Mitochondria-Mediated Cardiovascular Benefits of Sodium-Glucose Co-Transporter 2 Inhibitors
Abstract
:1. Introduction
2. Proposed Pharmacological Mechanisms of SGLT2 Inhibitor Effects
2.1. The Diuretic Hypothesis
2.2. The “Thrifty Substrate” Hypothesis
2.3. The Sodium Hypothesis
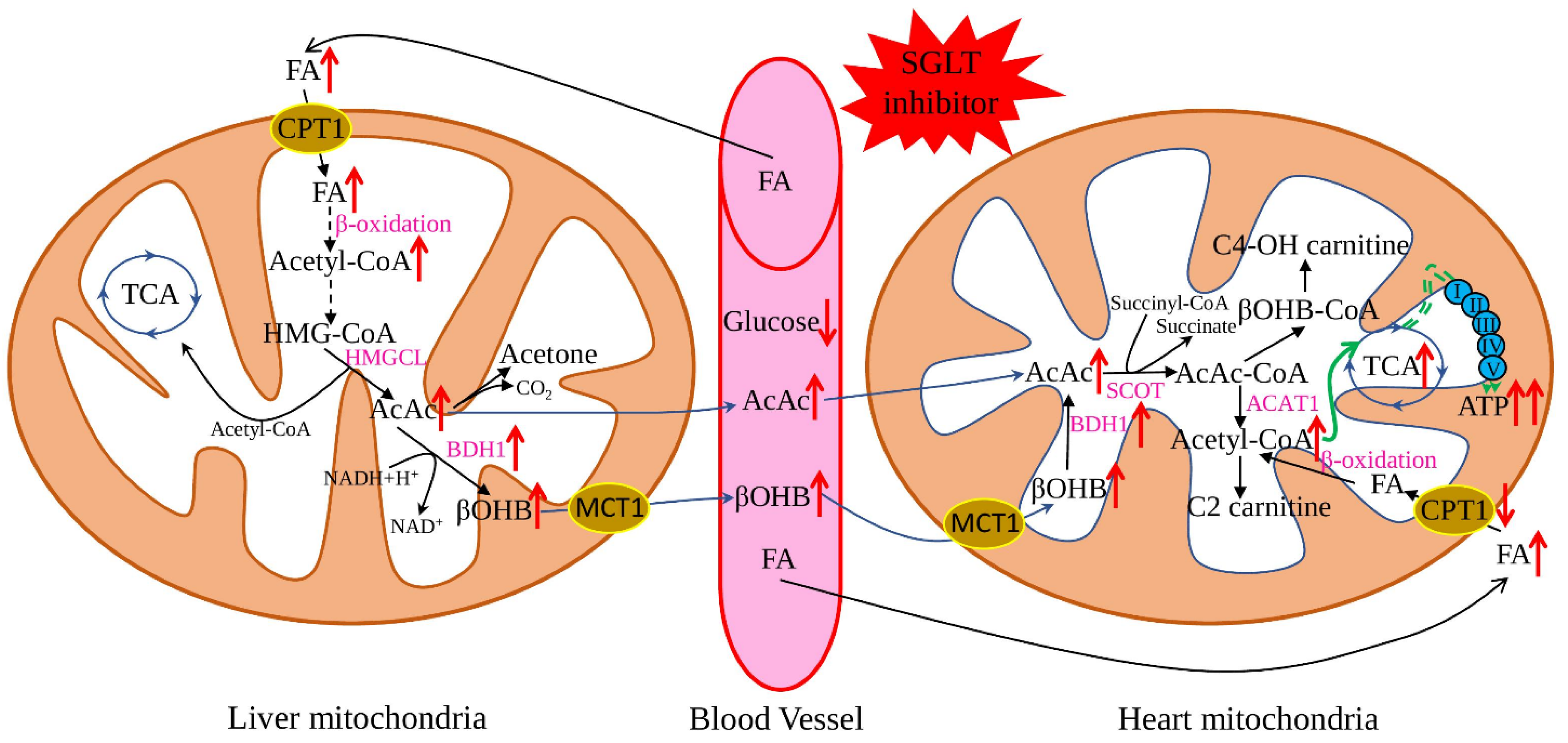
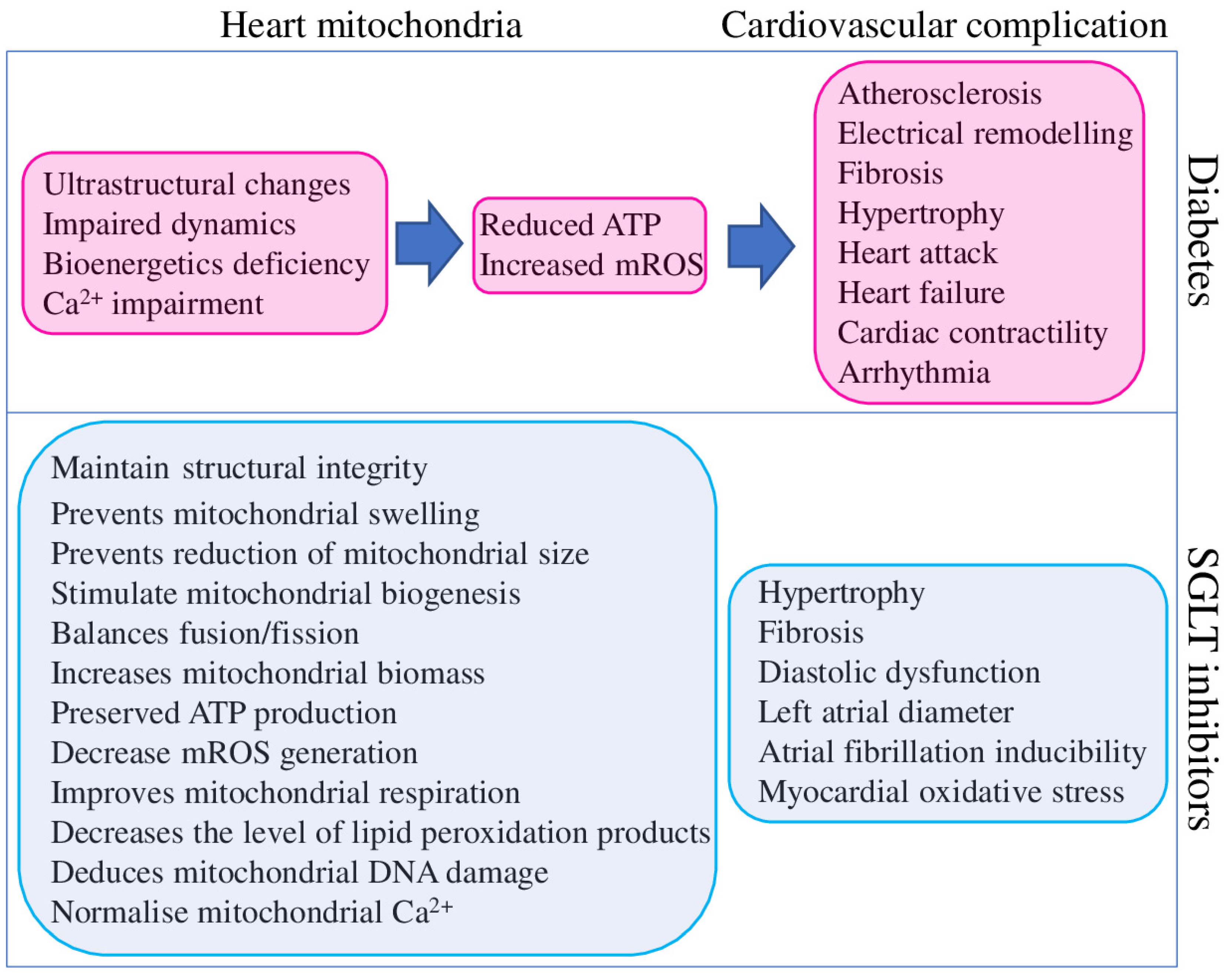
3. Effect of SGLT2 Inhibitors on Mitochondria
- mROS could directly activate CaMKII (Ca2+/calmodulin dependent kinase II), a multifunctional nodal regulator of many cellular pathways, including excitation-contraction coupling [108].
- increased mROS combined with hyperglycaemia provide persistent CaMKII activation, a major driver of arrhythmogenicity in diabetic hearts [109].
- chronic hyperglycaemia and CaMKII activation downregulate K+ channel expression and function in the NOX2-ROS-PKC (NADPH oxidase 2–ROS-protein kinase C) pathway, which increases arrhythmia risk [110].
- low ATP levels could suppress the activity of SERCA and Na+/K+-ATPase, which will alter Ca2+ homeostasis and increase arrhythmia risk [111].
- such pathological deficiencies could promote cardiomyocyte hypertrophy and interstitial fibrosis, two critical drivers of arrhythmia—reviewed in [112].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AcAc CoA | acetoacetyl CoA |
| ACAT1 | acetyl-CoA acetyltransferase |
| AF | atrial fibrillation |
| ATP | adenosine triphosphate |
| BDH1 | mitochondrial β-hydroxybutyrate dehydrogenase |
| C2-carnitine | acetylcarnitine, |
| C4-OH carnitine | hydroxy butyryl carnitine |
| CA | cardiac arrest |
| CACNA1C | voltage-dependent L-type calcium channel or LTCC |
| CaMKII | Ca2+/calmodulin dependent kinase II |
| CaT | Ca2+ transients |
| CMEC | cardiac microvascular endothelial cell |
| CPT1 | carnitine palmitoyltransferase 1 |
| CVD | cardiovascular diseases |
| DCMP | diabetic cardiomyopathy |
| DKD | diabetic kidney disease |
| DNM1L | dynamin 1 like |
| FA | fatty acids |
| FAM | fatty acid metabolism |
| FAO | fatty acid oxidation |
| FIS1 | fission, mitochondrial 1 |
| GSK-3α | glycogen synthase kinase-3α |
| HFD | high-fat diet |
| HfpEF | heart failure with a preserved ejection fraction |
| HMGCL | 3-hydroxy-3-methylglutaryl-coenzyme A lyase |
| HMG-CoA | 3-hydroxy-3-methtylglutaryl-CoA |
| hRPTCs | human renal proximal tubular cells |
| HSD | high-sucrose diet |
| LA | left atrial |
| LCCs | L-type Ca2+-channels |
| MCT1 | monocarboxylate transporter 1 |
| MCU | mitochondrial Ca2+ uniporter |
| MFN1, MFN2 | mitofusin 1 and 2 |
| NCLX | mitochondrial Na+/Ca2+ exchanger |
| MMP9 | matrix metalloproteinase 9 |
| mROS | mitochondrial reactive oxygen species |
| NCX1 | sarcolemmal Na+/Ca2+-Exchange Protein 1 |
| NHE | sarcolemmal Na+/H+ -exchanger |
| NKA | Na+/K+-ATPase |
| NOX2 | NADPH oxidase 2 |
| OPA1 | optic atrophy protein 1 |
| OS | oxidative stress |
| OXPHOS | oxidative phosphorylation |
| PCAL | permanent coronary artery ligation |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PKC | protein kinase C |
| PPAR-α | peroxisome proliferator-activated receptor alpha |
| RyR | ryanodine receptor |
| ScaEs | spontaneous Ca2+ release events |
| SCaEs | spontaneous Ca2+ release events |
| SCOT | Succinyl-CoA:3-oxoacid-CoA transferase |
| SIRT7 | NAD-dependent protein-lysine deacylase 7 |
| SERCA | sarco/endoplasmic reticulum Ca2+-ATPase |
| SGK1 | serum/glucocorticoid regulated kinase 1 |
| SGLTSOD2 | Na+-glucose co-transportersuperoxide dismutase 2 |
| TCA | tricarboxylic acid cycle |
| T2DMTFAM | type 2 diabetes mellitus transcription factor A, mitochondrial |
| βOHB | β-hydroxybutyrate |
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- American Diabetes Association. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S103–S123. [Google Scholar] [CrossRef] [Green Version]
- Summerhill, V.I.; Grechko, A.V.; Yet, S.F.; Sobenin, I.A.; Orekhov, A.N. The Atherogenic Role of Circulating Modified Lipids in Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 3561. [Google Scholar] [CrossRef] [Green Version]
- Laiteerapong, N.; Ham, S.A.; Gao, Y.; Moffet, H.H.; Liu, J.Y.; Huang, E.S.; Karter, A.J. The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care 2019, 42, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Ohkuma, T.; Cooper, M.; Harrap, S.; Mancia, G.; Poulter, N.; Wang, J.-G.; Zoungas, S.; Woodward, M.; Chalmers, J. Effects of Intensive Glycemic Control on Clinical Outcomes Among Patients With Type 2 Diabetes With Different Levels of Cardiovascular Risk and Hemoglobin A1c in the ADVANCE Trial. Diabetes Care 2020, 43, 1293–1299. [Google Scholar] [CrossRef]
- Ohkuma, T.; Chalmers, J.; Cooper, M.; Hamet, P.; Harrap, S.; Marre, M.; Mancia, G.; Poulter, N.; Woodward, M. The Comparative Effects of Intensive Glucose Lowering in Diabetes Patients Aged below or above 65 Years: Results from the ADVANCE Trial. Diabetes Obes. Metab. 2021, 23, 1292–1300. [Google Scholar] [CrossRef]
- Reaven, P.D.; Emanuele, N.V.; Wiitala, W.L.; Bahn, G.D.; Reda, D.J.; McCarren, M.; Duckworth, W.C.; Hayward, R.A. VADT Investigators Intensive Glucose Control in Patients with Type 2 Diabetes—15-Year Follow-Up. N. Engl. J. Med. 2019, 380, 2215–2224. [Google Scholar] [CrossRef]
- Crane, R.K. Intestinal Absorption of Sugars. Physiol. Rev. 1960, 40, 789–825. [Google Scholar] [CrossRef]
- Hediger, M.A.; Coady, M.J.; Ikeda, T.S.; Wright, E.M. Expression Cloning and CDNA Sequencing of the Na+/Glucose Co-Transporter. Nature 1987, 330, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of Renal Glucose Handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sędzikowska, A.; Szablewski, L. Human Glucose Transporters in Renal Glucose Homeostasis. Int. J. Mol. Sci. 2021, 22, 13522. [Google Scholar] [CrossRef] [PubMed]
- FDA. Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors; FDA: Silver Spring, MD, USA, 2018. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/sodium-glucose-cotransporter-2-sglt2-inhibitors (accessed on 6 March 2022).
- Vallon, V.; Thomson, S.C. Targeting Renal Glucose Reabsorption to Treat Hyperglycaemia: The Pleiotropic Effects of SGLT2 Inhibition. Diabetologia 2017, 60, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Xu, L.; Chen, P.; Jiang, H.; Shi, D.; Guo, M. Empagliflozin in Patients With Heart Failure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021, 8, 683281. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, J.R.L.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A Review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef]
- Grempler, R.; Thomas, L.; Eckhardt, M.; Himmelsbach, F.; Sauer, A.; Sharp, D.E.; Bakker, R.A.; Mark, M.; Klein, T.; Eickelmann, P. Empagliflozin, a Novel Selective Sodium Glucose Cotransporter-2 (SGLT-2) Inhibitor: Characterisation and Comparison with Other SGLT-2 Inhibitors. Diabetes Obes. Metab. 2012, 14, 83–90. [Google Scholar] [CrossRef]
- Kwak, S.H.; Hwang, Y.-C.; Won, J.C.; Bae, J.C.; Kim, H.J.; Suh, S.; Lee, E.Y.; Lee, S.; Kim, S.-Y.; Kim, J.H. Comparison of the Effects of Gemigliptin and Dapagliflozin on Glycaemic Variability in Type 2 Diabetes: A Randomized, Open-Label, Active-Controlled, 12-Week Study (STABLE II Study). Diabetes Obes. Metab. 2020, 22, 173–181. [Google Scholar] [CrossRef]
- McNeill, A.M.; Davies, G.; Kruger, E.; Kowal, S.; Reason, T.; Ejzykowicz, F.; Hannachi, H.; Cater, N.; McLeod, E. Ertugliflozin Compared to Other Anti-Hyperglycemic Agents as Monotherapy and Add-on Therapy in Type 2 Diabetes: A Systematic Literature Review and Network Meta-Analysis. Diabetes Ther. 2019, 10, 473–491. [Google Scholar] [CrossRef] [Green Version]
- Kanbay, M.; Tapoi, L.; Ureche, C.; Tanriover, C.; Cevik, E.; Demiray, A.; Afsar, B.; Cherney, D.Z.I.; Covic, A. Effect of Sodium-Glucose Cotransporter 2 Inhibitors on Hemoglobin and Hematocrit Levels in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. Urol. Nephrol. 2021, 54, 827–841. [Google Scholar] [CrossRef]
- Ott, C.; Jumar, A.; Striepe, K.; Friedrich, S.; Karg, M.V.; Bramlage, P.; Schmieder, R.E. A Randomised Study of the Impact of the SGLT2 Inhibitor Dapagliflozin on Microvascular and Macrovascular Circulation. Cardiovasc. Diabetol. 2017, 16, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bommel, E.J.M.; Muskiet, M.H.A.; van Baar, M.J.B.; Tonneijck, L.; Smits, M.M.; Emanuel, A.L.; Bozovic, A.; Danser, A.H.J.; Geurts, F.; Hoorn, E.J.; et al. The Renal Hemodynamic Effects of the SGLT2 Inhibitor Dapagliflozin Are Caused by Post-Glomerular Vasodilatation Rather than Pre-Glomerular Vasoconstriction in Metformin-Treated Patients with Type 2 Diabetes in the Randomized, Double-Blind RED Trial. Kidney Int. 2020, 97, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, X.; Zhang, J.; Li, Y.; Del Gobbo, L.C.; Zhai, S.; Song, Y. Elevated Serum Magnesium Associated with SGLT2 Inhibitor Use in Type 2 Diabetes Patients: A Meta-Analysis of Randomised Controlled Trials. Diabetologia 2016, 59, 2546–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkenfeld, A.L.; Jordan, J.; Dworak, M.; Merkel, T.; Burnstock, G. Myocardial Metabolism in Heart Failure: Purinergic Signalling and Other Metabolic Concepts. Pharmacol. Ther. 2019, 194, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Song, J.D.; Alves, T.C.; Befroy, D.E.; Perry, R.J.; Mason, G.F.; Zhang, X.-M.; Munk, A.; Zhang, Y.; Zhang, D.; Cline, G.W.; et al. Dissociation of Muscle Insulin Resistance from Alterations in Mitochondrial Substrate Preference. Cell Metabolism 2020, 32, 726–735.e5. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Salonen, J.T.; Zhelankin, A.V.; Melnichenko, A.A.; Kaikkonen, J.; Bobryshev, Y.V.; Orekhov, A.N. Low Density Lipoprotein-Containing Circulating Immune Complexes: Role in Atherosclerosis and Diagnostic Value. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Mereweather, L.J.; Montes Aparicio, C.N.; Heather, L.C. Positioning Metabolism as a Central Player in the Diabetic Heart. J. Lipid Atheroscler. 2020, 9, 92–109. [Google Scholar] [CrossRef]
- Strand, E.; Lysne, V.; Grinna, M.L.; Bohov, P.; Svardal, A.; Nygård, O.; Berge, R.K.; Bjørndal, B. Short-Term Activation of Peroxisome Proliferator-Activated Receptors α and γ Induces Tissue-Specific Effects on Lipid Metabolism and Fatty Acid Composition in Male Wistar Rats. PPAR Res. 2019, 2019, 8047627. [Google Scholar] [CrossRef] [Green Version]
- Sikder, K.; Shukla, S.K.; Patel, N.; Singh, H.; Rafiq, K. High Fat Diet Upregulates Fatty Acid Oxidation and Ketogenesis via Intervention of PPAR-γ. Cell. Physiol. Biochem. 2018, 48, 1317–1331. [Google Scholar] [CrossRef]
- Toral, M.; Romero, M.; Pérez-Vizcaíno, F.; Duarte, J.; Jiménez, R. Antihypertensive Effects of Peroxisome Proliferator-Activated Receptor-β/δ Activation. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H189–H200. [Google Scholar] [CrossRef] [Green Version]
- Papatheodorou, I.; Galatou, E.; Panagiotidis, G.-D.; Ravingerová, T.; Lazou, A. Cardioprotective Effects of PPARβ/δ Activation against Ischemia/Reperfusion Injury in Rat Heart Are Associated with ALDH2 Upregulation, Amelioration of Oxidative Stress and Preservation of Mitochondrial Energy Production. Int. J. Mol. Sci. 2021, 22, 6399. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Wang, M.; Ma, L.; Wang, Q.; Gao, P.; Tian, X.; Li, C.; Lu, L.; Li, C.; Wang, W.; et al. β-Elemene Blocks Lipid-Induced Inflammatory Pathways via PPARβ Activation in Heart Failure. Eur. J. Pharmacol. 2021, 910, 174450. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Sun, Q.; Lopaschuk, G.D. The Contribution of Cardiac Fatty Acid Oxidation to Diabetic Cardiomyopathy Severity. Cells 2021, 10, 3259. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.; Revin, V.; Sobenin, I.; Orekhov, A.; Bobryshev, Y. Vascular Endothelium: Functioning in Norm, Changes in Atherosclerosis and Current Dietary Approaches to Improve Endothelial Function. MRMC 2015, 15, 338–350. [Google Scholar] [CrossRef]
- Ferrannini, E.; Mark, M.; Mayoux, E. CV Protection in the EMPA-REG OUTCOME Trial: A “Thrifty Substrate” Hypothesis. Diabetes Care 2016, 39, 1108–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudaliar, S.; Alloju, S.; Henry, R.R. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care 2016, 39, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Tajima, T.; Yoshifuji, A.; Matsui, A.; Itoh, T.; Uchiyama, K.; Kanda, T.; Tokuyama, H.; Wakino, S.; Itoh, H. β-Hydroxybutyrate Attenuates Renal Ischemia-Reperfusion Injury through Its Anti-Pyroptotic Effects. Kidney Int. 2019, 95, 1120–1137. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Rong, X.; Yang, J.; Lu, Y. Evaluation of the Antioxidant Effects of Different Histone Deacetylase Inhibitors (HDACis) on Human Lens Epithelial Cells (HLECs) after UVB Exposure. BMC Ophthalmol. 2019, 19, 42. [Google Scholar] [CrossRef] [Green Version]
- Chriett, S.; Dąbek, A.; Wojtala, M.; Vidal, H.; Balcerczyk, A.; Pirola, L. Prominent Action of Butyrate over β-Hydroxybutyrate as Histone Deacetylase Inhibitor, Transcriptional Modulator and Anti-Inflammatory Molecule. Sci. Rep. 2019, 9, 742. [Google Scholar] [CrossRef] [Green Version]
- Szekeres, Z.; Toth, K.; Szabados, E. The Effects of SGLT2 Inhibitors on Lipid Metabolism. Metabolites 2021, 11, 87. [Google Scholar] [CrossRef]
- Pietschner, R.; Kolwelter, J.; Bosch, A.; Striepe, K.; Jung, S.; Kannenkeril, D.; Ott, C.; Schiffer, M.; Achenbach, S.; Schmieder, R.E. Effect of Empagliflozin on Ketone Bodies in Patients with Stable Chronic Heart Failure. Cardiovasc. Diabetol. 2021, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Yurista, S.R.; Silljé, H.H.W.; Oberdorf-Maass, S.U.; Schouten, E.-M.; Pavez Giani, M.G.; Hillebrands, J.-L.; van Goor, H.; van Veldhuisen, D.J.; de Boer, R.A.; Westenbrink, B.D. Sodium-Glucose Co-Transporter 2 Inhibition with Empagliflozin Improves Cardiac Function in Non-Diabetic Rats with Left Ventricular Dysfunction after Myocardial Infarction. Eur. J. Heart Fail. 2019, 21, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Empagliflozin’s Fuel Hypothesis: Not so Soon. Cell Metab. 2016, 24, 200–202. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Liu, T.; Husain, S.; Zhai, P.; Warren, J.S.; Hsu, C.-P.; Matsuda, T.; Phiel, C.J.; Cox, J.E.; Tian, B.; et al. Glycogen Synthase Kinase-3α Promotes Fatty Acid Uptake and Lipotoxic Cardiomyopathy. Cell Metab. 2019, 29, 1119–1134.e12. [Google Scholar] [CrossRef]
- Neubauer, S. The Failing Heart—An Engine out of Fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, R.; Møller, N.; Gormsen, L.C.; Tolbod, L.P.; Hansson, N.H.; Sorensen, J.; Harms, H.J.; Frøkiær, J.; Eiskjaer, H.; Jespersen, N.R.; et al. Cardiovascular Effects of Treatment With the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation 2019, 139, 2129–2141. [Google Scholar] [CrossRef]
- Takahara, S.; Soni, S.; Phaterpekar, K.; Kim, T.T.; Maayah, Z.H.; Levasseur, J.L.; Silver, H.L.; Freed, D.H.; Ferdaoussi, M.; Dyck, J.R.B. Chronic Exogenous Ketone Supplementation Blunts the Decline of Cardiac Function in the Failing Heart. ESC Heart Fail. 2021, 8, 5606–5612. [Google Scholar] [CrossRef]
- Byrne, N.J.; Soni, S.; Takahara, S.; Ferdaoussi, M.; Al Batran, R.; Darwesh, A.M.; Levasseur, J.L.; Beker, D.; Vos, D.Y.; Schmidt, M.A.; et al. Chronically Elevating Circulating Ketones Can Reduce Cardiac Inflammation and Blunt the Development of Heart Failure. Circ. Heart Fail. 2020, 13, e006573. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Orekhov, A.N.; Bobryshev, Y.V. Myeloid Dendritic Cells: Development, Functions, and Role in Atherosclerotic Inflammation. Immunobiology 2015, 220, 833–844. [Google Scholar] [CrossRef]
- Yurista, S.R.; Matsuura, T.R.; Silljé, H.H.W.; Nijholt, K.T.; McDaid, K.S.; Shewale, S.V.; Leone, T.C.; Newman, J.C.; Verdin, E.; van Veldhuisen, D.J.; et al. Ketone Ester Treatment Improves Cardiac Function and Reduces Pathologic Remodeling in Preclinical Models of Heart Failure. Circ. Heart Fail. 2021, 14, e007684. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, K.; Liang, L.; Liu, Y.; Song, F.; Ge, Q.; Fang, X.; Yu, T.; Huang, Z.; Jiang, L.; et al. Sodium–Glucose Co-Transporter 2 Inhibition With Empagliflozin Improves Cardiac Function After Cardiac Arrest in Rats by Enhancing Mitochondrial Energy Metabolism. Front. Pharmacol. 2021, 12, 758080. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.L.; Zhang, L.; Wagg, C.; Al Batran, R.; Gopal, K.; Levasseur, J.; Leone, T.; Dyck, J.R.B.; Ussher, J.R.; Muoio, D.M.; et al. Increased Ketone Body Oxidation Provides Additional Energy for the Failing Heart without Improving Cardiac Efficiency. Cardiovasc. Res. 2019, 115, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tao, H.; Cao, W.; Cao, L.; Lin, Y.; Zhao, S.-M.; Xu, W.; Cao, J.; Zhao, J.-Y. Ketogenic Diets Inhibit Mitochondrial Biogenesis and Induce Cardiac Fibrosis. Signal. Transduct. Target. Ther. 2021, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.-W.; Liang, Y.-L.; Zhang, Q.-X.; Wang, D.; Lei, M.-Z.; Qu, J.; He, X.-H.; Lei, Q.-Y.; Wang, Y.-P. Arginine Methylation of SIRT7 Couples Glucose Sensing with Mitochondria Biogenesis. EMBO Rep. 2018, 19, e46377. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Miki, T.; Kuno, A.; Mizuno, M.; Sato, T.; Tanno, M.; Yano, T.; Nakata, K.; Kimura, Y.; Abe, K.; et al. Empagliflozin, an SGLT2 Inhibitor, Reduced the Mortality Rate after Acute Myocardial Infarction with Modification of Cardiac Metabolomes and Antioxidants in Diabetic Rats. J. Pharmacol. Exp. Ther. 2019, 368, 524–534. [Google Scholar] [CrossRef] [Green Version]
- Monzo, L.; Sedlacek, K.; Hromanikova, K.; Tomanova, L.; Borlaug, B.A.; Jabor, A.; Kautzner, J.; Melenovsky, V. Myocardial Ketone Body Utilization in Patients with Heart Failure: The Impact of Oral Ketone Ester. Metabolism 2021, 115, 154452. [Google Scholar] [CrossRef]
- Brahma, M.K.; Ha, C.-M.; Pepin, M.E.; Mia, S.; Sun, Z.; Chatham, J.C.; Habegger, K.M.; Abel, E.D.; Paterson, A.J.; Young, M.E.; et al. Increased Glucose Availability Attenuates Myocardial Ketone Body Utilization. J. Am. Heart Assoc. 2020, 9, e013039. [Google Scholar] [CrossRef]
- Yurista, S.R.; Nguyen, C.T.; Rosenzweig, A.; de Boer, R.A.; Westenbrink, B.D. Ketone Bodies for the Failing Heart: Fuels That Can Fix the Engine? Trends Endocrinol. Metab. 2021, 32, 814–826. [Google Scholar] [CrossRef]
- Takahara, S.; Soni, S.; Maayah, Z.H.; Ferdaoussi, M.; Dyck, J.R.B. Ketone Therapy for Heart Failure: Current Evidence for Clinical Use. Cardiovasc. Res. 2021, 118, cvab068. [Google Scholar] [CrossRef]
- Despa, S. Myocyte [Na+]i Dysregulation in Heart Failure and Diabetic Cardiomyopathy. Front. Physiol. 2018, 9, 1303. [Google Scholar] [CrossRef] [Green Version]
- Trum, M.; Riechel, J.; Wagner, S. Cardioprotection by SGLT2 Inhibitors-Does It All Come Down to Na+? Int. J. Mol. Sci. 2021, 22, 7976. [Google Scholar] [CrossRef]
- Baartscheer, A.; Schumacher, C.A.; Wüst, R.C.I.; Fiolet, J.W.T.; Stienen, G.J.M.; Coronel, R.; Zuurbier, C.J. Empagliflozin Decreases Myocardial Cytoplasmic Na+ through Inhibition of the Cardiac Na+/H+ Exchanger in Rats and Rabbits. Diabetologia 2017, 60, 568–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.-I.; Chen, Y.-C.; Lin, Y.-K.; Chung, C.-C.; Lu, Y.-Y.; Kao, Y.-H.; Chen, Y.-J. Empagliflozin Attenuates Myocardial Sodium and Calcium Dysregulation and Reverses Cardiac Remodeling in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2019, 20, 1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habibi, J.; Aroor, A.R.; Sowers, J.R.; Jia, G.; Hayden, M.R.; Garro, M.; Barron, B.; Mayoux, E.; Rector, R.S.; Whaley-Connell, A.; et al. Sodium Glucose Transporter 2 (SGLT2) Inhibition with Empagliflozin Improves Cardiac Diastolic Function in a Female Rodent Model of Diabetes. Cardiovasc. Diabetol. 2017, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldatov, V.O.; Malorodova, T.N.; Balamutova, T.I.; Ksenofontov, A.O.; Dovgan, A.P.; Urozhevskaya, Z.S. Endothelial Dysfunction: Comparative Evaluation of Ultrasound Dopplerography, Laser Dopplerflowmetry and Direct Monitoring of Arterial Pressure for Conducting Pharmacological Tests in Rats. RRP 2018, 4, 73–80. [Google Scholar] [CrossRef]
- Sierra-Ramos, C.; Velazquez-Garcia, S.; Vastola-Mascolo, A.; Hernández, G.; Faresse, N.; Alvarez de la Rosa, D. SGK1 Activation Exacerbates Diet-Induced Obesity, Metabolic Syndrome and Hypertension. J. Endocrinol. 2020, 244, 149–162. [Google Scholar] [CrossRef]
- Arow, M.; Waldman, M.; Yadin, D.; Nudelman, V.; Shainberg, A.; Abraham, N.G.; Freimark, D.; Kornowski, R.; Aravot, D.; Hochhauser, E.; et al. Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Attenuates Diabetic Cardiomyopathy. Cardiovasc. Diabetol. 2020, 19, 7. [Google Scholar] [CrossRef]
- Puchenkova, O.A.; Nadezhdin, S.V.; Soldatov, V.O.; Zhuchenko, M.A.; Korshunova, D.S.; Kubekina, M.V.; Korshunov, E.N.; Korokina, L.V.; Golubinskaya, P.A.; Kulikov, A.L.; et al. STUDY OF Antiatherosclerotic And Endothelioprotective Activity of Peptide Agonists of Epor/Cd131 Heteroreceptor. Farm. Farmakol. 2020, 8, 100–111. [Google Scholar] [CrossRef]
- Uthman, L.; Baartscheer, A.; Bleijlevens, B.; Schumacher, C.A.; Fiolet, J.W.T.; Koeman, A.; Jancev, M.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; et al. Class Effects of SGLT2 Inhibitors in Mouse Cardiomyocytes and Hearts: Inhibition of Na+/H+ Exchanger, Lowering of Cytosolic Na+ and Vasodilation. Diabetologia 2018, 61, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Cefalo, C.M.A.; Cinti, F.; Moffa, S.; Impronta, F.; Sorice, G.P.; Mezza, T.; Pontecorvi, A.; Giaccari, A. Sotagliflozin, the First Dual SGLT Inhibitor: Current Outlook and Perspectives. Cardiovasc. Diabetol. 2019, 18, 20. [Google Scholar] [CrossRef] [Green Version]
- Zynquista Approved in EU for Certain Patients with Type I Diabetes. Available online: https://www.pharmatimes.com/news/zynquista_approved_in_eu_for_certain_patients_with_type_i_diabetes_1286004 (accessed on 6 March 2022).
- Bode, D.; Semmler, L.; Wakula, P.; Hegemann, N.; Primessnig, U.; Beindorff, N.; Powell, D.; Dahmen, R.; Ruetten, H.; Oeing, C.; et al. Dual SGLT-1 and SGLT-2 Inhibition Improves Left Atrial Dysfunction in HFpEF. Cardiovasc. Diabetol. 2021, 20, 7. [Google Scholar] [CrossRef]
- Karg, M.V.; Bosch, A.; Kannenkeril, D.; Striepe, K.; Ott, C.; Schneider, M.P.; Boemke-Zelch, F.; Linz, P.; Nagel, A.M.; Titze, J.; et al. SGLT-2-Inhibition with Dapagliflozin Reduces Tissue Sodium Content: A Randomised Controlled Trial. Cardiovasc. Diabetol. 2018, 17, 5. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.P.; Raff, U.; Kopp, C.; Scheppach, J.B.; Toncar, S.; Wanner, C.; Schlieper, G.; Saritas, T.; Floege, J.; Schmid, M.; et al. Skin Sodium Concentration Correlates with Left Ventricular Hypertrophy in CKD. J. Am. Soc. Nephrol. 2017, 28, 1867–1876. [Google Scholar] [CrossRef]
- Olgar, Y.; Turan, B. A Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Dapagliflozin Comparison with Insulin Shows Important Effects on Zn2+-Transporters in Cardiomyocytes from Insulin-Resistant Metabolic Syndrome Rats through Inhibition of Oxidative Stress 1. Can. J. Physiol. Pharmacol. 2019, 97, 528–535. [Google Scholar] [CrossRef]
- Gollmer, J.; Zirlik, A.; Bugger, H. Mitochondrial Mechanisms in Diabetic Cardiomyopathy. Diabetes Metab. J. 2020, 44, 33–53. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Zhang, W.; Liu, X. Mitochondrial Dysfunction and Mitochondrial Therapies in Heart Failure. Pharmacol. Res. 2022, 175, 106038. [Google Scholar] [CrossRef]
- Depaoli, M.R.; Hay, J.C.; Graier, W.F.; Malli, R. The Enigmatic ATP Supply of the Endoplasmic Reticulum. Biol. Rev. Camb. Philos. Soc. 2019, 94, 610–628. [Google Scholar] [CrossRef] [Green Version]
- Cabassi, A.; Miragoli, M. Altered Mitochondrial Metabolism and Mechanosensation in the Failing Heart: Focus on Intracellular Calcium Signaling. Int. J. Mol. Sci. 2017, 18, 1487. [Google Scholar] [CrossRef] [Green Version]
- Miller, W.L. Disorders in the Initial Steps of Steroid Hormone Synthesis. J. Steroid Biochem. Mol. Biol. 2017, 165, 18–37. [Google Scholar] [CrossRef]
- De Breda, C.N.S.; Davanzo, G.G.; Basso, P.J.; Saraiva Câmara, N.O.; Moraes-Vieira, P.M.M. Mitochondria as Central Hub of the Immune System. Redox. Biol. 2019, 26, 101255. [Google Scholar] [CrossRef]
- Dadsena, S.; King, L.E.; García-Sáez, A.J. Apoptosis Regulation at the Mitochondria Membrane Level. Biochimica et Biophysica Acta Biomembr. 2021, 1863, 183716. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogenic Control of Mitochondrial Function. Redox. Biol. 2020, 31, 101435. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, V.; Kirichenko, T.; Melnichenko, A.; Orekhova, V.; Ravani, A.; Poggio, P.; Sobenin, I.; Bobryshev, Y.; Orekhov, A. Anti-Atherosclerotic Effects of a Phytoestrogen-Rich Herbal Preparation in Postmenopausal Women. Int. J. Mol. Sci. 2016, 17, 1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, L.-D. Mitochondrial Biogenesis: An Update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial Dynamics: Overview of Molecular Mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef] [Green Version]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular Mechanisms and Physiological Functions of Mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Sobenin, I.A.; Bobryshev, Y.V. Plasmacytoid Dendritic Cells: Development, Functions, and Role in Atherosclerotic Inflammation. Front. Physiol. 2014, 5, 279. [Google Scholar] [CrossRef] [Green Version]
- Croteau, D.; Luptak, I.; Chambers, J.M.; Hobai, I.; Panagia, M.; Pimentel, D.R.; Siwik, D.A.; Qin, F.; Colucci, W.S. Effects of Sodium-Glucose Linked Transporter 2 Inhibition With Ertugliflozin on Mitochondrial Function, Energetics, and Metabolic Gene Expression in the Presence and Absence of Diabetes Mellitus in Mice. JAHA 2021, 10, e019995. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, S.; Zhu, P.; Hu, S.; Chen, Y.; Ren, J. Empagliflozin Rescues Diabetic Myocardial Microvascular Injury via AMPK-Mediated Inhibition of Mitochondrial Fission. Redox Biol. 2018, 15, 335–346. [Google Scholar] [CrossRef]
- Mizuno, M.; Kuno, A.; Yano, T.; Miki, T.; Oshima, H.; Sato, T.; Nakata, K.; Kimura, Y.; Tanno, M.; Miura, T. Empagliflozin Normalizes the Size and Number of Mitochondria and Prevents Reduction in Mitochondrial Size after Myocardial Infarction in Diabetic Hearts. Physiol. Rep. 2018, 6, e13741. [Google Scholar] [CrossRef]
- Durak, A.; Olgar, Y.; Degirmenci, S.; Akkus, E.; Tuncay, E.; Turan, B. A SGLT2 Inhibitor Dapagliflozin Suppresses Prolonged Ventricular-Repolarization through Augmentation of Mitochondrial Function in Insulin-Resistant Metabolic Syndrome Rats. Cardiovasc. Diabetol. 2018, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Meng, L.; Lee, S.; Tse, G.; Gong, M.; Zhang, Z.; Zhao, J.; Zhao, Y.; Li, G.; Liu, T. Empagliflozin, a Sodium Glucose Co-Transporter-2 Inhibitor, Alleviates Atrial Remodeling and Improves Mitochondrial Function in High-Fat Diet/Streptozotocin-Induced Diabetic Rats. Cardiovasc. Diabetol. 2019, 18, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.H.; Kim, S.H.; Kang, J.M.; Heo, J.H.; Kim, D.-J.; Park, S.H.; Sung, M.; Kim, J.; Oh, J.; Yang, D.H.; et al. Empagliflozin Attenuates Diabetic Tubulopathy by Improving Mitochondrial Fragmentation and Autophagy. Am. J. Physiol. Renal Physiol. 2019, 317, F767–F780. [Google Scholar] [CrossRef] [PubMed]
- Makrecka-Kuka, M.; Korzh, S.; Videja, M.; Vilks, K.; Cirule, H.; Kuka, J.; Dambrova, M.; Liepinsh, E. Empagliflozin Protects Cardiac Mitochondrial Fatty Acid Metabolism in a Mouse Model of Diet-Induced Lipid Overload. Cardiovasc. Drugs Ther. 2020, 34, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Li, J.; Takagaki, Y.; Kitada, M.; Nitta, K.; Takasu, T.; Kanasaki, K.; Koya, D. Ipragliflozin Improves Mitochondrial Abnormalities in Renal Tubules Induced by a High-Fat Diet. J. Diabetes Investig. 2018, 9, 1025–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Xu, C.; Xu, L.; Li, X.; Sun, H.; Xue, M.; Li, T.; Yu, X.; Sun, B.; Chen, L. Empagliflozin Improves Diabetic Renal Tubular Injury by Alleviating Mitochondrial Fission via AMPK/SP1/PGAM5 Pathway. Metabolism 2020, 111, 154334. [Google Scholar] [CrossRef]
- Sa-nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Jaiwongkam, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. SGLT2-Inhibitor and DPP-4 Inhibitor Improve Brain Function via Attenuating Mitochondrial Dysfunction, Insulin Resistance, Inflammation, and Apoptosis in HFD-Induced Obese Rats. Toxicol. Appl. Pharmacol. 2017, 333, 43–50. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Starinets, V.S.; Belosludtsev, M.N.; Mikheeva, I.B.; Dubinin, M.V.; Belosludtseva, N.V. Chronic Treatment with Dapagliflozin Protects against Mitochondrial Dysfunction in the Liver of C57BL/6NCrl Mice with High-Fat Diet/Streptozotocin-Induced Diabetes Mellitus. Mitochondrion 2021, 59, 246–254. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, C.-C.; Lin, M.-H.; Su, H.-T.; Ho, M.-Y.; Yeh, J.-K.; Tsai, M.-L.; Hsieh, I.-C.; Wen, M.-S. TLR9 Binding to Beclin 1 and Mitochondrial SIRT3 by a Sodium-Glucose Co-Transporter 2 Inhibitor Protects the Heart from Doxorubicin Toxicity. Biology 2020, 9, 369. [Google Scholar] [CrossRef]
- Goerg, J.; Sommerfeld, M.; Greiner, B.; Lauer, D.; Seckin, Y.; Kulikov, A.; Ivkin, D.; Kintscher, U.; Okovityi, S.; Kaschina, E. Low-Dose Empagliflozin Improves Systolic Heart Function after Myocardial Infarction in Rats: Regulation of MMP9, NHE1, and SERCA2a. Int. J. Mol. Sci. 2021, 22, 5437. [Google Scholar] [CrossRef]
- Song, Y.; Huang, C.; Sin, J.; de Germano, J.F.; Taylor, D.J.R.; Thakur, R.; Gottlieb, R.A.; Mentzer, R.M.; Andres, A.M. Attenuation of Adverse Postinfarction Left Ventricular Remodeling with Empagliflozin Enhances Mitochondria-Linked Cellular Energetics and Mitochondrial Biogenesis. Int. J. Mol. Sci. 2021, 23, 437. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Gill, E.K.; Abudalo, R.A.; Edgar, K.S.; Watson, C.J.; Grieve, D.J. Reactive Oxygen Species Signalling in the Diabetic Heart: Emerging Prospect for Therapeutic Targeting. Heart 2018, 104, 293–299. [Google Scholar] [CrossRef]
- Liu, C.; Ma, N.; Guo, Z.; Zhang, Y.; Zhang, J.; Yang, F.; Su, X.; Zhang, G.; Xiong, X.; Xing, Y. Relevance of Mitochondrial Oxidative Stress to Arrhythmias: Innovative Concepts to Target Treatments. Pharmacol. Res. 2022, 175, 106027. [Google Scholar] [CrossRef] [PubMed]
- Singh, H. Mitochondrial Ion Channels in Cardiac Function. Am. J. Physiol. Cell Physiol. 2021, 321, C812–C825. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.A.; Koval, O.M.; Li, J.; He, B.J.; Allamargot, C.; Gao, Z.; Luczak, E.D.; Hall, D.D.; Fink, B.D.; Chen, B.; et al. CaMKII Determines Mitochondrial Stress Responses in Heart. Nature 2012, 491, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Erickson, J.R.; Pereira, L.; Wang, L.; Han, G.; Ferguson, A.; Dao, K.; Copeland, R.J.; Despa, F.; Hart, G.W.; Ripplinger, C.M.; et al. Diabetic Hyperglycaemia Activates CaMKII and Arrhythmias by O-Linked Glycosylation. Nature 2013, 502, 372–376. [Google Scholar] [CrossRef]
- Hegyi, B.; Borst, J.M.; Bailey, L.R.J.; Shen, E.Y.; Lucena, A.J.; Navedo, M.F.; Bossuyt, J.; Bers, D.M. Hyperglycemia Regulates Cardiac K+ Channels via O-GlcNAc-CaMKII and NOX2-ROS-PKC Pathways. Basic Res. Cardiol. 2020, 115, 71. [Google Scholar] [CrossRef]
- De Marchi, U.; Castelbou, C.; Demaurex, N. Uncoupling Protein 3 (UCP3) Modulates the Activity of Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA) by Decreasing Mitochondrial ATP Production. J. Biol. Chem. 2011, 286, 32533–32541. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.S.H.; Goncalves, E. Atrial Fibrillation and Type 2 Diabetes: Prevalence, Etiology, Pathophysiology and Effect of Anti-Diabetic Therapies. Diabetes Obes. Metab. 2019, 21, 210–217. [Google Scholar] [CrossRef]

| Used Drug | Experimental System/Model Animal/Cell Culture | Cardiovascular Effect | Mitochondrial Effects | Other Effects/Notes | References |
|---|---|---|---|---|---|
| Empagliflozin | non-DM male rats after CA | increases LV function and survival time; reduces myocardial fibrosis, serum cardiac troponin I levels and myocardial OS after CA | maintains the structural integrity of myocardial mitochondria and increases mitochondrial activity after CA | increases circulating and myocardial ketone levels and heart BDH1 expression | [53] |
| Empagliflozin | DM rats after MI | the sizes of MI were comparable | increases myocardial levels of Sirt3 | increases glucose oxidation and ketone utilisation, SOD2 levels | [57] |
| Empagliflozin | in vitro culture of ventricular myocytes (rabbits and rats) | - | enhances [Ca2+]m | reduces [Na+]c and [Ca2+]c | [64] |
| Sotagliflozin | obese rats’ model of HFpEF | ameliorates LA enlargement in HFpEF in vivo; reduced the magnitude of SCaEs in-vitro LA cardiomyocytes | prevents mitochondrial swelling, enhances mitochondrial Ca2+ buffer capacity, improves mitochondrial fission and ROS production, averts Ca2+ accumulation upon glycolytic inhibition; increases NCX forward-mode activity | lowers diastolic [Ca2+] of CaT | [74] |
| Ertugliflozin | mice on HFD and HSD | beneficial for hallmarks of DCMP: LV hypertrophy, myocyte hypertrophy, myocardial interstitial fibrosis, and diastolic dysfunction | prevents mitochondrial dysfunction, preserves ATP production, and decreases mROS generation | positive enrichment of gene sets related to OXPHOS (oxidative phosphorylation) and FAM | [91] |
| Empagliflozin | streptozotocin-induced DM mice | improves diabetic myocardial structure and function, preserves cardiac microvascular barrier function and integrity, sustains eNOS phosphorylation and endothelium-dependent relaxation, improves microvessel density and perfusion | inhibits mitochondrial fission, suppresses mROS production | preserves CMEC barrier function and impedes CMEC senescence | [92] |
| Empagliflozin | myocardial tissues of the DM rats after MI | - | suppresses FIS1 and increases BNIP3 expression; prevents reduction of mitochondrial size and autophagic vacuole number; upregulates SOD2 and CAT expression | reduces blood glucose and triglycerides, increases lipid droplets in cardiomyocytes | [93] |
| Dapagliflozin | overweight insulin-resistant MetS-rats | augments the increased blood pressure, prolonged Q–R interval, and low heart rate with depressed LV function and relaxation of the aorta | preserves the depolarised mitochondrial membrane potential; normalises the expression of fusion-fission proteins and cytosolic Ca2+-homeostasis | increases voltage-gated Na+-currents and intracellular pH; normalises the cellular levels of increased OS, protein–thiol oxidation and ADP/ATP ratio in cardiomyocytes | [94] |
| Empagliflozin | streptozotocin- induced HFD DM rats | reduces left atrial diameter, interstitial fibrosis, and the incidence of AF inducibility | improves atrial mitochondrial respiratory function, mitochondrial membrane potential, and mitochondrial biogenesis | increases the expression of PGC-1a, NRF-1 and TFAM (Transcription Factor A, Mitochondrial) | [95] |
| Empagliflozin | streptozotocin-induced DM mice; hRPTCs | - | improves mitochondrial biogenesis and balances fusion–fission proteins expression; increases autophagy; reduces mROS and expression of apoptotic and fibrotic proteins in hRPTCs; normalises AMP/ATP ratios | suppresses SGLT2 expression and ameliorates renal morphological changes in the kidneys of DM mice | [96] |
| Empagliflozin | mice with HFD-induced lipid overload | - | normalises mitochondrial function in the heart via an increase in FAO and protects against HFD-induced disturbances in cardiac metabolism | increases palmitate uptake and decreases the accumulation of metabolites of incomplete FAO in cardiac tissues | [97] |
| Ipragliflozin | HFD mice | - | normalises mitochondrial morphology and fusion restores OPA1 and MFN2 expression; reduces mROS | ameliorates tubular vacuolation, dilatation and epithelial cell detachment | [98] |
| Empagliflozin | DM mice | - | alleviates mitochondrial fission via AMPK/SP1/PGAM5 pathway | renal protection in DKD | [99] |
| Dapagliflozin | HFD-induced obese rats | - | improves brain mitochondria function, insulin signalling, apoptosis and prevents cognitive decline | improves peripheral insulin sensitivity and hippocampal synaptic plasticity, reduces weight gain | [100] |
| Dapagliflozin | hepatocytes of HFD streptozotocin-induced DM mice | - | prevents mitochondrial swelling; normalises the mitochondrial size, mtDNA copy number and mitochondrial respiration; decreases the level of lipid peroxidation products in mitochondria | increases the expression of the MFN2 and DRP1 in the liver tissue; | [101] |
| Empagliflozin | human cardiomyocyte cells; CAG-RFP-EGFP-LC3, Becn1+/−, SIRT3-knock-out and TLR9-knock-out mice | protects against doxorubicin-induced cardiomyopathy through a mitochondrial TLR9-SIRT3 mechanism; increases autophagic flux in hearts and cardiomyocytes | increases the TLR9 activation and the abundance of SIRT3 in the mitochondria, which enhances the mitochondrial respiration rate and exerts its protection against ROS and apoptosis | - | [102] |
| Empagliflozin | non-DM rats with LV dysfunction after MI | increases the LV ejection fraction, attenuates cardiomyocyte hypertrophy, diminishes interstitial fibrosis and reduces myocardial OS | reduces mitochondrial DNA damage and stimulated mitochondrial biogenesis, normalises the myocardial uptake and oxidation of glucose and fatty acids | increases urine production two-fold without affecting creatinine clearance and serum electrolytes; increases circulating ketone levels and myocardial expression of the MCT1 and BDH1 | [44] |
| Empagliflozin | non-DM rats after MI | increases cardiac contractility and improves systolic heart function after MI; does not affect arterial stiffness, blood pressure, markers of fibrosis, and necroptosis; | NHE1 modulation decreases [Na+]c and [Ca2+]c levels while increasing the myocytes [Ca2+]m concentra-tion | inhibits MMP9, down-regulates NHE1 and upregulates SERCA2a expression | [103] |
| Empagliflozin | wild-type and Parkin−/− male mice after PCAL; H9C2 cells | attenuates PCAL-induced adverse remodelling | increases mitochondrial biomass, respiratory capacity, and markers of mitochondrial biogenesis; the mechanism is not entirely de-pendent on Parkin | - | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabravolski, S.A.; Zhuravlev, A.D.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. Mitochondria-Mediated Cardiovascular Benefits of Sodium-Glucose Co-Transporter 2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 5371. https://doi.org/10.3390/ijms23105371
Dabravolski SA, Zhuravlev AD, Kartuesov AG, Borisov EE, Sukhorukov VN, Orekhov AN. Mitochondria-Mediated Cardiovascular Benefits of Sodium-Glucose Co-Transporter 2 Inhibitors. International Journal of Molecular Sciences. 2022; 23(10):5371. https://doi.org/10.3390/ijms23105371
Chicago/Turabian StyleDabravolski, Siarhei A., Alexander D. Zhuravlev, Andrey G. Kartuesov, Evgeny E. Borisov, Vasily N. Sukhorukov, and Alexander N. Orekhov. 2022. "Mitochondria-Mediated Cardiovascular Benefits of Sodium-Glucose Co-Transporter 2 Inhibitors" International Journal of Molecular Sciences 23, no. 10: 5371. https://doi.org/10.3390/ijms23105371
APA StyleDabravolski, S. A., Zhuravlev, A. D., Kartuesov, A. G., Borisov, E. E., Sukhorukov, V. N., & Orekhov, A. N. (2022). Mitochondria-Mediated Cardiovascular Benefits of Sodium-Glucose Co-Transporter 2 Inhibitors. International Journal of Molecular Sciences, 23(10), 5371. https://doi.org/10.3390/ijms23105371








