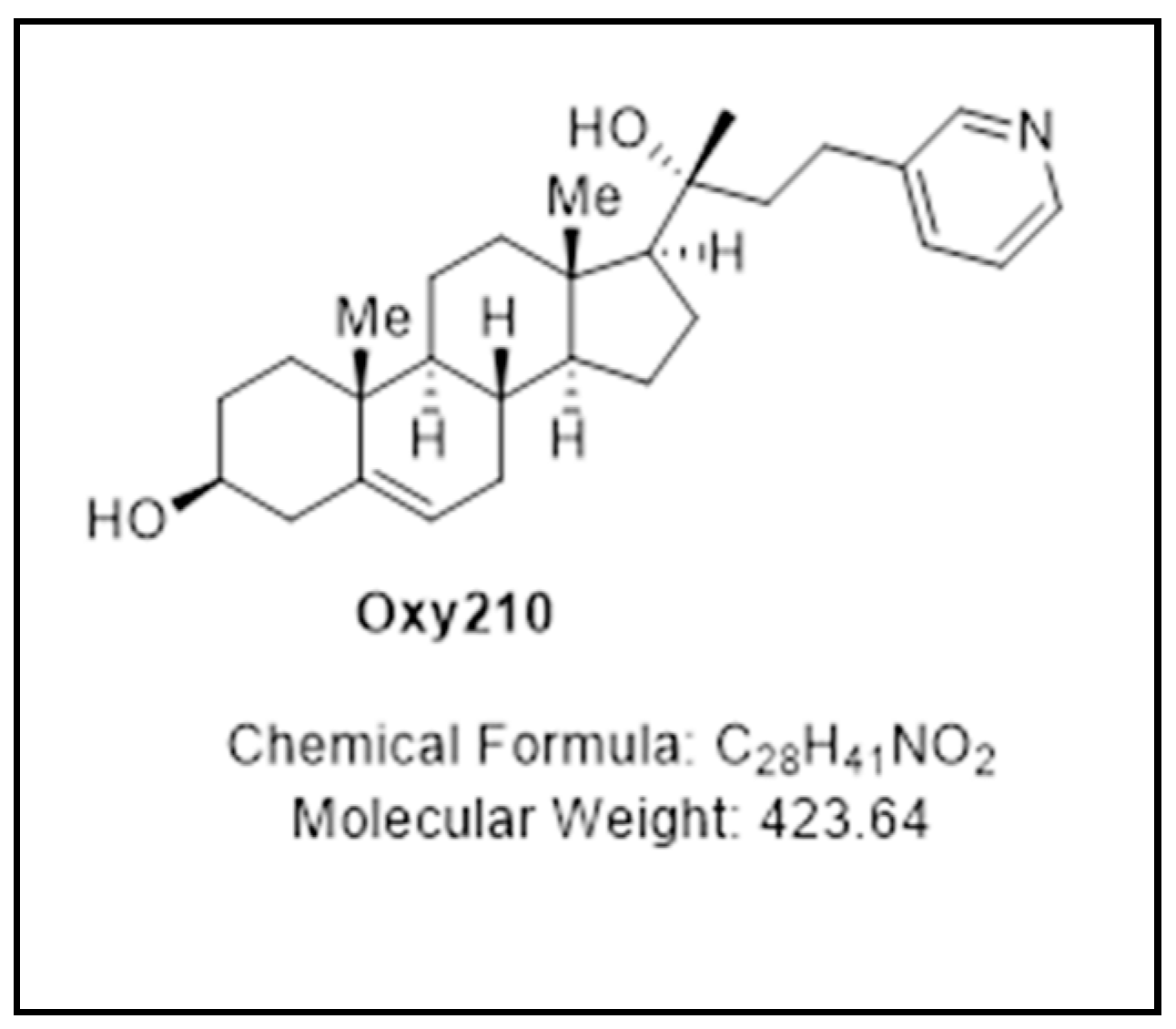
Oxy210, a Semi-Synthetic Oxysterol, Exerts Anti-Inflammatory Effects in Macrophages via Inhibition of Toll-like Receptor (TLR) 4 and TLR2 Signaling and Modulation of Macrophage Polarization
Abstract
:1. Introduction
2. Results
2.1. Oxy210 Inhibits White Adipose Tissue Inflammation
2.2. Oxy210 Inhibits LPS-Induced Expression of Inflammatory Genes in Mouse and Human Macrophages
2.3. Oxy210 Inhibits the Expression of Inflammatory Genes Induced by Synthetic TLR4 and TLR1/2 Agonists
2.4. Effect of Oxy210 on Cyclooxygenases and Steroid Hormone Receptors
2.5. Oxy210 Does Not Interfere with the Anti-Inflammatory Effects of TGF-β in Macrophages
2.6. Oxy210 Inhibits Both M1 and M2 Polarization of Macrophages
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Synthesis and Molecular Characterization of Semi-Synthetic Oxysterol Derivatives
4.2.1. Oxy43
4.2.2. Oxy133
4.2.3. Oxy210
4.2.4. Oxy234
4.3. Quantitative RT-PCR
4.4. TLR, NFκB, and AP-1 Activity Assays (Abeomics, Inc., San Diego, CA, USA)
4.5. COX1 & COX2 Activity Assays (BPS Bioscience, Inc., San Diego, CA, USA)
4.6. GR and MR Activity Assays (Eurofins DiscoverX, Fremont, CA, USA)
4.7. Western Blotting
4.8. Animal Studies
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinarello, C.A. Anti-inflammatory agents: Present and future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahesh, G.; Kumar, K.A.; Reddanna, P. Overview on the discovery and development of anti-inflammatory drugs: Should the focus be on synthesis or degradation of PGE2? J. Inflamm. Res. 2021, 14, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, D.I.; Okoro, U.C.; Ukoha, P.O.; Gupta, A.; Okafor, S.N. Novel anti-inflammatory and analgesic agents: Synthesis, molecular docking and in vivo studies. J. Enzyme Inhib. Med. Chem. 2018, 33, 405–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botz, B.; Bolcskei, K.; Helyes, Z. Challenges to develop novel anti-inflammatory and analgesic drugs. Wires Nanomed. Nanobiotechnol. 2017, 9, e1427. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.; Tintinger, G.R.; Ker, J.A.; Pannell, N. Adverse effects of biologic anti-inflammatory agents on the respiratory system: A review. Afr. J. Thorac. Crit. Care Med. 2021, 27, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2018, 19, 92. [Google Scholar] [CrossRef] [Green Version]
- Duque, G.A.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef] [Green Version]
- Palsson-Mcdermott, E.M.; O’Neill, L.A.J. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Chavez-Sanchez, L.; Madrid-Miller, A.; Chavez-Rueda, K.; Legorreta-Haquet, M.V.; Tesoro-Cruz, E.; Blanco-Favela, F. Activation of TLR2 and TLR4 by minimally modified low-density lipoprotein in human macrophages and monocytes triggers the inflammatory response. Hum. Immunol. 2010, 71, 737–744. [Google Scholar] [CrossRef]
- Menghini, R.; Campia, U.; Tesauro, M.; Marino, A.; Rovella, V.; Rodia, G.; Schinzari, F.; Tolusso, B.; di Daniele, N.; Federici, M.; et al. Toll-Like Receptor 4 Mediates Endothelial Cell Activation Through NF-kB but Is Not Associated with Endothelial Dysfunction in Patients with Rheumatoid Arthritis. PLoS ONE 2014, 9, e99053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadl, A.; Sharma, P.R.; Chen, W.; Agrawal, R.; Meher, A.K.; Rudraiah, S.; Grubbs, N.; Sharma, R.; Leitinger, N. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radic. Biol. Med. 2011, 51, 1903–1909. [Google Scholar] [CrossRef] [Green Version]
- Bochkov, V.; Gesslbauer, B.; Mauerhofer, C.; Philippova, M.; Erne, P.; Oskolkova, O.V. Pleiotropic effects of oxidized phospholipids. Free Radic. Biol. Med. 2017, 111, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, E.J.; Parker, A.E.; O’Neill, L.A.J. Targeting Toll-like receptors: Emerging therapeutics? Nat. Rev. 2010, 9, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Zaffaroni, L.; Peri, F. Recent advances on Toll-like receptor 4 modulation: New therapeutic perspectives. Future Med. Chem. 2018, 10, 461–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, S.; Li, H.; Wang, H.; Zhang, T.; Hutchinson, M.R.; Yin, H.; Wang, X. Small-molecule modulators of Toll-like receptors. Acc. Chem. Res. 2020, 19, 1046–1055. [Google Scholar] [CrossRef]
- Bruno, K.; Woller, S.A.; Miller, Y.I.; Yaksh, T.L.; Wallace, M.; Beaton, G.; Chakravarthy, K. Targeting Toll-like receptor-4 (TLR4)—Emerging therapeutic target for persistent pain states. Pain 2021, 159, 1908–1915. [Google Scholar] [CrossRef]
- Roshan, M.H.K.; Tambo, A.; Pace, N.P. The role of TLR2, TLR4, and TLR9 in the pathogenesis of atherosclerosis. Int. J. Inflamm. 2016, 2016, 1532832. [Google Scholar] [CrossRef] [Green Version]
- Fuster, J.J. TLR4 in atherogenesis. J. Am. Coll. Cardiol. 2018, 71, 1571–1573. [Google Scholar] [CrossRef]
- Valins, W.; Amini, S.; Berman, B. The expression of Toll-like receptors in dermatological diseases and the therapeutic effect of current and newer topical Toll-like receptor modulators. J. Clin. Aesthetic Dermatol. 2010, 3, 20–29. [Google Scholar]
- Stifano, G.; Affandi, A.J.; Mathes, A.L.; Rice, L.M.; Nakerakanti, S.; Nazari, B.; Lee, J.; Christmann, R.B.; Lafyatis, R. Chronic Toll-like receptor 4 stimulation in skin induces inflammation, macrophage activation, transforming growth factor beta signature gene expression, and fibrosis. Arthritis Res. Ther. 2014, 16, R136. [Google Scholar] [CrossRef] [Green Version]
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48. [Google Scholar] [CrossRef] [PubMed]
- McKernan, K.; Varghese, M.; Patel, R.; Singer, K. Role of TLR4 in the induction of inflammatory changes in adipocytes and macrophages. Adipocyte 2020, 9, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Calder, P.C. Obesity, inflammation, Toll-like receptor 4 and fatty acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vila, I.K.; Badin, P.; Marques, M.; Monbrun, L.; Lefort, C.; Mir, L.; Louche, K.; Bourlier, V.; Roussel, B.; Gui, P.; et al. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep. 2014, 7, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Sharifnia, T.; Antoun, J.; Verriere, T.G.C.; Suarez, G.; Wattacheril, J.; Wilson, K.T.; Peek, R.M.; Abumrad, N.N.; Flynn, C.R. Hepatic TLR4 signaling in NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G270–G278. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Seki, E. Toll-like receptors in liver fibrosis: Cellular crosstalk and mechanisms. Front. Physiol. 2012, 3, 138. [Google Scholar] [CrossRef] [Green Version]
- Seki, E.; De Minicis, S.; Osterreicher, C.H.; Kluwe, J.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 2007, 13, 1324–1332. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Sterols, oxysterols, and accessible cholesterol: Signalling for homeostasis, in immunity and during development. Front. Physiol. 2021, 12, 723224. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Oxysterol research: A brief review. Biochem. Soc. Trans. 2019, 47, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.J.; Sharpe, L.J.; Rogers, M.J. Oxysterols: From physiological tuners to pharmacological opportunities. Br. J. Pharmacol. 2020, 178, 3089–3103. [Google Scholar] [CrossRef]
- Choi, C.; Finlay, D.K. Diverse immunoregulatory roles of oxysterols—The oxidized cholesterol metabolites. Metabolites 2020, 10, 384. [Google Scholar] [CrossRef]
- Bah, S.Y.; Dickinson, P.; Forster, T.; Kampmann, B.; Ghazal, P. Immune oxysterols: Role in mycobacterial infection and inflammation. J. Steroid Biochem. Mol. Biol. 2017, 169, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, S.R.; Nargizyan, T.; Meliton, V.; Nachtergaele, S.; Rohatgi, R.; Stappenbeck, F.; Jung, M.E.; Johnson, J.S.; Aghdasi, B.; Tian, H.; et al. A novel osteogenic oxysterol compound for therapeutic development to promote bone growth: Activation of hedgehog signaling and osteogenesis through Smoothened binding. J. Bone Miner. Res. 2014, 29, 1872–1885. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Stappenbeck, F.; Parhami, F. Inhibition of hedgehog signaling in fibroblasts, pancreatic, and lung tumor cells by Oxy186, an oxysterol analogue with drug-like properties. Cells 2019, 8, 509. [Google Scholar] [CrossRef] [Green Version]
- Stappenbeck, F.; Wang, F.; Tang, L.; Zhang, Y.E.; Parhami, F. Inhibition of non-small cell lung cancer cells by Oxy210, an oxysterol-derivative that antagonizes TGFβ and hedgehog signaling. Cells 2019, 8, 1297. [Google Scholar] [CrossRef] [Green Version]
- Hui, S.T.; Wang, F.; Stappenbeck, F.; French, S.W.; Magyar, C.E.; Parhami, F.; Lusis, A.J. Oxy210, a novel inhibitor of hedgehog and TGF-β signalling, ameliorates hepatic fibrosis and hypercholesterolemia in mice. Endocrinol. Diabetes Metab. 2021, 4, e00296. [Google Scholar] [CrossRef]
- Hui, S.T.; Parks, B.W.; Org, E.; Norheim, F.; Che, N.; Pan, C.; Castellani, L.W.; Charugundla, S.; Dirks, D.L.; Psychogios, N.; et al. The genetic architecture of NAFLD among inbred strains of mice. eLife 2015, 4, e05607. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, X.; Chai, Y.; Yao, Y. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage polarization in chronic inflammatory diseases: Killers or builders? J. Immunol. Res. 2018, 2018, 8917804. [Google Scholar] [CrossRef]
- Toda, M.; Mizuguchi, S.; Minamiyama, Y.; Yamamoto-Oka, H.; Aota, T.; Kubo, S.; Nishiyama, N.; Shibata, T.; Takemura, S. Pirfenidone suppresses polarization to M2 phenotype macrophages and the fibrogenic activity of rat lung fibroblasts. J. Clin. Biochem. Nutr. 2018, 63, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Ying, H.; Fang, M.; Hang, Q.Q.; Chen, Y.; Qian, X.; Chen, M. Pirfenidone modulates macrophage polarization and ameliorates radiation-induced lung fibrosis by inhibiting the TGF-β1/Smad3 pathway. J. Cell. Mol. Med. 2021, 25, 8662–8675. [Google Scholar] [CrossRef]
- Braga, T.T.; Agudelo, J.S.H.; Camara, N.O.S. Macrophages during the fibrotic process: M2 as friend and foe. Front. Immunol. 2015, 6, 602. [Google Scholar] [CrossRef] [Green Version]
- Grunhut, J.; Wang, W.; Aykut, B.; Gakhal, I.; Torres-Hernandez, A.; Miller, G. Macrophages in nonalcoholic steatohepatitis: Friend or Foe? Eur. Med. J. Hepatol. 2018, 6, 100–109. [Google Scholar]
- Kishore, A.; Petrek, M. Roles of macrophage polarization and macrophage-derived miRNAs in pulmonary fibrosis. Front. Immunol. 2021, 12, 678457. [Google Scholar] [CrossRef]
- Lech, M.; Anders, H. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim. Et Biophys. Acta 2013, 1832, 989–997. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef]
- Guo, Z.; Song, J.; Hao, J.; Zhao, H.; Du, X.; Li, E.; Kuang, Y.; Yang, F.; Wang, W.; Deng, J.; et al. M2 macrophages promote NSCLC metastasis by upregulating CRYAB. Cell Death Dis. 2019, 10, 377. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2013, 18, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Ueha, S.; Shand, F.H.W.; Matsushima, K. Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front. Immunol. 2012, 3, 71. [Google Scholar] [CrossRef] [Green Version]
- Sisto, M.; Ribatti, D.; Lisi, S. Organ fibrosis and autoimmunity: The role of inflammation in TGFβ-dependent EMT. Biomolecules 2021, 11, 310. [Google Scholar] [CrossRef]
- Wu, B.; Sodji, Q.H.; Oyelere, A.K. Inflammation, fibrosis and cancer: Mechanisms, therapeutic options and challenges. Cancers 2022, 14, 552. [Google Scholar] [CrossRef]
- Dwyer, J.R.; Sever, N.; Carlson, M.; Nelson, S.F.; Beachy, P.A.; Parhami, F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J. Biol. Chem. 2007, 282, 8959–8968. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Meliton, V.; Amantea, C.M.; Hahn, T.J.; Parhami, F. 20(S)-hydroxycholesterol inhibits PPARγ expression and adipogenic differentiation of bone marrow stromal cells through a hedgehog-dependent mechanism. J. Bone Miner. Res. 2007, 22, 1711–1719. [Google Scholar] [CrossRef]
- Wang, F.; Stappenbeck, F.; Matsui, W.; Parhami, F. Inhibition of pancreatic cancer cell-induced hedgehog signaling by Liver X Receptor agonists and Oxy16, a naturally occurring oxysterol. J. Cell. Biochem. 2017, 118, 499–509. [Google Scholar] [CrossRef]
- Li, A.; Hokugo, A.; Segovia, L.A.; Yalom, A.; Rezzadeh, K.; Zhou, S.; Zhang, Z.; Parhami, F.; Stappenbeck, F.; Jarrahy, R. Oxy133, a novel osteogenic agent, promotes bone regeneration in an intramembranous bone-healing model. J. Tissue Eng. Regen. Med. 2017, 11, 1490–1499. [Google Scholar] [CrossRef]
- Hsu, L.Y.; Kampf, J.W.; Nordman, C.E. Structure and pseudosymmetry of cholesterol at 310 K. Acta Cryst. 2002, 58, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Byrne, E.F.X.; Sircar, R.; Miller, P.S.; Hedger, G.; Luchetti, G.; Nachtergaele, S.; Tully, M.D.; Mydock-McGrane, L.; Covey, D.F.; Rambo, R.P.; et al. Structural basis for Smoothened regulation by its extracellular domains. Nature 2016, 535, 517–522. [Google Scholar] [CrossRef]
- Huang, P.; Nedelcu, D.; Watanabe, M.; Jao, C.; Kim, Y.; Liu, J.; Salic, A. Cellular cholesterol directly activates Smoothened in hedgehog signaling. Cell 2016, 166, 1176–1187. [Google Scholar] [CrossRef] [Green Version]
- Wessler, S.; Krisch, L.M.; Elmer, D.P.; Aberger, F. From inflammation to gastric cancer—The importance of hedgehog/GLI signaling in Helicobacter pylori-induced inflammatory and neoplastic diseases. Cell Commun. Signal. 2017, 15, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fan, J.; Zeng, X.; Nie, M.; Luan, J.; Wang, Y.; Ju, D.; Yin, K. Hedgehog signaling in gastrointestinal carcinogenesis and the gastrointestinal tumor microenvironment. Acta Pharm. Sin. B. 2021, 11, 609–620. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Hanna, A.; Lama-Sherpa, T.; Metge, B.; Kammerud, S.C.; Benavides, G.A.; Kumar, A.; Alsheikh, H.A.; Mota, M.; Chen, D.; et al. Hedgehog signaling regulates metabolism and polarization of mammary tumor-associated macrophages. Cancer Res. 2021, 81, 5425–5437. [Google Scholar] [CrossRef]
- Matissek, S.J.; Han, W.; Hage, A.; Karbalivand, M.; Rajsbaum, R.; Elsawa, S.F. A novel mechanism of regulation of the transcription factor GLI3 by toll-like receptor signaling. bioRxiv 2021. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, G.; Wan, H.; Zhou, M. Hedgehog signaling regulates the expression levels of inflammatory mediators in cigarette-induced airway inflammation. Mol. Med. Rep. 2018, 17, 8557–8563. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.Y.; Chen, R.; Zhou, M.; Guo, Y. Hedgehog signaling modulates cigarette-induced COPD development. Exp. Ther. Med. 2021, 22, 729. [Google Scholar] [CrossRef]
- Duax, W.L.; Griffin, J.F.; Rohrer, D.C.; Weeks, C.M. Conformational analysis of sterols: Comparison of X-ray crystallographic observations with data from other sources. Lipids 1980, 15, 783–792. [Google Scholar] [CrossRef]
- Maschinot, C.A.; Corman, A.R.; DeBerardinis, A.M.; Hadden, M.K. Synthesis and evaluation of osteogenic oxysterols as hedgehog pathway activators. ChemMedChem 2016, 11, 679–686. [Google Scholar] [CrossRef]
- Osafo, N.; Agyare, C.; Obiri, D.D.; Antwi, A.O. Mechanism of Action of Nonsteroidal Anti-Inflammatory Drugs; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Willinger, T. Oxysterols in intestinal immunity and inflammation. J. Int. Med. 2019, 285, 367–380. [Google Scholar] [CrossRef]
- Shibata, N.; Glass, C.K. Macrophages, oxysterols and atherosclerosis. Circ. J. 2010, 74, 2045–2051. [Google Scholar] [CrossRef] [Green Version]
- Michael, D.R.; Ashlin, T.G.; Buckley, M.L.; Ramji, D.P. Liver X receptors, atherosclerosis and inflammation. Curr. Atheroscler. Rep. 2012, 14, 284–293. [Google Scholar] [CrossRef]
- Kang, J.; Rivest, S. Lipid metabolism and neuroinflammation in Alzheimer’s disease; a role for liver X receptors. Endocr. Rev. 2012, 33, 715–746. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Hong, C.; Rong, X.; Zhu, X.; Tarling, E.J.; Hedde, P.N.; Gratton, E.; Parks, J.; Tontonoz, P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. eLife 2015, 4, e08009. [Google Scholar] [CrossRef]
- Rodriguez-Diez, R.; Rayego-Mateos, S.; Orejudo, M.; Aroeira, L.S.; Selgas, R.; Ortiz, A.; Egido, J.; Ruiz-Ortega, M. TGF-Beta blockade increases renal inflammation caused by the C-terminal module of the CCN2. Mediators Inflamm. 2015, 2015, 506041. [Google Scholar] [CrossRef]
- Toma, I. Transforming growth factor-β and atherosclerosis: Interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2012, 347, 155–175. [Google Scholar] [CrossRef] [Green Version]
- Robertson, A.L.; Rudling, M.; Zhou, X.; Gorelink, L.; Flavell, R.A.; Hansson, G.K. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J. Clin. Investig. 2003, 112, 1342–1350. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.O.; Duarte, R.; Dix-Peek, T.; Dickens, C.; Naidoo, S.; Vachiat, A.; Grinter, S.; Manga, P.; Naicker, S. Transforming growth factor-β protects against inflammation-related atherosclerosis in South African CKD patients. Int. J. Nephrol. 2018, 2018, 8702372. [Google Scholar] [CrossRef] [Green Version]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef] [Green Version]
- Reinmuth, L.; Hsiao, C.; Hamann, J.; Rosenkilde, M.; Mackrill, J. Multiple targets for oxysterols in their regulation of the immune system. Cells 2021, 10, 2078. [Google Scholar] [CrossRef]
- Boi, S.K.; Fernandez-Zapico, E.; Elsawa, S.F. The transcription factor GLI3 is a novel candidate effector of Toll-Like Receptor 4 (TLR4) signaling in monocytes. Blood 2013, 122, 2269. [Google Scholar] [CrossRef]
- Matissek, S.J.; Elsawa, S.F. Regulation of GLI3 expression by TLR4 signaling. J. Immunol. 2019, 202 (Suppl. S1), 64.14. [Google Scholar]
- Matissek, S.J.; Koeppen, K.; Elsawa, S.F. TLR-TRIF signaling induces GLI3 to modulate inflammation. J. Immunol. 2020, 204 (Suppl. S1), 152.5. [Google Scholar]
- Leveen, P.; Larsson, J.; Ehinger, M.; Cilio, C.M.; Sundler, M.; Sjostrand, L.J.; Holmdahl, R.; Karlsson, S. Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood 2002, 100, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Gojova, A.; Marchiol-Fournigault, C.; Esposito, B.; Kamate, C.; Merval, R.; Fradelizi, D.; Tedgui, A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 2001, 89, 930–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, C.M.; Branagan, P.; McElvaney, N.G. Toll-like receptors as therapeutic targets in cystic fibrosis. Expert Opin. Ther. Targets 2008, 12, 1481–1495. [Google Scholar] [CrossRef]
- Vencken, S.F.; Greene, C.M. Toll-like receptors in cystic fibrosis: Impact of dysfunctional microRNA on innate immune responses in the cystic fibrosis lung. J. Innate Immun. 2016, 8, 541–549. [Google Scholar] [CrossRef]
- Yiu, W.H.; Lin, M.; Tang, S.C.W. Toll-like receptor activation: From renal inflammation to fibrosis. Kidney Int. 2014, 4, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Karampitsakos, T.; Woolard, T.; Bouros, D.; Tzouvelekis, A. Toll-like receptors in pathogenesis of pulmonary fibrosis. Eur. J. Pharmacol. 2017, 808, 35–43. [Google Scholar] [CrossRef]
- He, Z.; Zhu, Y.; Jiang, H. Inhibiting toll-like receptor 4 signaling ameliorates pulmonary fibrosis during acute lung injury induced by lipopolysaccharide: An experimental study. Respir. Res. 2009, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.K.; Chong, H.C.; Tan, E.; Tan, N.S. Getting ‘Smad’ about obesity and diabetes. Nutr. Diabetes 2012, 2, e29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBari, M.K.; Abbott, R.D. Adipose tissue fibrosis: Mechanisms, models, and importance. Int. J. Mol. Sci. 2020, 21, 6030. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, S.; Li, X.; Feng, J.; Du, J.; Guo, L.; Su, Y.; Zhou, J.; Ding, G.; Bai, Y.; et al. Aspirin inhibits LPS-induced macrophage activation via the NF-κB pathway. Sci. Rep. 2017, 7, 11549. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Tolmeijer, S.; Oskam, J.M.; Tonkens, T.; Meijer, A.H.; Schaaf, M.J.M. Glucocorticoids inhibit macrophage differentiation toward a pro-inflammatory phenotype upon wounding without affecting their migration. Dis. Model Mech. 2019, 12, dmm037887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellamri, N.; Morzadec, C.; Lecureur, V.; Joannes, A.; Wollin, L.; Jouneau, S.; Vernhet, L. Effects of Nintedanib on the M1 and M2a polarization of human macrophages. Eur. Respir. J. 2018, 52, PA5250. [Google Scholar]
- He, L.; Jhong, J.; Chen, Q.; Huang, K.; Strittmatter, K.; Kreuzer, J.; DeRan, M.; Wu, X.; Lee, T.; Slavov, N.; et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021, 37, 109955. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Marneros, A.G. Doxycycline inhibits polarization of macrophages to the proangiogenic M2-type and subsequent neovascularization. J. Biol. Chem. 2014, 289, 8019–8028. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, J.; Sui, L.; Xu, H.; Piao, Q.; Liu, Y.; Qu, X.; Sun, Y.; Song, L.; Li, D.; et al. Antibiotics induce polarization of pleural macrophages to M2-like phenotype in patients with tuberculous pleuritis. Sci. Rep. 2017, 7, 14982. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Hao, D.; Xu, W.; Li, J.; Li, X.; Shen, D.; Sheng, K.; Zhao, L.; Xu, W.; Gao, Z.; et al. β-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharmaceut. Biol. 2019, 57, 161–168. [Google Scholar] [CrossRef] [Green Version]

















| Gene | IC50 (µM) |
|---|---|
| IL-6 | 0.99 ± 0.36 |
| TNF-a | 1.67 ± 0.13 |
| iNOS | 1.15 ± 0.58 |
| MCP-1 (CCL-2) | 1.07 ± 0.07 |
| NLRP3 | 1.73 ± 0.33 |
| GR-Agonism | MR-Agonism | |
|---|---|---|
| Dexamethasone (10 µM) | 100% | - |
| Dexamethason (0.4 µM) | 100% | - |
| Aldosteron (1 µM) | - | 100% |
| Aldosteron (0.04 µM) | - | 100% |
| Oxy210 (10 µM) | 1% | −3.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Stappenbeck, F.; Tang, L.-Y.; Zhang, Y.E.; Hui, S.T.; Lusis, A.J.; Parhami, F. Oxy210, a Semi-Synthetic Oxysterol, Exerts Anti-Inflammatory Effects in Macrophages via Inhibition of Toll-like Receptor (TLR) 4 and TLR2 Signaling and Modulation of Macrophage Polarization. Int. J. Mol. Sci. 2022, 23, 5478. https://doi.org/10.3390/ijms23105478
Wang F, Stappenbeck F, Tang L-Y, Zhang YE, Hui ST, Lusis AJ, Parhami F. Oxy210, a Semi-Synthetic Oxysterol, Exerts Anti-Inflammatory Effects in Macrophages via Inhibition of Toll-like Receptor (TLR) 4 and TLR2 Signaling and Modulation of Macrophage Polarization. International Journal of Molecular Sciences. 2022; 23(10):5478. https://doi.org/10.3390/ijms23105478
Chicago/Turabian StyleWang, Feng, Frank Stappenbeck, Liu-Ya Tang, Ying E. Zhang, Simon T. Hui, Aldons J. Lusis, and Farhad Parhami. 2022. "Oxy210, a Semi-Synthetic Oxysterol, Exerts Anti-Inflammatory Effects in Macrophages via Inhibition of Toll-like Receptor (TLR) 4 and TLR2 Signaling and Modulation of Macrophage Polarization" International Journal of Molecular Sciences 23, no. 10: 5478. https://doi.org/10.3390/ijms23105478
APA StyleWang, F., Stappenbeck, F., Tang, L.-Y., Zhang, Y. E., Hui, S. T., Lusis, A. J., & Parhami, F. (2022). Oxy210, a Semi-Synthetic Oxysterol, Exerts Anti-Inflammatory Effects in Macrophages via Inhibition of Toll-like Receptor (TLR) 4 and TLR2 Signaling and Modulation of Macrophage Polarization. International Journal of Molecular Sciences, 23(10), 5478. https://doi.org/10.3390/ijms23105478






