Abstract
New tetrahydropyrazino[2,3-c]quinolin-5(6H)-ones were prepared from 3-chloroquinoline-2,4(1H,3H)-diones and ethylene diamine. In their reaction with HNCO, an unprecedented molecular rearrangement produced new types of hydantoin derivatives. All prepared compounds were characterized on the basis of their 1H, 13C, and 15N NMR and ESI mass spectra and some were authenticated by X-ray analysis of single crystalline material. A proposed mechanism for rearrangement is discussed in this essay. The CDK and ABL inhibition activity as well as in vitro cytotoxicity of the prepared compounds was also tested.
1. Introduction
The presence of an amino group is common in many biologically active compounds. The group of reactive compounds including an amino group (especially α-aminoketones with respect to their easily conversion) belongs to various heterocycles [1]. Suitable compounds of this type are 3-aminoquinoline-2,4-diones, which is our particular area of interest [2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Even if the occurrence of these compounds in the relevant literature was early rather than rare [16,17], we managed to prepare 3-aminoderivatives using 3-chloroquinolinediones and ammonium salts or primary amines [2]. Later, we proved that these compounds may also be prepared from 3-hydroxyquinoline-2,4-diones in reaction with ammonia or ammonium salts [14].
The biological activity of some 3-aminoquinoline-2,4-diones has been described [18]. 3-Amino-3-(4-fluorophenyl)-1H-quinoline-2,4-dione was demonstrated as effective against oxidative stress-related diseases [19] and suppresses reactive oxygen species [20,21]. A similar effect was exhibited by 3-amino-6-fluoro-3-(4-fluorophenyl)-1H-quinoline-2,4-dione [19].
We found that 3-aminoquinoline-2,4-diones were subject to molecular rearrangements after their reaction with urea [3,4,5], nitrourea [6,7], isocyanates [8], isothiocyanates [9,10,11], isothiocyanic acid [12,13,15], and isocyanic acid [13], creating a broad palette of new heterocyclic compounds, e.g., imidazo[1,5-c]quinazoline-3,5-diones, 3-(3-acylureido)-2,3-dihydro-1H-indol-2-ones, 4-alkylidene-1’H-spiro[imidazolidine-5,3’-indole]-2,2’-diones and spiro-linked imidazoline-2-thiones.
We also examined the reaction of 3-chloroquinolin-2,4-diones 1 with ethanolamine and found that the results were similar to those from the reaction of 1 with simple aliphatic amines, and 3-(2-hydroxyethylamino)quinoline-2,4-diones were obtained. Their reaction with isocyanic acid presented rearranged products that were structurally analogous to those listed above. However, their reaction with isothiocyanic acid proceeded differently and resulted in mainly non-rearranged compounds [15].
Considering these results, we decided to study the reactions of 3-chloroquinoline-2,4-diones 1 with 1,2-diamines. In the literature, most of the reactions reported are of α-haloketones with o-phenylenediamines. Surprisingly, reactions of tertiary α-bromoketones with aliphatic 1,2-diamines have only been described in one article [22].
In our previous paper [23], we described the reaction of N-1 unsubstituted 3-chloroquinolinediones with ethylene diamine. The results of this reaction were remarkable because we obtained two types of new quinazoline derivatives that did not react with isocyanic and isothiocyanic acids.
Hydantoin (systematically imidazolidine-2,4-dione) represents a structural motif that has been of interest to many researchers in recent years, not only chemically but also biologically [24,25,26,27]. Hydantoin-based compounds exhibit a broad range of biological activities, such as fungicidal, herbicidal, antitumor, anti-inflammatory, anti-HIV, hypolipidemic, antiarrhythmic, antiplatelet, and antihypertensive activities [28,29,30]. Some of these compounds have been approved for clinical use to treat many human diseases. For example, they act as muscle relaxants, anticonvulsants, or androgen receptor antagonists [28].
In this paper, we demonstrate that the reaction of ethylene diamine with N-1 substituted 3-chloroquinoline-2,4-diones proceeds smoothly without rearrangement to result in pyrazino[2,3-c]quinolin-5(6H)-ones 2. Moreover, new molecular rearrangement of the easily obtainable compounds 2 yielded two hitherto unknown types of potentially biologically active hydantoins during their reaction with isothiocyanic acid.
2. Results and Discussion
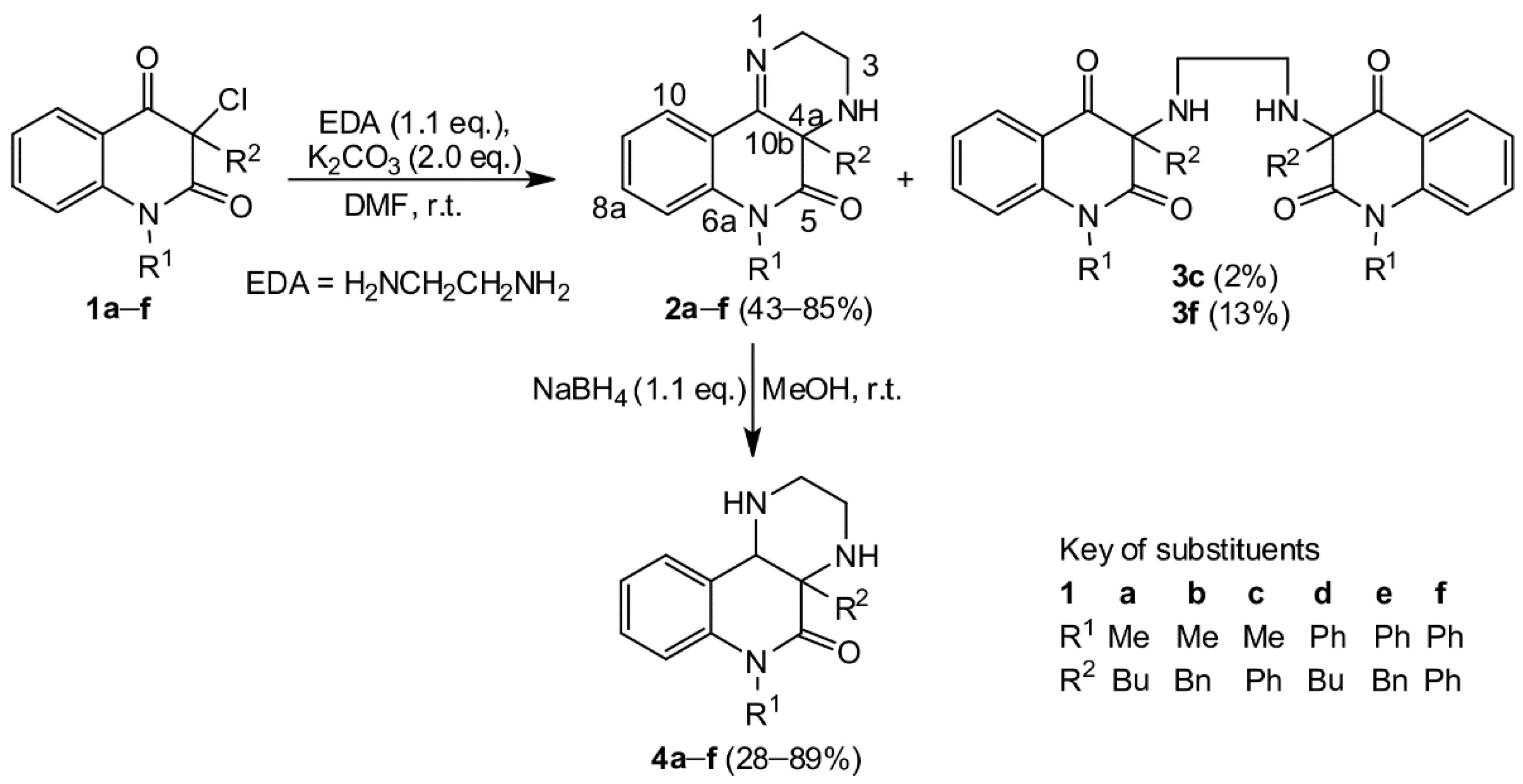
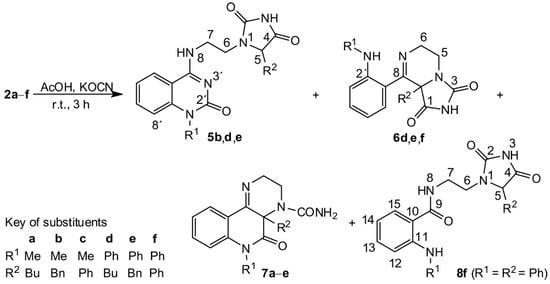
Our purpose was to study in detail the reaction connected with the isolation of a large quantity of minority compounds and to clarify the reaction mechanism. The reactions of 3-chloroquinolin-2,4-diones 1a–f with ethylene diamine were performed in DMF in the presence of powdered potassium carbonate. In a good yield, novel tricyclic pyrazino[2,3-c]quinolin-5(6H)-ones 2 were obtained (Scheme 1). In just two cases, a small quantity of dimeric compounds 3c and 3f was produced via double alkylation of ethylene diamine with the chloroderivatives 1c and 1f. Their 1H and 13C NMR spectra exhibited two sets of signals according to the presence of two observable diastereoisomers. Reaction of compounds 2 with sodium borohydride confirmed the presence of the imine group and led to the expected dihydroderivatives 4 (Scheme 1). Even though ethylene diamine is a strong base, we did not observe the formation of other compounds that would be products of a rearrangement analogous to rearrangement of 3-aminoquinolinediones. The NMR spectra and chemical shifts for the isolated compounds 2, 3, and 4 are presented in the Supplementary Materials (see Figures S1–S15 and Tables S1–S3, respectively). The reactions of compound 2 with potassium cyanate were carried out with a molar ratio 1:1.6 in a solution of acetic acid (Scheme 2, Table 1). Our first look at the IR and NMR spectra for the reaction products showed that at least three types of compounds were present. However, we were not able to determine the structure of the isolated compounds from their NMR spectra. Only a few isolated fragments were found, but it was impossible to determine how they were interconnected. Fortunately, after more unsuccessful experimentation, we managed to prepare a single crystal of the compound acquired from compound 2d. The structure of this compound (5d) was established by X-ray diffraction analysis (Figure 1). Although the structures of imidazolidine-2,4-dione (also a part of 5d skeleton) derivatives had been described crystallographically more than 170 times, derivatives with a longer hydrocarbon chain are absent from the literature. Moreover, the second part of the 5d molecule, a 1,2-dihydroquinazolin-2-one fragment, is scarcely reported [31,32].
Scheme 1.
The preparation and reduction of pyrazino [2,3-c]quinolin-5(6H)-ones 2.
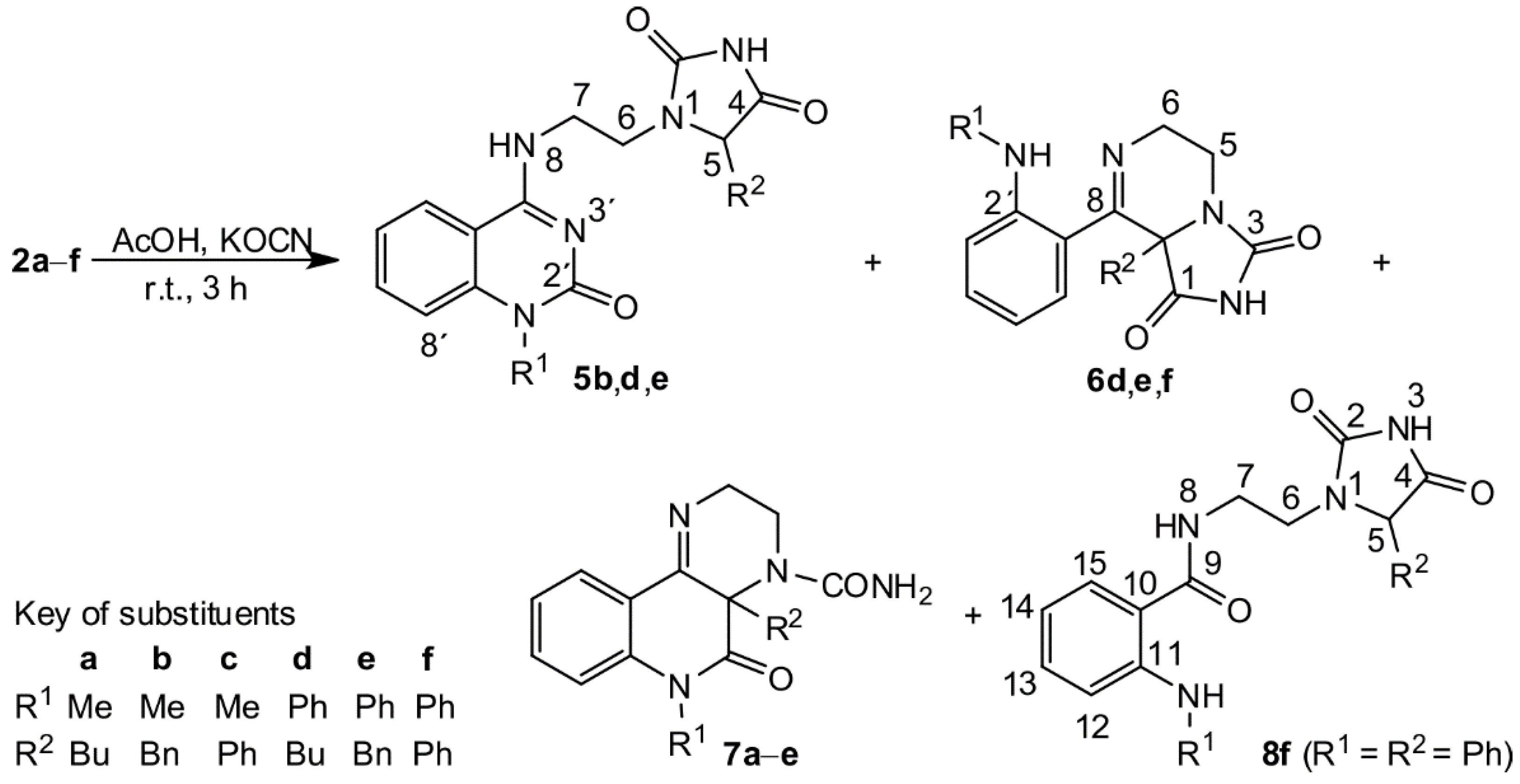
Scheme 2.
Reaction of compounds 2 with potassium cyanate.

Table 1.
Results of the reactions of compounds 2, 6 and 7.
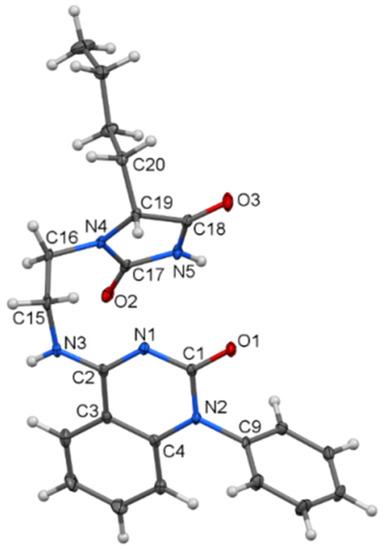
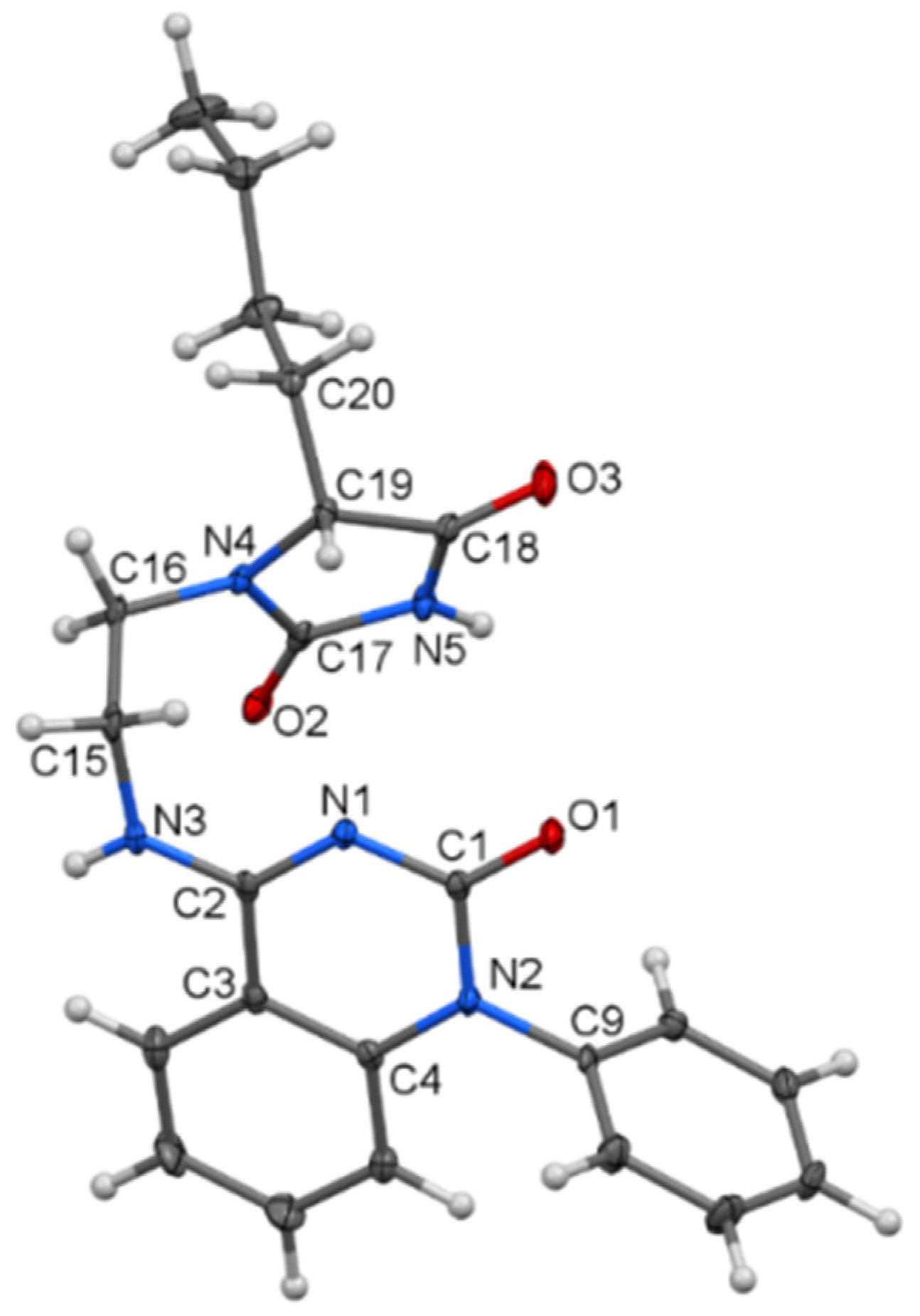
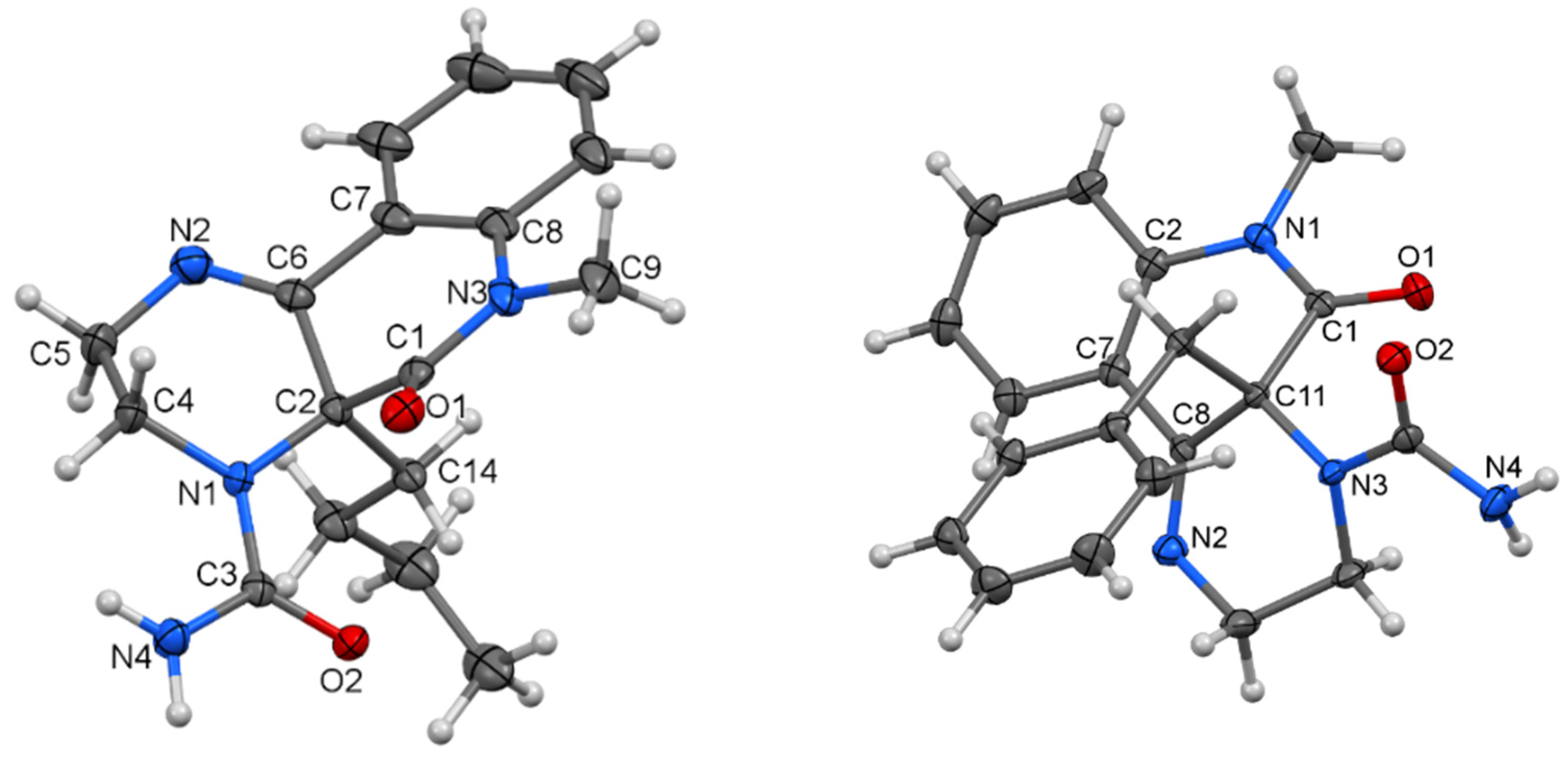
Figure 1.
Molecular structure of compound 5d—ORTEP diagram drawn with 40% probability level.
In 5d (Figure 1), the planes of the imidazolidine-2,4-dione and 1,2-dihydroquinazolin-2-one parts, which are separated by an iminoethane bridge, exhibit an interplanar angle of 26.16(9)°. The two molecules are interconnected by C=O···H–N bridges (see Supplementary Materials, Figure S29).
The structure of 5d is surprising because its creation requires the scission of the C(2)–C(3) bond in the starting compound 2d. We did not observe such a reaction at any time. The transformation of quinolinedione to a quinazolinedione skeleton was previously observed only in cases where the starting compound was N-unsubstituted, allowing the formation of a useful isocyanate intermediate [23].
Compound 5d consists of two bioactive moieties: 4-iminoquinazolin-2-one and substituted hydantoin. Several methods for the preparation of closely related quinazolin-4-ones [33] and quinazoline-2,4-diones [34] were recently described; however, none of them are remotely similar to the presented transformation. It must be pointed out that the reaction of compounds 2 with HNCO was carried out with a molar ratio 1:1.6 because we did not anticipate initially the reaction of compound 2 with more than one mole of isocyanic acid. Therefore, complete conversion of compounds 2 to 5 cannot be expected, but rather, only the formation of a mixture of products can proceed (Table 1). Using an excess of KNCO, the composition of the reaction products changed (Table 1), but at no time was the full conversion of 2 to 5 achieved.
Compounds 5b and 5e belong to the group of compounds produced by the reaction of 2 with two equivalents of HNCO that exhibited an absorption band at ca. 1770 cm−1 in the IR spectrum characteristic of hydantoins [35]. All their NMR data (see Supplementary Materials, Table S4 and Figures S16 and S18, respectively) are in the agreement with the proposed structure.
In addition to compound 5d, the next product was obtained from compound 2d. From ESI-MS and elemental analysis, it was determined that only one mole of HNCO was consumed. Its IR spectrum exhibited an absorption band at 1776 cm–1, indicative of the presence of a hydantoin ring [14], and a singlet at 11.2 ppm appeared in the 1H NMR spectrum pertaining to a NH proton in position 2 of the hydantoin moiety [36]. The fragment Ar―NH―Ph was also found, which bears witness to the opening of the quinolinone ring in 2d.
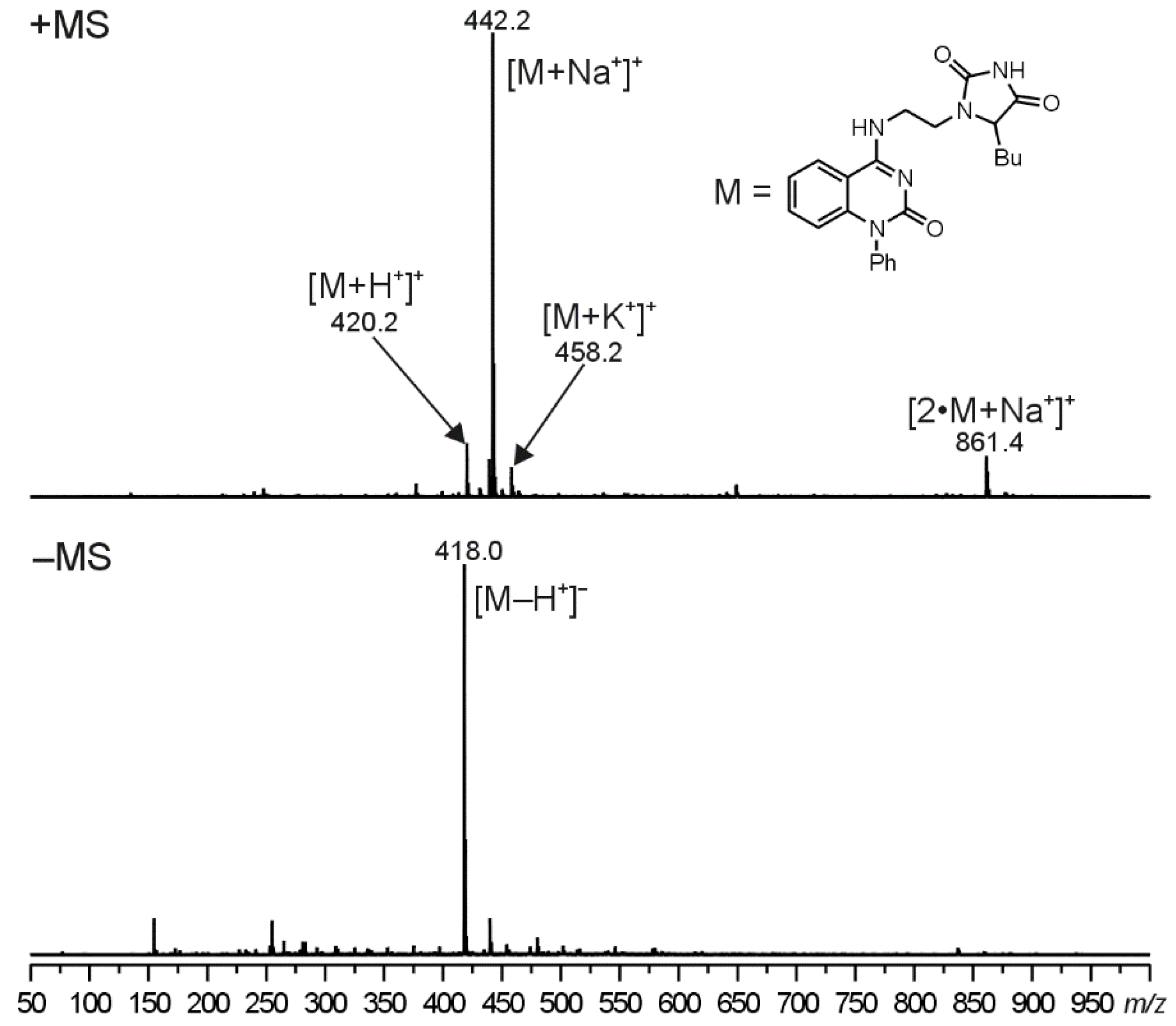
The molecular structure of compounds 5 were proved using ESI-MS/MS analyses. In the positive-ion first-order mass spectra, four singly charged ions were observed. The most abundant ion, assigned as a sodium adduct of the molecule ([M+Na+]+), was accompanied by two less intense signals at m/z corresponding to a protonated molecule ([M+H+]+) and a potassium adduct of the molecule ([M+K+]+). Moreover, a sodium adduct of the dimer ([2 M+Na+]+) was observed in the case of compounds 5. In the negative polarity mode, an ion assigned as a deprotonated molecule ([M+Na+]+) was formed. Illustrative ESI mass spectra for compound 5d can be seen in Figure 2 (ESI-MS spectra for compounds 5b and 5e are given in the Supplementary Materials, Figures S47 and S48, respectively).
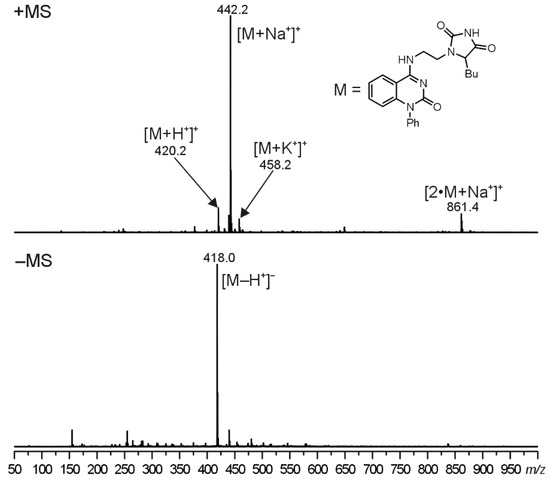
Figure 2.
The first-order positive and negative ion ESI-MS spectra for compound 5d. The assignments for the observed signals are shown in the brackets.
Compound 6d represents the second structural group of products produced from the reaction of 2 with only one mole of isocyanic acid that exhibited an IR absorption band at ca 1760 cm–1. Compounds 6e and 6f also pertain to this group. All these compounds display an absorption band at ca 1760 cm–1 in the IR spectrum and a broad signal at ca 11.1 ppm in their 1H NMR spectra. In their 13C NMR spectra (see Supplementary Materials, Table S5 and Figures S19–S21, respectively), quaternary carbons signals appeared at ca 68.9 ppm and, in their 15N NMR spectra, a signal adherent to the C=N group can be seen, much like that for the starting compound 2. Four nitrogen atoms were present in forenamed compounds. One belonged to a C=N group, the second was imidic, and the third pertained to a tertiary amino group. Therefore, the fourth nitrogen atom, which exhibited a singlet at ca 8 ppm in its 1H NMR spectrum, must be part of Ar―NH―R1 grouping. In both positive and negative ion ESI-MS spectra for compounds 6, the most abundant signal was observed at m/z corresponding to a (de)protonated molecule (see Supplementary Materials, Figures S49–S51).
The third product of the reaction of 2d with HCNO was a compound that did not have any IR absorption around 1760 cm–1 and, therefore, did not contain a hydantoin ring. Its 1H and 13C NMR spectra (see Supplementary Materials, Figure S25) were similar to 2d, but the presence of a CONH2 group in the results suggest the structure of 7d. The reaction of this compound with an excess of HNCO (Table 1) provided compound 5d, indicating that 7d is the first intermediate in the molecular rearrangement of 2d. It was found that compounds 7 resulted from all compounds 2 except 2f.
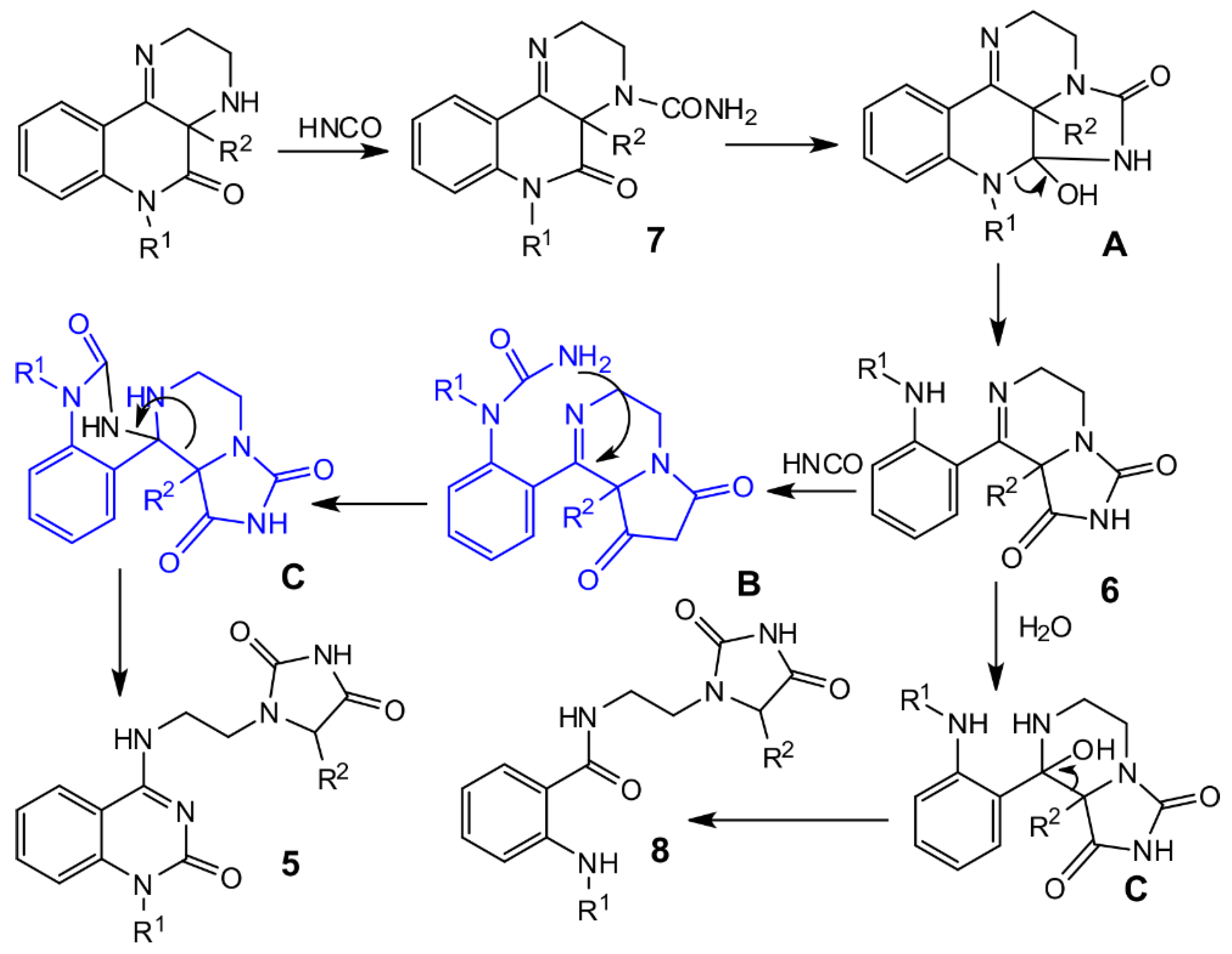
The molecular structure of compounds 7a (Figure 3, left) and 7b (Figure 3, right) were proven by X-ray analysis. The structures of 7a and 7b are characterized by the presence of substituted tricyclic systems where the π―electron conjugation is interrupted by the presence of a stereogenic center at C-2 (7a) and or C-11 (7b) as well as an ethylene bridge. The constitution of the tricyclic system in 7a is totally unknown. On the other hand, the characteristic interatomic distances and angles in both compounds that crystallize in achiral space groups P21/c and P-1, respectively, are essentially the same as previously known structures with the same type of functional groups and atom hybridization [37,38].
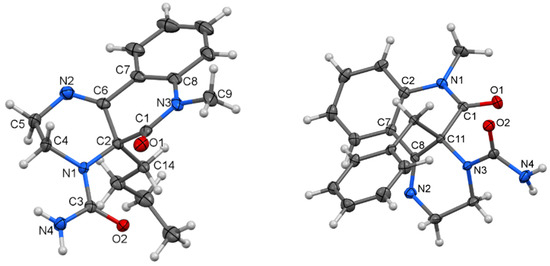
Figure 3.
Molecular structure of compound 7a (left) and 7b (right)—ORTEP diagrams drawn with 40% probability level.
Three molecules of 7a co-crystallize with two molecules of water to form an extensive system of H-bridges. In 7b, both optical isomers are interconnected by an NH···O=C bridging motif. Co-crystallized dichloromethane molecules occupy tunnels formed by the aromatic rings of the molecule. All the geometric parameters for all X-rayed structures are given in the Supplementary Materials (Figures S28–S33, Tables S8–S16). Compounds 7c and 7d exhibited anomalous behavior in the form of very broad signals when their NMR spectra were measured in DMSO-d6. Therefore, they were measured in CDCl3.
As in the case of the above-mentioned compounds, the structures of compounds 7 were confirmed using mass spectrometry. Except commonly observed ions, such as protonated molecules, sodium and potassium adducts of the molecule, and/or sodium adducts of the dimer, we observed a singly charged signal in the positive-ion first-order ESI mass spectra that was assigned as a [M+H+–HCNO]+ ion. Its presence can be explained, according to tandem mass spectrometry experiments, as a result of in-source fragmentation. ESI mass spectra for compounds 7 are given in the Supplementary Materials (see Figures S52–S56).
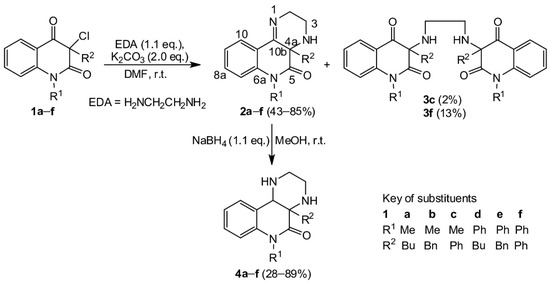
Compound 7 is primarily the product of the reaction between compound 2 and isocyanic acid, and therefore provides the starting compounds for the following molecular rearrangement to compounds 5 and 6. Our proposal for the reaction mechanism for rearrangement of compounds 2 is illustrated in Scheme 3. We suppose that addition of compound 2 to isocyanic acid produces compound 7, which is subsequently changed to compound 6 via the intermediate A. The reaction of compound 6 with isocyanic acid affords the intermediate B, which undergoes retro-Claisen condensation for the formation of compounds 5.
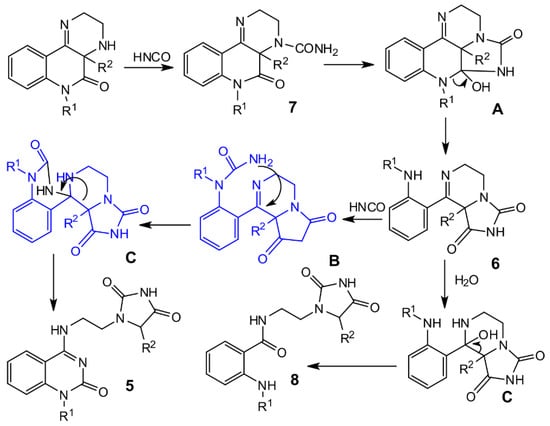
Scheme 3.
Proposed reaction mechanism.
One of the isolated products, prepared from 2f, was different from the compounds mentioned above. The fragment NCH2CH2N was present, but the compound did not contain the C=N group, and instead of a quaternary carbon atom, it contained a CHR group. The presence of an IR band at 1775 cm–1 in the IR spectrum and 11.2 ppm in the 1H NMR spectrum indicated that the hydantoin ring must be present. In the molecule that pointed to the structure 8f, the amide group was found (see Supplementary Materials, Table S7 and Figure S27). Not only IR and NMR, but also mass spectrometry provided clear evidence for the structure of compound 8f. Results for its ESI-MS analysis are given in Supplementary Materials (see Figure S57). The origin of this compound can be explained by the addition of water to compound 6f and following retro-Claisen condensation through intermediates B and C (Scheme 3).
As mentioned in the introduction, some compounds bearing a quinoline or hydantoin moiety are known to possess a wide range of biological activities. However, there are only few examples of compounds possessing both of the above-mentioned structural motifs. For example, Kumar and co-workers published the synthesis of new series of 7-chloroquinoline-thiohydantoin derivatives with potent antimalarial activity [39]. Quinoline and hydantoin derivatives are well-known for their anticancer activity, as recently described in several comprehensive reviews [28,40]. According to this fact, we decided to test the antiproliferative activity of compounds 5, 6, and 7 on two types of human tumor cell lines (K-562, chronic myelogenous leukemia and MV4;11, acute myelogenous leukemia). Moreover, the inhibitory potency of these compounds was assayed on two types of protein kinases, namely the recombinant heterodimeric complex CDK2/cyclin E and tyrosine-protein kinase ABL1. Unfortunately, no biological activity was observed for concentrations up to 10 µM.
3. Materials and Methods
3.1. General Data
Melting points were determined with a Kofler block. IR (KBr) spectra were recorded with a Smart OMNI-Transmission Nicolet iS10 spectrophotometer. The 1H, 13C, and 15N NMR spectra were recorded with a Bruker Avance III HD 500 spectrometer (500.13 MHz for 1H, 125.76 MHz for 13C, and 50.68 MHz for 15N) in DMSO-d6. 1H and 13C chemical shifts are given on the δ scale (ppm) and are referenced to internal TMS (δ = 0.0). 15N chemical shifts were referred to external neat CH3NO2 in a co-axial capillary (δ = 0.0). All 2D experiments (gradient-selected (gs)-COSY, gs-TOCSY, gs-HMQC, gs-HMQC-RELAY, gs-HMBC) were performed using the manufacturer’s software. Full-sets of diffraction data for 5d (yellow) and 7a and 7b (colorless) were collected at 150(2)K with a D8-Venture diffractometer (Bruker, Germany) equipped with Cu (Cu/Kα radiation; λ = 1.54178 Å) or Mo (Mo/Kα radiation; λ = 0.71073 Å) microfocus X-ray (IµS) sources, CMOS photon detector, and an Oxford Cryosystems cooling device was used for data collection. Experimental details are stated in Supporting Information. The frames were integrated with the Bruker SAINT Software package using a narrow-frame algorithm. Data were corrected for absorption effects using the Multi-Scan method (SADABS). The obtained data were treated by XT-version 2018/1 and SHELXL-2017/1 software implemented in an APEX3 v2016.5-0 (Bruker AXS) system [41]. The positive-ion EI mass spectra were measured on a QP-2010 instrument (Shimadzu, Japan) within the mass range m/z = 50–600 using a direct inlet probe (DI). Samples were dissolved in dichloromethane (30 μg·mL–1) and 10 μL of the solution was evaporated in a DI cuvette at 50 °C. The ion source temperature was 200 °C; the energy of electrons was 70 eV. Only signals exceeding a relative abundance of 5% are listed. The electrospray mass spectra (ESI-MS) were recorded using an amaZon X ion-trap mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with an electrospray ion source. All experiments were conducted in both positive and negative polarity mode. Individual samples (with a concentration of 500 ng·mL–1) were infused into the ESI source as methanol/water (1/1, v/v) solutions via a syringe pump with a constant flow rate of 3 μL·min–1. The other instrumental conditions were as follows: m/z range 50–1500, electrospray voltage of –4.2 kV (4.2 kV in negative polarity mode), capillary exit voltage of 140 V (–140 V in negative polarity mode), drying gas temperature of 220 °C, drying gas flow of 6.0 dm3·min–1, nebulizer pressure of 55.16 kPa. Nitrogen was used as the nebulizing and drying gases for all experiments. Tandem mass spectra were collected using collision-induced dissociation (CID) with He as the collision gas after isolating the required ions. Column chromatography was carried out on silica gel (Merck, grade 60, 70–230 mesh) using successive mixtures of chloroform/ethanol (in ratios from 99:1 to 8:2) (S1) or benzene/ethyl acetate (in ratios from 99:1 to 8:2) (S2). Reactions, the course of separation, and the purity of substances were monitored by TLC (elution systems: benzene/ethyl acetate (4:1) (S3), chloroform/ethanol (9:1 and 1:1) (S4 and S5), and chloroform/ethyl acetate (7:3) (S6)) on Alugram® SIL G/UV254 foils (Macherey-Nagel, Germany). Elemental analyses (C, H, N) were performed with an EA Flash EA 1112 Elemental Analyzer (Thermo Fisher Scientific, Waltham, MA, USA).
3.2. General Procedure for the Reaction of Compounds 1 with Ethylene Diamine
To the solution of compound 1 (1 mmol) in DMF (9 mL), pulverized potassium carbonate (276 mg, 2 mmol) and ethylene diamine (EDA) (0.1 mL, 1.1 mmol) were added and the mixture was stirred at room temperature. The course of the reaction was monitored with TLC. After the spot corresponding to compound 1 faded away, the reaction mixture was diluted with water (20 mL). The deposited product was filtered with suction, dried and crystallized with an appropriate solvent. In cases where the crude product was oily or waxy, the solution was extracted with chloroform (3 × 20 mL). The collected extracts were dried, evaporated to dryness, and the residue was separated by chromatography on a silica gel column.
- 4a-Butyl-6-methyl-2,3,4,4a-tetrahydro-pyrazino[2,3-c]quinolin-5(6H)-one (2a)
Compound was prepared from 1a and EDA with 53% yield, reaction time 6 h. White solid, mp 111–113 °C (ethyl acetate/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 50.2/3.82 and 3.49, C(3)H2 39.2/2.90 and 2.64, C(4a) 63.2, C(5) 171.7, C(6a) 139.1, C(7)H 114.9/7.21, C(8)H 131.4/7.49, C(9)H 123.3/7.18, C(10)H 125.1/7.65, C(10a) 123.9, C(10b) 162.7, C(1’(R1))H3 30.1/3.33, C(1’(R2))H2 39.0/1.45 and 1.31, C(2’(R2))H2 25.4/1.31 and 1.05, C(3’(R2))H2 22.0/1.05, C(4’(R2))H3 13.8/0.69 ppm. IR (cm–1) ν: 3346, 3042, 2959, 2936, 2872, 2825, 1676, 1645, 1602, 1462, 1431, 1410, 1367, 1347, 1315, 1297, 1279, 1265, 1229, 1192, 1125, 1109, 1056, 1044, 992, 961, 946, 840, 759, 740, 680, 654,627, 579, 532. ESI-MS (pos.) m/z (%): 565.2 [2·M + Na+]+ (7), 294.1 [M + K+]+ (19), 272.1 [M + Na+]+ (100), 216.0 [M + H+]+ (11). Anal. Calcd for C16H21N3O (271.36): C 70.82; H 7.80; N 15.49. Found: C 70.55; H 8.00; N 15.40.
- 6-Methyl-4a-benzyl-2,3,4,4a-tetrahydropyrazino[2,3-c]quinolin-5(6H)-one (2b)
Compound was prepared from 1b and EDA with 43% yield. Colorless solid, mp 102–106 °C (benzene/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 49.8/3.64 and 3.02, C(3)H2 38.8/2.88 and 2.45, C(4a) 64.1, C(5) 170.8, C(6a) 139.1, C(7)H 115.1/7.0,2 C(8)H 131.6/7.24, C(9)H 123.3/7.19, C(10)H 125.3/7.64, C(10a) 123.8, C(10b) 161.7, C(1’(R1))H3 30.1/3.35, C(1’(R2))H2 44.9/2.75 and 2.68, C(2’(R2)) 135.2, C(3’(R2))H 130.4/6.94, C(4’(R2))H 127.4/7.16, C(5’(R2))H 126.8/7.19 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −52.7, N(4) −351.7, N(6) −257.6 ppm. IR (cm–1) ν: 3329, 3066, 3028, 2942, 2905, 2838, 1668, 1633, 1603, 1497, 1472, 1455, 1438, 1416, 1357, 1339, 1298, 1270, 1226, 1202, 1159, 1129, 1076, 1062, 1047, 1010, 958, 903, 763, 699, 656, 620, 535, 504. ESI-MS (pos.) m/z (%): 633.2 [2·M + Na+]+ (6), 328.0 [M + Na+]+ (20), 306.0 [M + H+]+ (100), 214.9 [M + H+ − C7H7]+ (10). Anal. Calcd for C19H19N3O (305.37): C 74.73; H 6.27; N 13.76. Found: C 74.63; H 6.40; N 13.84.
- 6-Methyl-4a-phenyl-2,3,4,4a-tetrahydropyrazino[2,3-c]quinolin-5(6H)-one (2c)
Compound was prepared from 1c with 54% yield beside 3c. White solid, mp 178–182 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 49.9/3.96 and 3.81, C(3)H2 37.8/2.71 and 2.65, C(4a) 65.6, C(5) 169.2, C(6a) 138.8, C(7)H 115.1/7.12, C(8)H 131.5/7.43, C(9)H 123.4/7.14, C(10)H 125.1/7.81, C(10a) 124.2, C(10b) 160.6, C(1’(R1))H3 30.1/3.39, C(1’(R2)) 139.9, C(2’(R2))H 126.9/7.18, C(3’(R2)) 128.5/7.29, C(4’(R2))H 127.9/7.24 ppm. IR (cm–1) ν: 3358, 2933, 2838, 1671, 1638, 1602, 1469, 1446, 1417, 1362, 1297, 1150, 1124, 1080, 991, 896, 845, 763, 744, 706, 692, 661, 625, 569, 536, 506. EI-MS m/z (%): 292 (21), 291 (M+, 100), 290 (20), 262 (23), 261 (47), 160 (12), 132 (14), 131 (20), 104 (18), 77 (16). ESI-MS (pos.) m/z (%): 605.2 [2·M + Na+]+ (42), 583.2 [2·M + H+]+ (16), 314.1 [M + Na+]+ (18), 292.1 [M + H+]+ (100). Anal. Calcd for C18H17N3O (291.35): C 74.20; H 5.88; N 14.42. Found: C 73.99; H 5.98; N 14.24.
- 4a-Butyl-6-phenyl-2,3,4,4a-tetrahydropyrazino[2,3-c]quinolin-5(6H)-one (2d)
Compound was prepared from 1d and EDA with 85% yield. Colorless solid, mp 86–90 °C (hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 50.2/3.82 and 3.49, C(3)H2 39.2/2.90 and 2.64, C(4a) 63.2, C(5) 171.7, C(6a) 139.1, C(7)H 114.9/7.21, C(8)H 131.4/7.49, C(9)H 123.3/7.18, C(10)H 125.1/7.65, C(10a) 123.9, C(10b) 162.7, C(1’(R1)) 139.1, C(2’(R1))H overlap/7.25, C(3’(R1))H 130.2/7.57, C(4’(R1))H 128.7/7.50, C(1’(R2))H2 39.0/1.73 and 1.59, C(2’(R2))H2 25.4/1.38 and 1.15, C(3’(R2))H2 22.0/1.15, C(4’(R2))H3 13.9/0.77 ppm. IR (cm–1) ν: 3448, 3330, 2954, 2868, 1677, 1642, 1604, 1492, 1456, 1348, 1332, 1314, 1293, 1261, 1222, 1177, 1113, 1072, 999, 770, 697, 682, 656, 648, 610, 491. EI-MS: m/z (%) 334 (6), 333 (24), 291 (6), 290 (25), 277 (21), 276 (100), 275 (7), 262 (6), 77 (7), 57 (5). ESI-MS (pos.) m/z (%): 356.1 [M + Na+]+ (5), 334.2 [M + H+]+ (100), 278.1 [M + H+ – C4H8]+ (3). Anal. Calcd for C21H23N3O (333.43): C 75.65; H 6.95; N 12.60. Found: C 75.82; H 7.14; N 12.55.
- 4a-Benzyl-6-phenyl-2,3,4,4a-tetrahydropyrazino[2,3-c]quinolin-5(6H)-one (2e)
Compound was prepared from 1e and EDA with 51% yield, reaction time 3 h. White solid, mp 161–164 °C (benzene/cyclohexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 49.8/3.73 and 3.10, C(3)H2 38.6/2.95 and 2.56, C(4a) 64.4, C(5) 170.9, C(6a) 140.0, C(7)H 116.2/6.34, C(8)H 131.2/7.35, C(9)H 123.4/7.13, C810)H 125.6/7.73, C(10a) 123.4, C(10b) 161.5, C(1’(R1)) 137.9, C(2’(R1))H overlapped signals, C(3’(R1))H 130.1/7.52, C(4’(R1))H 128.7/7.51, C(1’(R2))H2 44.7/3.06 and 2.91, C(2’(R2)) 135.2, C(3’(R2))H 130.6/7.10, C(4’(R2))H 127.5/7.22, C(5’(R2))H 126.9/7.22 ppm. IR (cm–1) ν: 3354, 2925, 2897, 2836, 1690, 1638, 1600, 1492, 1460, 1351, 1315, 1275, 1214, 1164, 1073, 1003, 959, 877, 782, 766, 741, 702, 657, 632, 598. EI-MS: m/z (%): 367 (14), 277 (21), 276 (100), 91 (8), 77 (5). ESI-MS (pos.) m/z (%): 757.3 [2·M + Na+]+ (5), 390.1 [M + Na+]+ (21), 368.1 [M + H+]+ (100), 277.0 [M + H+ – C7H7]+ (5). Anal. Calcd for C24H21N3O (367.44): C 78.45; H 5.76; N 11.44. Found: C 78.41; H 5.88; N 11.43.
- 4a,6-Diphenyl-2,3,4,4a-tetrahydropyrazino[2,3-c]quinolin-5(6H)-one (2f)
Compound was prepared from 1f and EDA with 66% yield. Colorless solid, mp 156–160 °C (hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 49.9/3.97 and 3.94, C(3)H2 37.5/2.72 and 2.60, C(4a) 66.1, C(5) 169.4, C(6a) 139.7, C(7)H 116.9/6.09, C(8)H 131.2/7.16, C(9)H 123.5/7.08, C(10)H 125.5/7.83, C(10a) 123.7, C(10b) 160.2, C(1’(R1)) 137.8, C(2’(R1))H overlapped signals, C(3’(R1))H 130.3/7.56, C(4’(R1))H 129.5/7.52, C(1’(R2)) 139.4, C(2’(R2))H 127.0/7.26, C(3’(R2))H 129.5/7.35 C(4’(R2))H 128.7/7.28 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −53.0, N(4) −339.3, N(6) −234.3 ppm. IR (cm–1) ν: 3442, 2908, 2820, 1681, 1638, 1602, 1493, 1458, 1422, 1355, 1335, 1261, 1261, 1160, 1109, 995, 936, 893, 758, 740, 719, 700, 663, 627, 574, 516. EI-MS: m/z (%): 354 (26), 353 (100), 352 (16), 338 (6), 324 (13), 323 (26), 296 (5), 250 (7), 249 (5), 248 (5), 222 (7), 221 (9), 194 (14), 193 (6), 149 (6), 131 (8), 104 (11), 103 (6), 77 (17), 71 (5), 66 (5), 57 (9), 55 (6), 51 (7), 43 (10). ESI-MS (pos.) m/z (%): 354.1 [M + H+]+ (100). Anal. Calcd for C23H19N3O (353.42): C 78.16; H 5.42; N 11.89: Found: C 78.06; H 5.50; N 11.88.
- 3,3’-(Ethane-1,2-diyl)bis(azanediyl)bis(1-methyl-3-phenylquinoline)-2,4(1H,3H)-dione (3c)
Compound was prepared from 1c with 2% yield beside 2c. Yellowish solid, mp 198–210 °C (benzene-hexane). 1H and 13C chemical shifts in DMSO-d6: C(2) 171.32 and 171.23, C(3) 76.98 and 76.96, C(4) 193.27 and 192.72, C(4a) 120.71 and 120.60, C(5)H 127.48 and 127.39/7.75, C(6)H 123.29 and 123.27/7.17, C(7)H 136.41 and 136.39/7.69, C(8)H 115.99 and 115.96/7.39, C(8a) 142.3, NH 2.60, CH2 45.38 and 45.17/2.59 and 2.48, C(1’(R1)) 139.63 and 139.43 C(1’(R2)) 137.78 and 137.44, other C/H signals exist as broadened overlapped signals resonating at 126.9–131.2/7.12–7.8 ppm. IR (cm–1) ν: 3333, 3064, 3033, 2946, 2854, 1703, 1666, 1602, 1472, 1417, 1354, 1303, 1254, 1185, 1114, 1034, 994, 911, 864, 764, 699, 684, 637, 600, 533, 495. ESI-MS (pos.) m/z (%): 581.2 [M + Na+]+ (44), 559.2 [M + H+]+ (100). ESI-MS (neg.) m/z (%): 575.1 [M – H+ + H2O]– (100), 557.1 [M – H+]– (37). Anal. Calcd for C34H30N4O4 (558.63): C 73.10; H 5.41; N 10.03. Found: C 73.41; H 5.68; N 9.95.
- 3,3’-(Ethane-1,2-diyl)bis(azanediyl)bis(1,3-diphenylquinoline)-2,4(1H,3H)-dione (3f)
Compound was prepared from 1f with 13% yield. Colorless solid, mp 262–268 °C (benzene/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2) 171.33 and 171.26, C(3) 77.23 and 77.19, C(4) 193.10 and 192.64, C(4a) 120.50 and 120.44, C(6)H 123.48 and 123.40, C(7)H 135.93/7.46, C(8)H 116.59 and 116.55/6.32, C(8a) 142.3, N 2.60, CH2 45.33 and 45.17/2.58 and 2.51, C(1’(R1))H3 30.03 and 30.00/3.53 and 3.50, C(1’(R2)) 137.91 and 137.86, C(2’(R2))H 126.69 and 126.64/7.31, C(3’(R2))H 128.84 and 128.79/7.28 C(4’(R2))H 128.66 and 128.62/7.28 ppm. IR (cm–1) ν: 3442, 3063, 2927, 2858, 1707, 1673, 1600, 1492, 1461, 1337, 1303, 1249, 1192, 1173, 1158, 1113, 1072, 1031, 1002, 981, 902, 820, 762, 747, 719, 703, 650, 609, 576, 539, 516. ESI-MS (pos.) m/z (%): 1387.5 [2·M + Na+]+ (11), 1365.4 [2·M + H+]+ (6), 721.2 [M + K+]+ (7), 705.3 [M + Na+]+ (31), 683.3 [M + H+]+ (100) Anal. Calcd for C44H34N4O4 (682.77): C 77.40; H 5.02; N 8.21. Found: C 76.98; H 5.13; N 8.36.
3.3. General Procedure for the Reduction of Compounds 2 with NaBH4
To the solution of compound 2 (1.5 mmol) in methanol (20 mL), NaBH4 (67 mg, 1.7 mmol) was added over 5 min. The mixture was stirred for 1.5–3 h at room temperature and then poured onto 20 mL of crushed ice. Hydrochloric acid (35%, 0.28 mL) was added, and after 5 min, 5% NaHCO3. The alkaline reaction mixture was extracted with chloroform (3 × 25 mL), dried and evaporated to dryness. The residue was crystallized with an appropriate solvent.
- 4a-Butyl-6-methyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4a)
Compound was prepared from 2a with 28% yield. Colorless solid, mp 145–149 °C (hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 45.4/2.90 and 2.68, C(3)H2 39.9/2.65 and 2.61, C(4a) 56.9, C(5) 171.6, C(6a) 138.1, C(7)H 114.1/7.06, C(8)H 127.6/7.27, C(9)H 123.4/7.27, C(10)H 122.7/7.06, C(10a) 127.4, C(10b)H 58.2/3.87, C(1’(R1))H3 29.3/3.24, C(2’(R1))H 129.2/7.29, C(3’(R1))H 130.1/7.50, C(4’(R1))H 129.0/7.50, C(1’(R2))H2 22.6/1.96 and 0.57, C(2’(R2))H2 24.2/1.14 and 0.86, C(3’(R2))H2 22.4/1.05, C(4’(R2))H3 14.0/0.73 ppm. IR (cm–1) ν: 3369, 3064, 3040, 2951, 2928, 2862, 2801, 1666, 1601, 1497, 1470, 1443, 1418, 1357, 1294, 1275, 1233, 1203, 1156, 1124, 1040, 983, 958, 887, 874, 847, 824, 758, 684, 632, 593, 548, 537. ESI-MS (pos.) m/z (%): 547.2 [2·M + H+]+ (7), 274.1 [M + H+]+ (100). Anal. Calcd for C16H23N3O (273.37): C 70.30; H 8.48; N 15.37. Found: C 70.53; H 8.34; N 15.23.
- 4a-Benzyl-6-methyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4b)
Compound was prepared from 2b with 30% yield. Colorless solid, mp 207–210 °C (ethanol). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 45.5/3.11 and 3.03, C(3)H2 40.1/2.77 and 2.71, C(4a) 58.6, C(5) 170.4, C(6a) 138.2, C(7)H 114.3/7.13, C(8)H 127.8/7.37, C(9)H 123.5/7.32, C(10)H 122.8/7.13, C(10a) 127.3, C(10b)H 58.4/3.98, C(1’(R1))H3 29.3/3.24, C(1’(R2))H2 29.7/3.21 and 2.04, C(2’(R2)) 136.7, C(3’(R2))H 129.7/6.80, C(4’(R2))H 127.9/7.15, C(5’(R2))H 126.1/7.15 ppm. IR (cm–1) ν: 3295, 3070, 3024, 2969, 2940, 2898, 2835, 2814, 2769, 2721, 1665, 1604, 1504, 1479, 1470, 1459, 1422, 1367, 1336, 1318, 1284, 1238, 1145, 1132, 1118, 1082, 1050, 991, 973, 862, 827, 764, 730, 702, 691, 662, 643, 504. ESI-MS (pos.) m/z (%): 637.2 [2·M + Na+]+ (4), 330.1 [M + Na+]+ (9), 308.1 [M + H+]+ (100). Anal. Calcd for C19H21N3O (307.39): C 74.69; H 6.89; N 13.69. Found: C 74.57; H 7.05; N 13.61.
- 6-Methyl-4a-phenyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4c)
Compound was prepared from 2c with 89% yield. Colorless solid, mp 216–218 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 46.1/2.99 and 2.87, C(3)H2 40.4/2.60 and 2.32, C(4a) 59.7, C(5) 170.4, C(6a) 138.0, C(7)H 114.7/6.95, C(8)H 127.4/7.20, C(9)H 123.1/7.13, C(10)H 122.8/7.49, C(10a) 12714, C(10b)H 58.0/4.25, C(1’(R1))H3 29.8/3.23, C(1’(R2)) 137.4, C(2’(R2))H 129.1/7.460.86, C(3’(R2)) 127.3/7.05, C(4’(R2))H 126.7/7.05 ppm. IR (cm–1) ν: 3067, 2954, 2922, 2802, 1668, 1601, 1495, 1470, 1448, 1412, 1355, 1306, 1271, 1155, 1140, 1117, 1042, 981, 951, 816, 771, 756, 719, 705, 679, 694, 600, 543. ESI-MS (pos.) m/z (%): 587.2 [2·M + H+]+ (7), 316.0 [M + Na+]+ (8), 294.1 [M + H+]+ (100). Anal. Calcd for C18H19N3O (293.36): C 73.69; H 6.53; N 14.32. Found: C 73.60; H 6.70; N 14.32.
- 4a-Butyl-6-phenyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4d)
Compound was prepared from 2d with 62% yield. Colorless solid, mp 120–124 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 45.4/2.96 and 2.74, C(3)H2 39.7/2.73 and 2.66, C(4a) 57.5, C(5) 171.8, C(6a) 138.6, C(7)H 115.5/6.15, C(8)H 127.4/7.07, C(9)H 123.9/7.04, C(10)H 123.0/7.44, C(10a) 127.1, C(10b)H 58.2/4.15, C(1’(R1)) 139.2, C(2’(R1))H 129.2/7.15, C(3’(R1))H 130.0/7.54, C(4’(R1))H 128.2/7.44, C(1’(R2))H2 22.7/2.08 and 0.89, C(2’(R2))H2 24.3/1.26 and 1.02, C(3’(R2))H2 22.5/1.18, C(4’(R2))H3 14.1/0.80 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −344.6, N(4) −354.2, N(6) −235.0 ppm. IR (cm–1) ν: 3251, 3206, 3064, 2958, 2932, 2872, 1709, 1666, 1604, 1494, 1461, 1405, 1379, 1353, 1300, 1266, 1201, 1158, 1141, 1105, 1048, 929, 872, 838, 757, 696, 667, 564. ESI-MS (pos.) m/z (%): 671.3 [2·M + H+]+ (11), 358.1 [M + Na+]+ (5), 336.1 [M + H+]+ (100). Anal. Calcd for C20H21N3O (335.40): C 71.62; H 6.31; N 12.53. Found: C 71.79; H 6.48; N 12.43.
- 4a-Benzyl-6-phenyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4e)
Compound was prepared from 2e with 80% yield. Colorless solid, mp 182–184 °C (benzene/hexane) 1H and 13C chemical shifts in DMSO-d6: C(2)H2 45.5/3.08 and 2.82, C(3)H2 40.4/3.15 and 2.74, C(4a) 58.8, C(5) 170.4, C(6a) 138.5, C(7)H 116.1/6.29, C(8)H 127.5/7.14, C(9)H 123.8/7.14, C(10)H 123.1/7.44, C(10a) 127.4, C(10b)H 58.6/4.27, C(1’(R1)) 139.2, C(2’(R1))H 129.1/7.16, C(3’(R1))H 129.8/7.52, C(4’(R1))H 128.0/7.43, C(1’(R2))H2 29.8/3.36 and 2.27, C(2’(R2)) 136.7, C(3’(R2))H 129.9/7.04, C(4’(R2))H 128.1/7.26, C(5’(R2))H 126.2/7.16 ppm. IR (cm–1) ν: 3287, 3268, 3059, 3019, 2913, 2851, 1690, 1603, 1489, 1449, 1347, 1335, 1289, 1277, 1233, 1193, 1153, 1123, 1080, 1029, 897, 951, 899, 874, 755, 724, 695, 639, 595, 556. ESI-MS (pos.) m/z (%): 761.3 [2·M + Na+]+ (5), 739.3 [2·M + H+]+ (18), 392.1 [M + Na+]+ (10), 370.1 [M + H+]+ (100). Anal. Calcd for C24H23N3O (369.46): C 78.02; H 6.27; N 11.37. Found: C 77.97; H 6.25; N 11.28.
- 4a,6-Diphenyl-1,2,3,4,4a,10b-hexahydropyrazino[2,3-c]quinolin-5(6H)-one (4f)
Compound was prepared from 2f with 80% yield. Colorless solid, mp 174–179 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 45.5/3.09 and 2.96, C(3)H2 40.1/2.70 and 2.43, C(4a) 60.3, C(5) 170.2, C(6a) 138.2, C(7)H 115.8/6.01, C(8)H 127.1/6.98, C(9)H 123.3/7.11, C(10)H 123.2/7.53, C(10a) 127.8, C(10b)H 57.5/4.55, C(1’(R1)) 138.2, C(2’(R1))H 128.7/7.08, C(3’(R1))H 129.9/7.52, C(4’(R1))H 128.2/7.43, C(1’(R2)) 136.9, C(2’(R2))H 129.3/7.64, C(3’(R2))H 127.5/7.16, C(4’(R2))H 127.1/7.16 ppm. IR (cm–1) ν: 3261, 2943, 2904, 2851, 1674, 1604, 1467, 1452, 1346, 1291, 1144, 1072, 772, 755, 717, 698, 648, 586, 550. ESI-MS (pos.) m/z (%): 356.2 [M + H+]+ (100). Anal. Calcd for C23H21N3O (355.43): C 77.72; H 5.96; N 11.82. Found: C 77.59; H 6.00; N 11.69.
3.4. General Procedure for the Reaction of Compounds 2 with Isocyanic Acid
To the solution of 2 (1.5 mmol) in acetic acid (4.5 mL), potassium cyanate (0.195 g, 2.4 mmol) was added, and the mixture was stirred for 3 h at room temperature. The mixture was poured onto crushed ice (20 mL) and extracted with chloroform (5 × 15 mL). The collected extracts were dried and evaporated to dryness. The residue was chromatographed on a silica gel column.
- 5-Benzyl-1-{2-[(1-methyl-2-oxo-2,3-dihydroquinazolin-4(1H)-ylidene)amino]ethyl}imida-zolidine-2,4-dione (5b)
Compound was prepared from 2b with 18% yield beside 7b. Colorless solid, mp 209–215 °C (ethyl acetate). 1H and 13C chemical shifts in DMSO-d6: C(2) 156.5, C(4) 173.8, C(5)H 60.6/4.55, C(6)H2 38.7/3.61, C(7)H2 38.1/3.85 and 3.17, C(2’) 155.2, C(4’) 160.0, C(4a’) 109.8, C(5’)H 123.7/7.95, C(6’)H 121.0/7.20, C(7’)H 133.9/7.67, C(8’)H 114.4/7.34, C(8a’) 142.8, C(1’(R1))H3 30.0/3.43, C(2’(R1))H3 30.0/3.43 C(3’(R1))H3 30.0/3.43 C(4’(R1))H3, 30.0/3.43, C(1’(R2))H2 33.4/3.14 and 3.01, C(2’(R2)) 135.4, C(3’(R2))H 128.2/7.10, C(4’(R2))H 129.4/7.20, C(5’(R2))H 126.7/7.20 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −286.8, N(3)H n.o./10.53 1J(15N, 1H) 95.2 Hz, N(8)H −286.8/8.37 1J(15N, 1H) 88.5 Hz, N(1’) −262.9, N(3’) −171.2 ppm. IR (cm–1) ν: 3400, 3129, 3030, 2939, 2746, 1761, 1709, 1619, 1597, 1565, 1543, 1497, 1456, 1419, 1352, 1329, 1263, 1234, 1173, 1138, 1127, 1095, 1036, 1005, 946, 872, 851, 768, 749, 702, 681, 650, 621, 594, 535. ESI-MS (pos.) m/z (%): 805.3 [2·M + Na+]+ (8), 430.1 [M + K+]+ (5), 414.1 [M + Na+]+ (100), 392.1 [M + H+]+ (22). ESI-MS (neg.) m/z (%): 390.0 [M – H+]– (100). Anal. Calcd for C21H21N5O3 (391.16): C 64.44; H 5.41; N 17.89. Found: C 64.63; H 5.70; N 17.89.
- 5-Butyl-1-{2-[(2-oxo-1-phenyl-2,3-dihydroquinazolin-4(1H)-ylidene)amino]ethyl}imidazolidine-2,4-dione (5d)
Compound was prepared from 2d in 17% yield beside 6d and 7d. Yellowish solid, mp 247–250 °C (benzene/cyclohexane). 1H and 13C chemical shifts in DMSO-d6: C(2) 156.9, C(4) 174.6, C(5)H 59.9/4.26, C(6)H2 38.8/3.67, C(7)H2 38.6/3.82 and 3.24, C(2’) 154.6, C(4’) 160.6, C(4a’) 109.4, C(5’)H 123.7/8.04, C(6’)H 121.4/7.19, C(7’)H 133.6/7.45, C(8’)H 115.0/6.40, C(8a’) 147.7, C(1’(R1)) 138.2, C(2’(R1))H 128.3/7.26 C(3’(R1))H 129.9/7.58, C(4’(R1))H 128.3/7.49, C(1’(R2))H2 27.4/1.76 and 1.73, C(2’(R2))H2 135.4, C(3’(R2)) H2 21.9/1.20, C(4’(R2)) H3 13.8/0.79 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −286.4, N(3)H n.o./10.78 1J(15N, 1H) 90.8 Hz, N(8)H −285.0/8.58 1J(15N, 1H) 92.4 Hz, N(1’) −241.2, N(3’) −171.4 ppm. IR (cm–1) ν: 3335, 3063, 2956, 2871, 1771, 1703, 1640, 1599, 1565, 1537, 1492, 1453, 1418, 1390, 1354, 1327, 1263, 1230, 1183, 1156, 1138, 1113, 1086, 1070, 973, 877, 813, 764, 748, 704, 675, 654, 613, 562, 547, 510. ESI-MS (pos.) m/z (%): 861.4 [2·M + Na+]+ (8), 458.2 [M + K+]+ (6), 442.2 [M + Na+]+ (100), 420.2 [M + H+]+ (11). ESI-MS (neg.) m/z (%): 418.0 [M – H+]– (100). Anal. Calcd for C23H25N5O3 (419.48): C 65.85; H 6.01; N 16.70. Found: C 65.74; H 6.07; N 16.57. Using the excess of KOCN (3 equiv.), 17% of 5d, 8% of 6d and 31% of 7d was obtained. Using the excess of KOCN (3 equiv.), compound 5d was prepared in 57% yield from 6d and in 33% yield from 7d.
- 5-Benzyl-1-{2-[(2’-oxo-1’-phenyl-1,2-dihydroquinazolin-4-yl)amino]ethyl}imidazolidine-2,4-dione (5e)
Compound was prepared from 2e with 22% yield beside 6e and 7e. Colorless solid, mp 181–192 °C (ethyl acetate). After recrystallization from ethanol, the mp increased to 280–283 °C without any change in its IR spectrum. 1H and 13C chemical shifts in DMSO-d6: C(2) 156.6, C(4) 173.8, C(5)H 60.6/4.60, C(6)H2 38.8/3.69, C(7)H2 38.2/3.82 and 3.20, C(2’) 154.5, C(4’) 160.6, C(4a’) 109.4, C(5’)H 123.6/8.02, C(6’)H 121.3/7.21, C(7’)H 133.5/7.49, C(8’)H 115.0/6.41, C(8a’) 143.7, C(1’(R1)) 138.2, C(2’(R1))H 128.2/7.24, C(3’(R1))H 129.9/7.59, C(4’(R1))H 128.2/7.50, C(1’(R2))H2 30.9/3.20 and 3.05, C(2’(R2)) 135.4, C(3’(R2))H 128.2/7.14, C(4’(R2))H 129.4/7.28, C(5’(R2))H 126.7/7.22 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −287.6, N(3)H −232.2/10.58 1J(15N, 1H) 94.6 Hz, N(8)H −285.0/8.56 1J(15N, 1H) 92.7 Hz, N(1’) −241.1, N(3’) −171.7 ppm. IR (cm–1) ν: 3331, 3065, 2936, 1770, 1712, 1653, 1641, 1615, 1600, 1538, 1488, 1454, 1423, 1355, 1330, 1225, 1184, 1156, 1131, 1084, 1030, 775, 753, 707, 675, 622, 541, 509. ESI-MS (pos.) m/z (%): 929.4 [2·M + Na+]+ (4), 492.2 [M + K+]+ (11), 476.2 [M + Na+]+ (100), 454.2 [M + H+]+ (16). ESI-MS (neg.) m/z (%): 452.0 [M – H+]– (100). Anal. Calcd for C26H23N5O3 (453.49): C 68.86; H 5.11; N 15.44. Found: C 68.67; H 5.56; N 15.22. Using the excess of KOCN (4 equiv.), 50% of 5e, 6% of 6e and 29% of 7e was prepared from 2e.
- 8a-Butyl-8-[2-(phenylamino)phenyl]-5,6-dihydroimidazo[1,5-a]pyrazine-1,3(2H,8aH)-dione (6d)
Compound was prepared from 2d with 13% yield. Colorless solid, mp 187–190 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(1) 170.6, C(3) 156.2, C(5)H2 46.4/3.82, C(6)H2 33.9/3.82 and 3.15, C(8) 163.1, C(8a) 68.0, C(1’) 128.3, C(2’) 141.7, C(3’)H 117.9/7.10, C(4’)H 128.7/7.19, C(5’)H 119.7/6.86, C(6’)H 129.3/7.10, C(1’(R1)) 143.6, C(2’(R1))H 118.6/7.02, C(3’(R1))H 128.7/7.19, C(4’(R1))H 120.3/6.83, C(1’(R2))H2 32.7/1.94, C(2’(R2))H2 24.7/1.15 and 0.98, C(3’(R2)) H2 21.7/1.15, C(4’(R2)) H3 13.7/0.74 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(2)H n.o./11.01, N(4)H −290.8, N(7) −53.7, N(2’)H −295.9/7.15 1J(15N, 1H) 90.3 Hz. IR (cm–1) ν: 3302, 3046, 2958, 2871, 2732, 1776, 1719, 1640, 1593, 1508, 1455, 1419, 1303, 1127, 1115, 1070, 1021, 912, 890, 859, 756, 699, 678, 627, 578, 534, 499. ESI-MS (pos.) m/z (%): 399.2 [M + Na+]+ (12), 377.2 [M + H+]+ (100). ESI-MS (neg.) m/z (%): 375.0 [M – H+]– (100). Anal. Calcd for C22H24N4O2 (376.45): C 70.19; H 6.43; N 14.88. Found: C 70.30; H 6.58; N 14.53.
- 8a-Benzyl-8-[2-(phenylamino)phenyl]-5,6-dihydroimidazo[1,5-a]pyrazine-1,3(2H,8aH)-dione (6e)
Compound was prepared from 2e with 14% yield. Colorless solid, mp 227–230 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(1) 169.9, C(3) 155.8, C(5)H2 46.6/3.93 and 3.86, C(6)H2 34.0/3.78 and 3.42, C(8) 162.9, C(8a) 68.3, C(1’) 128.2, C(2’) 142.1, C(3’)H 117.7/7.12, C(4’)H 128.9/7.22, C(5’)H 120.1/6.93, C(6’)H 129.4/7.22,C(1’(R1)) 143.5, C(2’(R1))H 119.1/7.02, C(3’(R1))H 128.7/7.21, C(4’(R1))H 120.5/6.83, C(1’(R2))H2 38.5/3.26 and 3.36, C(2’(R2)) 133.8, C(3’(R2))H 131.2/7.02, C(4’(R2))H 128.3/7.20, C(5’(R2))H 127.1/7.22 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(2)H n.o./10.68, N(4)H −291.4, N(7) −50.6, N(2’)H −295.6/7.37 1J(15N, 1H) 90.4 Hz. IR (cm–1) ν: 3323, 3032, 2932, 2731, 1770, 1720, 1645, 1595, 1510, 1496, 1478, 1454, 1411, 1302, 1137, 1067, 1039, 929, 753, 700, 689, 663, 597, 560, 535, 491. ESI-MS (pos.) m/z (%): 433.2 [M + Na+]+ (7), 411.2 [M + H+]+ (100). ESI-MS (neg.) m/z (%): 409.0 [M – H+]– (100). Anal. Calcd for C25H22N4O2 (410.47): C 73.15; H 5.40; N 13.65. Found: C 73.12; H 5.55; N 13.81. Using an excess of KOCN (3 equiv.), 6% of 6e, 29% of 7e, and 30% of 5e was obtained.
- 8a-Phenyl-8-[2-(phenylamino)phenyl]-5,6-dihydroimidazo[1,5-a]pyrazine-1,3(2H,8aH)-dione (6f)
Compound was prepared from 2f and KOCN (3 equiv.) with 20% yield beside 8f. Colorless solid, mp 190–192 °C (ethyl acetate/hexane). 1H and 13C chemical shifts in DMSO-d6: C(1) 170.3, C(3) 155.4, C(5)H2 45.5/4.03 and 3.55, C(6)H2 36.2/3.48 and 3.34, C(8) 165.0, C(8a) 68.9, C(1’) 125.3, C(2’) 138.8, C(3’)H 118.9/7.19, C(4’)H 130.8/7.23, C(5’)H 119.4/6.82, C(6’)H 131.6/7.25,C(1’(R1)) 142.8, C(2’(R1))H 118.9/7.01, C(3’(R1))H 128.9/7.22, C(4’(R1))H 120.9/6.89, C(1’(R2)) 137.3, C(2’(R2))H 126.5/7.39, C(3’(R2))H 128.9/7.42, C(4’(R2))H 130.0/7.42 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(2)H n.o./11.05, N(4)H −285.5, N(7) −58.2, N(2’)H −292.2/8.85 1J(15N, 1H) 87.2 Hz. IR (cm–1) ν: 3181, 3061, 2925, 2849, 2740, 1775, 1718, 1594, 1570, 1497, 1451, 1310, 1220, 1167, 1124, 1070, 1031, 963, 913, 889, 853, 751, 696, 594, 549. ESI-MS (pos.) m/z (%): 815.2 [2·M + Na+]+ (4), 419.1 [M + Na+]+ (18), 397.1 [M + H+]+ (100). ESI-MS (neg.) m/z (%): 813.2 [2·M – 2·H + Na+]− (33), 395.0 [M – H+]– (100). Anal. Calcd for C24H20N4O2 (396.43): C 72.71; H 5.08; N 14.13. Found: C 72.29; H 5.06; N 14.08.
- 4a-Butyl-6-methyl-5-oxo-2,3,5,6-tetrahydropyrazino[2,3-c]quinoline-4(4aH)-carboxamide (7a)
Compound was prepared from 2a and KOCN (1.4 equiv.) with 47% yield. Using an excess of KNCO (4 equiv.), 7a was prepared with 57% yield. Colorless solid, mp 155–158 °C (ethyl acetate/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 n.o/3.94, C(3)H2 40.1/3.80 and 3.49, CO 159.9, C(4a) n.o., C(5) 168.5, C(6a) 139.3, C(7)H 114.8/7.20, C(8)H 131.7/7.51, C(9)H 122.8/7.13, C(10)H 125.2/7.74, C(10a) 124.6, C(10b) 162.5, C(1’(R1))H3 30.3/3.31, C(1’(R2))H2 30.3/1.58, C(2’(R2))H2 25.2/1.19 and 0.85, C(3’(R2))H2 21.7/1.03, C(4’(R2))H3 13.7/0.66 ppm. IR (cm–1) ν: 3350. 3193, 2954, 2856, 1684, 1644, 1621, 1603, 1461, 1402, 1358, 1310, 1255, 1223, 1138, 1059, 1041, 1008, 992, 966, 941, 862, 750, 727, 699, 676, 595, 559. ESI-MS (pos.) m/z (%): 651.3 [2·M + Na+]+ (19), 353.0 [M + K+]+ (6), 337.1 [M + Na+]+ (77), 315.1 [M + H+]+ (100), 272.0 [M + H+ – HCNO]+ (16). ESI-MS (neg.) m/z (%): 312.9 [M – H+]– (100). Anal. Calcd for C17H22N4O2 (314.38): C 64.95; H 7.05; N 17.82. Found: C 64.75; H 7.22; N 17.71.
- 4a-Benzyl-6-methyl-5-oxo-2,3,5,6-tetrahydropyrazino[2,3-c]quinolone-4-(4aH)-carbox- amide (7b)
Compound was prepared from 2b with 63% yield beside 5b. Colorless solid, mp 146–151 °C (ethyl acetate/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 48.6/3.66, C(3)H2 40.4/3.61 and 2.74, CO 160.3, C(4a) 69.2, C(5) 167.8, C(6a) 139.6, C(7)H 115.1/7.20, C(8)H 131.9/7.53, C(9)H 123.0/7.20, C(10)H 125.9/7.75, C(10a) 122.9, C(10b) 161.1 C(1’(R1))H3 30.7/3.35, C(1’(R2))H2 39.7/2.60 and 2.32, C(2’(R2)) 135.4, C(3’(R2))H 129.8/6.91, C(4’(R2))H 127.5/7.20, C(5’(R2))H 129.8/7.20 ppm. IR (cm–1) ν: 3447, 2942, 1686, 1663, 1647, 1602, 1493, 1470, 1430, 1360, 1297, 1270, 1224, 1138, 1059, 1039, 1011, 973, 951, 919, 875, 760, 704, 620, 556, 504. ESI-MS (pos.) m/z (%): 719.3 [2·M + Na+]+ (18), 387.1 [M + K+]+ (12), 371.1 [M + Na+]+ (85), 349.1 [M + H+]+ (100), 306.1 [M + H+ – HCNO]+ (32). Anal. Calcd for C 68.95; H 5.73; N 16.08. Found: C 68.81; H 5.92; N 15.82.
- 6-Methyl-5-oxo-4a-phenyl-2,3,5,6-tetrahydropyrazino[2,3-c]quinoline-4(4aH)-carbox- amide (7c)
Compound was prepared from 2c with 32% yield. Using the excess of KOCN (4 equiv.), 7c was prepared in 68% yield. Yellowish solid, mp 209–211 °C (chloroform). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 50.4/4.21, C(3)H2 40.1/3.48 and 2.77, CO 160.7, C(4a) 68.8, C(5) 171.5, C(6a) 137.5, C(7)H 116.4/6.42, C(8)H 131.4/7.27, C(9)H 124.2/7.17, C(10)H 126.2/7.96, C(10a) 123.1, C(10b) 161.3, C(1’(R1)) 139.2, C(2’(R1))H 129.2/7.29, C(3’(R1))H 130.1/7.50, C(4’(R1))H 129.0/7.50, C(1’(R2))H2 34.8/3.19 and 1.95, C(2’(R2))H2 25.2/1.36 and 1.06, C(3’(R2))H2 22.1/1.21, C(4’(R2))H3 13.7/0.81 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −54.1, N(4) −296.1, NH2 −301.9/5.21 1J(15N, 1H) 83.6 Hz. IR (cm–1) ν: 3422, 3352, 3305, 3247, 3197, 3062, 2953, 1689, 1663, 1626, 1601, 1471, 1406, 1361, 1297, 1170, 1127, 1079, 1029, 1010, 933, 903, 886, 821, 762, 696, 633, 554. ESI-MS (pos.) m/z (%): 691.2 [2·M + Na+]+ (9), 373.0 [M + K+]+ (17), 357.0 [M + Na+]+ (100), 335.1 [M + H+]+ (84), 292.0 [M + H+ – HCNO]+ (13). Anal. Calcd for C19H18N4O2 (334.37): C 68.25; H 5.43; N 16.76. Found: C 68.21; H 5.50; N 16.79.
- 4a-Butyl-5-oxo-6-phenyl-2,3,5,6-tetrahydropyrazino[2,3-c]quinoline-4(4aH)-carboxamide(7d)
Compound was prepared from 2d with 38% yield beside 5d and 6d. Colorless solid, mp 144–152 °C (benzene/hexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 50.4/4.21, C(3)H2 40.1/3.48 and 2.77, CO 160.7, C(4a) 68.8, C(5) 171.5, C(6a) 137.5, C(7)H 116.4/6.42, C(8)H 131.4/7.27, C(9)H 124.2/7.17, C(10)H 126.2/7.96, C(10a) 123.1, C(10b) 161.3, C(1’(R1)) 139.2, C(2’(R1))H 129.2/7.29, C(3’(R1))H 130.1/7.50, C(4’(R1))H 129.0/7.50, C(1’(R2))H2 34.8/3.19 and 1.95, C(2’(R2))H2 25.2/1.36 and 1.06, C(3’(R2))H2 22.1/1.21, C(4’(R2))H3 13.7/0.81 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −54.1, N(4) −296.1, NH2 −301.9/5.21 1J(15N, 1H) 83.6 Hz. IR (cm–1) ν: 3434, 3398, 3215, 2955, 2851, 1700, 1662, 1645, 1607, 1489, 1459, 1428, 1350, 1330, 1313, 1301, 1257, 1217, 1173, 1163, 1131, 1052, 1030, 1009, 955, 877, 801, 769, 756, 701, 679, 646, 603, 570, 511, 490. ESI-MS (pos.) m/z (%): 775.4 [2·M + Na+]+ (8), 415.2 [M + K+]+ (10), 399.2 [M + Na+]+ (84), 377.2 [M + H+]+ (100). ESI-MS (neg.) m/z (%): 375.0 [M – H+]– (100). Anal. Calcd for C22H24N4O2 (376.45): C 70.19; H 6.43; N 14.89. Found: C 70.51; H 6.41; N 14.52.
- 4a-Benzyl-5-oxo-6-phenyl-2,3,5,6-tetrahydropyrazino[2,3-c]quinoline-4(4aH)-carbox- amide (7e)
Compound was prepared from 2e with 49% yield beside 5e and 6e. Colorless solid, mp 197–200 °C (benzene/cyclohexane). 1H and 13C chemical shifts in DMSO-d6: C(2)H2 48.5/3.66, C(3)H2 40.4/3.74 and 2.74, CO 160.3, C(4a) 69.2, C(5) 167.7, C(6a) 138.4, C(7)H 115.9/6.24, C(8)H 131.5/7.32, C(9)H 123.0/7.19, C(10)H 127.0/7.84, C(10a) 122.5, C(10b) 161.4, C(1’(R1)) 139.2, C(2’(R1))H 129.2 and 128.2/7.19, C(3’(R1))H 130.1/7.56, C(4’(R1))H 129.0/7.50, C(1’(R2))H2 34.8/3.19 and 1.95, C(2’(R2))H2 25.2/1.36 and 1.06, C(3’(R2))H2 22.1/1.21, C(4’(R2))H3 13.7/0.81 ppm. IR (cm–1) ν: 3427, 3196, 3063, 2938, 2850, 1703, 1666, 1602, 1492, 1460, 1420, 1359, 1336, 1310, 1298, 1267, 1217, 1179, 1074, 1046, 1032, 877, 845, 792, 754, 704, 641, 606, 575, 560, 544, 497. ESI-MS (pos.) m/z (%): 843.4 [2·M + Na+]+ (6), 449.1 [M + K+]+ (16), 433.2 [M + Na+]+ (85), 411.2 [M + H+]+ (100), 368.2 [M + H+ – HCNO]+ (6). ESI-MS (neg.) m/z (%): 409.0 [M – H+]– (100). Anal. Calcd for C25H22N4O2 (410.46): C 73.15; H 5.40; N 13.65. Found: C 73.28; H 5.92; N 13.51.
- N-[2-(2,4-Dioxo-5-phenylimidazolidin-1-yl)ethyl]-2-(phenylamino)benzamide (8f)
Compound was prepared from 2f with 18% yield beside 6f. Colorless solid, mp 160–165 °C (benzene). 1H and 13C chemical shifts in DMSO-d6: C(2) 158.8, C(4) 172.9, C(5)H2 64.3/2.22, C(6)H2 36.6/3.38 and 3.31, C(7)H2 39.6/3.77, C(9) 169.1, C(10) 119.0, C(11) 144.0, C(12)H 114.9/7.29, C(13)H 131.9/7.32, C(14)H 118.1/6.83, C(15) 128.9/7.57, C(1’(R2)) 141.5, C(2’(R2))H 119.4/7.15, C(3’(R2))H 129.4/7.30, C(4’(R2))H 121.7/6.97, C(1’(R2)) 133.8, C(2’(R2))H 127.6/7.16, C(3’(R2))H 129.1/7.36, C(4’(R2))H 128.8/7.36 ppm. 15N chemical shifts and 1J(15N, 1H) coupling constants in DMSO-d6: N(1) −284.5, N(3)H −235.5./11.03 1J(15N, 1H) 94.6 Hz, N(8)H −269.0/9.44 1J(15N, 1H) 91.5 Hz, N(11)H −292.2/8.68 1J(15N, 1H) 89.5 Hz. IR (cm–1) ν: 3390, 3353, 3228, 3068, 2939, 2740, 1775, 1722, 1629, 1589, 1512, 1447, 1421, 1383, 1329, 1310, 1282, 1222, 1167, 1157, 1120, 1078, 1053, 1027, 963, 942, 895, 841, 751, 701, 628, 580, 516. ESI-MS (pos.) m/z (%): 851.3 [2·M + Na+]+ (11), 453.2 [M + K+]+ (26), 437.2 [M + Na+]+ (100), 415.2 [M + H+]+ (31). ESI-MS (neg.) m/z (%): 413.0 [M – H+]– (100). Anal. Calcd for C24H22N4O3 (414.46): C 69.55; H 5.35; N 13.52. Found: C 69.99; H 5.78; N 13.17.
3.5. CDK and ABL Inhibition Assay
CDK2/cyclin E and ABL1 activity was assayed as previously described [42,43]. Briefly, the kinase was assayed with [γ-33P]ATP and suitable peptide substrates in a reaction buffer (60 mM HEPES-NaOH, pH 7.5, 3 mM MgCl2, 3 mM MnCl2, 3 μM Na-orthovanadate, 1.2 mM DTT, 2.5 μg/50 μL PEG20.000). The reactions were stopped by adding 5 µL of 3% aq. H3PO4. Aliquots were spotted onto P-81 phosphocellulose, washed with 0.5% aq. H3PO4 and air-dried. Kinase inhibition was quantified using an FLA-7000 digital image analyzer. The concentration of the test compound required to reduce kinase activity by 50% was determined from a dose-response curves and reported as the IC50 value.
3.6. In Vitro Cytotoxicity
Cell lines K562 and MV4;11 were obtained from the European Collection of Cell Cultures. The cell lines were cultivated in Dulbecco’s Modified Eagle medium supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C in 5% CO2. For the viability assays, cells were seeded into 96-well plates (5000 cells per well), and after the preincubation period, were treated in triplicate with six different doses of each compound for 72 h. After treatment, a resazurin (Sigma-Aldrich) solution was added for four hours, and the fluorescence of resorufin formed in live cells was measured at 544 nm/590 nm (excitation/emission) using a Fluoroskan Ascent microplate reader (Labsystems). The IC50 value, the drug concentration that was lethal for 50% of the cells, was calculated from the dose–response curve.
4. Conclusions
In conclusion, the tetrahydropyrazino[2,3-c]quinolin-5(6H)-ones 2 react with isocyanic acid to give (2-oxo-2,3-dihydroquinazolin-4(1H)-ylidene)-amino)ethyl) imidazolidine-2,4-diones 5, 2-(phenylamino)phenyl)-5,6-dihydroimidazo[1,5-a]pyrazine-diones 6, and 5-oxo-tetrahydropyrazinoquinoline-4-carboxamides 7. The molecular structures of the isolated compounds were suggested according to 1H, 13C and 15N NMR and electrospray-ionization mass spectrometry experiments. The structures of compounds 5d, 7a, and 7b were proved using X-ray analysis of crystalline material. Moreover, we proposed a mechanism for the molecular rearrangement of starting compounds 2 providing two hitherto unknown hydantoin-based derivatives 5 and 6. Retro-Claisen condensation seems to be a key step in the formation of the corresponding compounds. The presented work extends the set of compounds containing a hydantoin structural motif and offers a new approach for their synthesis. According to the previously described anticancer activity of several hydantoin-based derivatives [28], we decided to screen compounds 5, 6, and 7 for antiproliferative activity by using two cancer cell lines, K-562 and MV4;11. The inhibitory potency of these compounds for two types of protein kinases (CDK2/cyclin E and ABL1) was also assayed. However, no biological activity was observed for the tested molecules.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms23105481/s1.
Author Contributions
Individual contributions of authors are as follows: Conceptualization, A.K. and M.R.; methodology, A.K., A.L., A.R. and M.R.; investigation, A.K., A.L., F.K., A.R. and M.R.; writing and original draft preparation, A.K., A.L., A.R. and M.R.; writing, review and editing, A.K., A.L., A.R. and M.R.; visualization, A.K., A.R. and M.R.; supervision, A.K.; project administration, A.K.; funding acquisition, A.K. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Internal Funding Agency of Tomas Bata University in Zlín (IGA/FT/2020/007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank H. Geržová (Department of Chemistry, Faculty of Technology, Tomas Bata University in Zlín) for technical help. The authors also gratefully acknowledge Vladimír Kryštof (Department of Experimental Biology, Faculty of Science, Palacký University Olomouc) for the biological activity testing. A. L. thanks the University of Hradec Králové for the support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Erian, A.W.; Sherif, S.M.; Gaber, H.M. The Chemistry of α-Haloketones and Their Utility in Heterocyclic Synthesis. Molecules 2003, 8, 793–865. [Google Scholar] [CrossRef] [Green Version]
- Kafka, S.; Klásek, A.; Polis, J.; Košmrlj, J. Syntheses of 3-Aminoquionoline-2,4(1H,3H)-diones. Heterocycles 2002, 57, 1659–1682. [Google Scholar]
- Klásek, A.; Kořistek, K.; Lyčka, A.; Holčapek, M. Unprecendented reactivity of 3-amino-1H,3H-quionoline-2,4-diones with urea: An efficient synthesis of 2,6-dihydro-imidazo[1,5-c]quinazoline-3,5-diones. Tetrahedron 2003, 59, 1283–1288. [Google Scholar] [CrossRef]
- Klásek, A.; Kořistek, K.; Lyčka, A.; Holčapek, M. Reaction of 1-alkyl/aryl-3amino-1H,3H-quinoline-2,4-diones with urea. Synthetic route to novel 3-(3-acylureido)-2,3-dihydro-1H-indol-2-ones, 4-alkylidene-1’H-spiro[imidazolidine-5,3‘-indole]-2,2’-diones, and 3,3a-dihydro-5H-imidazo[4,5-c]quinoline-2,4-diones. Tetrahedron 2003, 59, 5279–5288. [Google Scholar]
- Klásek, A.; Lyčka, A.; Holčapek, M.; Hoza, I. Reaction of 3-aminoquinoline-2,4-diones with nitrourea. Synthetic route to novel 3-ureidoqunoline-2,4-diones and imidazo[4,5-c]qunoline-2,4-diones. Tetrahedron 2004, 60, 9953–9961. [Google Scholar] [CrossRef]
- Klásek, A.; Lyčka, A.; Holčapek, M.; Kovář, M.; Hoza, I. Molecular Rearrangement of 1-Substituted 3-Aminoquinoline-2,4-diones and Their Reaction with Urea and Nitrourea. Synthesis and Transformations of Reaction Intermediates. J. Het. Chem. 2006, 43, 1251–1260. [Google Scholar] [CrossRef]
- Klásek, A.; Lyčka, A.; Holčapek, M.; Hoza, I. Reaction of 3-Aminoquinoline-2,4-diones with Isocyanates. Synthesis of Novel 3-(3’-Alkyl/arylureido)quinoline-2,4-diones and Their Cyclic Carbinolamide Isomers. J. Het. Chem. 2006, 43, 203–211. [Google Scholar] [CrossRef]
- Klásek, A.; Lyčka, A.; Holčapek, M. Molecular rearrangement of 1-substituted 9b-hydroxy-3,3a,5,9b-tetrahydro-1H-imidazo[4,5-c]quinoline-2,4-diones—An unexpected pathway to new indole and imidazolinone derivatives. Tetrahedron 2007, 63, 7059–7069. [Google Scholar] [CrossRef]
- Prucková, Z.; Klásek, A.; Lyčka, A.; Mikšík, I.; Růžička, A. Synthesis of 2-thioxoimidazolines via reaction of 1-unsubstituted 3-aminoquinoline-2,4-diones with isothiocyanates. Tetrahedron 2009, 65, 9103–9115. [Google Scholar] [CrossRef]
- Klásek, A.; Mrkvička, V.; Lyčka, A.; Mikšík, I.; Růžička, A. Reaction of 1-substituted 3-aminoquinoline-2,4-diones with isothiocyanates. An easy pathway to generate novel 2-thioxo-1’H-spiro[imidazoline-5,3’-indole]-2,2’-diones. Tetrahedron 2009, 65, 4908–4916. [Google Scholar] [CrossRef]
- Klásek, A.; Lyčka, A.; Mikšík, I.; Růžička, A. Reaction of 3-phenyl-3-aminoquinoline-2,4-diones with isothiocyanates. Facile access to novel spiro-linked 2-thioxoimidazolidine-oxindoles and imidazoline-2-thiones. Tetrahedron 2010, 66, 2015–2025. [Google Scholar] [CrossRef]
- Mrkvička, V.; Lyčka, A.; Rudolf, O.; Klásek, A. Reaction of 3-aminoquinoline-2,4-diones with isothiocyanic acid—An easy pathway to thioxo derivatives of imidazo[1,5-c]quinazolin-5-ones and imidazo[4,5-c]quinolin-4-ones. Tetrahedron 2010, 66, 8441–8445. [Google Scholar] [CrossRef]
- Mrkvička, V.; Rudolf, O.; Lyčka, A.; Klásek, A. Reaction of 1-substituted 3-aminoquinolinediones with isocyanic and isothiocyanic acid. Tetrahedron 2011, 67, 2407–2413. [Google Scholar] [CrossRef]
- Klásek, A.; Rudolf, O.; Rouchal, M.; Lyčka, A. Reaction of 3-Hydroxyquinoline-2,4-diones with Inorganic Thiocyanates in the Presence of Ammonium or Alkylammonium Ions: The Unexpected Replacement of a Hydroxy Group by an Amino Group. Helv. Chim. Acta 2015, 98, 318–335. [Google Scholar] [CrossRef]
- Klásek, A.; Lyčka, A.; Rouchal, M.; Bartošík, R. Reaction of 1-substituted 3-(2-hydroxyethylamino)quinoline-2,4(1H,3H)-diones with isothiocyanic acid. Chem. Heterocycl. Comp. 2020, 56, 566–571. [Google Scholar] [CrossRef]
- Laschober, R.; Stadlbauer, W. Synthesis of 3-heptyl- and 3-nonyl-2,4-(1H,3H)-quinolinediones. Liebigs Ann. Chem. 1990, 1083–1086. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/jlac.1990199001195 (accessed on 12 April 2022).
- Podesva, C.; Vagi, K.; Solomon, C. Synthesis and chemistry of 1-methyl-3-imino-4-hydroxy-4-phenyl-6-chloro-1,2,3,4-tetrahydroquinoline-2-one. Can. J. Chem. 1968, 46, 2263–2269. [Google Scholar] [CrossRef]
- Elshaier, Y.A.M.M.; Aly, A.A.; El-Aziz, M.A.; Fathy, H.M.; Brown, A.B.; Ramadan, M. A review on the synthesis of heteroannulated quionolones and their biological activities. Mol. Divers. 2021. [Google Scholar] [CrossRef]
- Shin, Y.S.; Song, S.J.; Kang, S.U.; Hwang, H.S.; Choi, J.W.; Lee, B.H.; Jung, Y.-S.; Kim, C.-H. A novel synthetic compound, 3-amino-3-(4-fluoro-phenyl)-1H-qunoline-2,4-dione, inhibits cisplatin-induced hearing loss by the suppression of reactive oxygen species: In vitro and in vivo study. Neuroscience 2013, 232, 1–12. [Google Scholar] [CrossRef]
- Cifuentes-Pagano, M.E.; Meijles, D.N.; Pagano, P.J. Nox Inhibitors & Therapies: Rational Design of Peptidic and Small Molecule Inhibitors. Curr. Pharm. Design 2015, 21, 6032–6035. [Google Scholar]
- Mittal, R.; Debs, L.H.; Nguyen, D.; Patel, A.P.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; Liu, X.Z. Signaling in the Auditory System: Impications in Hair Cell Regeneration and Hearing Function. J. Cell. Physiol. 2017, 232, 2710–2721. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Hatakeda, K.; Ito, S.; Asano, T.; Toda, T. Formation of Bis(2-oxazolidinone) Derivatives by Reaction of 2-Methoxy-3,3-dimethyl-2-phenyloxirane or α-bromoisobutyrophenone with Carbon Dioxide and Aliphatic α,ω-Diamines. Bull. Chem. Soc. Jpn. 1986, 59, 1629–1631. [Google Scholar] [CrossRef] [Green Version]
- Klásek, A.; Lyčka, A.; Rouchal, M. Completely dissimilar: The reactivity of 1-unsubstituted 3-chloroquinoline-2,4-diones with ethylene diamine and ethanolamine to form new molecular rearrangements. Arkivoc 2020, vi, 209–219. [Google Scholar] [CrossRef]
- Kumar, V. Designed Synthesis of Diversely Substituted Hydantoins and Hydantoin-Based Hybrid Molecules: A Personal Account. Synlett 2021, 32, 1897–1910. [Google Scholar] [CrossRef]
- Kalník, M.; Gabko, P.; Bella, M.; Koóš, M. The Bucherer-Bergs Multicomponent Synthesis of Hydantoins–Excellence in Simplicity. Molecules 2021, 26, 4024. [Google Scholar] [CrossRef]
- Roy, A.; Sarkar, T.; Datta, S.; Maiti, A.; Chakrabarti, M.; Mondal, T.; Mondal, C.; Banerjee, A.; Roy, S.; Mukherjee, S.; et al. Structure-based discovery of (S)-2-amino-6-(4-fluorobenzyl)-5,6,11,11a-tetrahydro-1H-imidazo[1‘,5‘:1,6]pyrido[3,4-b]indole-1,3-(2H)-dione as low nanomolar, orally bioavailable autotaxin inhibitor. Chem. Biol. Drug Des. 2022, 99, 496–503. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Zhao, Z.; Nie, Z.; Yao, Z.; Ren, W.; Yang, X.; Hou, X.; Fang, H. Design, synthesis and biological evaluation of hydantoin derivatives as Mcl-1 selective inhibitors. Bioorganic Chem. 2022, 121, 105643. [Google Scholar] [CrossRef]
- Cho, S.; Kim, S.-H.; Shin, D. Recent applications of hydantoin and thiohydantoin in medicinal chemistry. Eur. J. Med. Chem. 2019, 164, 517–545. [Google Scholar] [CrossRef]
- Machado, L.; Spengler, G.; Evaristo, M.; Handzlik, J.; Molnár, J.; Viveiros, M.; Kiec-Kononowicz, K.; Amaral, L. Biological Activity of Twenty-three Hydantoin Derivatives on Intrinsic Efflux Pump System of Salmonella enterica serovar Enteritidis NCTC 13349. In Vivo 2011, 25, 769–772. [Google Scholar]
- Konnert, L.; Lamaty, F.; Martinez, J.; Colacino, E. Recent Advances in the Synthesis of Hydantoins: The State of the Art of a Valuable Scaffold. Chem. Rev. 2017, 117, 13757–13809. [Google Scholar] [CrossRef]
- Calestani, G.; Leardini, R.; McNab, H.; Nanni, D.; Zanardi, G. Thermal decomposition of tert-butyl o-(phenoxy)- and o-(anilino)-phenyliminoxyperacetates. J. Chem. Soc. Perkin Trans. 1 1998, 1813–1824. [Google Scholar] [CrossRef]
- Mahajan, M.P.; Sondhi, S.M.; Ralhan, N.K. Studies in Heterocyclics. VI. Synthesis of Thiazolo-Benzo-Triazepines. Bull. Chem. Soc. Jpn. 1976, 49, 2609–2610. [Google Scholar] [CrossRef] [Green Version]
- Anil, S.M.; Shobith, R.; Kiran, K.R.; Swaroop, T.R.; Mallesha, N.; Sadashiva, M.P. Facile synthesis of 1,4-benzodiazepine-2,5-diones and quinazolinones from amino acids as anti-tubercular agents. New. J. Chem. 2019, 43, 182–187. [Google Scholar] [CrossRef]
- Beutner, G.L.; Hsiao, Y.; Razler, T.; Simmons, E.M.; Wertjes, W. Nickel-Catalyzed Synthesis of Quinazolinediones. Org. Lett. 2017, 19, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, R.A.; Fiedler, S.L. Infrared study of five- and six-membered type cyclic imides. Vib. Spectrosc. 1995, 8, 365–386. [Google Scholar] [CrossRef]
- Ösz, E.; Szilágyi, L.; Marton, J. Structural analysis of hydantoins and 2-thiohydantoins in solution using 13C, 1H NMR coupling constants. J. Mol. Struct. 1998, 442, 267–274. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G. Tables of Bond Lenghts determined by X-ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc.-Perkin Trans. 2 1987, S1–S19. Available online: https://pubs.rsc.org/en/content/articlelanding/1987/p2/p298700000s1 (accessed on 12 April 2022).
- Allen, F.H.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Typical interatomic distances: Organic compounds. Int. Tables Crystallogr. 2006, C, 790–811. [Google Scholar]
- Raj, R.; Mehra, V.; Gut, J.; Rosenthal, P.J.; Wicht, K.J.; Egan, T.J.; Hopper, M.; Wrischnik, L.; Kirkwood, M.L.; Kumar, V. Discovery of highly selective 7-chloroquinoline-thiohydantoins with potent antimalarial activity. Eur. J. Med. Chem. 2014, 84, 425–432. [Google Scholar] [CrossRef]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gucký, T.; Jorda, R.; Zatloukal, M.; Bazgier, V.; Berka, K.; Řezníčková, E.; Béres, T.; Strnad, M.; Kryštof, V. A Novel Series of Highly Potent 2,6,9-Trisubstituted Purine Cyclin-Dependent Kinase Inhibitors. J. Med. Chem. 2013, 56, 6234–6247. [Google Scholar] [CrossRef] [PubMed]
- Jorda, R.; Havlíček, L.; McNae, I.W.; Walkinshaw, M.D.; Voller, J.; Šturc, A.; Navrátilová, J.; Kuzma, M.; Mistrík, M.; Bártek, J.; et al. Pyrazolo[4,3-d]pyrimidine Bioisostere of Roscovitine: Evaluation of a Novel Selective Inhibitor of Cyclin-Dependent Kinases with Antiproliferative Activity. J. Med. Chem. 2011, 54, 2980–2993. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).