Glassy-like Metal Oxide Particles Embedded on Micrometer Thicker Alginate Films as Promising Wound Healing Nanomaterials
Abstract
:1. Introduction
2. Results and Discussion
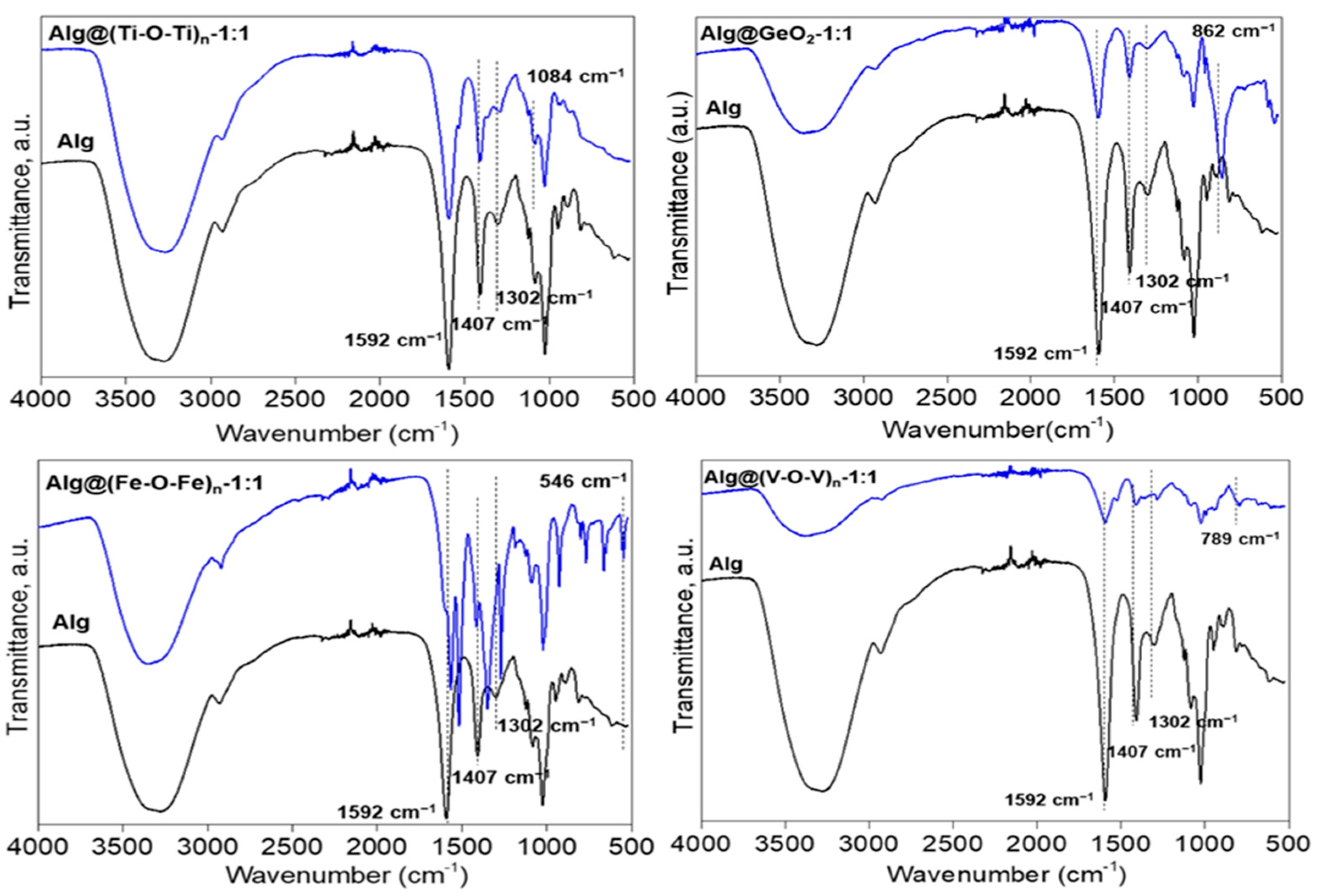
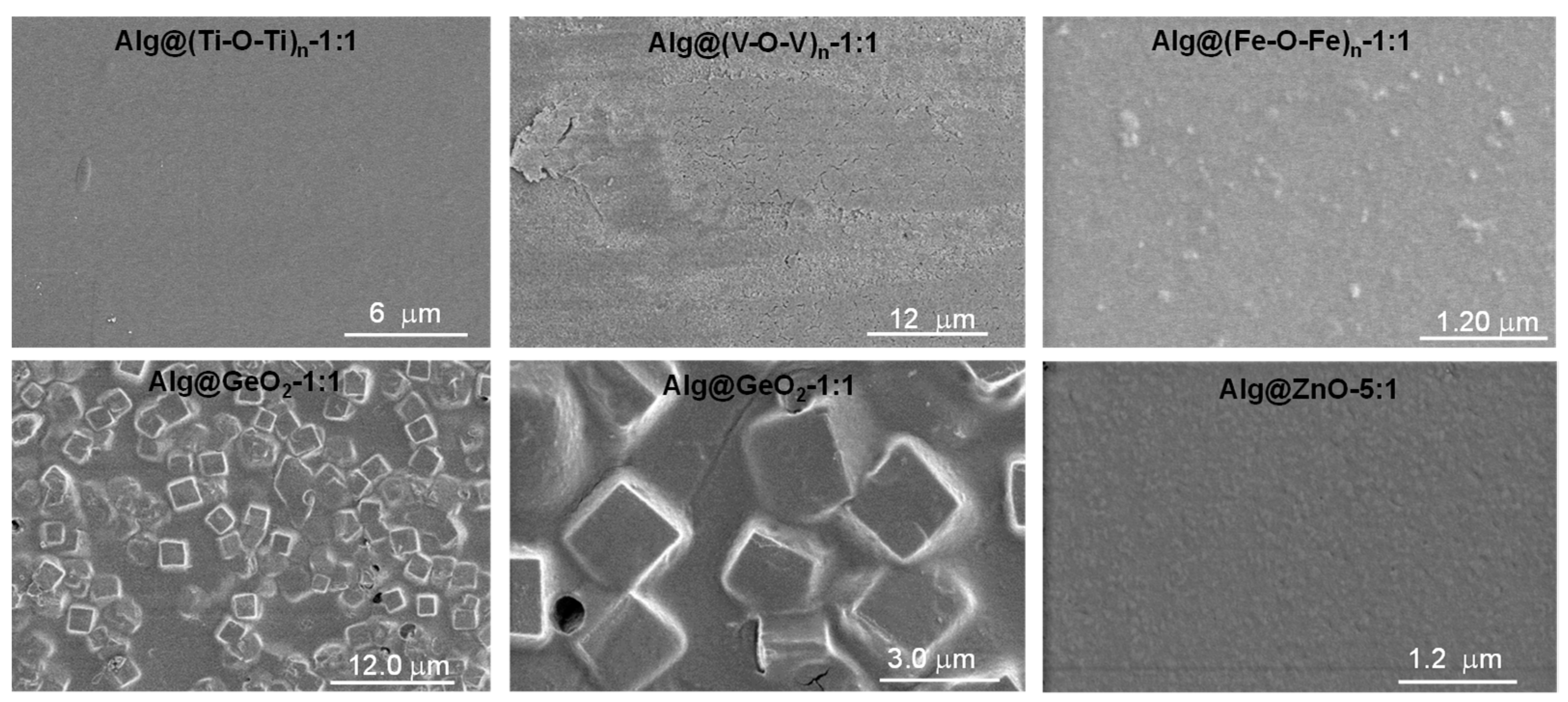
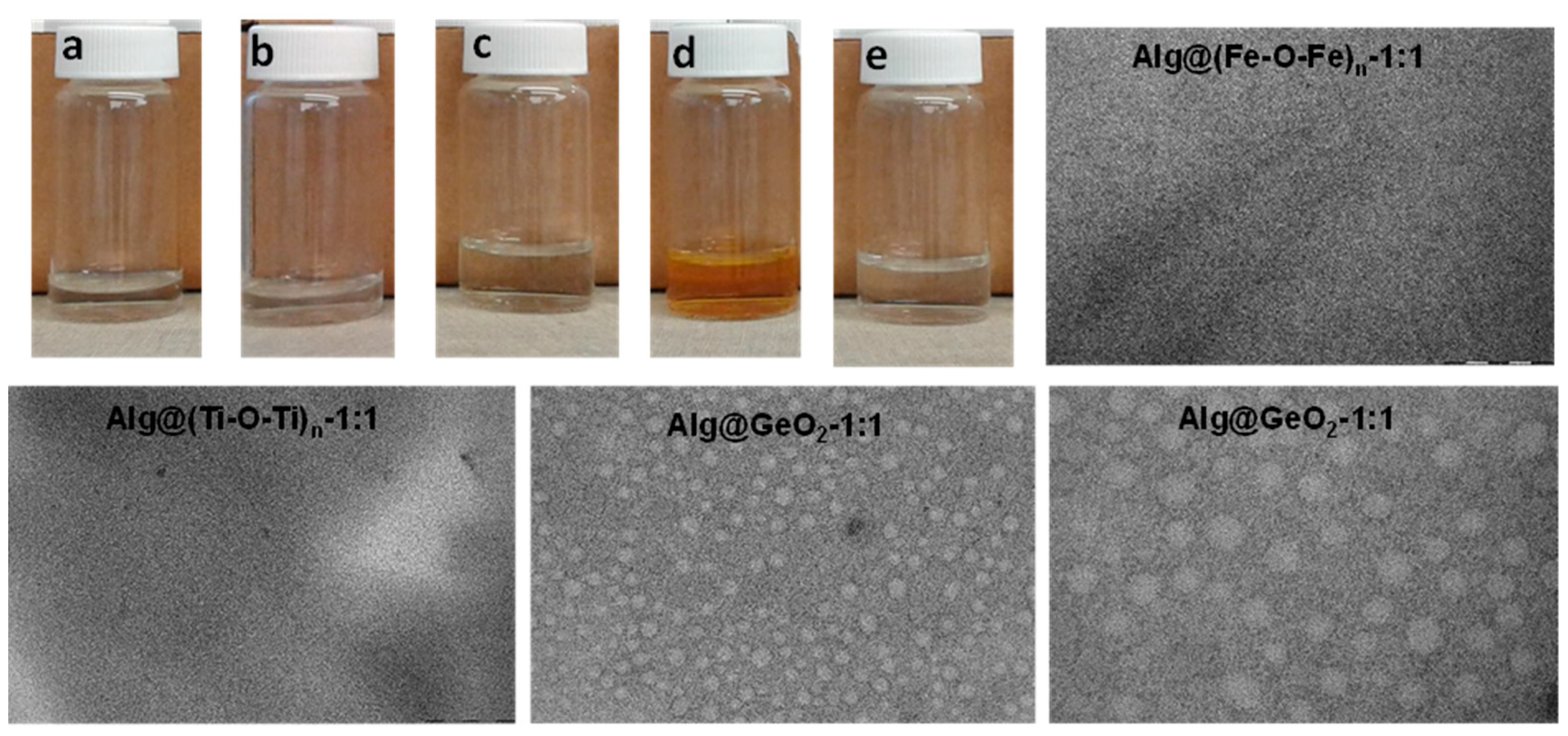
2.1. Preparation and Characterisation of Alg@(M-O-M)n Films
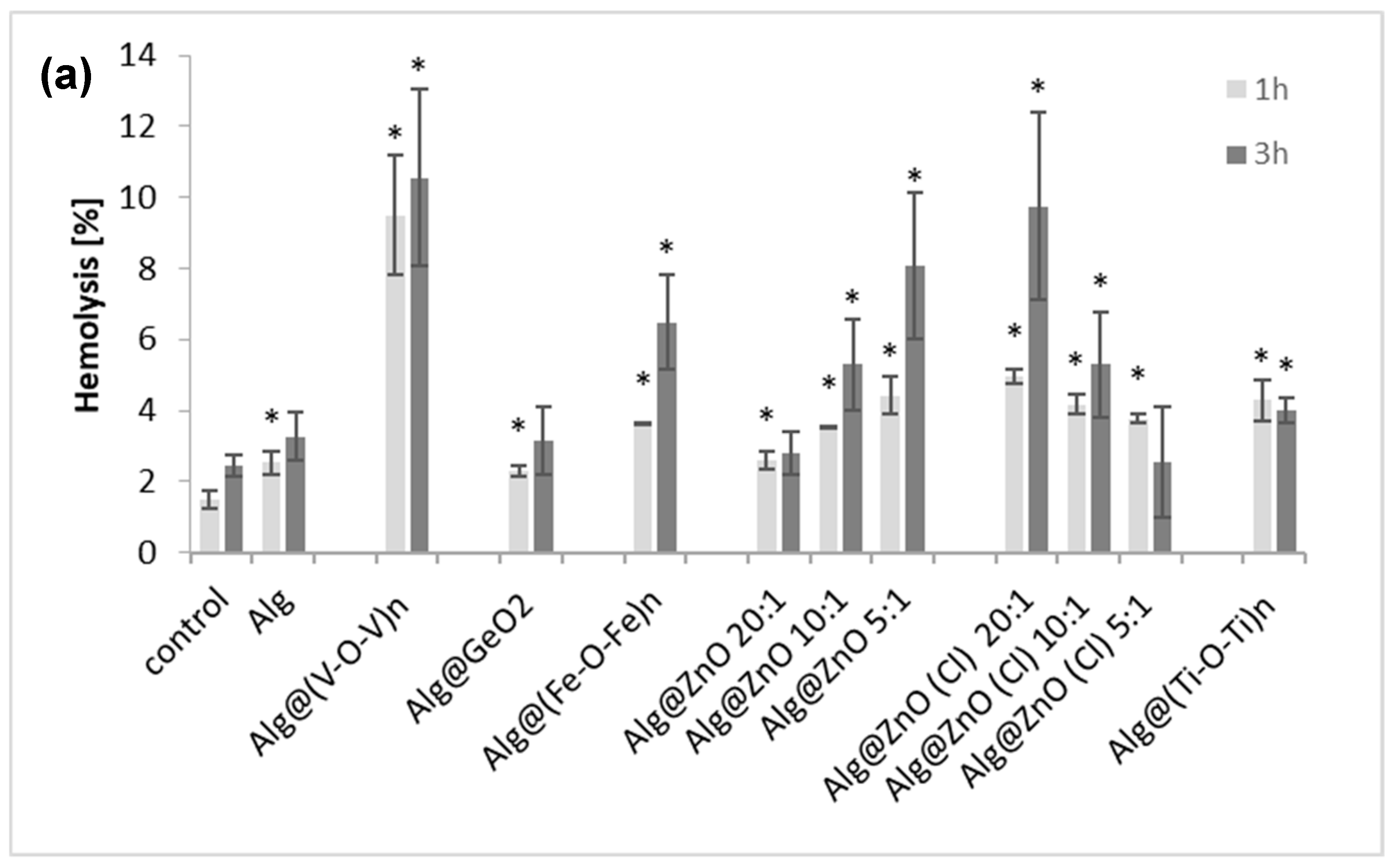
2.2. Hemolytic Activity and Hb Adsorption
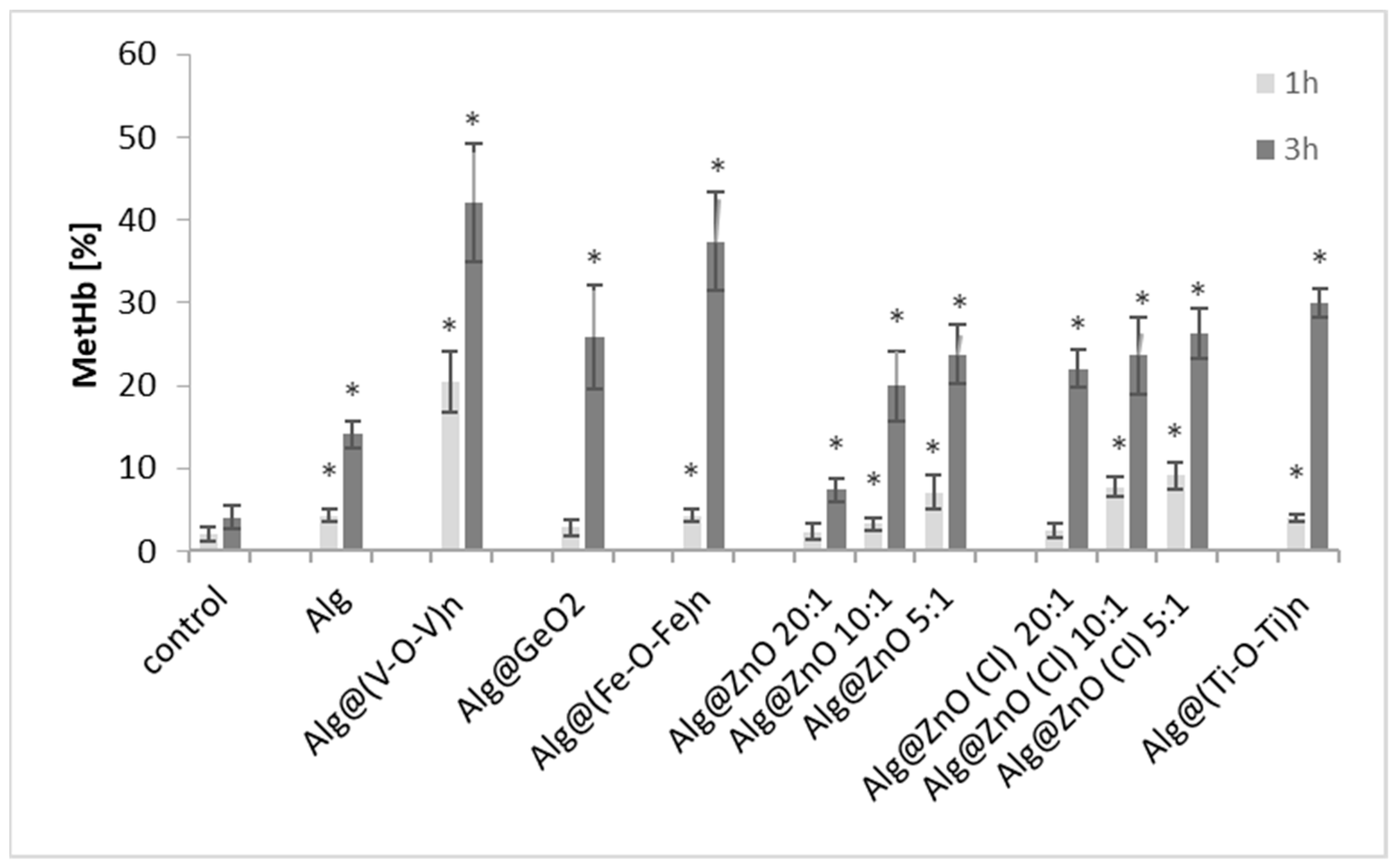
2.3. Methemoglobin
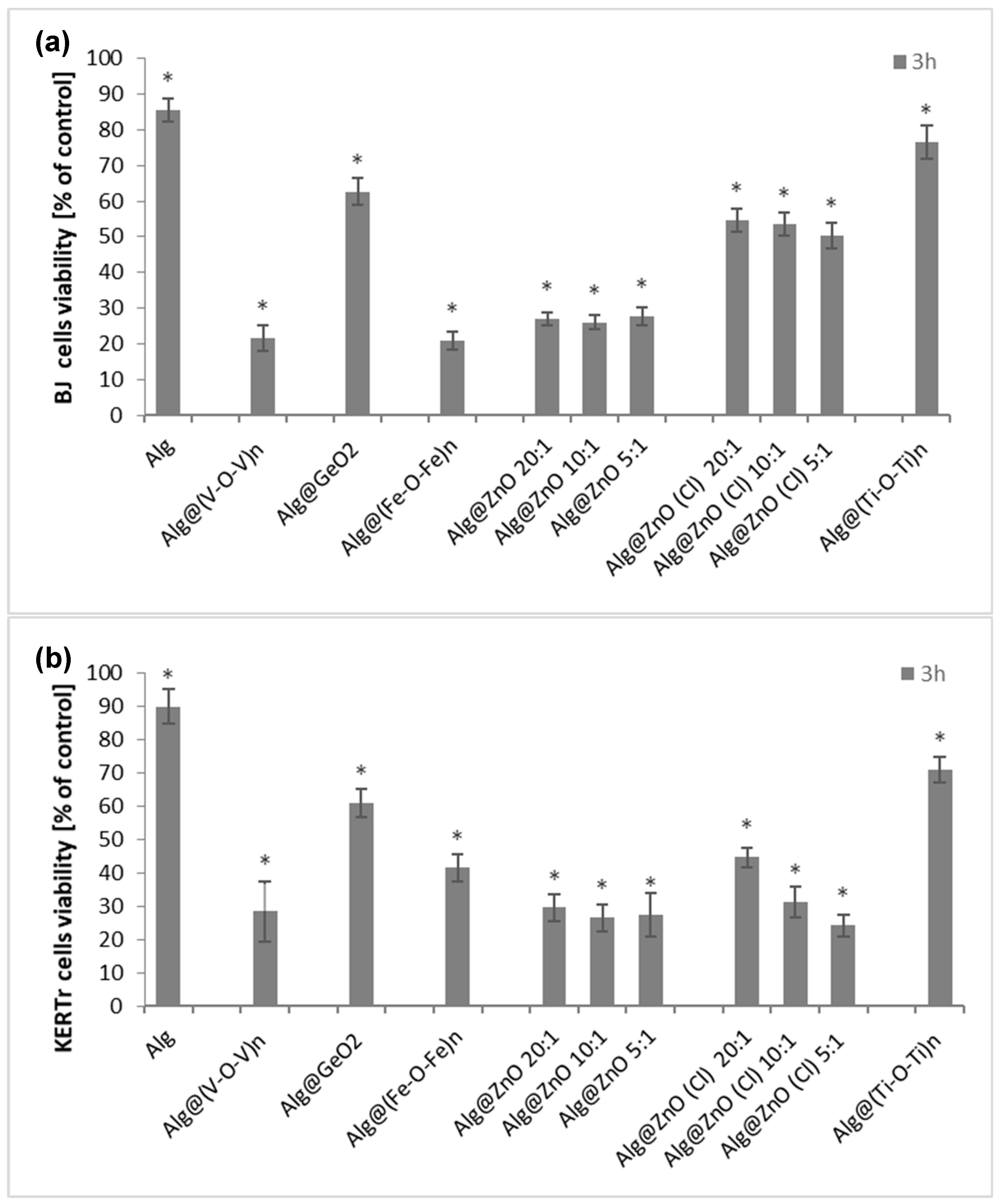
2.4. Cytotoxicity (MTT)
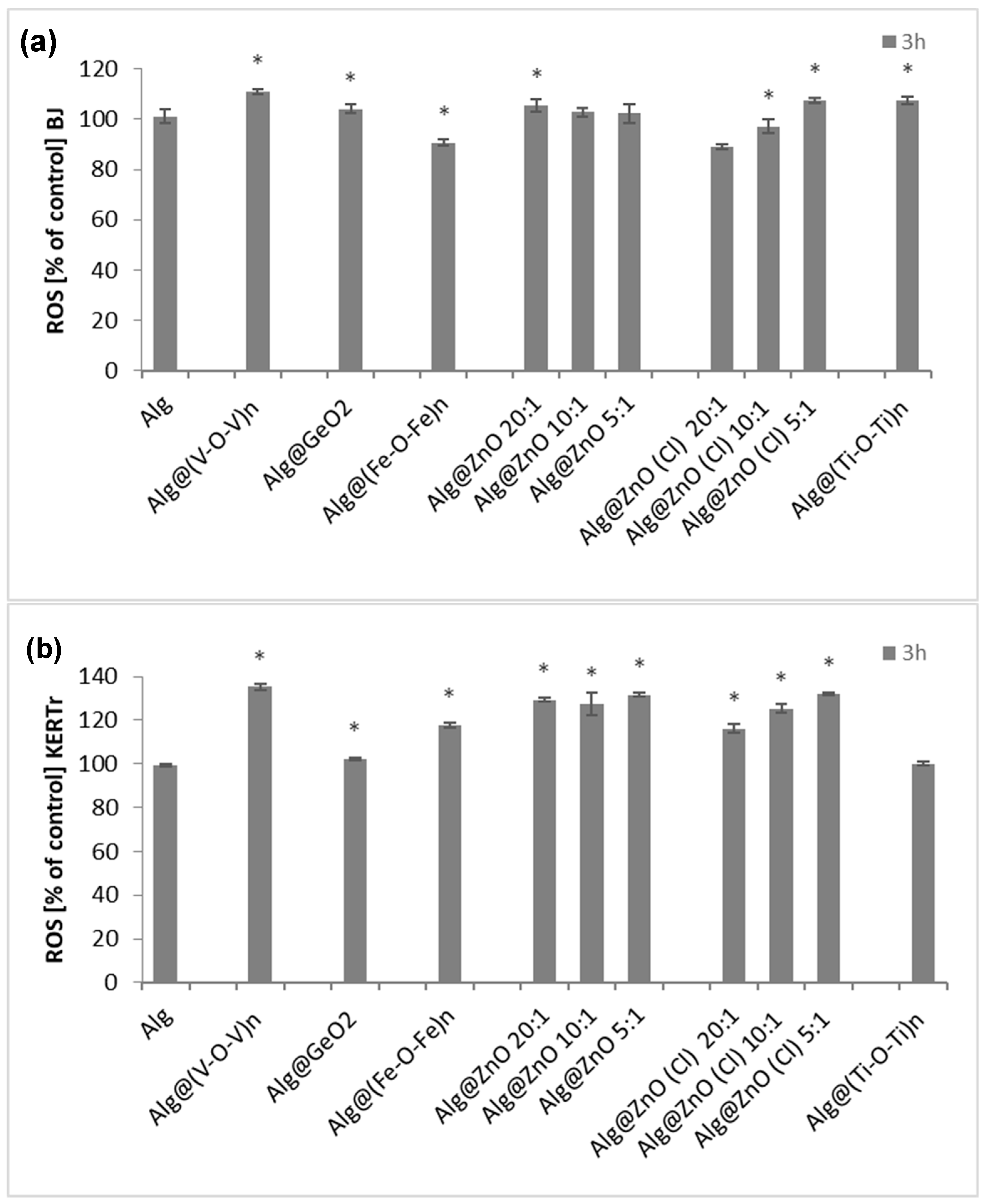
2.5. ROS Generation
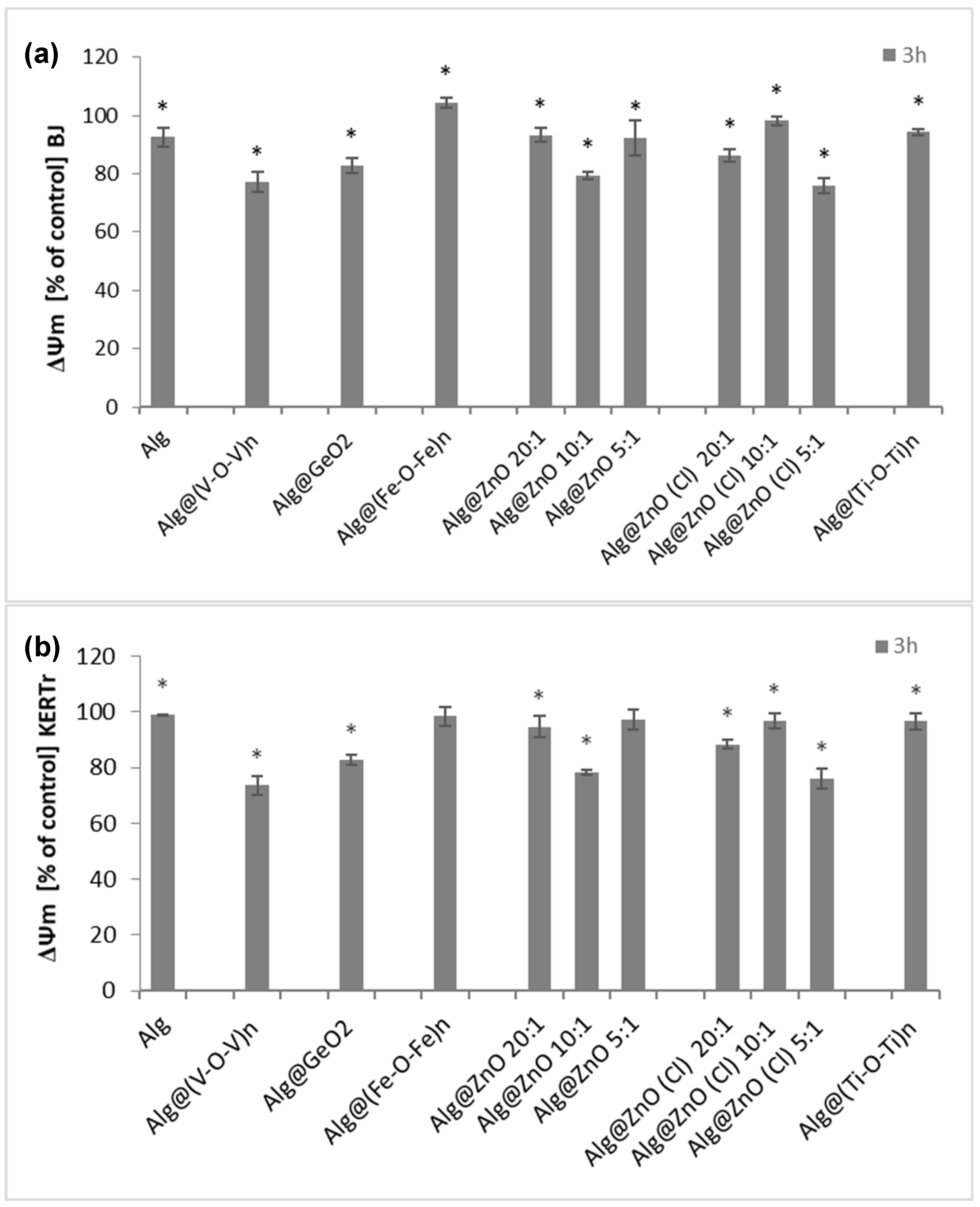
2.6. Assessment of Mitochondrial Membrane Potential (ΔΨm)
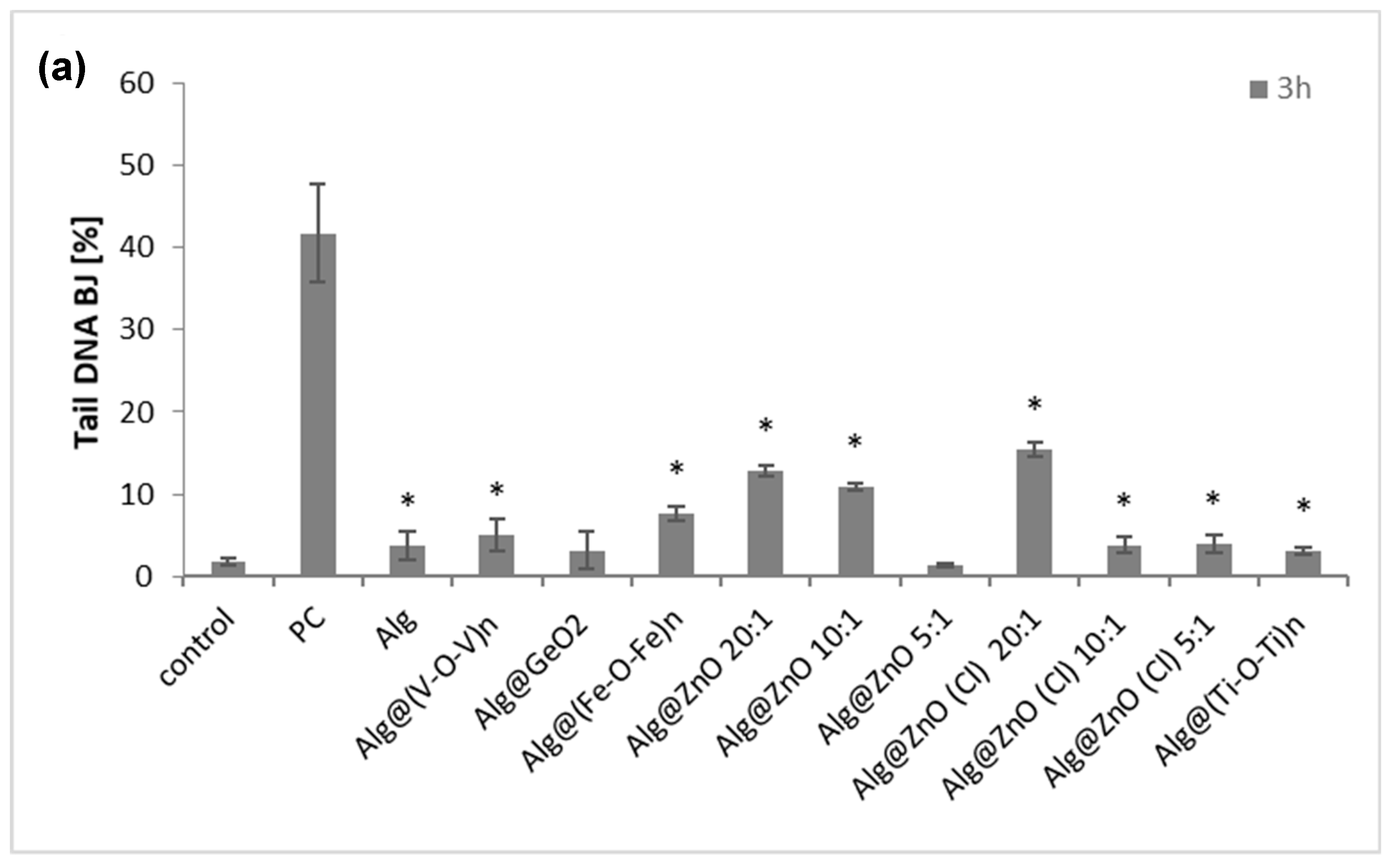
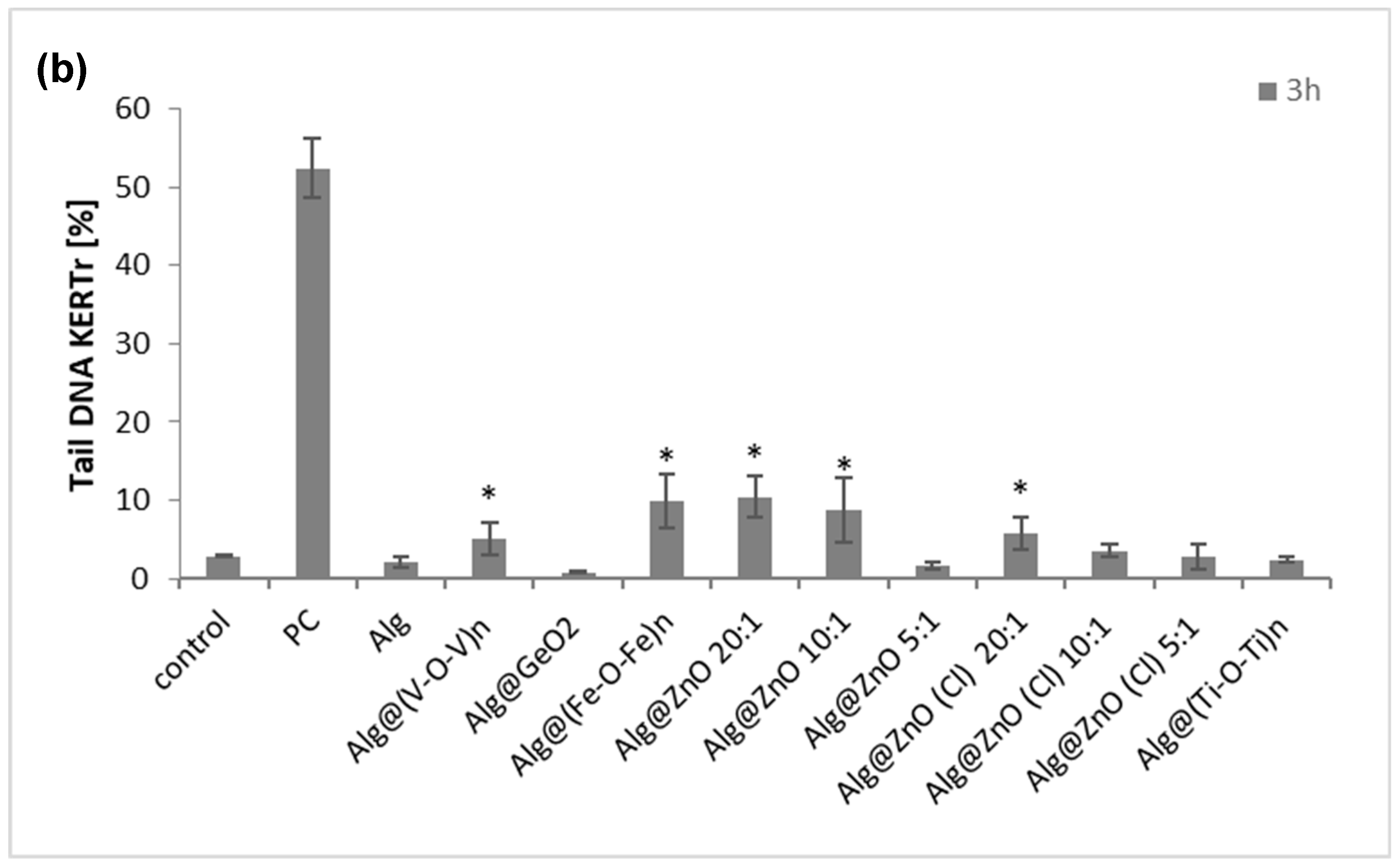
2.7. Genotoxicity (Comet Assay)
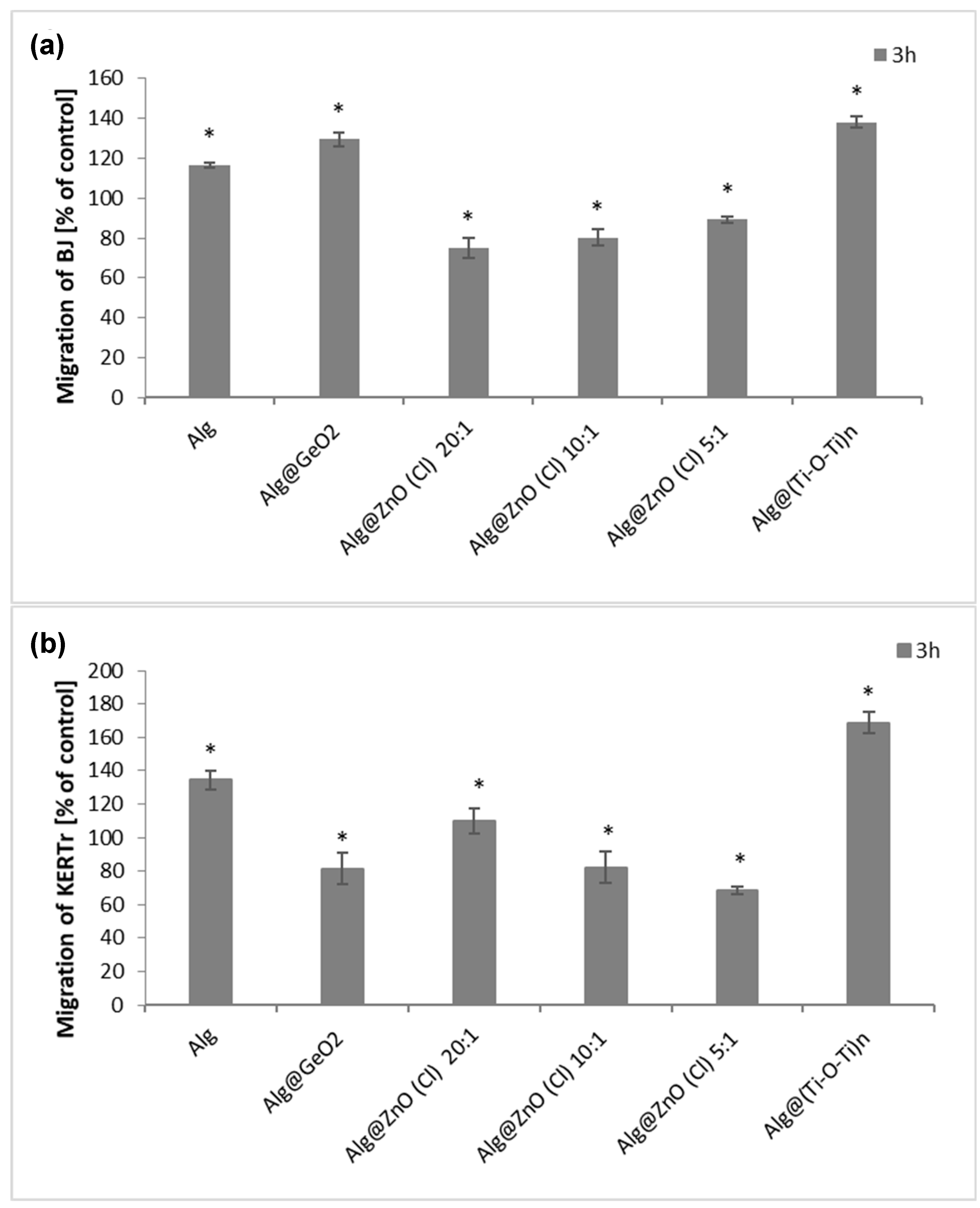
2.8. Migration Cells Fibroblasts and Kerationcytes
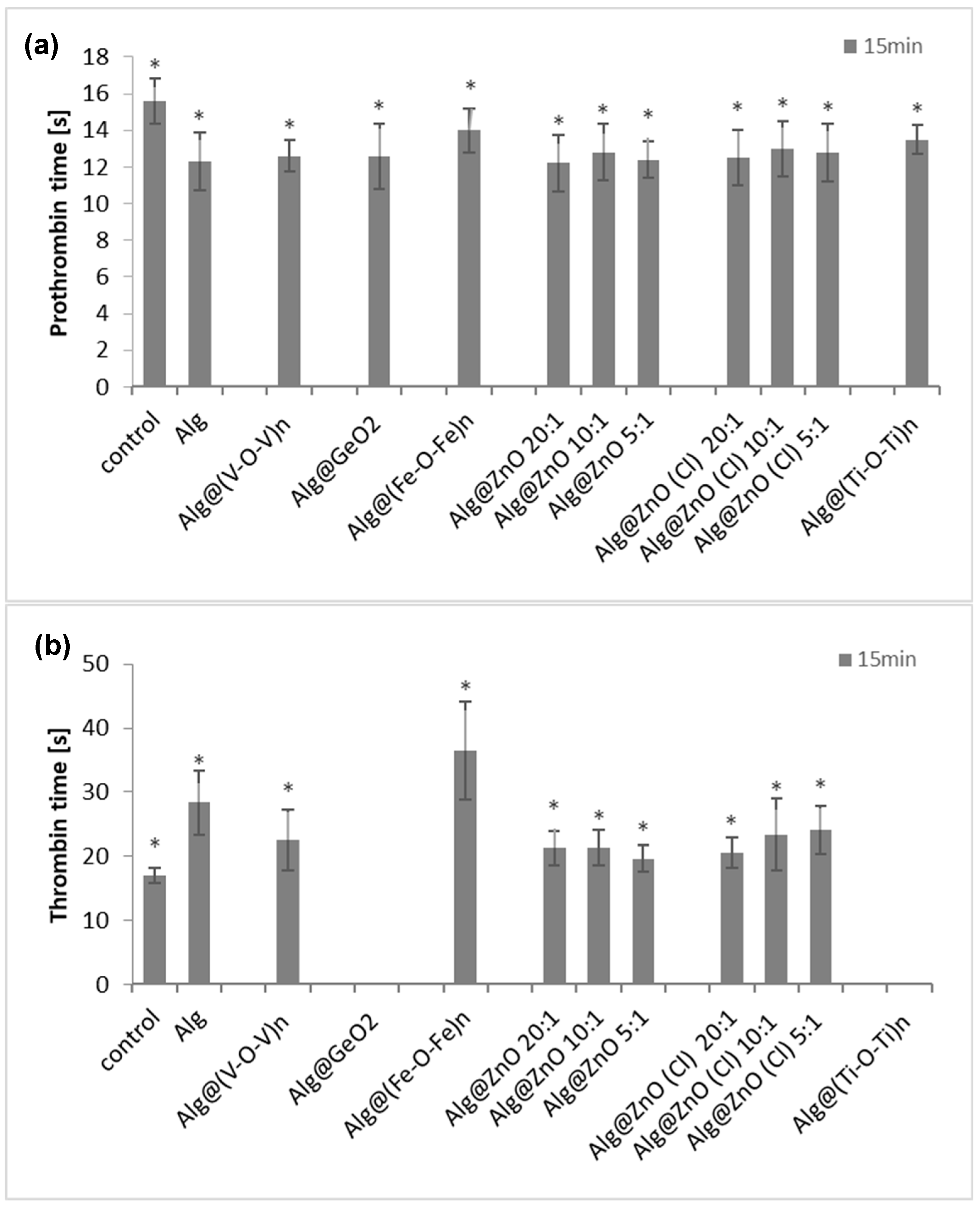
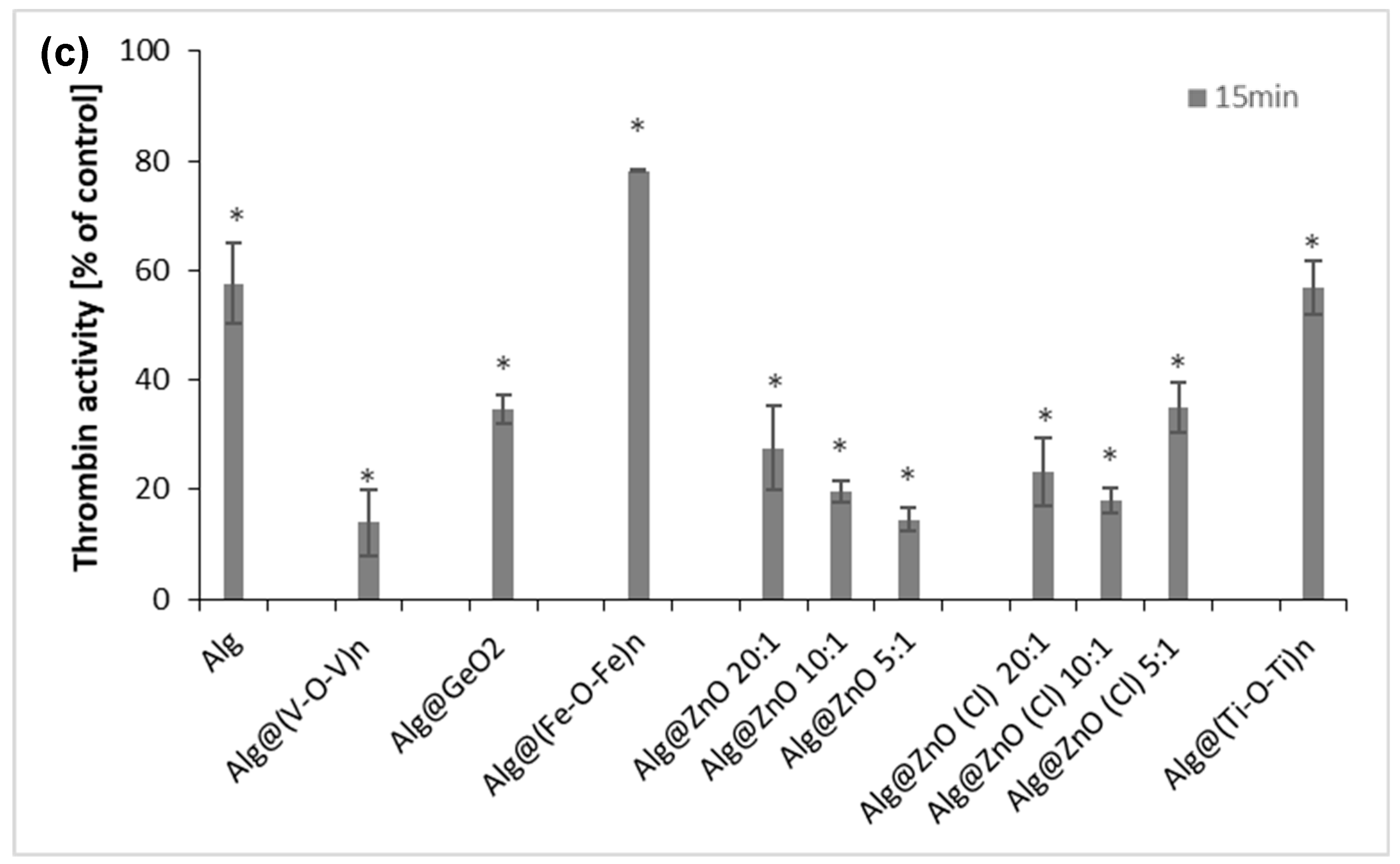
2.9. Prothrombin Time (PT), Thrombin Time (TT) and Amidolytic Activity of Thrombin
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Characterisation Techniques
3.3. Preparation of Alginate-Metal Oxide Films
3.4. Materials for Biological Studies
3.5. Hemolysis Assay
- As represents the absorbance of the sample
- Ac represents the absorbance of the erythrocytes in water (100% of hemolysis).
3.6. The Adsorption of Hemoglobin (Hb)
- As is the absorbance of the sample containing the alginate composites
- Ac is the absorbance of the control (hemoglobin without alginate composites).
3.7. Methemoglobin
- A630—the absorbance of a control sample and sample with composites at 630 nm,
- A700—the absorbance of a control sample and sample with composites at 700 nm,
- A630*—the absorbance of a control sample and sample with composites treated with potassium ferricyanide—100% met-Hb at 630 nm,
- A700*—the absorbance of a control sample and sample with composites treated with potassium ferricyanide—100% met-Hb at 700 nm.
3.8. Cell Culture
3.9. Cytotoxicity Assay
3.10. Measurement of Reactive Oxygen Species (ROS)
3.11. Assessment of Mitochondrial Membrane Potential (ΔΨm)
- ΔΨm—mitochondrial transmembrane potential directly proportional to the fluorescence coefficient,
- Fd—dimer fluorescence,
- Fm—monomer fluorescence.
3.12. Genotoxicity
3.13. Cell Migration
3.14. Measurements of Prothrombin Time (PT)
3.15. Measurements of Thrombin Time (TT)
3.16. Amidolytic Activity of Thrombin
3.17. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, L.; Feng, Y.; Wei, K.; Xu, X.; Liu, R.; Chen, G. Carbohydrate-Based Macromolecular Biomaterials. Chem. Rev. 2021, 121, 10950–11029. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M.; Carvalho, A.J.F.; Trovatti, E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef]
- Wrońska, N.; Anouar, A.; El Achaby, M.; Zawadzka, K.; Kędzierska, M.; Miłowska, K.; Katir, N.; Draoui, K.; Różalska, S.; Piwoński, I.; et al. Chitosan-Functionalized Graphene Nanocomposite Films: Interfacial Interplay and Biological Activity. Materials 2020, 13, 998. [Google Scholar] [CrossRef] [Green Version]
- Frindy, S.; Primo, A.; Qaiss, A.; El Kacem Bouhfid, R.; Lahcini, M.; Garcia, H.; Bousmina, M.; El Kadib, A. Insightful understanding of the role of clay topology on the stability of biomimetic hybrid chitosan-clay thin films and CO2-dried porous aerogel microspheres. Carbohydr. Polym. 2016, 146, 353–361. [Google Scholar] [CrossRef]
- Blilid, S.; Kędzierska, M.; Miłowska, K.; Wrońska, N.; El Achaby, M.; Katir, N.; Belamie, E.; Alonso, B.; Lisowska, K.; Lahcini, M.; et al. Phosphorylated Micro- and Nanocellulose-Filled Chitosan Nanocomposites as Fully Sustainable, Biologically Active Bioplastics. ACS Sustain. Chem. Eng. 2020, 8, 18354–18365. [Google Scholar] [CrossRef]
- El Kadib, A. Green and functional aerogels by macromolecular and textural engineering of chitosan microspheres. Chem. Rec. 2020, 20, 753–772. [Google Scholar] [CrossRef]
- El Kadib, A.; Bousmina, M.; Brunel, D. Recent Progress in Chitosan Bio-Based Soft Nanomaterials. J. Nanosci. Nanotechnol. 2014, 14, 308–331. [Google Scholar] [CrossRef]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef]
- Teng, K.; An, Q.; Chen, Y.; Zhang, Y.; Zhao, Y. Recent Development of Alginate-Based Materials and Their Versatile Functions in Biomedicine, Flexible Electronics, and Environmental Uses. ACS Biomater. Sci. Eng. 2021, 7, 1302–1337. [Google Scholar] [CrossRef]
- Agulhon, P.; Robitzer, M.; David, L.; Quignard, F. Structural Regime Identification in Ionotropic Alginate Gels: Influence of the Cation Nature and Alginate Structure. Biomacromolecules 2012, 13, 215–220. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Agulhon, P.; Grams, J.; Wąchała, M.; Wojciechowska, J.; Świerczyński, D.; Cacciaguerra, T.; Tanchoux, N.; Quignard, F. Synthesis of TiO2–ZrO2 Mixed Oxides via the Alginate Route: Application in the Ru Catalytic Hydrogenation of Levulinic Acid to Gamma-Valerolactone. Energies 2019, 12, 4706. [Google Scholar] [CrossRef] [Green Version]
- Agulhon, P.; Robitzer, M.; Habas, J.-P. Quignard, FInfluence of both cation and alginate nature on the rheological behavior of transition metal alginate gels. Carbohydr. Polym. 2014, 112, 525–531. [Google Scholar] [CrossRef]
- Milowska, K.; Rybczynska, A.; Mosiolek, J.; Durdyn, J.; Szewczyk, E.M.; Katir, N.; Brahmi, Y.; Majoral, J.-P.; Bousmina, M.; Bryszewska, M.; et al. Biological Activity of Mesoporous Dendrimer-Coated Titanium Dioxide: Insight on the Role of the Surface-Interface Composition and the Framework Crystallinity. ACS Appl. Mater. Interface 2015, 7, 19994–20003. [Google Scholar] [CrossRef]
- Delumeau, L.-V.; Asgarimoghaddam, H.; Alkie, T.; Jones, A.J.B.; Lum, S.; Mistry, K.; Aucoin, M.G.; DeWitte-Orr, S.; Musselman, K.P. Effectiveness of antiviral metal and metal oxide thin-film coatings against human coronavirus 229E. APL Mater. 2021, 9, 111114. [Google Scholar] [CrossRef]
- Hammi, N.; Wrońska, N.; Katir, N.; Lisowska, K.; Marcotte, N.; Cacciaguerra, T.; Bryszewska, M.; El Kadib, A. Supramolecular Chemistry-Driven Preparation of Nanostructured, Transformable, and Biologically Active Chitosan-Clustered Single, Binary, and Ternary Metal Oxide Bioplastics. ACS Appl. Bio Mater. 2019, 2, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Guari, Y.; Larionova, J.; Molvinger, K.; Folch, B.; Guérin, C. Magnetic water-soluble cyano-bridged metal coordination nano-polymers. Chem. Commun. 2006, 24, 2613–2615. [Google Scholar] [CrossRef]
- Anicic, N.; Vukomanovic, M.; Suvorov, D. Design of a multifunctional vanadium pentoxide/polymer biocomposite for implant-coating applications. RSC Adv. 2017, 7, 38647–38658. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, M.V.; Juaristi, J.A.; Alvarez, M.A.; Brandan, N.C. Characteristics of in vivo murine erythropoietic response to sodium orthovanadate. Chem.-Biol. Interact. 2005, 156, 55–68. [Google Scholar] [CrossRef]
- Hogan, G.R. Peripheral erythrocyte levels, hemolysis and three vanadium compounds. Experientia 1990, 46, 444–446. [Google Scholar] [CrossRef]
- Shirsekar, P.P.; Kanhe, N.; Mathe, V.L.; Lahir, Y.K.; Dongre, P.M. Interaction of Zinc oxides nanoparticles with human red blood cells. Bionano Front. 2016, 9, 99–104. [Google Scholar]
- Khan, M.; Husain, A.; Ahmad, M. Comparative study of the cytotoxic and genotoxic potentials of zinc oxide and titanium dioxide nanoparticles. Toxicol. Rep. 2015, 2, 765–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keceli, S.A.; Alaynali, H.A. Study on the Evaluation of the Cytotoxicity of Al2O3, Nb2O5, Ta2O5, TiO2 and ZrO2. Turk. J. Eng. Environ. Sci. 2004, 28, 49–54. [Google Scholar]
- Horie, M.; Nishio, K.; Fujita, K.; Endoh, S.; Miyauchi, A.; Saito, Y.; Yoshida, Y. Protein Adsorption of Ultrafine Metal Oxide and Its Influence on Cytotoxicity toward Cultured Cells. Chem. Res. Toxicol. 2009, 22, 543–553. [Google Scholar] [CrossRef]
- Horie, M.; Fujita, K.; Kato, H.; Endoh, S.; Nishio, K.; Komaba, L.K.; Iwahashi, H. Association of the physical and chemical properties and the cytotoxicity of metal oxide nanoparticles: Metal ion release, adsorption ability and specific surface area. Metallomics 2012, 4, 350. [Google Scholar] [CrossRef]
- García-Hevia, L.; Valiente, R.; Martín-Rodríguez, R.; Renero-Lecuna, C.; González, J.; Rodríguez-Fernández, L.; Aguado, F.; Villegas, J.C.; Fanarraga, M.L. Nano-ZnO lead to tubulin macro tube assembly and actin-bundling triggering, cytoskeletal catastrophe and cell necrosis. Nanoscale 2016, 8, 10963–10973. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Nishio, K.; Fujita, K.; Kato, H.; Endoh, S.; Suzuki, M.; Nakamura, A.; Miyauchi, A.; Kinugasa, S.; Yamamoto, K.; et al. Cellular responses by stable and uniform ultrafine titanium dioxide particles in culture-medium dispersions when secondary particle size was 100 nm or less. Toxicol. In Vitro 2010, 24, 1629–1638. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Zhang, S.; Zhong, M.; Fan, H. Ferrite Nanoparticles-Based Reactive Oxygen Species-Mediated Cancer Therapy. Front. Chem. 2021, 9, 651053. [Google Scholar] [CrossRef] [PubMed]
- Labouta, H.I.; Schneider, M. Interaction of inorganic nanoparticles with the skin barrier: Current status and critical review. Nanomedicine 2013, 9, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Shokri, N.; Javar, H.A. Comparison of calcium phosphate and zinc oxide nanoparticles as dermal penetration enhancers for albumin. Indian J. Pharm. Sci. 2015, 77, 694–704. [Google Scholar] [CrossRef]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef]
- Rizk, M.Z.; Ali, S.A.; Hamed, M.A.; El-Rigal, N.S.; Alya, H.F.; Sala, H.H. Toxicity of titanium dioxide nanoparticles: Effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomed. Pharmacother. 2017, 90, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.C.; Shelton, R.M.; Grover, L.M. Reversible mitotic and metabolic inhibition following the encapsulation of fibroblasts in alginate hydrogels. Biomaterials 2009, 32, 6435–6443. [Google Scholar] [CrossRef] [PubMed]
- Grigore, A.; Sarker, B.; Fabry, B.; Boccaccini, A.R.; Detsch, R. Behavior of Encapsulated MG-63 Cells in RGD and Gelatine-Modified Alginate Hydrogels. Tissue Eng. Part A 2014, 20, 15–16, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Pokhrel, S.; Xia, T.; Gilbert, B.; Ji, Z.; Schowalter, M.; Rosenauer, A.; Damoiseaux, R.; Bradley, K.A.; Madler, L.; et al. Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano 2010, 4, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Strianese, M.; Basile, A.; Mazzone, A.; Morello, S.; Turco, M.C.; Pellecchia, C. Therapeutic potential of a pyridoxal-based vanadium (IV) complex showing selective cytotoxicity for cancer vs. healthy cells. J. Cell. Physiol. 2013, 228, 2202–2209. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Reductive Stress in Inflammation-Associated Diseases and the Pro-Oxidant Effect of Antioxidant Agents. Int. J. Mol. Sci. 2017, 18, 2098. [Google Scholar] [CrossRef]
- Starkov, A.A.; Fiskum, G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD (P) H redox state. J. Neurochem. 2003, 86, 1101–1107. [Google Scholar] [CrossRef]
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Decavanadate induces mitochondrial membrane depolarization and inhibits oxygen consumption. J. Inorg. Biochem. 2007, 101, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Mcausland, T.M.; Vloten, J.P.V.; Santry, L.A.; Guilleman, M.M.; Rghei, A.D.; Ferreira, E.M.; Ingrao, J.C.; Arulanandam, R.; Major, P.P.; Susta, L. Combining vanadyl sulfate with Newcastle disease virus potentiates rapid innate immune-mediated regression with curative potential in murine cancer models. Mol. Ther. Oncol. 2021, 20, 306–324. [Google Scholar] [CrossRef]
- Gopalan, R.C.; Osman, I.F.; Amani, A.; De Matas, M.; Anderson, D. The effect of zinc oxide and titanium dioxide nanoparticles in the Comet assay with UVA photoactivation of human sperm and lymphocytes. Nanotoxicology 2009, 3, 33–39. [Google Scholar] [CrossRef]
- Xie, H.; Mason, M.M.; Wise, J.P. Genotoxicity of metal nanoparticles. Rev. Environ. Health 2011, 26, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Klien, K.; Godnić-Cvar, J. Genotoxicity of Metal Nanoparticles: Focus on In Vivo Studies. Arch. Ind. Hyg. Toxicol. 2012, 63, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seabra, A.B.; Pasquôto, T.; Ferrarini, A.C.F.; Santos, M.D.C.; Haddad, P.S.; De Lima, R. Preparation, Characterization, Cytotoxicity, and Genotoxicity Evaluations of Thiolated-and S-Nitrosated Superparamagnetic Iron Oxide Nanoparticles: Implications for Cancer Treatment. Chem. Res. Toxicol. 2014, 27, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Alhadlaq, H.A.; Alam, J.; Khan, M.A.; Ali, D.; Alarafi, S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr. Pharm. Des. 2013, 19, 6681. [Google Scholar] [CrossRef]
- Magdolenova, Z.; Drlickova, M.; Henjum, K.; Runden-Pran, E.; Tulinska, J.; Bilanicova, D.; Pojana, G.; Kazimirova, A.; Barancokova, M.; Kuricova, M. Coating-dependent induction of cytotoxicity and genotoxicity of iron oxide nanoparticles. Nanotoxicology 2013, 9, 44. [Google Scholar] [CrossRef]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 2009, 28, 185. [Google Scholar] [CrossRef]
- Weng, Y.; Wang, M.; Liu, W. Repair of experimental alveolar bone defects by tissue-engineered bone. J. Tissue Eng. 2006, 6, 1503. [Google Scholar] [CrossRef]
- Dar, A.; Shachar, M.; Leor, J. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol. Bioeng. 2002, 80, 305. [Google Scholar] [CrossRef]
- Glicklis, R.; Shapiro, L.; Agbaria, R. Hepatocyte behavior within three-dimensional porous alginate scaffolds. Biotechnol. Bioeng. 2000, 67, 344. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Neufurth, M.; Tolba, E.; Schepler, H.; Xiao, S.; Müller, W.E.G. Biomimetic Alginate/Gelatin Cross-Linked Hydrogels Supplemented with Polyphosphate for Wound Healing Applications. Molecules 2020, 25, 5210. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, B.; Chouhan, G.; Moiemen, N.S.; Grover, L.M. Advances in keratinocyte delivery in burn wound care. Adv. Drug Deliv. Rev. 2018, 123, 18–32. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [Green Version]
- Lechtenberg, B.C.; Freund, S.M.; Huntington, J.A. An ensemble view of thrombin allostery. J. Biol. Chem. 2012, 393, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.T.; Zanardelli, S.; Chion, C.K.; Lane, D.A. The central role of thrombin in hemostasis. J. Thromb. Haemost. 2007, 5, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska, M.; Blilid, S.; Miłowska, K.; Kołodziejczyk-Czepas, J.; Katir, N.; Lahcini, M.; El Kadib, A.; Bryszewska, M. Insight into Factors Influencing Wound Healing Using Phosphorylated Cellulose-Filled Chitosan Nanocomposite Films. Int. J. Mol. Sci. 2021, 22, 11386. [Google Scholar] [CrossRef]
- Mossman, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Myhre, O.; Andresen, J.M.; Aarnes, H.; Fonnum, F. Evaluation of the Probes 2′,7′-Dichlorofluorescin Diacetate, Luminol, and Lucigenin as Indicators of Reactive Oxygen Species Formation. Biochem. Pharmacol. 2003, 65, 1575–1582. [Google Scholar] [CrossRef]
- Salvioli, S.; Ardizzoni, A.; Franceschi, C.; Cossarizza, A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: Implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997, 411, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Blasiak, J.; Kowalik, J. A comparison of the in vitro genotoxicity of tri-and hexavalent chromium. Mutat. Res. 2000, 469, 135–145. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kędzierska, M.; Hammi, N.; Kolodziejczyk-Czepas, J.; Katir, N.; Bryszewska, M.; Milowska, K.; El Kadib, A. Glassy-like Metal Oxide Particles Embedded on Micrometer Thicker Alginate Films as Promising Wound Healing Nanomaterials. Int. J. Mol. Sci. 2022, 23, 5585. https://doi.org/10.3390/ijms23105585
Kędzierska M, Hammi N, Kolodziejczyk-Czepas J, Katir N, Bryszewska M, Milowska K, El Kadib A. Glassy-like Metal Oxide Particles Embedded on Micrometer Thicker Alginate Films as Promising Wound Healing Nanomaterials. International Journal of Molecular Sciences. 2022; 23(10):5585. https://doi.org/10.3390/ijms23105585
Chicago/Turabian StyleKędzierska, Marta, Nisrine Hammi, Joanna Kolodziejczyk-Czepas, Nadia Katir, Maria Bryszewska, Katarzyna Milowska, and Abdelkrim El Kadib. 2022. "Glassy-like Metal Oxide Particles Embedded on Micrometer Thicker Alginate Films as Promising Wound Healing Nanomaterials" International Journal of Molecular Sciences 23, no. 10: 5585. https://doi.org/10.3390/ijms23105585
APA StyleKędzierska, M., Hammi, N., Kolodziejczyk-Czepas, J., Katir, N., Bryszewska, M., Milowska, K., & El Kadib, A. (2022). Glassy-like Metal Oxide Particles Embedded on Micrometer Thicker Alginate Films as Promising Wound Healing Nanomaterials. International Journal of Molecular Sciences, 23(10), 5585. https://doi.org/10.3390/ijms23105585





