Maternal High-Energy Diet during Pregnancy and Lactation Impairs Neurogenesis and Alters the Behavior of Adult Offspring in a Phenotype-Dependent Manner
Abstract
1. Introduction
2. Results
- ➢
- CN—control group of mice characterized by normal weight (±20 g) and physiological percentage of body fat deposits (7–8%).
- ➢
- CL—control group of mice characterized by reduced weight and reduced body fat deposits (<7%).
- ➢
- EXN—experimental group of mice characterized by normal weight and moderately elevated body fat deposits (8–11%), obesity-induction resistant experimental mice.
- ➢
- EXF—experimental group of mice characterized by markedly elevated weight (±24 g) and body fat deposits (>11%).
2.1. The SVZ/RMS Thickness Is Increased in Obese Mice
2.2. Adult Neurogenesis in the SVZ/RMS Is Altered in Mice with Elevated/Decreased Body Fat and Weight in a Phenotype-Dependent Manner
2.2.1. Cell Proliferation
2.2.2. Cell Degeneration
2.2.3. Cell Differentiation
2.3. Locomotor Activity Is Reduced in Mice with Elevated/Lowered Body Fat and weight
2.4. Overfeeding during Prenatal and Early Postnatal Development Enhances Anxiety-Related Behavior
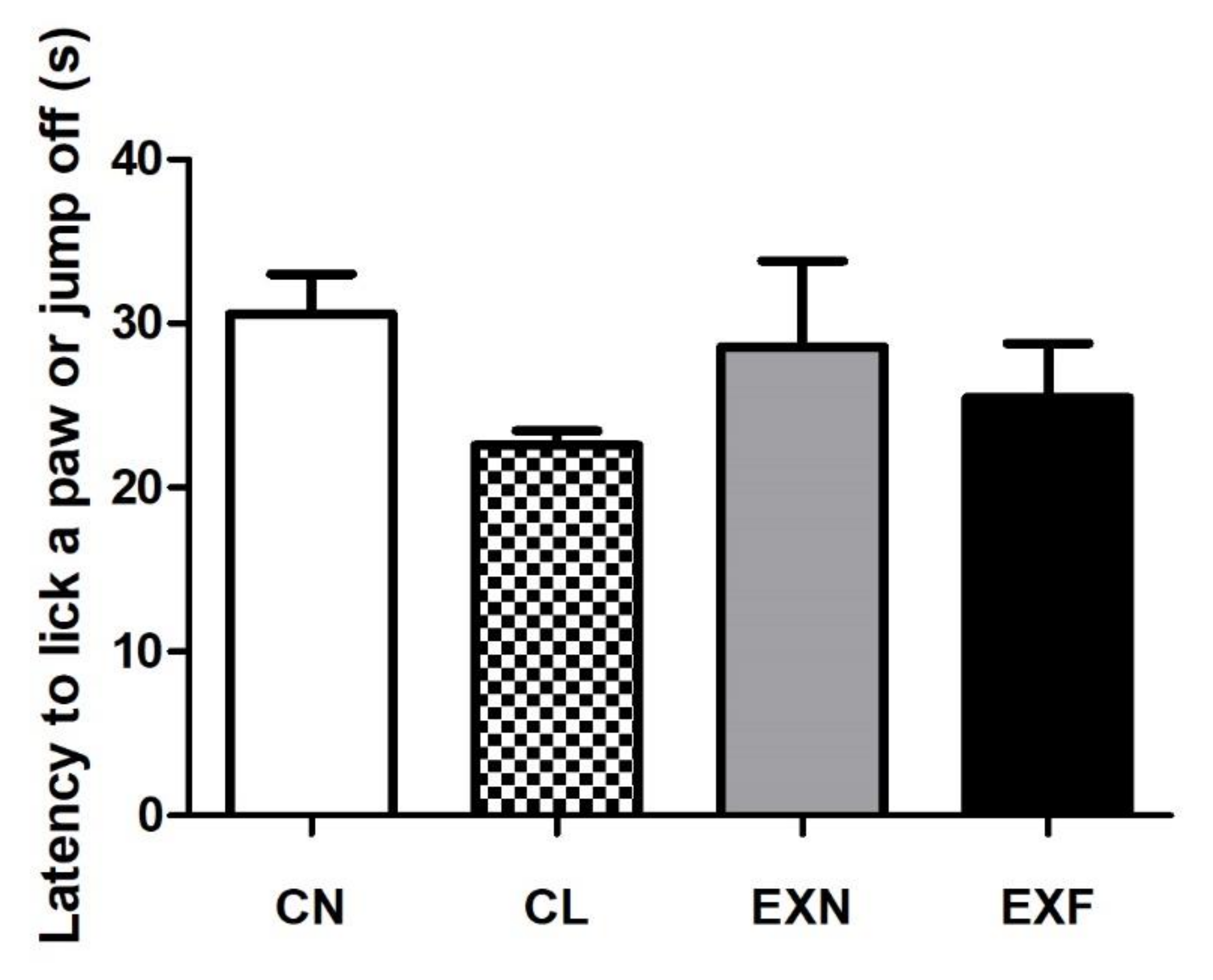
2.5. Mice with Various Amounts of Body Fat Show Comparable Performances in the Hot Plate Test
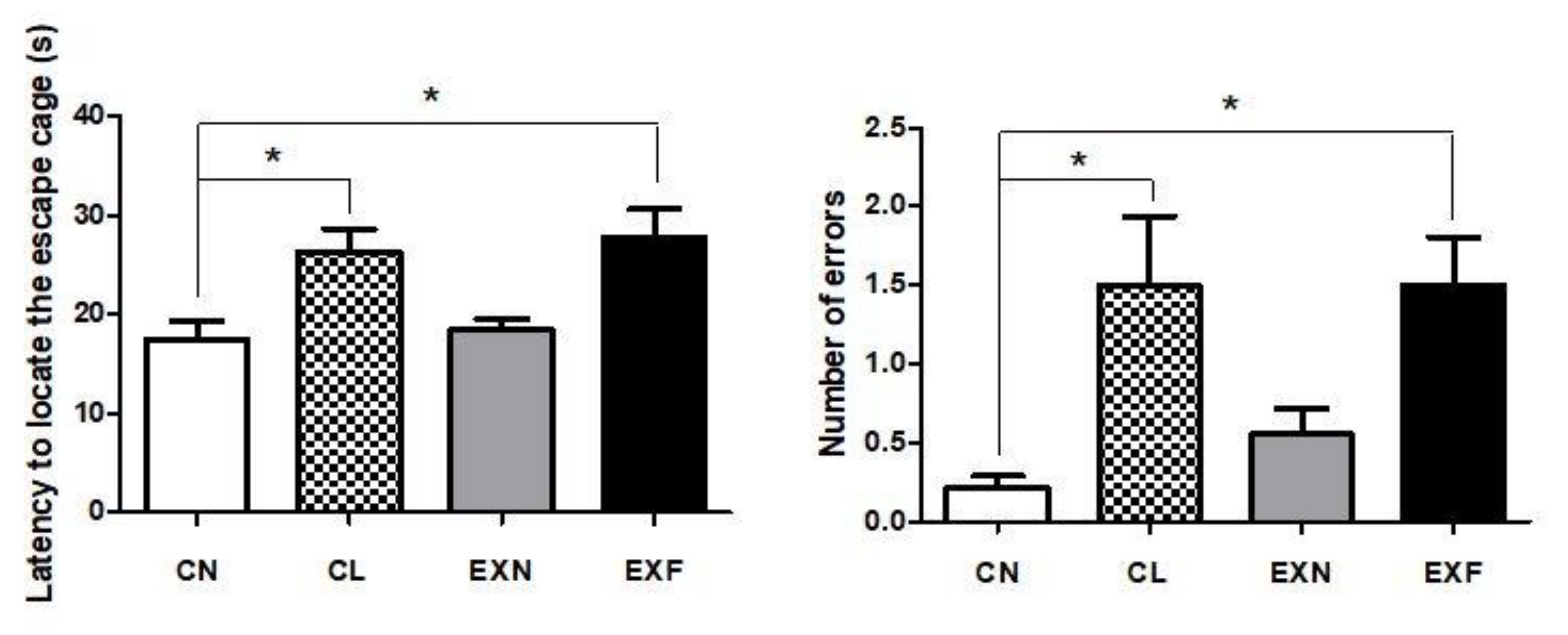
2.6. Maternal HED Exposure Impairs Spatial Memory and Learning in a Phenotype-Dependent Manner
3. Discussion
3.1. The SVZ/RMS Thickness Is Increased in Obese Mice
3.2. Adult neurogenesis in the SVZ/RMS Is Altered in Mice with Elevated/Decreased Body Fat and Weight in a Phenotype-Dependent Manner
3.3. Mice with Various Amounts of Body Fat Show Comparable Performances in the Hot Plate Test
3.4. Overfeeding during Prenatal and Early Postnatal Development Enhances Anxiety-Related Behavior, Reduces Locomotor Activity and Impairs Spatial Memory and Learning in Adult Offspring
4. Material and Methods
4.1. Animal Model
- control group (C, n = 7)—during the gestation period (21 days) and the lactation period (21 days from birth of pups to weaning) the dams were fed a standard pellet diet (M1, Ricmanice, Czech Republic, 3.2 kcal/g) ad libitum.
- experimental group (EX, n = 12)—during the gestation period (21 days) and the lactation period (21 days from birth of pups to weaning) the dams were fed a standard pellet diet (M1) plus Ensure Plus high-energy nutritional product (Abbott Laboratories, Lake Bluff, IL, USA, 1.5 kcal/mL) ad libitum.
- females acquired from control dams:
- ➢
- CN—control group of mice characterized by normal weight (±20g) and physiological percentage of body fat deposits (7–8%).
- ➢
- CL—control group of mice characterized by reduced weight and reduced body fat deposits (<7%).
- females acquired from experimental dams:
- ➢
- EXN—experimental group of mice characterized by normal weight and moderately elevated body fat deposits (8–11%), obesity-induction resistant experimental mice.
- ➢
- EXF—experimental group of mice characterized by markedly elevated weight (±24 g) and body fat deposits (>11%).
4.2. Open Field
4.3. Hot Plate Test
4.4. Barnes Maze
4.5. Tissue Processing
4.6. Image Analysis and Quantification
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Limitations of the Study
References
- Thompson, D.L. The Costs of Obesity: What Occupational Health Nurses Need to Know. AAOHN J. 2007, 55, 265–270. [Google Scholar] [CrossRef]
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef]
- Huynh, M.J.; Wang, Y.; Chang, S.L.; Tully, K.H.; Chung, B.I.; Wright, J.L.; Mossanen, M. The Cost of Obesity in Radical Cystectomy. Urol. Oncol. 2020, 38, 932.e9–932.e14. [Google Scholar] [CrossRef]
- Cheung, P.C.; Cunningham, S.A.; Naryan, K.M.V.; Kramer, M.R. Childhood Obesity Incidence in the United States: A Systematic Review. Child. Obes. 2016, 12, 1–11. [Google Scholar] [CrossRef]
- Ahirwar, R.; Mondal, P.R. Prevalence of Obesity in India: A Systematic Review. Diabetes Metab. Syndr. 2019, 13, 318–321. [Google Scholar] [CrossRef]
- Lemamsha, H.; Randhawa, G.; Papadopoulos, C. Prevalence of Overweight and Obesity among Libyan Men and Women. Biomed Res. Int. 2019, 2019, 8531360. [Google Scholar] [CrossRef]
- Matta, J.; Carette, C.; Rives Lange, C.; Czernichow, S. French and Worldwide Epidemiology of Obesity. Presse Med. 2018, 47, 434–438. [Google Scholar] [CrossRef]
- Qi, Y.; Yu, Y.; Li, K.; Chen, Z.; Liu, Y.; Zhang, H. Prevalence of Obesity among Primary Students between 2009 to 2014 in China: A Meta-Analysis. Nutr. Hosp. 2014, 31, 185–190. [Google Scholar] [CrossRef]
- Janssen, F.; Bardoutsos, A.; Vidra, N. Obesity Prevalence in the Long-Term Future in 18 European Countries and in the USA. Obes. Facts 2020, 13, 514–527. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, Regional and National Prevalence of Overweight and Obesity in Children and Adults 1980–2013: A Systematic Analysis. Lancet 2014, 384, 766. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Keller, J.N.; Morrison, C.D. Obesity and Vulnerability of the CNS. Biochim. Biophys. Acta—Mol. Basis Dis. 2009, 1792, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. High Fat Diet and Its Effects on Cognitive Health: Alterations of Neuronal and Vascular Components of Brain. Physiol. Behav. 2021, 240, 113528. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J. The Role of Western Diets and Obesity in Peripheral Immune Cell Recruitment and Inflammation in the Central Nervous System. Brain Behav. Immun.—Health 2021, 16, 100298. [Google Scholar] [CrossRef]
- Daoust, J.; Schaffer, J.; Zeighami, Y.; Dagher, A.; García-García, I.; Michaud, A. White Matter Integrity Differences in Obesity: A Meta-Analysis of Diffusion Tensor Imaging Studies. Neurosci. Biobehav. Rev. 2021, 129, 133–141. [Google Scholar] [CrossRef]
- Samant, N.P.; Gupta, G.L. Adiponectin: A Potential Target for Obesity-Associated Alzheimer’s Disease. Metab. Brain Dis. 2021, 36, 1565–1572. [Google Scholar] [CrossRef]
- López-Taboada, I.; González-Pardo, H.; Conejo, N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020, 11, 564413. [Google Scholar] [CrossRef]
- Yeomans, M.R. Adverse Effects of Consuming High Fat–Sugar Diets on Cognition: Implications for Understanding Obesity. Proc. Nutr. Soc. 2017, 76, 455–465. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a Medical Problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Cadena-Burbano, E.V.; Cavalcanti, C.C.L.; Lago, A.B.; Benjamim, R.d.A.C.; Oliveira, T.R.d.P.; Silva, J.M.; Manhães-De-Castro, R.; Da Silva Aragão, R. A Maternal High-Fat/High-Caloric Diet Delays Reflex Ontogeny during Lactation but Enhances Locomotor Performance during Late Adolescence in Rats. Nutr. Neurosci. 2019, 22, 98–109. [Google Scholar] [CrossRef]
- Boitard, C.; Etchamendy, N.; Sauvant, J.; Aubert, A.; Tronel, S.; Marighetto, A.; Layé, S.; Ferreira, G. Juvenile, but Not Adult Exposure to High-Fat Diet Impairs Relational Memory and Hippocampal Neurogenesis in Mice. Hippocampus 2012, 22, 2095–2100. [Google Scholar] [CrossRef]
- Boitard, C.; Cavaroc, A.; Sauvant, J.; Aubert, A.; Castanon, N.; Layé, S.; Ferreira, G. Impairment of Hippocampal-Dependent Memory Induced by Juvenile High-Fat Diet Intake Is Associated with Enhanced Hippocampal Inflammation in Rats. Brain Behav. Immun. 2014, 40, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Jonas, W.; Iggena, D.; Empl, L.; Rivalan, M.; Wiedmer, P.; Spranger, J.; Hellweg, R.; Winter, Y.; Steiner, B. Exercise Prevents High-Fat Diet-Induced Impairment of Flexible Memory Expression in the Water Maze and Modulates Adult Hippocampal Neurogenesis in Mice. Neurobiol. Learn. Mem. 2016, 131, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Zuloaga, K.L.; Johnson, L.A.; Roese, N.E.; Marzulla, T.; Zhang, W.; Nie, X.; Alkayed, F.N.; Hong, C.; Grafe, M.R.; Pike, M.M.; et al. High Fat Diet-Induced Diabetes in Mice Exacerbates Cognitive Deficit Due to Chronic Hypoperfusion. J. Cereb. Blood Flow Metab. 2016, 36, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Suhett, C.P.; Graham, A.; Chen, Y.; Deuster, P. Behavioral Changes in Male Mice Fed a High-Fat Diet Are Associated with IL-1β Expression in Specific Brain Regions. Physiol. Behav. 2017, 169, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.Y.; Yau, S.Y. From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions. Int. J. Mol. Sci. 2020, 22, 201. [Google Scholar] [CrossRef]
- Knight, E.M.; Martins, I.V.A.; Gümüsgöz, S.; Allan, S.M.; Lawrence, C.B. High-Fat Diet-Induced Memory Impairment in Triple-Transgenic Alzheimer’s Disease (3xTgAD) Mice Is Independent of Changes in Amyloid and Tau Pathology. Neurobiol. Aging 2014, 35, 1821–1832. [Google Scholar] [CrossRef]
- Kim, D.G.; Krenz, A.; Toussaint, L.E.; Maurer, K.J.; Robinson, S.A.; Yan, A.; Torres, L.; Bynoe, M.S. Non-Alcoholic Fatty Liver Disease Induces Signs of Alzheimer’s Disease (AD) in Wild-Type Mice and Accelerates Pathological Signs of AD in an AD Model. J. Neuroinflamm. 2016, 13, 1. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.Y.; Kim, E.; Seo, K.Y.; Kang, Y.J.; Kim, J.Y.; Kim, C.H.; Song, H.T.; Saksida, L.M.; Lee, J.E. Assessment of Cognitive Impairment in a Mouse Model of High-Fat Diet-Induced Metabolic Stress with Touchscreen-Based Automated Battery System. Exp. Neurobiol. 2018, 27, 277–286. [Google Scholar] [CrossRef]
- Yang, T.Y.; Gao, Z.; Liang, N.C. Sex-Dependent Wheel Running Effects on High Fat Diet Preference, Metabolic Outcomes, and Performance on the Barnes Maze in Rats. Nutrients 2020, 12, 2721. [Google Scholar] [CrossRef]
- Sharma, S.; Fulton, S. Diet-Induced Obesity Promotes Depressive-like Behaviour That Is Associated with Neural Adaptations in Brain Reward Circuitry. Int. J. Obes. 2013, 37, 382–389. [Google Scholar] [CrossRef]
- Biyong, E.F.; Alfos, S.; Dumetz, F.; Helbling, J.C.; Aubert, A.; Brossaud, J.; Foury, A.; Moisan, M.P.; Layé, S.; Richard, E.; et al. Dietary Vitamin A Supplementation Prevents Early Obesogenic Diet-Induced Microbiota, Neuronal and Cognitive Alterations. Int. J. Obes. 2021, 45, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Cheke, L.G.; Bonnici, H.M.; Clayton, N.S.; Simons, J.S. Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia 2017, 96, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.J.; Kaakoush, N.O.; Bertoldo, M.J.; Westbrook, R.F.; Morris, M.J. Intermittent Cafeteria Diet Identifies Fecal Microbiome Changes as a Predictor of Spatial Recognition Memory Impairment in Female Rats. Transl. Psychiatry 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative Stress, Insulin Signaling, and Diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Kothari, V.; Luo, Y.; Tornabene, T.; O’Neill, A.M.; Greene, M.W.; Geetha, T.; Babu, J.R. High Fat Diet Induces Brain Insulin Resistance and Cognitive Impairment in Mice. Biochim. Biophys. Acta—Mol. Basis Dis. 2017, 1863, 499–508. [Google Scholar] [CrossRef]
- Kivipelto, M.; Ngandu, T.; Fratiglioni, L.; Viitanen, M.; Kåreholt, I.; Winblad, B.; Helkala, E.L.; Tuomilehto, J.; Soininen, H.; Nissinen, A. Obesity and Vascular Risk Factors at Midlife and the Risk of Dementia and Alzheimer Disease. Arch. Neurol. 2005, 62, 1556–1560. [Google Scholar] [CrossRef]
- Fruh, S.M. Obesity: Risk Factors, Complications, and Strategies for Sustainable Long-Term Weight Management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.M.; Schwarzschild, M.A.; Hernán, M.A.; Willett, W.C.; Ascherio, A. Obesity and the Risk of Parkinson’s Disease. Am. J. Epidemiol. 2004, 159, 547–555. [Google Scholar] [CrossRef]
- Miola, A.; Pinna, M.; Manchia, M.; Tondo, L.; Baldessarini, R.J. Overweight in Mood Disorders: Effects on Morbidity and Treatment Response. J. Affect. Disord. 2022, 297, 169–175. [Google Scholar] [CrossRef]
- Markowitz, S.; Friedman, M.A.; Arent, S.M. Understanding the Relation between Obesity and Depression: Causal Mechanisms and Implications for Treatment. Clin. Psychol. Sci. Pract. 2008, 15, 1–20. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, Obesity, and Depression. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Danat, I.M.; Clifford, A.; Partridge, M.; Zhou, W.; Bakre, A.T.; Chen, A.; McFeeters, D.; Smith, T.; Wan, Y.; Copeland, J.; et al. Impacts of Overweight and Obesity in Older Age on the Risk of Dementia: A Systematic Literature Review and a Meta-Analysis. J. Alzheimer’s Dis. 2019, 70, S87–S99. [Google Scholar] [CrossRef] [PubMed]
- Albanese, E.; Launer, L.J.; Egger, M.; Prince, M.J.; Giannakopoulos, P.; Wolters, F.J.; Egan, K. Body Mass Index in Midlife and Dementia: Systematic Review and Meta-Regression Analysis of 589,649 Men and Women Followed in Longitudinal Studies. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 8, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Jacques, A.; Chaaya, N.; Beecher, K.; Ali, S.A.; Belmer, A.; Bartlett, S. The Impact of Sugar Consumption on Stress Driven, Emotional and Addictive Behaviors. Neurosci. Biobehav. Rev. 2019, 103, 178–199. [Google Scholar] [CrossRef]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-Sweetened Beverages and Weight Gain in Children and Adults: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Davidson, T.L. Western Diet Consumption and Cognitive Impairment: Links to Hippocampal Dysfunction and Obesity. Physiol. Behav. 2011, 103, 59–68. [Google Scholar] [CrossRef]
- Noble, E.E.; Hsu, T.M.; Liang, J.; Kanoski, S.E. Early-Life Sugar Consumption Has Long-Term Negative Effects on Memory Function in Male Rats. Nutr. Neurosci. 2019, 22, 273–283. [Google Scholar] [CrossRef]
- Yeh, S.H.H.; Shie, F.S.; Liu, H.K.; Yao, H.H.; Kao, P.C.; Lee, Y.H.; Chen, L.M.; Hsu, S.M.; Chao, L.J.; Wu, K.W.; et al. A High-Sucrose Diet Aggravates Alzheimer’s Disease Pathology, Attenuates Hypothalamic Leptin Signaling, and Impairs Food-Anticipatory Activity in APPswe/PS1dE9 Mice. Neurobiol. Aging 2020, 90, 60–74. [Google Scholar] [CrossRef]
- Kim, S.; Shou, J.; Abera, S.; Ziff, E.B. Sucrose Withdrawal Induces Depression and Anxiety-like Behavior by Kir2.1 Upregulation in the Nucleus Accumbens. Neuropharmacology 2018, 130, 10–17. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The Metabolic Syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Caroline Mcmillen, I.; Robinson, J.S.; Caroline, I. Developmental Origins of the Metabolic Syndrome: Prediction, Plasticity, and Programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef] [PubMed]
- Plagemann, A. Perinatal Nutrition and Hormone-Dependent Programming of Food Intake. Horm. Res. 2006, 65, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H.; Breier, B.H.; Cutfield, W.S.; Hofman, P.L.; Gluckman, P.D. Fetal Origins of Hyperphagia, Obesity, and Hypertension and Postnatal Amplification by Hypercaloric Nutrition. Am. J. Physiol.-Endocrinol. Metab. 2000, 279, E83–E87. [Google Scholar] [CrossRef] [PubMed]
- Lagisz, M.; Blair, H.; Kenyon, P.; Uller, T.; Raubenheimer, D.; Nakagawa, S. Little Appetite for Obesity: Meta-Analysis of the Effects of Maternal Obesogenic Diets on Offspring Food Intake and Body Mass in Rodents. Int. J. Obes. 2015, 39, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Mendes-da-Silva, C.; Giriko, C.Á.; Mennitti, L.V.; Hosoume, L.F.; Souto, T.d.S.; da Silva, A.V. Maternal High-Fat Diet during Pregnancy or Lactation Changes the Somatic and Neurological Development of the Offspring. Arq. Neuropsiquiatr. 2014, 72, 136–144. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Lupu, D.S. High Fat Diet-Induced Maternal Obesity Alters Fetal Hippocampal Development. Int. J. Dev. Neurosci. 2009, 27, 627–633. [Google Scholar] [CrossRef]
- Johnson, S.A.; Javurek, A.B.; Painter, M.S.; Murphy, C.R.; Conard, C.M.; Gant, K.L.; Howald, E.C.; Ellersieck, M.R.; Wiedmeyer, C.E.; Vieira-Potter, V.J.; et al. Effects of a Maternal High-Fat Diet on Offspring Behavioral and Metabolic Parameters in a Rodent Model. J. Dev. Orig. Health Dis. 2017, 8, 75–88. [Google Scholar] [CrossRef]
- Nicholas, K.R.; Hartmann, P.E. Milk Secretion in the Rat: Progressive Changes in Milk Composition during Lactation and Weaning and the Effect of Diet. Comp. Biochem. Physiol. A Comp. Physiol. 1991, 98, 535–542. [Google Scholar] [CrossRef]
- Del Prado, M.; Delgado, G.; Villalpando, S. Maternal Lipid Intake during Pregnancy and Lactation Alters Milk Composition and Production and Litter Growth in Rats. J. Nutr. 1997, 127, 458–462. [Google Scholar] [CrossRef]
- Trottier, G.; Koski, K.G.; Brun, T.; Toufexis, D.; Richard, D.; Walker, C.D. Increased Fat Intake during Lactation Modifies Hypothalamic-Pituitary-Adrenal Responsiveness in Developing Rat Pups: A Possible Role for Leptin. Endocrinology 1998, 139, 3704–3711. [Google Scholar] [CrossRef][Green Version]
- Jones, H.N.; Woollett, L.A.; Barbour, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. High-Fat Diet before and during Pregnancy Causes Marked up-Regulation of Placental Nutrient Transport and Fetal Overgrowth in C57/BL6 Mice. FASEB J. 2009, 23, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.Q.; Gaysinskaya, V.; Karatayev, O.; Leibowitz, S.F. Maternal High-Fat Diet and Fetal Programming: Increased Proliferation of Hypothalamic Peptide-Producing Neurons That Increase Risk for Overeating and Obesity. J. Neurosci. 2008, 28, 12107–12119. [Google Scholar] [CrossRef] [PubMed]
- Peleg-Raibstein, D.; Luca, E.; Wolfrum, C. Maternal High-Fat Diet in Mice Programs Emotional Behavior in Adulthood. Behav. Brain Res. 2012, 233, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Férézou-Viala, J.; Roy, A.F.; Sérougne, C.; Gripois, D.; Parquet, M.; Bailleux, V.; Gertler, A.; Delplanque, B.; Djiane, J.; Riottot, M.; et al. Long-Term Consequences of Maternal High-Fat Feeding on Hypothalamic Leptin Sensitivity and Diet-Induced Obesity in the Offspring. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2007, 293, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Xavier, S.; Soch, A.; Younesi, S.; Malik, S.; Spencer, S.J.; Sominsky, L. Maternal diet before and during pregnancy modulates microglial activation and neurogenesis in the postpartum rat brain. Brain Behav. Immun. 2021, 98, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Lépinay, A.L.; Larrieu, T.; Joffre, C.; Acar, N.; Gárate, I.; Castanon, N.; Ferreira, G.; Langelier, B.; Guesnet, P.; Brétillon, L.; et al. Perinatal High-Fat Diet Increases Hippocampal Vulnerability to the Adverse Effects of Subsequent High-Fat Feeding. Psychoneuroendocrinology 2015, 53, 82–93. [Google Scholar] [CrossRef]
- Tozuka, Y.; Wada, E.; Wada, K. Diet-induced Obesity in Female Mice Leads to Peroxidized Lipid Accumulations and Impairment of Hippocampal Neurogenesis during the Early Life of Their Offspring. FASEB J. 2009, 23, 1920–1934. [Google Scholar] [CrossRef]
- Christie, B.R.; Cameron, H.A. Neurogenesis in the Adult Hippocampus. Hippocampus 2006, 16, 199–207. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H. Neurogenesis in the Adult Hippocampus. Novartis Found. Symp. 2000, 231, 220–235. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Seri, B.; Doetsch, F. Identification of Neural Stem Cells in the Adult Vertebrate Brain. Brain Res. Bull. 2002, 57, 751–758. [Google Scholar] [CrossRef]
- Kokoeva, M.V.; Yin, H.; Flier, J.S. Evidence for Constitutive Neural Cell Proliferation in the Adult Murine Hypothalamus. J. Comp. Neurol. 2007, 505, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F.; Alvarez-Buylla, A. Network of Tangential Pathways for Neuronal Migration in Adult Mammalian Brain. Proc. Natl. Acad. Sci. USA 1996, 93, 14895–14900. [Google Scholar] [CrossRef] [PubMed]
- Peretto, P.; Merighi, A.; Fasolo, A.; Bonfanti, L. The Subependymal Layer in Rodents: A Site of Structural Plasticity and Cell Migration in the Adult Mammalian Brain. Brain Res. Bull. 1999, 49, 221–243. [Google Scholar] [CrossRef]
- Lievajová, K.; Blaško, J.; Martončíková, M.; Cigánková, V.; Račeková, E. Delayed Maturation and Altered Proliferation within the Rat Rostral Migratory Stream Following Maternal Deprivation. Eur. J. Histochem. 2011, 55, 182–186. [Google Scholar] [CrossRef][Green Version]
- Poulose, S.M.; Miller, M.G.; Scott, T.; Shukitt-Hale, B. Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv. Nutr. 2017, 8, 804–811. [Google Scholar] [CrossRef]
- Wojtowicz, J.M. Irradiation as an Experimental Tool in Studies of Adult Neurogenesis. Hippocampus 2006, 16, 261–266. [Google Scholar] [CrossRef]
- Leone, L.; Fusco, S.; Mastrodonato, A.; Piacentini, R.; Barbati, S.A.; Zaffina, S.; Pani, G.; Podda, M.V.; Grassi, C. Epigenetic Modulation of Adult Hippocampal Neurogenesis by Extremely Low-Frequency Electromagnetic Fields. Mol. Neurobiol. 2014, 49, 1472–1486. [Google Scholar] [CrossRef]
- Edlow, A.G. Maternal Obesity and Neurodevelopmental and Psychiatric Disorders in Offspring. Prenat. Diagn. 2017, 37, 95–110. [Google Scholar] [CrossRef]
- Park, H.R.; Park, M.; Choi, J.; Park, K.Y.; Chung, H.Y.; Lee, J. A High-Fat Diet Impairs Neurogenesis: Involvement of Lipid Peroxidation and Brain-Derived Neurotrophic Factor. Neurosci. Lett. 2010, 482, 235–239. [Google Scholar] [CrossRef]
- Klein, C.; Jonas, W.; Wiedmer, P.; Schreyer, S.; Akyüz, L.; Spranger, J.; Hellweg, R.; Steiner, B. High-Fat Diet and Physical Exercise Differentially Modulate Adult Neurogenesis in the Mouse Hypothalamus. Neuroscience 2019, 400, 146–156. [Google Scholar] [CrossRef]
- Desai, M.; Ferrini, M.G.; Han, G.; Narwani, K.; Ross, M.G. Maternal High Fat Diet Programs Male Mice Offspring Hyperphagia and Obesity: Mechanism of Increased Appetite Neurons via Altered Neurogenic Factors and Nutrient Sensor AMPK. Nutrients 2020, 12, 3326. [Google Scholar] [CrossRef]
- Pencea, V.; Bingaman, K.D.; Freedman, L.J.; Luskin, M.B. Neurogenesis in the Subventricular Zone and Rostral Migratory Stream of the Neonatal and Adult Primate Forebrain. Exp. Neurol. 2001, 172, 1–16. [Google Scholar] [CrossRef]
- Mobley, A.S.; Bryant, A.K.; Richard, M.B.; Brann, J.H.; Firestein, S.J.; Greer, C.A. Age-Dependent Regional Changes in the Rostral Migratory Stream. Neurobiol. Aging 2013, 34, 1873–1881. [Google Scholar] [CrossRef]
- Kirschenbaum, B.; Doetsch, F.; Lois, C.; Alvarez-Buylla, A. Adult Subventricular Zone Neuronal Precursors Continue to Proliferate and Migrate in the Absence of the Olfactory Bulb. J. Neurosci. 1999, 19, 2171–2180. [Google Scholar] [CrossRef]
- Szele, F.G.; Chesselet, M.F. Cortical Lesions Induce an Increase in Cell Number and PSA-NCAM Expression in the Subventricular Zone of Adult Rats. J. Comp. Neurol. 1996, 368, 439–454. [Google Scholar] [CrossRef]
- Weinstein, D.E.; Burrola, P.; Kilpatrick, T.J. Increased Proliferation of Precursor Cells in the Adult Rat Brain after Targeted Lesioning. Brain Res. 1996, 743, 11–16. [Google Scholar] [CrossRef]
- Leventopoulos, M.; Rüedi-Bettschen, D.; Knuesel, I.; Feldon, J.; Pryce, C.R.; Opacka-Juffry, J. Long-Term Effects of Early Life Deprivation on Brain Glia in Fischer Rats. Brain Res. 2007, 1142, 119–126. [Google Scholar] [CrossRef]
- Fabricius, K.; Wörtwein, G.; Pakkenberg, B. The Impact of Maternal Separation on Adult Mouse Behaviour and on the Total Neuron Number in the Mouse Hippocampus. Brain Struct. Funct. 2008, 212, 403–416. [Google Scholar] [CrossRef]
- Huot, R.L.; Plotsky, P.M.; Lenox, R.H.; McNamara, R.K. Neonatal Maternal Separation Reduces Hippocampal Mossy Fiber Density in Adult Long Evans Rats. Brain Res. 2002, 950, 52–63. [Google Scholar] [CrossRef]
- Carnell, S.; Kim, Y.; Pryor, K. Fat Brains, Greedy Genes, and Parent Power: A Biobehavioural Risk Model of Child and Adult Obesity. Int. Rev. Psychiatry 2012, 24, 189–199. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Kanzari, A.; Guérin, S.; Randuineau, G.; Coquery, N. A Maternal Western Diet during Gestation and Lactation Modifies Offspring’s Microglial Cell Density and Morphology in the Hippocampus and Prefrontal Cortex in Yucatan Minipigs. Neurosci. Lett. 2020, 739, 135395. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Yang, Z. Perinatal Undernutrition Attenuates Field Excitatory Postsynaptic Potentials and Influences Dendritic Spine Density and Morphology in Hippocampus of Male Rat Offspring. Neuroscience 2013, 244, 31–41. [Google Scholar] [CrossRef]
- Lin, C.; Lin, Y.Y.; Luo, J.; Yu, J.R.; Cheng, Y.N.; Wu, X.Y.; Lin, L.; Lin, Y.S. Maternal High-Fat Diet Multigenerationally Impairs Hippocampal Synaptic Plasticity and Memory in Male Rat Offspring. Endocrinology 2021, 162, bqaa214. [Google Scholar] [CrossRef]
- Bedi, K.S. Nutritional Effects on Neuron Numbers. Nutr. Neurosci. 2003, 6, 141–152. [Google Scholar] [CrossRef]
- Bertrand, P.C.; O’Kusky, J.R.; Innis, S.M. Maternal Dietary (n-3) Fatty Acid Deficiency Alters Neurogenesis in the Embryonic Rat Brain. J. Nutr. 2006, 136, 1570–1575. [Google Scholar] [CrossRef]
- Hwang, I.K.; Kim, I.Y.; Kim, D.W.; Yoo, K.Y.; Kim, Y.N.; Yi, S.S.; Won, M.H.; Lee, I.S.; Yoon, Y.S.; Seong, J.K. Strain-Specific Differences in Cell Proliferation and Differentiation in the Dentate Gyrus of C57BL/6N and C3H/HeN Mice Fed a High Fat Diet. Brain Res. 2008, 1241, 1–6. [Google Scholar] [CrossRef]
- Ferreira, A.; Castro, J.P.; Andrade, J.P.; Dulce Madeira, M.; Cardoso, A. Cafeteria-Diet Effects on Cognitive Functions, Anxiety, Fear Response and Neurogenesis in the Juvenile Rat. Neurobiol. Learn. Mem. 2018, 155, 197–207. [Google Scholar] [CrossRef]
- Robison, L.S.; Albert, N.M.; Camargo, L.A.; Anderson, B.M.; Salinero, A.E.; Riccio, D.A.; Abi-Ghanem, C.; Gannon, O.J.; Zuloaga, K.L. High-Fat Diet-Induced Obesity Causes Sex-Specific Deficits in Adult Hippocampal Neurogenesis in Mice. eNeuro 2020, 7. [Google Scholar] [CrossRef]
- Rojczyk-Gołębiewska, E.; Pałasz, A.; Wiaderkiewicz, R. Hypothalamic Subependymal Niche: A Novel Site of the Adult Neurogenesis. Cell. Mol. Neurobiol. 2014, 34, 631–642. [Google Scholar] [CrossRef]
- Moraes, J.C.; Coope, A.; Morari, J.; Cintra, D.E.; Roman, E.A.; Pauli, J.R.; Romanatto, T.; Carvalheira, J.B.; Oliveira, A.L.R.; Saad, M.J.; et al. High-Fat Diet Induces Apoptosis of Hypothalamic Neurons. PLoS ONE 2009, 4, e5045. [Google Scholar] [CrossRef]
- Cutler, R.R.; Kokovay, E. Rejuvenating Subventricular Zone Neurogenesis in the Aging Brain. Curr. Opin. Pharmacol. 2020, 50, 1–8. [Google Scholar] [CrossRef]
- Apple, D.M.; Mahesula, S.; Fonseca, R.S.; Zhu, C.; Kokovay, E. Calorie Restriction Protects Neural Stem Cells from Age-Related Deficits in the Subventricular Zone. Aging 2019, 11, 115–126. [Google Scholar] [CrossRef]
- Rivera, P.; Romero-Zerbo, Y.; Pavón, F.J.; Serrano, A.; López-Ávalos, M.D.; Cifuentes, M.; Grondona, J.M.; Bermúdez-Silva, F.J.; Fernández-Llebrez, P.; de Fonseca, F.R.; et al. Obesity-Dependent Cannabinoid Modulation of Proliferation in Adult Neurogenic Regions. Eur. J. Neurosci. 2011, 33, 1577–1586. [Google Scholar] [CrossRef]
- Matarredona, E.R.; Murillo-Carretero, M.; Moreno-López, B.; Estrada, C. Nitric Oxide Synthesis Inhibition Increases Proliferation of Neural Precursors Isolated from the Postnatal Mouse Subventricular Zone. Brain Res. 2004, 995, 274–284. [Google Scholar] [CrossRef]
- Moreno-López, B.; Romero-Grimaldi, C.; Noval, J.A.; Murillo-Carretero, M.; Matarredona, E.R.; Estrada, C. Nitric Oxide Is a Physiological Inhibitor of Neurogenesis in the Adult Mouse Subventricular Zone and Olfactory Bulb. J. Neurosci. 2004, 24, 85–95. [Google Scholar] [CrossRef]
- Romero-Grimaldi, C.; Gheusi, G.; Lledo, P.M.; Estrada, C. Chronic Inhibition of Nitric Oxide Synthesis Enhances Both Subventricular Zone Neurogenesis and Olfactory Learning in Adult Mice. Eur. J. Neurosci. 2006, 24, 2461–2470. [Google Scholar] [CrossRef]
- Torroglosa, A.; Murillo-Carretero, M.; Romero-Grimaldi, C.; Matarredona, E.R.; Campos-Caro, A.; Estrada, C. Nitric Oxide Decreases Subventricular Zone Stem Cell Proliferation by Inhibition of Epidermal Growth Factor Receptor and Phosphoinositide-3-Kinase/Akt Pathway. Stem Cells 2007, 25, 88–97. [Google Scholar] [CrossRef]
- Carreira, B.P.; Morte, M.I.; Santos, A.I.; Lourenço, A.S.; Ambrósio, A.F.; Carvalho, C.M.; Araújo, I.M. Nitric Oxide from Inflammatory Origin Impairs Neural Stem Cell Proliferation by Inhibiting Epidermal Growth Factor Receptor Signaling. Front. Cell. Neurosci. 2014, 8, 343. [Google Scholar] [CrossRef]
- Ungvari, Z.; Parrado-Fernandez, C.; Csiszar, A.; De Cabo, R. Mechanisms Underlying Caloric Restriction and Lifespan Regulation: Implications for Vascular Aging. Circ. Res. 2008, 102, 519–529. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity Is Associated with Hypothalamic Injury in Rodents and Humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- McNay, D.E.G.; Briançon, N.; Kokoeva, M.V.; Maratos-Flier, E.; Flier, J.S. Remodeling of the Arcuate Nucleus Energy-Balance Circuit Is Inhibited in Obese Mice. J. Clin. Investig. 2012, 122, 142–152. [Google Scholar] [CrossRef]
- Blaschke, A.J.; Staley, K.; Chun, J. Widespread Programmed Cell Death in Proliferative and Postmitotic Regions of the Fetal Cerebral Cortex. Development 1996, 122, 1165–1174. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Besson, M.; Guérin, S.; Coquery, N.; Randuineau, G.; Kanzari, A.; Quesnel, H.; Bonhomme, N.; Bolhuis, J.E.; Kemp, B.; et al. A Maternal Western Diet during Gestation and Lactation Modifies Offspring’s Microbiota Activity, Blood Lipid Levels, Cognitive Responses, and Hippocampal Neurogenesis in Yucatan Pigs. FASEB J. 2017, 31, 2037–2049. [Google Scholar] [CrossRef]
- Mercer, J.G.; Archer, Z.A. Putting the Diet Back into Diet-Induced Obesity: Diet-Induced Hypothalamic Gene Expression. Eur. J. Pharmacol. 2008, 585, 31–37. [Google Scholar] [CrossRef]
- Levin, B.E.; Keesey, R.E. Defense of Differing Body Weight Set Points in Diet-Induced Obese and Resistant Rats. Am. J. Physiol. 1998, 274, R412–R419. [Google Scholar] [CrossRef]
- Hill, J.O.; Peters, J.C. Environmental Contributions to the Obesity Epidemic. Science 1998, 280, 1371–1374. [Google Scholar] [CrossRef]
- Hwang, L.L.; Wang, C.H.; Li, T.L.; Chang, S.D.; Lin, L.C.; Chen, C.P.; Chen, C.T.; Liang, K.C.; Ho, I.K.; Yang, W.S.; et al. Sex Differences in High-Fat Diet-Induced Obesity, Metabolic Alterations and Learning, and Synaptic Plasticity Deficits in Mice. Obesity 2010, 18, 463–469. [Google Scholar] [CrossRef]
- Nakandakari, S.C.B.R.; Muñoz, V.R.; Kuga, G.K.; Gaspar, R.C.; Sant’Ana, M.R.; Pavan, I.C.B.; da Silva, L.G.S.; Morelli, A.P.; Simabuco, F.M.; da Silva, A.S.R.; et al. Short-Term High-Fat Diet Modulates Several Inflammatory, ER Stress, and Apoptosis Markers in the Hippocampus of Young Mice. Brain. Behav. Immun. 2019, 79, 284–293. [Google Scholar] [CrossRef]
- Hersey, M.; Woodruff, J.L.; Maxwell, N.; Sadek, A.T.; Bykalo, M.K.; Bain, I.; Grillo, C.A.; Piroli, G.G.; Hashemi, P.; Reagan, L.P. High-Fat Diet Induces Neuroinflammation and Reduces the Serotonergic Response to Escitalopram in the Hippocampus of Obese Rats. Brain. Behav. Immun. 2021, 96, 63–72. [Google Scholar] [CrossRef]
- Miller, A.A.; Spencer, S.J. Obesity and Neuroinflammation: A Pathway to Cognitive Impairment. Brain. Behav. Immun. 2014, 42, 10–21. [Google Scholar] [CrossRef]
- Galvão, R.P.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Brain-Derived Neurotrophic Factor Signaling Does Not Stimulate Subventricular Zone Neurogenesis in Adult Mice and Rats. J. Neurosci. 2008, 28, 13368–13383. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Kim, Y.; Kim, S.; Sato, T.; Kojima, M.; Park, S. Ghrelin Stimulates Proliferation, Migration and Differentiation of Neural Progenitors from the Subventricular Zone in the Adult Mice. Exp. Neurol. 2014, 252, 75–84. [Google Scholar] [CrossRef]
- Larsen, C.M.; Grattan, D.R. Prolactin-Induced Mitogenesis in the Subventricular Zone of the Maternal Brain during Early Pregnancy Is Essential for Normal Postpartum Behavioral Responses in the Mother. Endocrinology 2010, 151, 3805–3814. [Google Scholar] [CrossRef] [PubMed]
- Melchior, M.; Kuhn, P.; Poisbeau, P. The Burden of Early Life Stress on the Nociceptive System Development and Pain Responses. Eur. J. Neurosci. 2021, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Salberg, S.; Yamakawa, G.R.; Griep, Y.; Bain, J.; Beveridge, J.K.; Sun, M.; McDonald, S.J.; Shultz, S.R.; Brady, R.D.; Wright, D.K.; et al. Pain in the Developing Brain: Early Life Factors Alter Nociception and Neurobiological Function in Adolescent Rats. Cereb. Cortex Commun. 2021, 2, tgab014. [Google Scholar] [CrossRef]
- Loredo-Pérez, A.A.; Montalvo-Blanco, C.E.; Hernández-González, L.I.; Anaya-Reyes, M.; Fernández Del Valle-Laisequilla, C.; Reyes-García, J.G.; Acosta-González, R.I.; Martínez-Martínez, A.; Villarreal-Salcido, J.C.; Vargas-Muñoz, V.M.; et al. High-Fat Diet Exacerbates Pain-like Behaviors and Periarticular Bone Loss in Mice with CFA-Induced Knee Arthritis. Obesity 2016, 24, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Tramullas, M.; Finger, B.C.; Dinan, T.G.; Cryan, J.F. Obesity Takes Its Toll on Visceral Pain: High-Fat Diet Induces Toll-Like Receptor 4-Dependent Visceral Hypersensitivity. PLoS ONE 2016, 11, e0155367. [Google Scholar] [CrossRef]
- Torensma, B.; Thomassen, I.; van Velzen, M.; in ’t Veld, B.A. Pain Experience and Perception in the Obese Subject Systematic Review (Revised Version). Obes. Surg. 2016, 26, 631–639. [Google Scholar] [CrossRef]
- Tashani, O.A.; Astita, R.; Sharp, D.; Johnson, M.I. Body Mass Index and Distribution of Body Fat Can Influence Sensory Detection and Pain Sensitivity. Eur. J. Pain 2017, 21, 1186–1196. [Google Scholar] [CrossRef]
- Torensma, B.; Oudejans, L.; van Velzen, M.; Swank, D.; Niesters, M.; Dahan, A. Pain Sensitivity and Pain Scoring in Patients with Morbid Obesity. Surg. Obes. Relat. Dis. 2017, 13, 788–795. [Google Scholar] [CrossRef]
- Chin, S.H.; Huang, W.L.; Akter, S.; Binks, M. Obesity and Pain: A Systematic Review. Int. J. Obes. 2020, 44, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Nousen, E.K.; Chamlou, K.A.; Grove, K.L. The Impact of Maternal High-Fat Diet Consumption on Neural Development and Behavior of Offspring. Int. J. Obes. Suppl. 2012, 2, S7–S13. [Google Scholar] [CrossRef] [PubMed]
- Tozuka, Y.; Kumon, M.; Wada, E.; Onodera, M.; Mochizuki, H.; Wada, K. Maternal Obesity Impairs Hippocampal BDNF Production and Spatial Learning Performance in Young Mouse Offspring. Neurochem. Int. 2010, 57, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; de Vega, W.C.; St-Cyr, S.; Pan, P.; McGowan, P.O. Perinatal High Fat Diet Alters Glucocorticoid Signaling and Anxiety Behavior in Adulthood. Neuroscience 2013, 240, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ogrodnik, M.; Zhu, Y.; Langhi, L.G.P.; Tchkonia, T.; Krüger, P.; Fielder, E.; Victorelli, S.; Ruswhandi, R.A.; Giorgadze, N.; Pirtskhalava, T.; et al. Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metab. 2019, 29, 1061–1077.e8. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Dziura, J.; Burgert, T.S.; Tamborlane, W.V.; Taksali, S.E.; Yeckel, C.W.; Allen, K.; Lopes, M.; Savoye, M.; Morrison, J.; et al. Obesity and the Metabolic Syndrome in Children and Adolescents. N. Engl. J. Med. 2004, 350, 2362–2374. [Google Scholar] [CrossRef]
- Whitaker, R.C. Predicting Preschooler Obesity at Birth: The Role of Maternal Obesity in Early Pregnancy. Pediatrics 2004, 114, e29–e36. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic Syndrome in Childhood: Association with Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef]
- Sasaki, A.; de Vega, W.; Sivanathan, S.; St-Cyr, S.; McGowan, P. Maternal High-Fat Diet Alters Anxiety Behavior and Glucocorticoid Signaling in Adolescent Offspring. Neuroscience 2014, 272, 92–101. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Tsang, V. Enduring Consequences of Maternal Obesity for Brain Inflammation and Behavior of Offspring. FASEB J. 2010, 24, 2104–2115. [Google Scholar] [CrossRef]
- Rodriguez, J.S.; Rodríguez-González, G.L.; Reyes-Castro, L.A.; Ibáñez, C.; Ramírez, A.; Chavira, R.; Larrea, F.; Nathanielsz, P.W.; Zambrano, E. Maternal Obesity in the Rat Programs Male Offspring Exploratory, Learning and Motivation Behavior: Prevention by Dietary Intervention Pre-Gestation or in Gestation. Int. J. Dev. Neurosci. 2012, 30, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Cordner, Z.A.; Tamashiro, K.L.K. Effects of High-Fat Diet Exposure on Learning & Memory. Physiol. Behav. 2015, 152, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Garciá, G.; Guzmán-Quevedo, O.; Da Silva Aragaõ, R.; Bolanõs-Jiménez, F. Early Malnutrition Results in Long-Lasting Impairments in Pattern-Separation for Overlapping Novel Object and Novel Location Memories and Reduced Hippocampal Neurogenesis. Sci. Rep. 2016, 6, 21275. [Google Scholar] [CrossRef] [PubMed]
- Kubandová, J.; Fabian, D.; Burkuš, J.; Čikoš, S.; Czikková, S.; Mozeš, S.; Šefčíková, Z.; Koppel, J. Two-Generation Diet-Induced Obesity Model Producing Mice with Increased Amount of Body Fat in Early Adulthood. Physiol. Res. 2014, 63, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Fabianová, K.; Martončíková, M.; Fabian, D.; Blaško, J.; Račeková, E. Diverse Effect of Different Odor Stimuli on Behavior and Fos Protein Production in the Olfactory System Neurogenic Region of Adult Rats. Behav. Brain Res. 2014, 265, 38–48. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. Mood and Anxiety Related Phenotypes in Mice. In Neuromethods; Humana Press: Totowa, NJ, USA, 2009; Volume 42, pp. 1–20. [Google Scholar] [CrossRef]
- Mogil, J.S.; Wilson, S.G.; Bon, K.; Lee, S.E.; Chung, K.; Raber, P.; Pieper, J.O.; Hain, H.S.; Belknap, J.K.; Hubert, L.; et al. Heritability of Nociception I: Responses of 11 Inbred Mouse Strains on 12 Measures of Nociception. Pain 1999, 80, 67–82. [Google Scholar] [CrossRef]
- Kesby, J.P.; Kim, J.J.; Scadeng, M.; Woods, G.; Kado, D.M.; Olefsky, J.M.; Jeste, D.V.; Achim, C.L.; Semenova, S. Spatial Cognition in Adult and Aged Mice Exposed to High-Fat Diet. PLoS ONE 2015, 10, e0140034. [Google Scholar] [CrossRef]
- Račeková, E.; Lievajová, K.; Danko, J.; Martončíková, M.; Flešárová, S.; Almašiová, V.; Orendáčová, J. Maternal Separation Induced Alterations of Neurogenesis in the Rat Rostral Migratory Stream. Cell. Mol. Neurobiol. 2009, 29, 811–819. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and Eosin Staining of Tissueand Cell Sections. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]

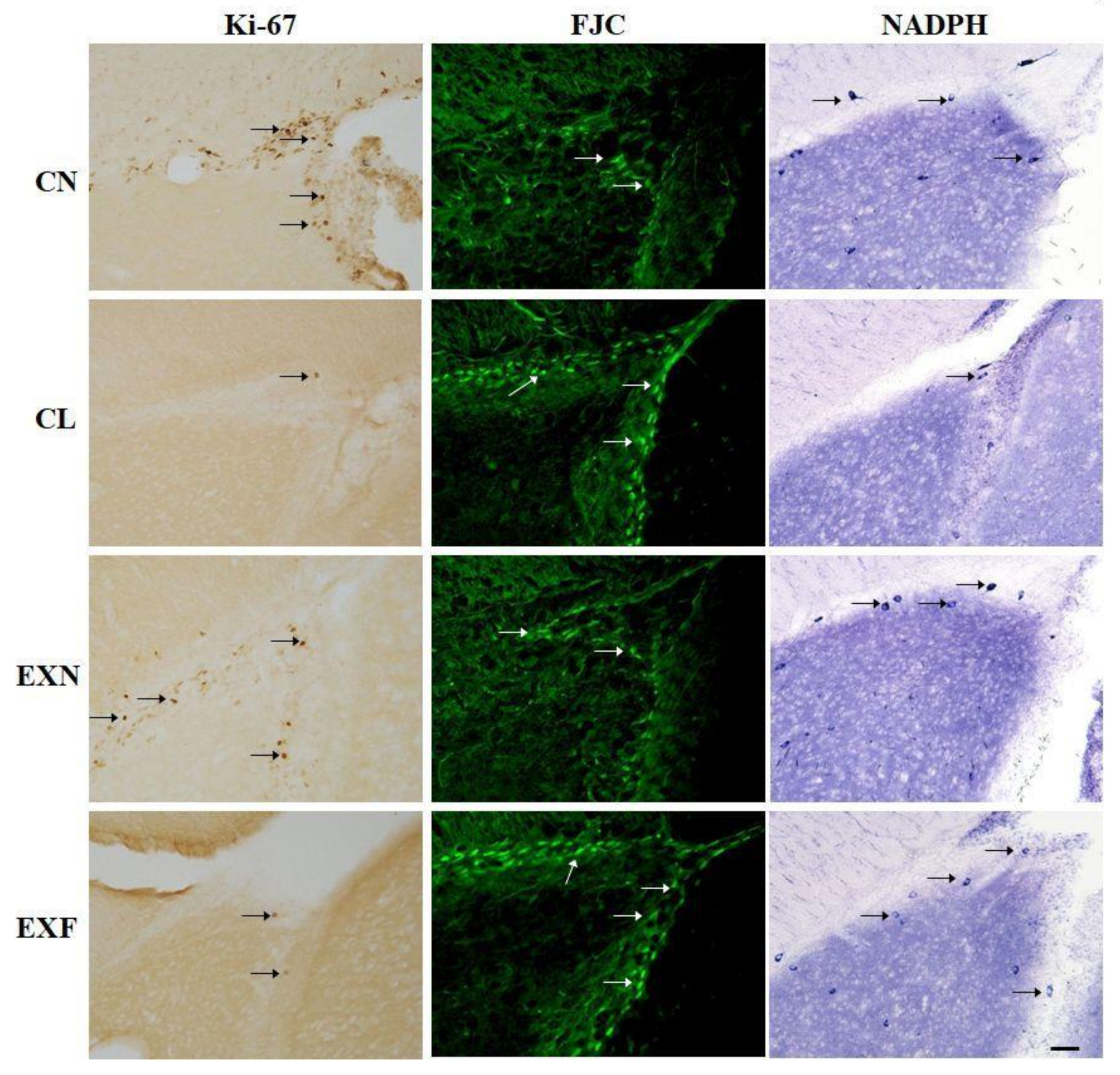

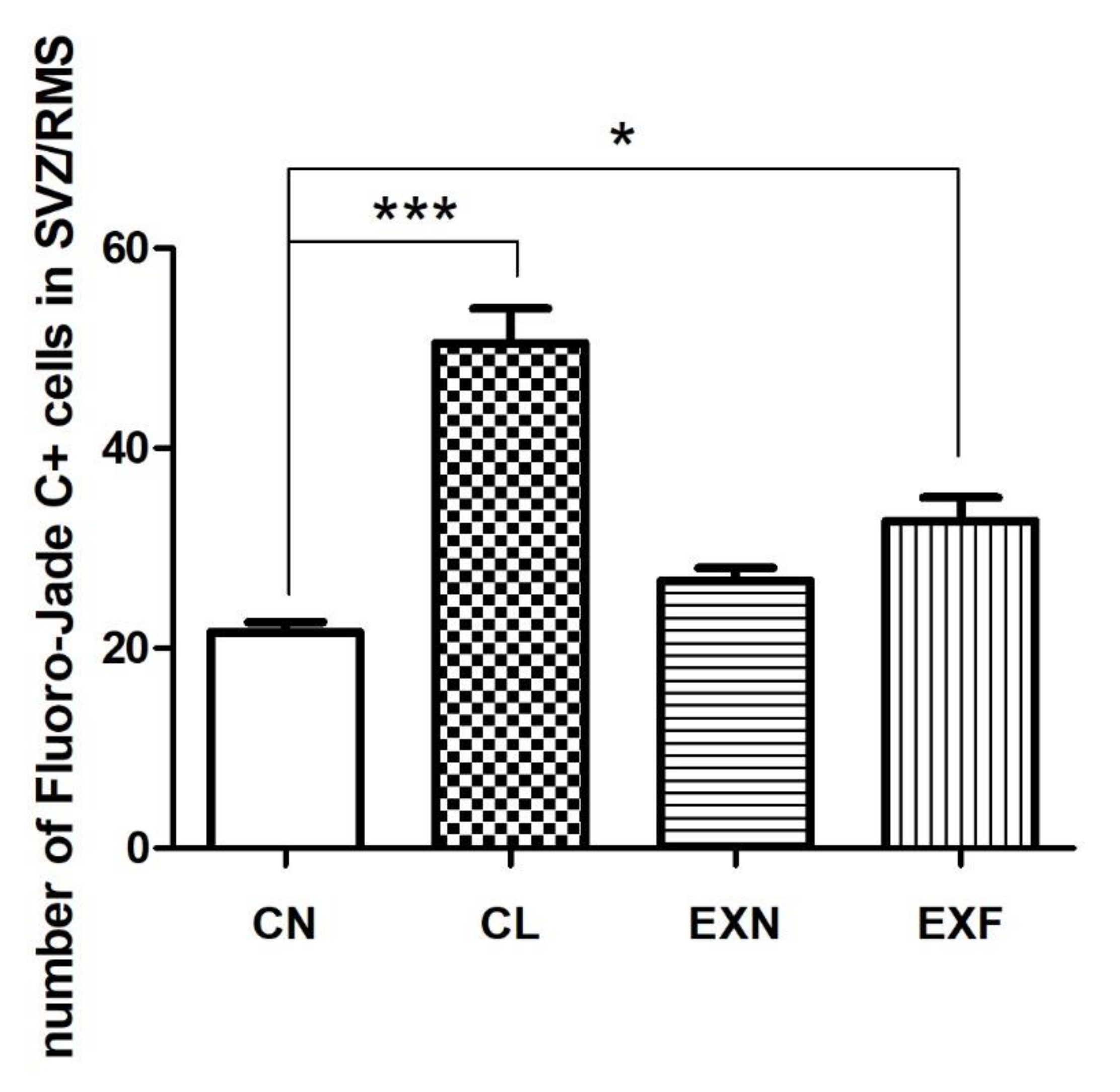
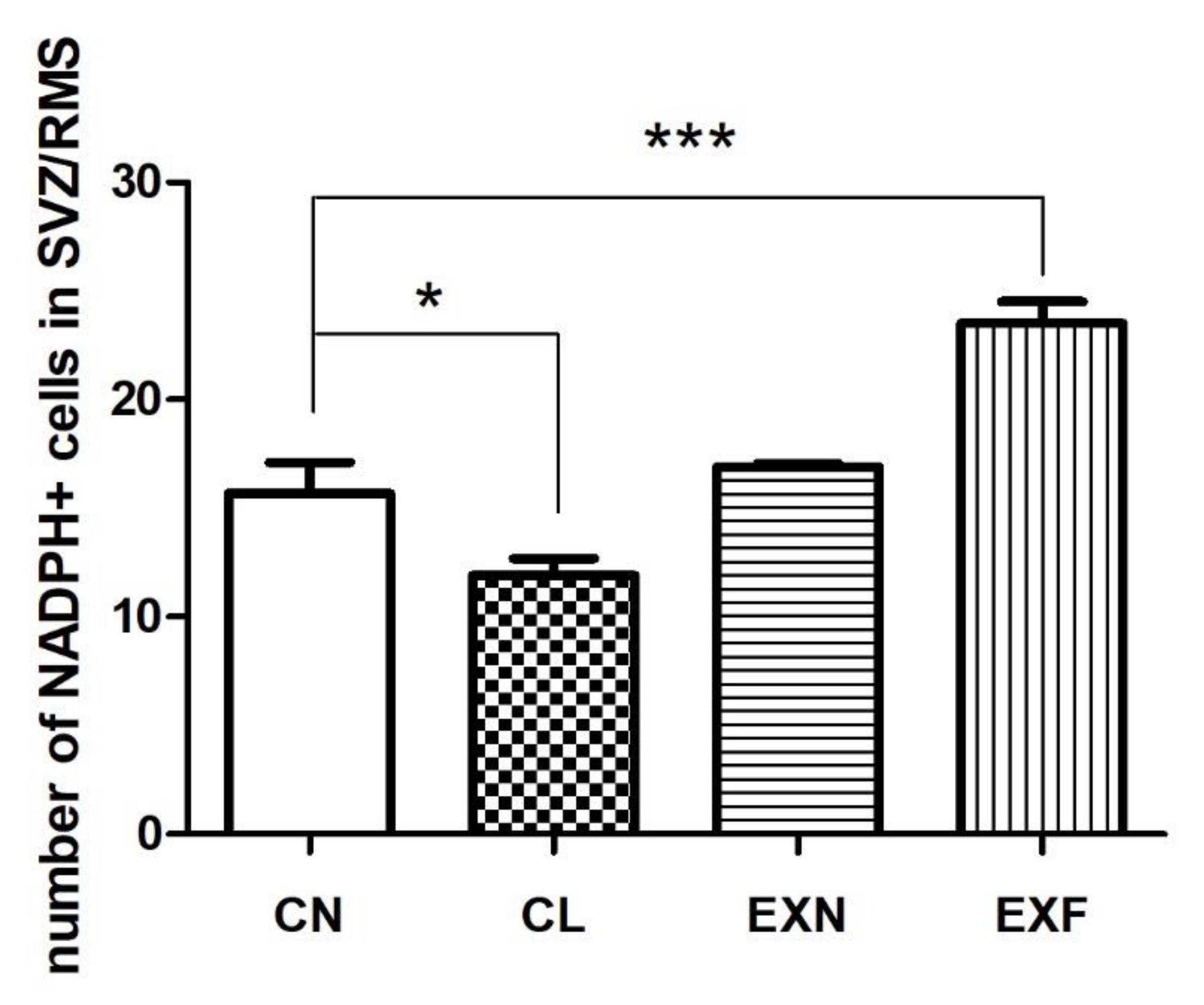

| Group (% of Body Fat) | CN (Control; 7–8%) (n = 6) | CL (<7%) (n = 6) | EXN (8–11%) (n = 6) | EXF (>11%) (n = 6) |
|---|---|---|---|---|
| Brain Area | ||||
| SVZ (µm) | 6.80 ± 1.03 | 7.37 ± 0.54 | 8.23 ± 0.74 | 13.70 ± 1.14 *** |
| vertical arm of the RMS (µm) | 3.47 ± 0.35 | 5.40 ± 0.62 * | 5.27 ± 0.31 * | 5.32 ± 0.45 * |
| elbow of the RMS (µm) | 18.13 ± 0.51 | 20.83 ± 0.73 * | 20.27 ± 0.23 * | 20.48 ± 0.59 * |
| horizontal arm of the RMS (µm) | 9.95 ± 0.85 | 8.43 ± 0.25 | 8.10 ± 1.10 | 9.38 ± 0.44 |
| Group (% of Body Fat) | CN (Control; 7–8%) (n = 6) | CL (<7%) (n = 6) | EXN (8–11%) (n = 6) | EXF (>11%) (n = 6) |
|---|---|---|---|---|
| Brain Area | ||||
| SVZ | 6.66 ± 0.62 | 1.90 ± 0.44 *** | 5.18 ± 0.43 | 1.98 ± 0.62 *** |
| vertical arm of the RMS | 3.04 ± 0.55 | 1.60 ± 0.45 | 2.62 ± 0.20 | 1.13 ± 0.47 * |
| elbow of the RMS | 5.50 ± 1.01 | 2.61 ± 0.24 * | 5.36 ± 0.70 | 2.23 ± 0.52 * |
| horizontal arm of the RMS | 2.88 ± 0.40 | 1.89 ± 0.15 | 2.13 ± 0.55 | 1.09 ± 0.26 * |
| Group (% of Body Fat) | CN (Control; 7–8%) (n = 6) | CL (<7%) (n = 6) | EXN (8–11%) (n = 6) | EXF (>11%) (n = 6) |
|---|---|---|---|---|
| Brain Area | ||||
| SVZ | 6.72 ± 1.16 | 17.79 ± 1.49 *** | 7.98 ± 0.57 | 19.87 ± 1.50 *** |
| vertical arm of the RMS | 5.13 ± 0.57 | 10.55 ± 0.97 *** | 5.30 ± 0.40 | 2.77 ± 0.39 |
| Elbow of the RMS | 5.85 ± 0.37 | 14.25 ± 1.43 *** | 8.23 ± 0.79 | 5.43 ± 0.67 |
| Horizontal arm of the RMS | 3.87 ± 0.36 | 8.53 ± 0.84 *** | 5.55 ± 0.56 | 3.82 ± 0.82 |
| Group (% of Body Fat) | CN (Control; 7–8%) (n = 6) | CL (<7%) (n = 6) | EXN (8–11%) (n = 6) | EXF (>11%) (n = 6) |
|---|---|---|---|---|
| Brain Area | ||||
| SVZ | 9.74 ± 1.27 | 8.62 ± 0.68 | 12.55 ± 0.42 | 14.06 ± 1.22 * |
| vertical arm of the RMS | 1.64 ± 0.06 | 1.62 ± 0.10 | 1.55 ± 0.42 | 1.97 ± 0.16 |
| elbow of the RMS | 1.63 ± 0.35 | 1.51 ± 0.24 | 2.43 ± 0.08 | 2.78 ± 0.19 * |
| horizontal arm of the RMS | 2.25 ± 0.23 | 1.58 ± 0.34 | 1.65 ± 0.07 | 3.70 ± 0.23 ** |
| Group (% of Body Fat) | CN (Control; 7–8%) (n = 6) | CL (<7%) (n = 6) | EXN (8–11%) (n = 6) | EXF (>11%) (n = 6) |
|---|---|---|---|---|
| Behavioral Measures | ||||
| The distance travelled (cm) | 3666 ± 198.10 | 2737 ± 143.70 * | 3179 ± 234.10 | 2846 ± 212.40 * |
| Mean velocity (cm/s) | 11.74 ± 0.64 | 10.55 ± 1.00 | 11.09 ± 0.97 | 10.62 ± 0.79 |
| Group (% of Body Fat) | CN (Control; 7–8%) (n = 6) | CL (<7%) (n = 6) | EXN (8–11%) (n = 6) | EXF (>11%) (n = 6) |
|---|---|---|---|---|
| Behavioral Measures | ||||
| The distance travelled (cm) | 200.70 ± 17.04 | 132.7 ± 14.68 * | 211.30 ± 21.36 | 140.70 ± 8.13 * |
| Mean velocity (cm/s) | 13.54 ± 084 | 12.53 ± 1.20 | 13.39 ± 1.07 | 12.52 ± 0.99 |
| Group (% of Body Fat) | CN (Control; 7–8%) (n = 6) | CL (<7%) (n = 6) | EXN (8–11%) (n = 6) | EXF (>11%) (n = 6) |
|---|---|---|---|---|
| Anxiety Scores | ||||
| Number of grooms | 2.00 ± 0.37 | 1.83 ± 0.31 | 1.50 ± 0.22 | 1.17 ± 0.17 |
| Grooming (s) | 13.83 ± 0.65 | 12.50 ± 1.69 | 9.83 ± 2.47 | 9.33 ± 1.05 |
| Latency to groom (s) | 72.94 ± 2.98 | 154.4 ±5.27 *** | 71.57 ± 5.66 | 145.0 ± 6.12 *** |
| Number of rears | 60.50 ± 3.34 | 31.67 ± 5.53 | 42.33 ± 4.35 * | 41.83 ± 2.41 * |
| Rearing (s) | 72.67 ± 2.45 | 42.50 ± 5.14 ** | 53.50 ± 6.85 | 62.83 ± 4.20 |
| Number of freezes | 3.5 ± 0.43 | 18 ± 4.32 *** | 6.7 ± 0.81 | 15.3 ± 3.27 ** |
| Neophobia (s) | 42.32 ± 2.45 | 139.19 ± 4.19 *** | 39.31 ± 1.45 | 142.23 ± 5.43 *** |
| Middle point crossings | 8.17 ± 1.28 | 2.17 ± 0.48 *** | 4.00 ± 0.77 * | 3.83 ± 0.83 * |
| Time spent by the walls (s) | 3.10 ± 0.28 | 3.79 ± 0.15 | 5.81 ± 0.19 *** | 7.78 ± 0.23 *** |
| Thigmotaxis | 1.57 ± 0.25 | 1.03 ± 0.08 | 0.33 ± 0.03 *** | 0.05 ± 0.01 *** |
| Defecation | 0.50 ± 0.22 | 2.83 ± 0.65 * | 1.83 ± 0.65 | 2.50 ± 0.22 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabianová, K.; Babeľová, J.; Fabian, D.; Popovičová, A.; Martončíková, M.; Raček, A.; Račeková, E. Maternal High-Energy Diet during Pregnancy and Lactation Impairs Neurogenesis and Alters the Behavior of Adult Offspring in a Phenotype-Dependent Manner. Int. J. Mol. Sci. 2022, 23, 5564. https://doi.org/10.3390/ijms23105564
Fabianová K, Babeľová J, Fabian D, Popovičová A, Martončíková M, Raček A, Račeková E. Maternal High-Energy Diet during Pregnancy and Lactation Impairs Neurogenesis and Alters the Behavior of Adult Offspring in a Phenotype-Dependent Manner. International Journal of Molecular Sciences. 2022; 23(10):5564. https://doi.org/10.3390/ijms23105564
Chicago/Turabian StyleFabianová, Kamila, Janka Babeľová, Dušan Fabian, Alexandra Popovičová, Marcela Martončíková, Adam Raček, and Enikő Račeková. 2022. "Maternal High-Energy Diet during Pregnancy and Lactation Impairs Neurogenesis and Alters the Behavior of Adult Offspring in a Phenotype-Dependent Manner" International Journal of Molecular Sciences 23, no. 10: 5564. https://doi.org/10.3390/ijms23105564
APA StyleFabianová, K., Babeľová, J., Fabian, D., Popovičová, A., Martončíková, M., Raček, A., & Račeková, E. (2022). Maternal High-Energy Diet during Pregnancy and Lactation Impairs Neurogenesis and Alters the Behavior of Adult Offspring in a Phenotype-Dependent Manner. International Journal of Molecular Sciences, 23(10), 5564. https://doi.org/10.3390/ijms23105564






