types. Cytoskeleton 2012, 69, 337–370. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, C.C.; Weber, A.; Bondad, M.; Pennise, C.R.; Fowler, V.M. Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature 1995, 377, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Kostyukova, A.S.; Choy, A.; Rapp, B.A. Tropomodulin binds two tropomyosins: A novel model for actin filament capping. Biochemistry 2006, 45, 12068–12075. [Google Scholar] [CrossRef]
- Sussman, M.A.; Welch, S.; Cambon, N.; Klevitsky, R.; Hewett, T.E.; Price, R.; Witt, S.A.; Kimball, T.R. Myofibril degeneration caused by tropomodulin overexpression leads to dilated cardiomyopathy in juvenile mice. J. Clin. Investig. 1998, 101, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gokhin, D.S.; Tierney, M.T.; Sui, Z.; Sacco, A.; Fowler, V.M. Calpain-mediated proteolysis of tropomodulin isoforms leads to thin filament elongation in dystrophic skeletal muscle. Mol. Biol. Cell 2014, 25, 852–865. [Google Scholar] [CrossRef] [PubMed]
- Gokhin, D.S.; Ochala, J.; Domenighetti, A.A.; Fowler, V.M. Tropomodulin 1 directly controls thin filament length in both wild-type and tropomodulin 4-deficient skeletal muscle. Development 2015, 142, 4351–4362. [Google Scholar] [CrossRef]
- Fowler, V.M.; Dominguez, R. Tropomodulins and Leiomodins: Actin Pointed End Caps and Nucleators in Muscles. Biophys. J. 2017, 112, 1742–1760. [Google Scholar] [CrossRef] [PubMed]
- Chereau, D.; Boczkowska, M.; Skwarek-Maruszewska, A.; Fujiwara, I.; Hayes, D.B.; Rebowski, G.; Lappalainen, P.; Pollard, T.D.; Dominguez, R. Leiomodin is an actin filament nucleator in muscle cells. Science 2008, 320, 239–243. [Google Scholar] [CrossRef]
- Tsukada, T.; Pappas, C.T.; Moroz, N.; Antin, P.B.; Kostyukova, A.S.; Gregorio, C.C. Leiomodin-2 is an antagonist of tropomodulin-1 at the pointed end of the thin filaments in cardiac muscle. J. Cell Sci. 2010, 123, 3136–3145. [Google Scholar] [CrossRef]
- Yuen, M.; Sandaradura, S.A.; Dowling, J.J.; Kostyukova, A.S.; Moroz, N.; Quinlan, K.G.; Lehtokari, V.L.; Ravenscroft, G.; Todd, E.J.; Ceyhan-Birsoy, O.; et al. Leiomodin-3 dysfunction results in thin filament disorganization and nemaline myopathy. J. Clin. Investig. 2014, 124, 4693–4708. [Google Scholar] [CrossRef]
- Boczkowska, M.; Rebowski, G.; Kremneva, E.; Lappalainen, P.; Dominguez, R. How Leiomodin and Tropomodulin use a common fold for different actin assembly functions. Nat. Commun. 2015, 6, 8314. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mo, K.; Tian, H.; Chu, C.; Sun, S.; Tian, L.; Ding, S.; Li, T.-R.; Wu, X.; Liu, F.; et al. Lmod2 piggyBac mutant mice exhibit dilated cardiomyopathy. Cell Biosci. 2016, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Ahrens-Nicklas, R.C.; Pappas, C.T.; Farman, G.P.; Mayfield, R.M.; Larrinaga, T.M.; Medne, L.; Ritter, A.; Krantz, I.D.; Murali, C.; Lin, K.Y.; et al. Disruption of cardiac thin filament assembly arising from a mutation in LMOD2: A novel mechanism of neonatal dilated cardiomyopathy. Sci. Adv. 2019, 5, eaax2066. [Google Scholar] [CrossRef]
- Cenik, B.K.; Garg, A.; McAnally, J.R.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N.; Liu, N. Severe myopathy in mice lacking the MEF2/SRF-dependent gene leiomodin-3. J. Clin. Investig. 2015, 125, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Halim, D.; Wilson, M.P.; Oliver, D.; Brosens, E.; Verheij, J.B.; Han, Y.; Nanda, V.; Lyu, Q.; Doukas, M.; Stoop, H.; et al. Loss of LMOD1 impairs smooth muscle cytocontractility and causes megacystis microcolon intestinal hypoperistalsis syndrome in humans and mice. Proc. Natl. Acad. Sci. USA 2017, 114, E2739–E2747. [Google Scholar] [CrossRef] [PubMed]
- Skwarek-Maruszewska, A.; Boczkowska, M.; Zajac, A.L.; Kremneva, E.; Svitkina, T.; Dominguez, R.; Lappalainen, P. Different localizations and cellular behaviors of leiomodin and tropomodulin in mature cardiomyocyte sarcomeres. Mol. Biol. Cell 2010, 21, 3352–3361. [Google Scholar] [CrossRef][Green Version]
- Tolkatchev, D.; Smith, G.E., Jr.; Schultz, L.E.; Colpan, M.; Helms, G.L.; Cort, J.R.; Gregorio, C.C.; Kostyukova, A.S. Leiomodin creates a leaky cap at the pointed end of actin-thin filaments. PLoS Biol. 2020, 18, e3000848. [Google Scholar] [CrossRef]
- Pappas, C.T.; Farman, G.P.; Mayfield, R.M.; Konhilas, J.P.; Gregorio, C.C. Cardiac-specific knockout of Lmod2 results in a severe reduction in myofilament force production and rapid cardiac failure. J. Mol. Cell. Cardiol. 2018, 122, 88–97. [Google Scholar] [CrossRef]
- Bai, J.; Hartwig, J.H.; Perrimon, N. SALS, a WH2-Domain-Containing Protein, Promotes Sarcomeric Actin Filament Elongation from Pointed Ends during Drosophila Muscle Growth. Dev. Cell 2007, 13, 828–842. [Google Scholar] [CrossRef]
- Tolkatchev, D.; Gregorio, C.C.; Kostyukova, A.S. The role of leiomodin in actin dynamics: A new road or a secret gate. FEBS J. 2021. [Google Scholar] [CrossRef]
- Ly, T.; Moroz, N.; Pappas, C.T.; Novak, S.M.; Tolkatchev, D.; Wooldridge, D.; Mayfield, R.M.; Helms, G.; Gregorio, C.C.; Kostyukova, A.S. The N-terminal tropomyosin- and actin-binding sites are important for leiomodin 2’s function. Mol. Biol. Cell 2016, 27, 2565–2575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tóth, M.Á.; Majoros, A.K.; Vig, A.T.; Migh, E.; Nyitrai, M.; Mihály, J.; Bugyi, B. Biochemical Activities of the Wiskott-Aldrich Syndrome Homology Region 2 Domains of Sarcomere Length Short (SALS) Protein*. J. Biol. Chem. 2016, 291, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Watanabe-Takano, H.; Suetsugu, S.; Kurita, S.; Tsujita, K.; Kimura, S.; Karatsu, T.; Takenawa, T.; Endo, T. Nebulin and N-WASP cooperate to cause IGF-1-induced sarcomeric actin filament formation. Science 2010, 330, 1536–1540. [Google Scholar] [CrossRef]
- Boczkowska, M.; Yurtsever, Z.; Rebowski, G.; Eck, M.J.; Dominguez, R. Crystal Structure of Leiomodin 2 in Complex with Actin: A Structural and Functional Reexamination. Biophys. J. 2017, 113, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Paunola, E.; Mattila, P.K.; Lappalainen, P. WH2 domain: A small, versatile adapter for actin monomers. FEBS Lett. 2002, 513, 92–97. [Google Scholar] [CrossRef]
- Conley, C.A.; Fritz-Six, K.L.; Almenar-Queralt, A.; Fowler, V.M. Leiomodins: Larger members of the tropomodulin (Tmod) gene family. Genomics 2001, 73, 127–139. [Google Scholar] [CrossRef]
- Garg, A.; O’Rourke, J.; Long, C.; Doering, J.; Ravenscroft, G.; Bezprozvannaya, S.; Nelson, B.R.; Beetz, N.; Li, L.; Chen, S.; et al. KLHL40 deficiency destabilizes thin filament proteins and promotes nemaline myopathy. J. Clin. Investig. 2014, 124, 3529–3539. [Google Scholar] [CrossRef]
- Szatmári, D.; Bugyi, B.; Ujfalusi, Z.; Grama, L.; Dudás, R.; Nyitrai, M. Cardiac leiomodin2 binds to the sides of actin filaments and regulates the ATPase activity of myosin. PLoS ONE 2017, 12, e0186288. [Google Scholar] [CrossRef]
- Li, F.; Barton, E.R.; Granzier, H. Deleting nebulin’s C-terminus reveals its importance to sarcomeric structure and function and is sufficient to invoke nemaline myopathy. Hum. Mol. Genet. 2019, 28, 1709–1725. [Google Scholar] [CrossRef]
- Yamamoto, D.L.; Vitiello, C.; Zhang, J.; Gokhin, D.S.; Castaldi, A.; Coulis, G.; Piaser, F.; Filomena, M.C.; Eggenhuizen, P.J.; Kunderfranco, P.; et al. The nebulin SH3 domain is dispensable for normal skeletal muscle structure but is required for effective active load bearing in mouse. J. Cell Sci. 2013, 126, 5477–5489. [Google Scholar] [CrossRef]
- Kremneva, E.; Makkonen, M.H.; Skwarek-Maruszewska, A.; Gateva, G.; Michelot, A.; Dominguez, R.; Lappalainen, P. Cofilin-2 controls actin filament length in muscle sarcomeres. Dev. Cell 2014, 31, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Yu, S.F.; Chin, S.M.; Soffar, D.B.; Windner, S.E.; Goode, B.L.; Baylies, M.K. Cofilin Loss in Drosophila Muscles Contributes to Muscle Weakness through Defective Sarcomerogenesis during Muscle Growth. Cell Rep. 2020, 32, 107893. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Parast, M.; Alberico, C.; Benian, G.M.; Ono, S. Specific requirement for two ADF/cofilin isoforms in distinct actin-dependent processes in Caenorhabditis elegans. J. Cell Sci. 2003, 116, 2073–2085. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Sato, N.; Nakagaki, T.; Abe, H.; Ono, S.; Obinata, T. Two mouse cofilin isoforms, muscle-type (MCF) and non-muscle type (NMCF), interact with F-actin with different efficiencies. J. Biochem. 2005, 138, 519–526. [Google Scholar] [CrossRef]
- Gurniak, C.B.; Chevessier, F.; Jokwitz, M.; Jönsson, F.; Perlas, E.; Richter, H.; Matern, G.; Boyl, P.P.; Chaponnier, C.; Fürst, D.; et al. Severe protein aggregate myopathy in a knockout mouse model points to an essential role of cofilin2 in sarcomeric actin exchange and muscle maintenance. Eur. J. Cell Biol. 2014, 93, 252–266. [Google Scholar] [CrossRef]
- Agrawal, P.B.; Greenleaf, R.S.; Tomczak, K.K.; Lehtokari, V.L.; Wallgren-Pettersson, C.; Wallefeld, W.; Laing, N.G.; Darras, B.T.; Maciver, S.K.; Dormitzer, P.R.; et al. Nemaline myopathy with minicores caused by mutation of the CFL2 gene encoding the skeletal muscle actin-binding protein, cofilin-2. Am. J. Hum. Genet. 2007, 80, 162–167. [Google Scholar] [CrossRef]
- Ockeloen, C.W.; Gilhuis, H.J.; Pfundt, R.; Kamsteeg, E.J.; Agrawal, P.B.; Beggs, A.H.; Hama-Amin, A.D.; Diekstra, A.; Knoers, N.V.; Lammens, M.; et al. Congenital myopathy caused by a novel missense mutation in the CFL2 gene. Neuromuscul. Disord. NMD 2012, 22, 632–639. [Google Scholar] [CrossRef]
- Vartiainen, M.K.; Mustonen, T.; Mattila, P.K.; Ojala, P.J.; Thesleff, I.; Partanen, J.; Lappalainen, P. The three mouse actin-depolymerizing factor/cofilins evolved to fulfill cell-type-specific requirements for actin dynamics. Mol. Biol. Cell 2002, 13, 183–194. [Google Scholar] [CrossRef]
- Nomura, K.; Ono, K.; Ono, S. CAS-1, a C. elegans cyclase-associated protein, is required for sarcomeric actin assembly in striated muscle. J. Cell Sci. 2012, 125, 4077–4089. [Google Scholar] [CrossRef]
- Effendi, K.; Yamazaki, K.; Mori, T.; Masugi, Y.; Makino, S.; Sakamoto, M. Involvement of hepatocellular carcinoma biomarker, cyclase-associated protein 2 in zebrafish body development and cancer progression. Exp. Cell Res. 2013, 319, 35–44. [Google Scholar] [CrossRef]
- Field, J.; Vojtek, A.; Ballester, R.; Bolger, G.; Colicelli, J.; Ferguson, K.; Gerst, J.; Kataoka, T.; Michaeli, T.; Powers, S.; et al. Cloning and characterization of CAP, the S. cerevisiae gene encoding the 70 kd adenylyl cyclase-associated protein. Cell 1990, 61, 319–327. [Google Scholar] [CrossRef]
- Moriyama, K.; Yahara, I. Human CAP1 is a key factor in the recycling of cofilin and actin for rapid actin turnover. J. Cell Sci. 2002, 115, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.; Little, K.; Talarico, L.; Quintero-Monzon, O.; Goode, B.L. A central role for the WH2 domain of Srv2/CAP in recharging actin monomers to drive actin turnover in vitro and in vivo. Cytoskeleton 2010, 67, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Kotila, T.; Wioland, H.; Enkavi, G.; Kogan, K.; Vattulainen, I.; Jégou, A.; Romet-Lemonne, G.; Lappalainen, P. Mechanism of synergistic actin filament pointed end depolymerization by cyclase-associated protein and cofilin. Nat. Commun. 2019, 10, 5320. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Chung, J.; Kondev, J.; Gelles, J.; Goode, B.L. Synergy between Cyclase-associated protein and Cofilin accelerates actin filament depolymerization by two orders of magnitude. Nat. Commun. 2019, 10, 5319. [Google Scholar] [CrossRef]
- Purde, V.; Busch, F.; Kudryashova, E.; Wysocki, V.H.; Kudryashov, D.S. Oligomerization Affects the Ability of Human Cyclase-Associated Proteins 1 and 2 to Promote Actin Severing by Cofilins. Int. J. Mol. Sci. 2019, 20, 5647. [Google Scholar] [CrossRef]
- Kotila, T.; Kogan, K.; Enkavi, G.; Guo, S.; Vattulainen, I.; Goode, B.L.; Lappalainen, P. Structural basis of actin monomer re-charging by cyclase-associated protein. Nat. Commun. 2018, 9, 1892. [Google Scholar] [CrossRef]
- Bertling, E.; Hotulainen, P.; Mattila, P.K.; Matilainen, T.; Salminen, M.; Lappalainen, P. Cyclase-associated protein 1 (CAP1) promotes cofilin-induced actin dynamics in mammalian nonmuscle cells. Mol. Biol. Cell 2004, 15, 2324–2334. [Google Scholar] [CrossRef]
- Peche, V.; Shekar, S.; Leichter, M.; Korte, H.; Schröder, R.; Schleicher, M.; Holak, T.A.; Clemen, C.S.; Ramanath, Y.B.; Pfitzer, G.; et al. CAP2, cyclase-associated protein 2, is a dual compartment protein. Cell. Mol. Life Sci. CMLS 2007, 64, 2702–2715. [Google Scholar] [CrossRef]
- Colpan, M.; Iwanski, J.; Gregorio, C.C. CAP2 is a regulator of actin pointed end dynamics and myofibrillogenesis in cardiac muscle. Commun. Biol. 2021, 4, 365. [Google Scholar] [CrossRef]
- Kepser, L.J.; Damar, F.; De Cicco, T.; Chaponnier, C.; Prószyński, T.J.; Pagenstecher, A.; Rust, M.B. CAP2 deficiency delays myofibril actin cytoskeleton differentiation and disturbs skeletal muscle architecture and function. Proc. Natl. Acad. Sci. USA 2019, 116, 8397–8402. [Google Scholar] [CrossRef] [PubMed]
- Peche, V.S.; Holak, T.A.; Burgute, B.D.; Kosmas, K.; Kale, S.P.; Wunderlich, F.T.; Elhamine, F.; Stehle, R.; Pfitzer, G.; Nohroudi, K.; et al. Ablation of cyclase-associated protein 2 (CAP2) leads to cardiomyopathy. Cell. Mol. LifeSci. CMLS 2013, 70, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Field, J.; Ye, D.Z.; Shinde, M.; Liu, F.; Schillinger, K.J.; Lu, M.; Wang, T.; Skettini, M.; Xiong, Y.; Brice, A.K.; et al. CAP2 in cardiac conduction, sudden cardiac death and eye development. Sci. Rep. 2015, 5, 17256. [Google Scholar] [CrossRef] [PubMed]
- Aspit, L.; Levitas, A.; Etzion, S.; Krymko, H.; Slanovic, L.; Zarivach, R.; Etzion, Y.; Parvari, R. CAP2 mutation leads to impaired actin dynamics and associates with supraventricular tachycardia and dilated cardiomyopathy. J. Med. Genet. 2019, 56, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.M. Regulation of actin filament length in erythrocytes and striated muscle. Curr. Opin. Cell Biol. 1996, 8, 86–96. [Google Scholar] [CrossRef]
- Wang, K.; Wright, J. Architecture of the sarcomere matrix of skeletal muscle: Immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J. Cell Biol. 1988, 107, 2199–2212. [Google Scholar] [CrossRef]
- Maruyama, K.; Matsuno, A.; Higuchi, H.; Shimaoka, S.; Kimura, S.; Shimizu, T. Behaviour of connectin (titin) and nebulin in skinned muscle fibres released after extreme stretch as revealed by immunoelectron microscopy. J. Muscle Res. Cell Motil. 1989, 10, 350–359. [Google Scholar] [CrossRef]
- Pierobon-Bormioli, S.; Betto, R.; Salviati, G. The organization of titin (connectin) and nebulin in the sarcomeres: An immunocytolocalization study. J. Muscle Res. Cell Motil. 1989, 10, 446–456. [Google Scholar] [CrossRef]
- Kruger, M.; Wright, J.; Wang, K. Nebulin as a length regulator of thin filaments of vertebrate skeletal muscles: Correlation of thin filament length, nebulin size, and epitope profile. J. Cell Biol. 1991, 115, 97–107. [Google Scholar] [CrossRef]
- Jin, J.P.; Wang, K. Nebulin as a giant actin-binding template protein in skeletal muscle sarcomere. Interaction of actin and cloned human nebulin fragments. FEBS Lett. 1991, 281, 93–96. [Google Scholar] [CrossRef]
- Wright, J.; Huang, Q.Q.; Wang, K. Nebulin is a full-length template of actin filaments in the skeletal muscle sarcomere: An immunoelectron microscopic study of its orientation and span with site-specific monoclonal antibodies. J. Muscle Res. Cell Motil. 1993, 14, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Labeit, S.; Kolmerer, B. The complete primary structure of human nebulin and its correlation to muscle structure. J. Mol. Biol. 1995, 248, 308–315. [Google Scholar] [CrossRef]
- Lehtokari, V.L.; Pelin, K.; Sandbacka, M.; Ranta, S.; Donner, K.; Muntoni, F.; Sewry, C.; Angelini, C.; Bushby, K.; Van den Bergh, P.; et al. Identification of 45 novel mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Hum. Mutat. 2006, 27, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.B.; Lehtokari, V.L.; Quijano-Roy, S.; Monnier, N.; Claeys, K.G.; Carlier, R.Y.; Pellegrini, N.; Orlikowski, D.; Barois, A.; Laing, N.G.; et al. Core-rod myopathy caused by mutations in the nebulin gene. Neurology 2009, 73, 1159–1161. [Google Scholar] [CrossRef]
- Scoto, M.; Cullup, T.; Cirak, S.; Yau, S.; Manzur, A.Y.; Feng, L.; Jacques, T.S.; Anderson, G.; Abbs, S.; Sewry, C.; et al. Nebulin (NEB) mutations in a childhood onset distal myopathy with rods and cores uncovered by next generation sequencing. Eur. J. Hum. Genet. EJHG 2013, 21, 1249–1252. [Google Scholar] [CrossRef]
- Pappas, C.T.; Bliss, K.T.; Zieseniss, A.; Gregorio, C.C. The Nebulin family: An actin support group. Trends Cell Biol. 2011, 21, 29–37. [Google Scholar] [CrossRef]
- Ottenheijm, C.A.C.; Granzier, H.; Labeit, S. The sarcomeric protein nebulin: Another multifunctional giant in charge of muscle strength optimization. Front. Physiol 2012, 3, 37. [Google Scholar] [CrossRef][Green Version]
- Labeit, S.; Gibson, T.; Lakey, A.; Leonard, K.; Zeviani, M.; Knight, P.; Wardale, J.; Trinick, J. Evidence that nebulin is a protein-ruler in muscle thin filaments. FEBS Lett. 1991, 282, 313–316. [Google Scholar] [CrossRef]
- Donner, K.; Sandbacka, M.; Lehtokari, V.L.; Wallgren-Pettersson, C.; Pelin, K. Complete genomic structure of the human nebulin gene and identification of alternatively spliced transcripts. Eur. J. Hum. Genet. EJHG 2004, 12, 744–751. [Google Scholar] [CrossRef]
- Laitila, J.; Hanif, M.; Paetau, A.; Hujanen, S.; Keto, J.; Somervuo, P.; Huovinen, S.; Udd, B.; Wallgren-Pettersson, C.; Auvinen, P.; et al. Expression of multiple nebulin isoforms in human skeletal muscle and brain. Muscle Nerve 2012, 46, 730–737. [Google Scholar] [CrossRef]
- Marttila, M.; Hanif, M.; Lemola, E.; Nowak, K.J.; Laitila, J.; Grönholm, M.; Wallgren-Pettersson, C.; Pelin, K. Nebulin interactions with actin and tropomyosin are altered by disease-causing mutations. Skelet. Muscle 2014, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Pappas, C.T.; Bhattacharya, N.; Cooper, J.A.; Gregorio, C.C. Nebulin interacts with CapZ and regulates thin filament architecture within the Z-disc. Mol. Biol. Cell 2008, 19, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- McElhinny, A.S.; Kolmerer, B.; Fowler, V.M.; Labeit, S.; Gregorio, C.C. The N-terminal end of nebulin interacts with tropomodulin at the pointed ends of the thin filaments. J. Biol. Chem. 2001, 276, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Buck, D.; Hudson, B.D.; Ottenheijm, C.A.; Labeit, S.; Granzier, H. Differential splicing of the large sarcomeric protein nebulin during skeletal muscle development. J. Struct. Biol. 2010, 170, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Grange, M.; Pospich, S.; Wagner, T.; Kho, A.L.; Gautel, M.; Raunser, S. Structures from intact myofibrils reveal mechanism of thin filament regulation through nebulin. Science 2022, 375, eabn1934. [Google Scholar] [CrossRef] [PubMed]
- Bang, M.-L.; Li, X.; Littlefield, R.; Bremner, S.; Thor, A.; Knowlton, K.U.; Lieber, R.L.; Chen, J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J. Cell Biol. 2006, 173, 905–916. [Google Scholar] [CrossRef]
- Witt, C.C.; Burkart, C.; Labeit, D.; McNabb, M.; Wu, Y.; Granzier, H.; Labeit, S. Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J. 2006, 25, 3843–3855. [Google Scholar] [CrossRef]
- Ottenheijm, C.A.C.; Witt, C.C.; Stienen, G.J.; Labeit, S.; Beggs, A.H.; Granzier, H. Thin filament length dysregulation contributes to muscle weakness in nemaline myopathy patients with nebulin deficiency. Hum. Mol. Genet. 2009, 18, 2359–2369. [Google Scholar] [CrossRef]
- Pappas, C.T.; Krieg, P.A.; Gregorio, C.C. Nebulin regulates actin filament lengths by a stabilization mechanism. J. Cell Biol. 2010, 189, 859–870. [Google Scholar] [CrossRef]
- Gokhin, D.S.; Fowler, V.M. A two-segment model for thin filament architecture in skeletal muscle. Nat. Rev. Mol. Cell Biol. 2013, 14, 113–119. [Google Scholar] [CrossRef]
- Kiss, B.; Lee, E.J.; Ma, W.; Li, F.W.; Tonino, P.; Mijailovich, S.M.; Irving, T.C.; Granzier, H.L. Nebulin stiffens the thin filament and augments cross-bridge interaction in skeletal muscle. Proc. Natl. Acad. Sci. USA 2018, 115, 10369–10374. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Gohlke, J.; Tonino, P.; Hourani, Z.; Kolb, J.; Strom, J.; Alekhina, O.; Smith, J.E., 3rd; Ottenheijm, C.; Gregorio, C.; et al. Nebulin and Lmod2 are critical for specifying thin-filament length in skeletal muscle. Sci. Adv. 2020, 6, eabc1992. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Zhang, J.Q.; Nguyen, T.P.; Herrera, A.H.; Paterson, B.; Horowits, R. Complete cDNA sequence and tissue localization of N-RAP, a novel nebulin-related protein of striated muscle. Cell Motil. Cytoskelet. 1997, 38, 75–90. [Google Scholar] [CrossRef]
- Schreiber, V.; Moog-Lutz, C.; Régnier, C.H.; Chenard, M.-P.; Boeuf, H.; Vonesch, J.-L.; Tomasetto, C.; Rio, M.-C. Lasp-1, a Novel Type of Actin-Binding Protein Accumulating in Cell Membrane Extensions. Mol. Med. 1998, 4, 675–687. [Google Scholar] [CrossRef]
- Li, B.; Zhuang, L.; Trueb, B. Zyxin interacts with the SH3 domains of the cytoskeletal proteins LIM-nebulette and Lasp-1. J. Biol. Chem. 2004, 279, 20401–20410. [Google Scholar] [CrossRef]
- Wu, H.; Reynolds, A.B.; Kanner, S.B.; Vines, R.R.; Parsons, J.T. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol. 1991, 11, 5113–5124. [Google Scholar] [CrossRef]
- Moncman, C.L.; Wang, K. Nebulette: A 107 kD nebulin-like protein in cardiac muscle. Cell Motil. Cytoskelet. 1995, 32, 205–225. [Google Scholar] [CrossRef]
- Bang, M.L.; Chen, J. Roles of Nebulin Family Members in the Heart. Circ. J. 2015, 79, 2081–2087. [Google Scholar] [CrossRef]
- Kazmierski, S.T.; Antin, P.B.; Witt, C.C.; Huebner, N.; McElhinny, A.S.; Labeit, S.; Gregorio, C.C. The complete mouse nebulin gene sequence and the identification of cardiac nebulin. J. Mol. Biol. 2003, 328, 835–846. [Google Scholar] [CrossRef]
- Moncman, C.L.; Wang, K. Functional dissection of nebulette demonstrates actin binding of nebulin-like repeats and Z-line targeting of SH3 and linker domains. Cell Motil Cytoskelet. 1999, 44, 1–22. [Google Scholar] [CrossRef]
- Arimura, T.; Nakamura, T.; Hiroi, S.; Satoh, M.; Takahashi, M.; Ohbuchi, N.; Ueda, K.; Nouchi, T.; Yamaguchi, N.; Akai, J.; et al. Characterization of the human nebulette gene: A polymorphism in an actin-binding motif is associated with nonfamilial idiopathic dilated cardiomyopathy. Hum. Genet. 2000, 107, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Purevjav, E.; Varela, J.; Morgado, M.; Kearney, D.L.; Li, H.; Taylor, M.D.; Arimura, T.; Moncman, C.L.; McKenna, W.; Murphy, R.T.; et al. Nebulette mutations are associated with dilated cardiomyopathy and endocardial fibroelastosis. J. Am. Coll. Cardiol. 2010, 56, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Maiellaro-Rafferty, K.; Wansapura, J.P.; Mendsaikhan, U.; Osinska, H.; James, J.F.; Taylor, M.D.; Robbins, J.; Kranias, E.G.; Towbin, J.A.; Purevjav, E. Altered regional cardiac wall mechanics are associated with differential cardiomyocyte calcium handling due to nebulette mutations in preclinical inherited dilated cardiomyopathy. J. Mol. Cell. Cardiol. 2013, 60, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Perrot, A.; Tomasov, P.; Villard, E.; Faludi, R.; Melacini, P.; Lossie, J.; Lohmann, N.; Richard, P.; De Bortoli, M.; Angelini, A.; et al. Mutations in NEBL encoding the cardiac Z-disk protein nebulette are associated with various cardiomyopathies. Arch. Med. Sci. AMS 2016, 12, 263–278. [Google Scholar] [CrossRef]
- Ogut, O.; Hossain, M.M.; Jin, J.P. Interactions between nebulin-like motifs and thin filament regulatory proteins. J. Biol. Chem. 2003, 278, 3089–3097. [Google Scholar] [CrossRef]
- Esham, M.; Bryan, K.; Milnes, J.; Holmes, W.B.; Moncman, C.L. Expression of nebulette during early cardiac development. Cell Motil. Cytoskelet. 2007, 64, 258–273. [Google Scholar] [CrossRef]
- Mastrototaro, G.; Liang, X.; Li, X.; Carullo, P.; Piroddi, N.; Tesi, C.; Gu, Y.; Dalton, N.D.; Peterson, K.L.; Poggesi, C.; et al. Nebulette knockout mice have normal cardiac function, but show Z-line widening and up-regulation of cardiac stress markers. Cardiovasc. Res. 2015, 107, 216–225. [Google Scholar] [CrossRef]
- Fernandes, I.; Schöck, F. The nebulin repeat protein Lasp regulates I-band architecture and filament spacing in myofibrils. J. Cell Biol. 2014, 206, 559–572. [Google Scholar] [CrossRef]
- Wang, K.; McClure, J.; Tu, A. Titin: Major myofibrillar components of striated muscle. Proc. Natl. Acad. Sci. USA 1979, 76, 3698–3702. [Google Scholar] [CrossRef]
- Fürst, D.O.; Osborn, M.; Nave, R.; Weber, K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: A map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J. Cell Biol. 1988, 106, 1563–1572. [Google Scholar] [CrossRef]
- Trombitás, K.; Jin, J.P.; Granzier, H. The mechanically active domain of titin in cardiac muscle. Circ. Res. 1995, 77, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Kellermayer, M.S.; Smith, S.B.; Granzier, H.L.; Bustamante, C. Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science 1997, 276, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Yoshioka, T.; Higuchi, H.; Ohashi, K.; Kimura, S.; Natori, R. Connectin filaments link thick filaments and Z lines in frog skeletal muscle as revealed by immunoelectron microscopy. J. Cell Biol. 1985, 101, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Whiting, A.; Wardale, J.; Trinick, J. Does titin regulate the length of muscle thick filaments? J. Mol. Biol. 1989, 205, 263–268. [Google Scholar] [CrossRef]
- Bennett, P.M.; Gautel, M. Titin Domain Patterns Correlate with the Axial Disposition of Myosin at the End of the Thick Filament. J. Mol. Biol. 1996, 259, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.; Rees, M.; Gautel, M. The Axial Alignment of Titin on the Muscle Thick Filament Supports Its Role as a Molecular Ruler. J. Mol. Biol. 2020, 432, 4815–4829. [Google Scholar] [CrossRef]
- Tskhovrebova, L.; Trinick, J. Titin and Nebulin in Thick and Thin Filament Length Regulation. Subcell. Biochem. 2017, 82, 285–318. [Google Scholar] [CrossRef]
- Gregorio, C.C.; Trombitás, K.; Centner, T.; Kolmerer, B.; Stier, G.; Kunke, K.; Suzuki, K.; Obermayr, F.; Herrmann, B.; Granzier, H.; et al. The NH2 terminus of titin spans the Z-disc: Its interaction with a novel 19-kD ligand (T-cap) is required for sarcomeric integrity. J. Cell Biol. 1998, 143, 1013–1027. [Google Scholar] [CrossRef]
- Peckham, M.; Young, P.; Gautel, M. Constitutive and variable regions of Z-disk titin/connectin in myofibril formation: A dominant-negative screen. Cell Struct. Funct. 1997, 22, 95–101. [Google Scholar] [CrossRef][Green Version]
- Linke, W.A.; Ivemeyer, M.; Mundel, P.; Stockmeier, M.R.; Kolmerer, B. Nature of PEVK-titin elasticity in skeletal muscle. Proc. Natl. Acad. Sci. USA 1998, 95, 8052–8057. [Google Scholar] [CrossRef]
- Opitz, C.A.; Kulke, M.; Leake, M.C.; Neagoe, C.; Hinssen, H.; Hajjar, R.J.; Linke, W.A. Damped elastic recoil of the titin spring in myofibrils of human myocardium. Proc. Natl. Acad. Sci. USA 2003, 100, 12688–12693. [Google Scholar] [CrossRef]
- Granzier, H.L.; Labeit, S. Titin and its associated proteins: The third myofilament system of the sarcomere. Adv. Protein Chem. 2005, 71, 89–119. [Google Scholar] [CrossRef]
- Muhle-Goll, C.; Habeck, M.; Cazorla, O.; Nilges, M.; Labeit, S.; Granzier, H. Structural and functional studies of titin’s fn3 modules reveal conserved surface patterns and binding to myosin S1—A possible role in the Frank-Starling mechanism of the heart. J. Mol. Biol. 2001, 313, 431–447. [Google Scholar] [CrossRef]
- Freiburg, A.; Gautel, M. A molecular map of the interactions between titin and myosin-binding protein C. Implications for sarcomeric assembly in familial hypertrophic cardiomyopathy. Eur. J. Biochem. 1996, 235, 317–323. [Google Scholar] [CrossRef]
- Obermann, W.M.; Gautel, M.; Weber, K.; Fürst, D.O. Molecular structure of the sarcomeric M band: Mapping of titin and myosin binding domains in myomesin and the identification of a potential regulatory phosphorylation site in myomesin. EMBO J. 1997, 16, 211–220. [Google Scholar] [CrossRef]
- Kontrogianni-Konstantopoulos, A.; Ackermann, M.A.; Bowman, A.L.; Yap, S.V.; Bloch, R.J. Muscle giants: Molecular scaffolds in sarcomerogenesis. Physiol. Rev. 2009, 89, 1217–1267. [Google Scholar] [CrossRef]
- Tonino, P.; Kiss, B.; Strom, J.; Methawasin, M.; Smith, J.E.; Kolb, J.; Labeit, S.; Granzier, H. The giant protein titin regulates the length of the striated muscle thick filament. Nat. Commun. 2017, 8, 1041. [Google Scholar] [CrossRef]
- Prado, L.G.; Makarenko, I.; Andresen, C.; Krüger, M.; Opitz, C.A.; Linke, W.A. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J. Gen. Physiol. 2005, 126, 461–480. [Google Scholar] [CrossRef]
- Guo, W.; Bharmal, S.J.; Esbona, K.; Greaser, M.L. Titin diversity—Alternative splicing gone wild. J. Biomed. Biotechnol. 2010, 2010, 753675. [Google Scholar] [CrossRef]
- Greaser, M.L.; Pleitner, J.M. Titin isoform size is not correlated with thin filament length in rat skeletal muscle. Front. Physiol. 2014, 5, 35. [Google Scholar] [CrossRef][Green Version]
- Greaser, M.L.; Warren, C.M.; Esbona, K.; Guo, W.; Duan, Y.; Parrish, A.M.; Krzesinski, P.R.; Norman, H.S.; Dunning, S.; Fitzsimons, D.P.; et al. Mutation that dramatically alters rat titin isoform expression and cardiomyocyte passive tension. J. Mol. Cell. Cardiol. 2008, 44, 983–991. [Google Scholar] [CrossRef]
- Methawasin, M.; Hutchinson, K.R.; Lee, E.J.; Smith, J.E., 3rd; Saripalli, C.; Hidalgo, C.G.; Ottenheijm, C.A.; Granzier, H. Experimentally increasing titin compliance in a novel mouse model attenuates the Frank-Starling mechanism but has a beneficial effect on diastole. Circulation 2014, 129, 1924–1936. [Google Scholar] [CrossRef]
- Hooper, S.L.; Thuma, J.B. Invertebrate muscles: Muscle specific genes and proteins. Physiol. Rev. 2005, 85, 1001–1060. [Google Scholar] [CrossRef]
- Reedy, M.C.; Beall, C. Ultrastructure of developing flight muscle in Drosophila. I. Assembly of myofibrils. Dev. Biol. 1993, 160, 443–465. [Google Scholar] [CrossRef]
- Chun, M.; Falkenthal, S. Ifm(2)2 is a myosin heavy chain allele that disrupts myofibrillar assembly only in the indirect flight muscle of Drosophila melanogaster. J. Cell Biol. 1988, 107, 2613–2621. [Google Scholar] [CrossRef]
- Bernstein, S.I.; O’Donnell, P.T.; Cripps, R.M. Molecular Genetic Analysis of Muscle Development, Structure, and Function in Drosophila. In International Review of Cytology; Jeon, K.W., Friedlander, M., Jarvik, J., Eds.; Academic Press: Cambridge, MA, USA, 1993; Volume 143, pp. 63–152. [Google Scholar]
- Vigoreaux, J.O.; Saide, J.D.; Valgeirsdottir, K.; Pardue, M.L. Flightin, a novel myofibrillar protein of Drosophila stretch-activated muscles. J. Cell Biol. 1993, 121, 587–598. [Google Scholar] [CrossRef]
- Ayer, G.; Vigoreaux, J.O. Flightin is a myosin rod binding protein. Cell Biochem. Biophys. 2003, 38, 41–54. [Google Scholar] [CrossRef]
- Reedy, M.C.; Bullard, B.; Vigoreaux, J.O. Flightin is essential for thick filament assembly and sarcomere stability in Drosophila flight muscles. J. Cell Biol. 2000, 151, 1483–1500. [Google Scholar] [CrossRef]
- Contompasis, J.L.; Nyland, L.R.; Maughan, D.W.; Vigoreaux, J.O. Flightin Is Necessary for Length Determination, Structural Integrity, and Large Bending Stiffness of Insect Flight Muscle Thick Filaments. J. Mol. Biol. 2010, 395, 340–348. [Google Scholar] [CrossRef]
- Gasek, N.S.; Nyland, L.R.; Vigoreaux, J.O. The Contributions of the Amino and Carboxy Terminal Domains of Flightin to the Biomechanical Properties of Drosophila Flight Muscle Thick Filaments. Biology 2016, 5, 16. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szikora, S.; Görög, P.; Mihály, J. The Mechanisms of Thin Filament Assembly and Length Regulation in Muscles. Int. J. Mol. Sci. 2022, 23, 5306. https://doi.org/10.3390/ijms23105306
Szikora S, Görög P, Mihály J. The Mechanisms of Thin Filament Assembly and Length Regulation in Muscles. International Journal of Molecular Sciences. 2022; 23(10):5306. https://doi.org/10.3390/ijms23105306
Chicago/Turabian StyleSzikora, Szilárd, Péter Görög, and József Mihály. 2022. "The Mechanisms of Thin Filament Assembly and Length Regulation in Muscles" International Journal of Molecular Sciences 23, no. 10: 5306. https://doi.org/10.3390/ijms23105306
APA StyleSzikora, S., Görög, P., & Mihály, J. (2022). The Mechanisms of Thin Filament Assembly and Length Regulation in Muscles. International Journal of Molecular Sciences, 23(10), 5306. https://doi.org/10.3390/ijms23105306







