Insights into the Mechanism of Human Deiodinase 1
Abstract
:1. Introduction
2. Results
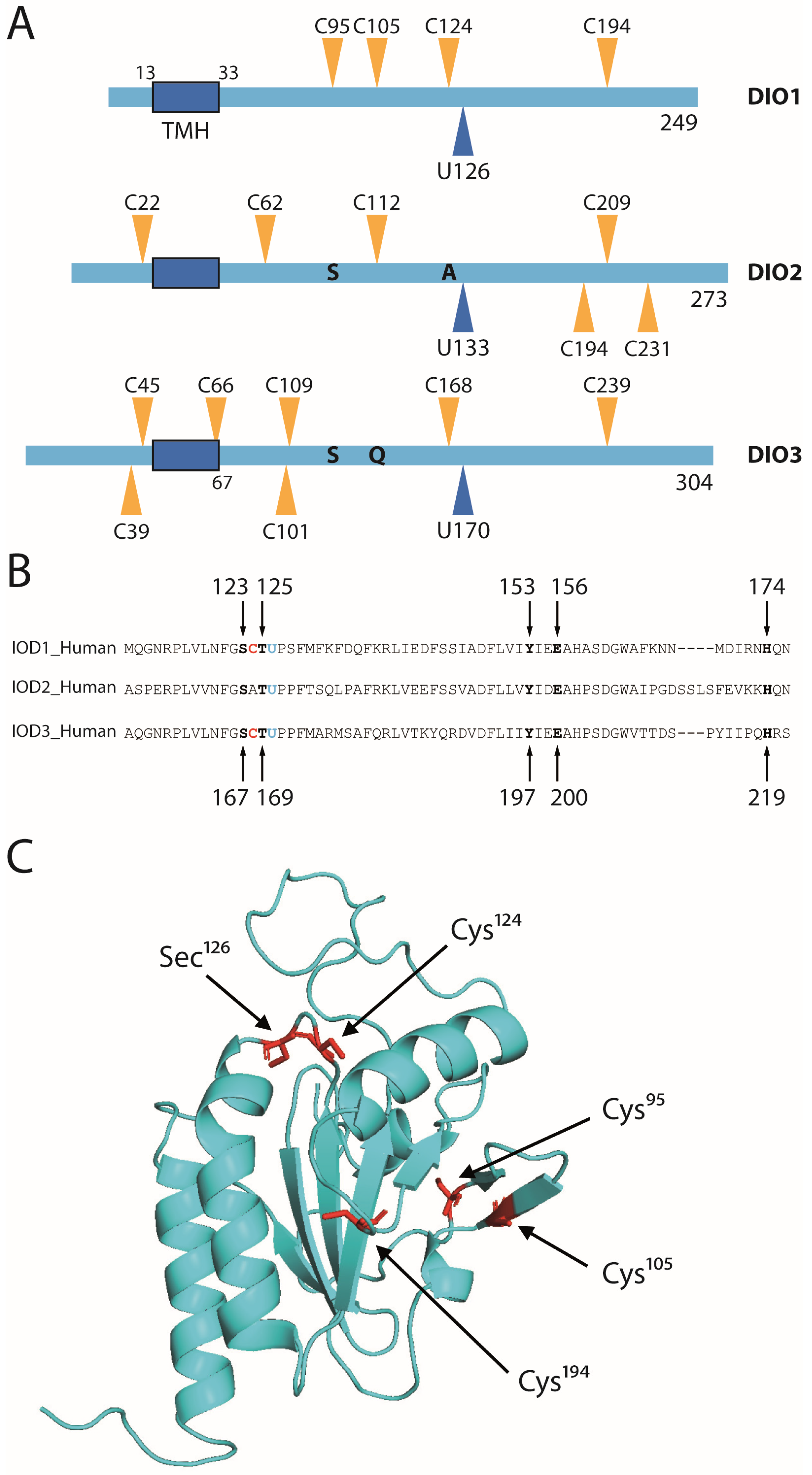
2.1. Conserved Amino Acids among Deiodinase Isoenzymes
2.2. Is There an Intermolecular Disulfide in the Mammalian DIO1 Homodimer?
2.3. Conserved Cysteines in the Mechanism of Reduction of DIO1
2.4. Analysis of Cysteine Disulfides in Recombinant DIO1U126C by Mass Spectrometry
2.5. Cys124 Is Essential for Reduction with GSH in DIO1 Containing Selenocysteine
2.6. New Evidence for a Proton Relay Pathway from Solvent to Substrate in DIO1
3. Discussion
3.1. The Mechanism: Re-Reduction of the Oxidized Deiodinase
3.2. The Mechanism: Proton Relay to the Active Center?
3.3. The Dimer
4. Materials and Methods
4.1. Cloning and Site-Directed Mutagenesis
4.2. Cell Culture
4.3. Bacmid Formation in Sf9 Cells
4.4. Transfection of Hi5 Cells for Recombinant Protein Expression of DIO1 Variants and Protein Preparation
4.5. Transfection of COS7 Cells
4.6. Western Blotting
4.7. Radioenzymatic Assay for 5′-Deiodination
4.8. Detection of Disulfide Bonds
4.9. Mass-Spectrometry-Based Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A





References
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dentice, M.; Marsili, A.; Zavacki, A.; Larsen, P.R.; Salvatore, D. The deiodinases and the control of intracellular thyroid hormone signaling during cellular differentiation. Biochim. Biophys. Acta 2013, 1830, 3937–3945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, S.; Raja, K.; Schweizer, U.; Mugesh, G. Chemistry and biology in the biosynthesis and action of thyroid hormones. Angew. Chem. Int. Ed. 2016, 55, 7606–7630. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J.; Maia, A.L.; Kieffer, J.D.; Harney, J.W.; Larsen, P.R. Substitution of cysteine for selenocysteine in type I iodothyronine deiodinase reduces the catalytic efficiency of the protein but enhances its translation. Endocrinology 1992, 131, 1848–1852. [Google Scholar] [CrossRef]
- Berry, M.J.; Banu, L.; Larsen, P.R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature 1991, 349, 438–440. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Klootwijk, W.; Visser, T.J. Substitution of cysteine for selenocysteine in the catalytic center of type III iodothyronine deiodinase reduces catalytic efficiency and alters substrate preference. Endocrinology 2003, 144, 2505–2513. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, U.; Schlicker, C.; Braun, D.; Kohrle, J.; Steegborn, C. Crystal structure of mammalian selenocysteine-dependent iodothyronine deiodinase suggests a peroxiredoxin-like catalytic mechanism. Proc. Natl. Acad. Sci. USA 2014, 111, 10526–10531. [Google Scholar] [CrossRef] [Green Version]
- Bayse, C.A.; Rafferty, E.R. Is halogen bonding the basis for iodothyronine deiodinase activity? Inorg. Chem. 2010, 49, 5365–5367. [Google Scholar] [CrossRef]
- Bhat, G.B.; Iwase, K.; Hummel, B.C.; Walfish, P.G. Kinetic characteristics of a thioredoxin-activated rat hepatic and renal low-Km iodothyronine 5′-deiodinase. Biochem. J. 1989, 258, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Croteau, W.; Bodwell, J.E.; Richardson, J.M.; Germain, D.L.S. Conserved Cysteines in the Type 1 Deiodinase Selenoprotein Are Not Essential for Catalytic Activity. J. Biol. Chem. 1998, 273, 25230–25236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, A.; Rosenberg, I.N. Iodothyronine 5’-deiodinase in rat kidney microsomes. Kinetic behavior at low substrate concentrations. J. Clin. Investig. 1984, 74, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Aerts, G.; Arrojo e Drigo, R.; Van Herck, S.L.J.; Sammels, E.; Mirebeau-Prunier, D.; Gereben, B.; Zeöld, A.; Harney, J.W.; Huang, S.A.; Mulcahey, M.A.; et al. Knockdown of the Type 3 Iodothyronine Deiodinase (D3) Interacting Protein Peroxiredoxin 3 Decreases D3-Mediated Deiodination in Intact Cells. Endocrinology 2009, 150, 5171–5180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steegborn, C.; Schweizer, U. Structure and Mechanism of Iodothyronine Deiodinases-What We Know, What We Don’t Know, and What Would Be Nice to Know. Exp. Clin. Endocrinol. Diabetes J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2020, 128, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Bayse, C.A.; Marsan, E.S.; Garcia, J.R.; Tran-Thompson, A.T. Thyroxine binding to type III iodothyronine deiodinase. Sci. Rep. 2020, 10, 15401. [Google Scholar] [CrossRef]
- Sagar, G.D.V.; Gereben, B.; Callebaut, I.; Mornon, J.-P.; Zeöld, A.; da Silva, W.S.; Luongo, C.; Dentice, M.; Tente, S.M.; Freitas, B.C.G.; et al. Ubiquitination-Induced Conformational Change within the Deiodinase Dimer Is a Switch Regulating Enzyme Activity. Mol. Cell. Biol. 2007, 27, 4774–4783. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, G.G.J.M.; Klootwijk, W.; Visser, T.J. Expression of recombinant membrane-bound type I iodothyronine deiodinase in yeast. J. Mol. Endocrinol. 2005, 34, 865–878. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Kester, M.H.; Peeters, R.P.; Visser, T.J. Biochemical mechanisms of thyroid hormone deiodination. Thyroid 2005, 15, 787–798. [Google Scholar] [CrossRef]
- Goto, K.; Sonoda, D.; Shimada, K.; Sase, S.; Kawashima, T. Modeling of the 5′-deiodination of thyroxine by iodothyronine deiodinase: Chemical corroboration of a selenenyl iodide intermediate. Angew. Chem. Int. Ed. Engl. 2010, 49, 545–547. [Google Scholar] [CrossRef]
- Masuda, R.; Kimura, R.; Karasaki, T.; Sase, S.; Goto, K. Modeling the Catalytic Cycle of Glutathione Peroxidase by Nuclear Magnetic Resonance Spectroscopic Analysis of Selenocysteine Selenenic Acids. J. Am. Chem. Soc. 2021, 143, 6345–6350. [Google Scholar] [CrossRef]
- Callebaut, I.; Curcio-Morelli, C.; Mornon, J.P.; Gereben, B.; Buettner, C.; Huang, S.; Castro, B.; Fonseca, T.L.; Harney, J.W.; Larsen, P.R.; et al. The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase clan GH-A-like structure. J. Biol. Chem. 2003, 278, 36887–36896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.C.; Harney, J.W.; Berry, M.J.; Larsen, P.R. The role of the active site cysteine in catalysis by type 1 iodothyronine deiodinase. Endocrinology 1997, 138, 5452–5458. [Google Scholar] [CrossRef] [PubMed]
- Manna, D.; Mugesh, G. Regioselective deiodination of thyroxine by iodothyronine deiodinase mimics: An unusual mechanistic pathway involving cooperative chalcogen and halogen bonding. J. Am. Chem. Soc. 2012, 134, 4269–4279. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.S.; Didierjean, C.; Navrot, N.; Panjikar, S.; Mulliert, G.; Rouhier, N.; Jacquot, J.P.; Aubry, A.; Shawkataly, O.; Corbier, C. Crystal structures of a poplar thioredoxin peroxidase that exhibits the structure of glutathione peroxidases: Insights into redox-driven conformational changes. J. Mol. Biol. 2007, 370, 512–529. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J. Identification of essential histidine residues in rat type I iodothyronine deiodinase. J. Biol. Chem. 1992, 267, 18055–18059. [Google Scholar] [CrossRef]
- Curcio-Morelli, C.; Gereben, B.; Zavacki, A.M.; Kim, B.W.; Huang, S.; Harney, J.W.; Larsen, P.R.; Bianco, A.C. In vivo dimerization of types 1, 2, and 3 iodothyronine selenodeiodinases. Endocrinology 2003, 144, 937–946. [Google Scholar] [CrossRef] [Green Version]
- Sagar, G.D.; Gereben, B.; Callebaut, I.; Mornon, J.P.; Zeold, A.; Curcio-Morelli, C.; Harney, J.W.; Luongo, C.; Mulcahey, M.A.; Larsen, P.R.; et al. The thyroid hormone-inactivating deiodinase functions as a homodimer. Mol. Endocrinol. 2008, 22, 1382–1393. [Google Scholar] [CrossRef] [Green Version]
- Renko, K.; Schache, S.; Hoefig, C.S.; Welsink, T.; Schwiebert, C.; Braun, D.; Becker, N.P.; Koehrle, J.; Schomburg, L. An improved non-radioactive screening method identifies genistein and xanthohumol as potent inhibitors of iodothyronine deiodinases. Thyroid 2015, 25, 962–968. [Google Scholar] [CrossRef]
- Bac-to-Bac® Baculovirus Expression System. An Efficient Cloning and Site-Specific Transposition System to Generate Recombinant Baculovirus for High-Level Protein Expression. Catalog Nos. A11101, A11100. Version A. Available online: http://tools.thermofisher.com/content/sfs/manuals/bactobac_topo_exp_system_man.pdf (accessed on 1 January 2022).
- Philipps, B.; Rotmann, D.; Wicki, M.; Mayr, L.M.; Forstner, M. Time reduction and process optimization of the baculovirus expression system for more efficient recombinant protein production in insect cells. Protein Expr. Purif. 2005, 42, 211–218. [Google Scholar] [CrossRef]
- Kinne, A.; Roth, S.; Biebermann, H.; Kohrle, J.; Gruters, A.; Schweizer, U. Surface translocation and tri-iodothyronine uptake of mutant MCT8 proteins are cell type-dependent. J. Mol. Endocrinol. 2009, 43, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Partis, M.; Griffiths, D.G.; Roberts, G.; Beechey, R.B. Cross-linking of protein by ω-maleimido alkanoylN-hydroxysuccinimido esters. J. Protein Chem. 1983, 2, 263–276. [Google Scholar] [CrossRef]
- Burns, J.A.; Butler, J.C.; Moran, J.; Whitesides, G.M. Selective reduction of disulfides by tris(2-carboxyethyl)phosphine. J. Org. Chem. 1991, 56, 2648–2650. [Google Scholar] [CrossRef]
- Rosenfeld, J.; Capdevielle, J.; Guillemot, J.C.; Ferrara, P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 1992, 203, 173–179. [Google Scholar] [CrossRef]
- Jenö, P.; Mini, T.; Moes, S.; Hintermann, E.; Horst, M. Internal sequences from proteins digested in polyacrylamide gels. Anal. Biochem. 1995, 224, 75–82. [Google Scholar] [CrossRef] [PubMed]
- DiANNA 1.1. Available online: http://clavius.bc.edu/~clotelab/DiANNA/ (accessed on 1 January 2020).
- Pino, L.K.; Searle, B.C.; Bollinger, J.G.; Nunn, B.; MacLean, B.; MacCoss, M.J. The Skyline ecosystem: Informatics for quantitative mass spectrometry proteomics. Mass Spectrom. Rev. 2020, 39, 229–244. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Ruiz, A.; Braun, D.; Pflug, S.; Brol, A.; Sylvester, M.; Steegborn, C.; Schweizer, U. Insights into the Mechanism of Human Deiodinase 1. Int. J. Mol. Sci. 2022, 23, 5361. https://doi.org/10.3390/ijms23105361
Rodriguez-Ruiz A, Braun D, Pflug S, Brol A, Sylvester M, Steegborn C, Schweizer U. Insights into the Mechanism of Human Deiodinase 1. International Journal of Molecular Sciences. 2022; 23(10):5361. https://doi.org/10.3390/ijms23105361
Chicago/Turabian StyleRodriguez-Ruiz, Alfonso, Doreen Braun, Simon Pflug, Alexander Brol, Marc Sylvester, Clemens Steegborn, and Ulrich Schweizer. 2022. "Insights into the Mechanism of Human Deiodinase 1" International Journal of Molecular Sciences 23, no. 10: 5361. https://doi.org/10.3390/ijms23105361
APA StyleRodriguez-Ruiz, A., Braun, D., Pflug, S., Brol, A., Sylvester, M., Steegborn, C., & Schweizer, U. (2022). Insights into the Mechanism of Human Deiodinase 1. International Journal of Molecular Sciences, 23(10), 5361. https://doi.org/10.3390/ijms23105361





