Activation of Focal Adhesion Kinase Restores Simulated Microgravity-Induced Inhibition of Osteoblast Differentiation via Wnt/Β-Catenin Pathway
Abstract
:1. Introduction
2. Results
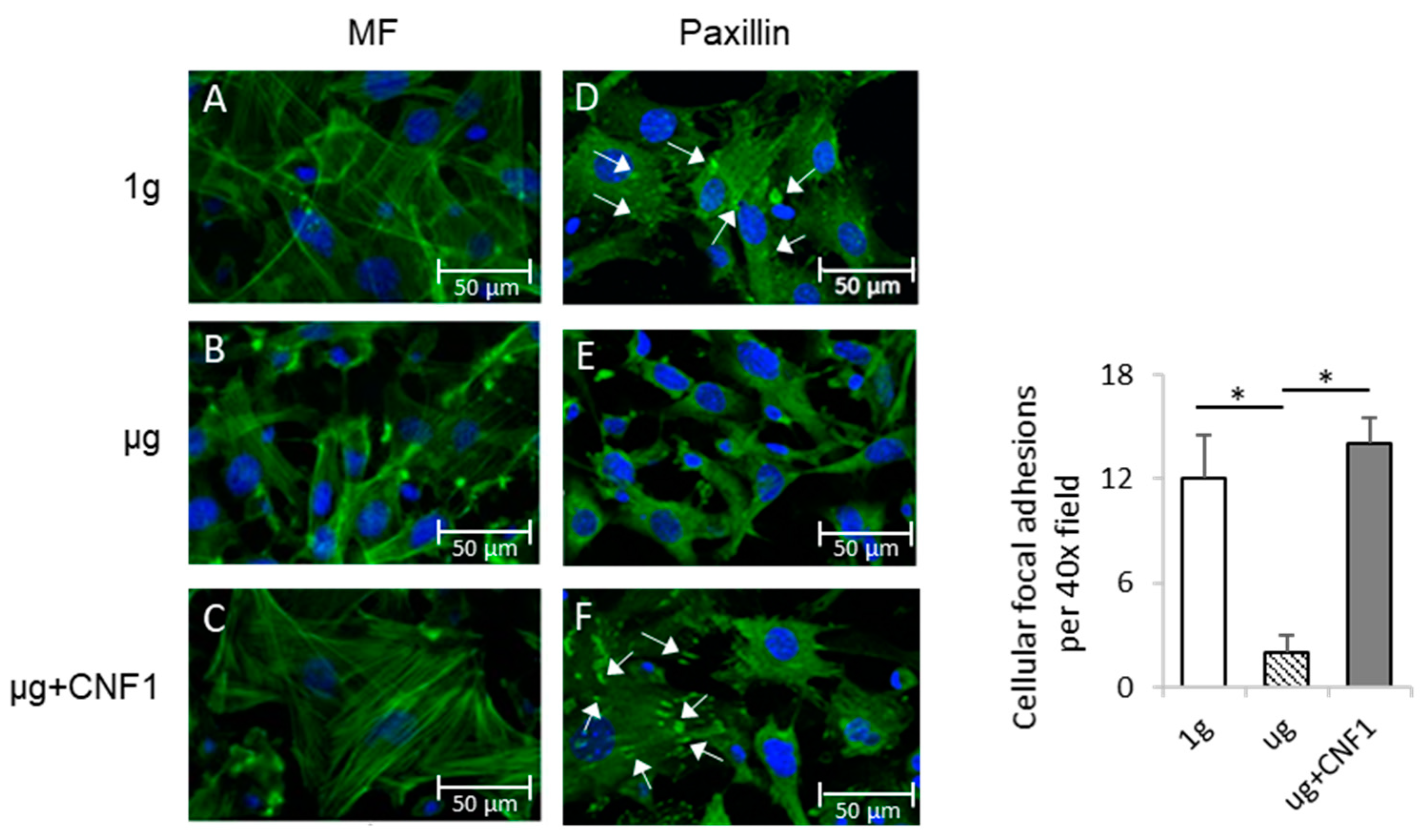
2.1. Simulated Microgravity Alters Cytoskeleton Structures and Reduces Focal Adhesions (FAs)
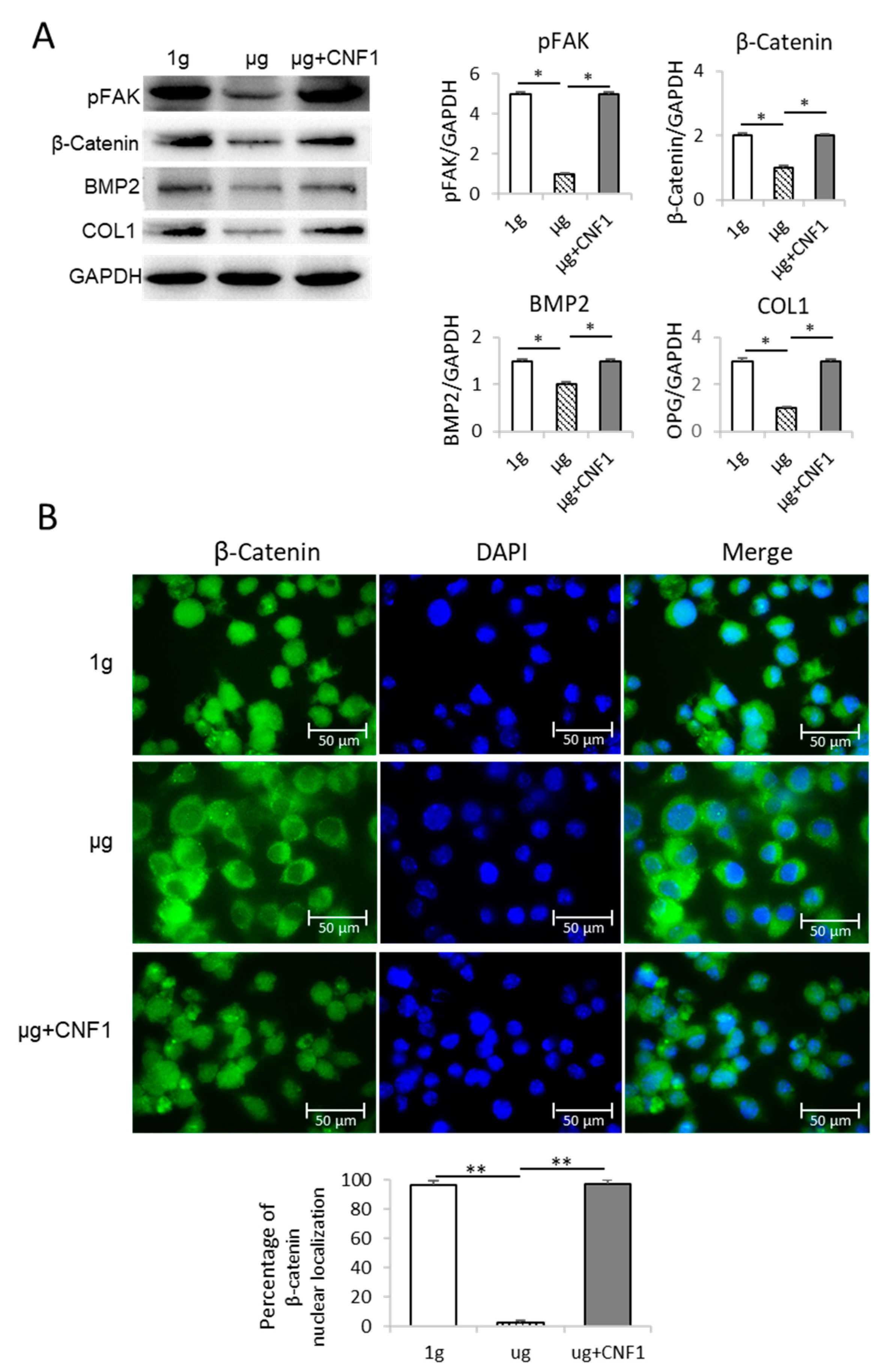
2.2. Simulated Microgravity Inhibits FAK Signaling
2.3. Simulated Microgravity Suppresses Expression of Wnt/β-catenin, BMP2 and COL1
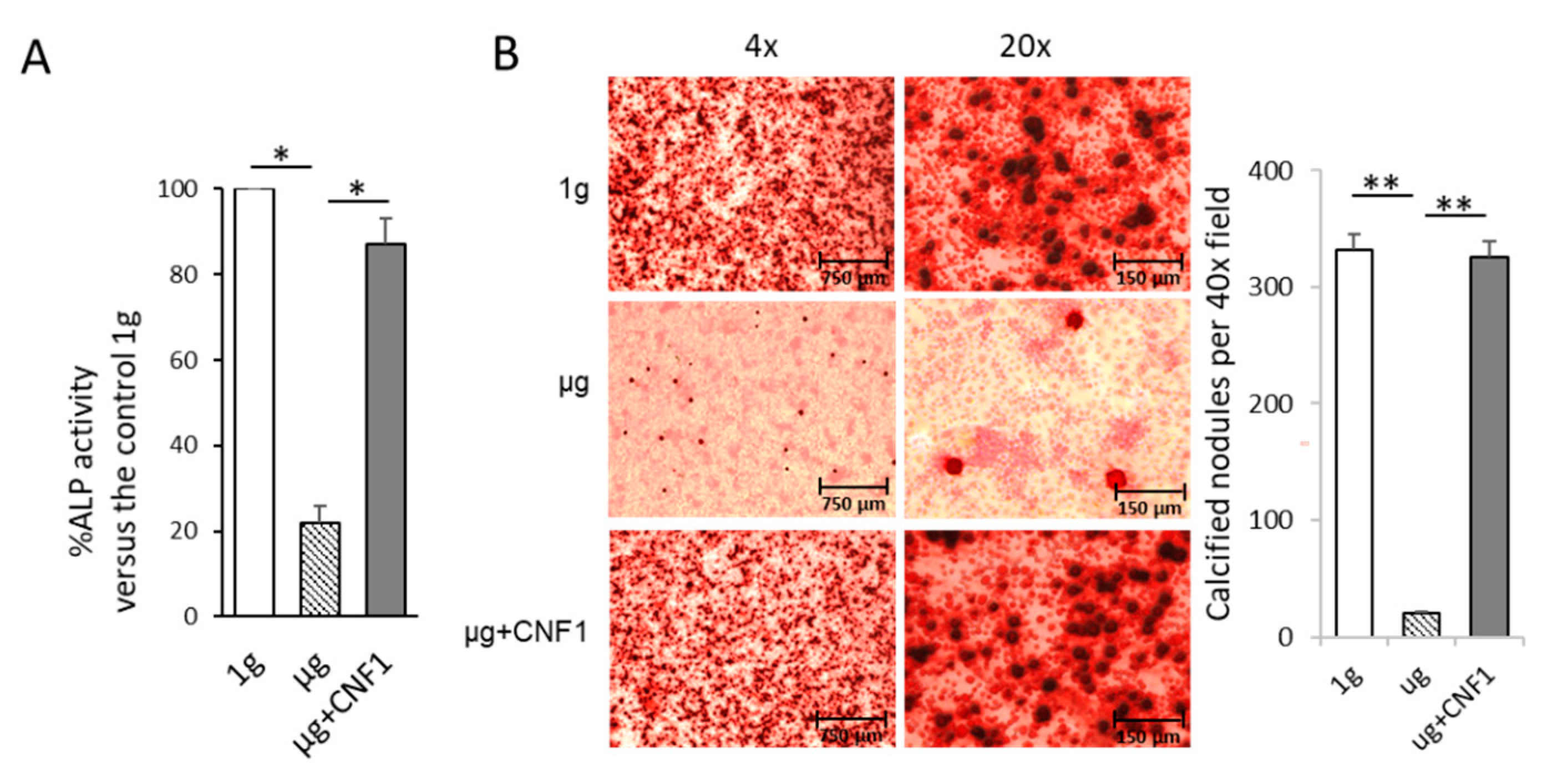
2.4. Simulated Microgravity Reduces ALP Activity and Matrix Mineralization
2.5. CNF1 Restores SMG-Induced Alterations in Cytoskeleton Structure, Focal Adhesions, Gene Expression and Osteoblast Differentiation/Maturation
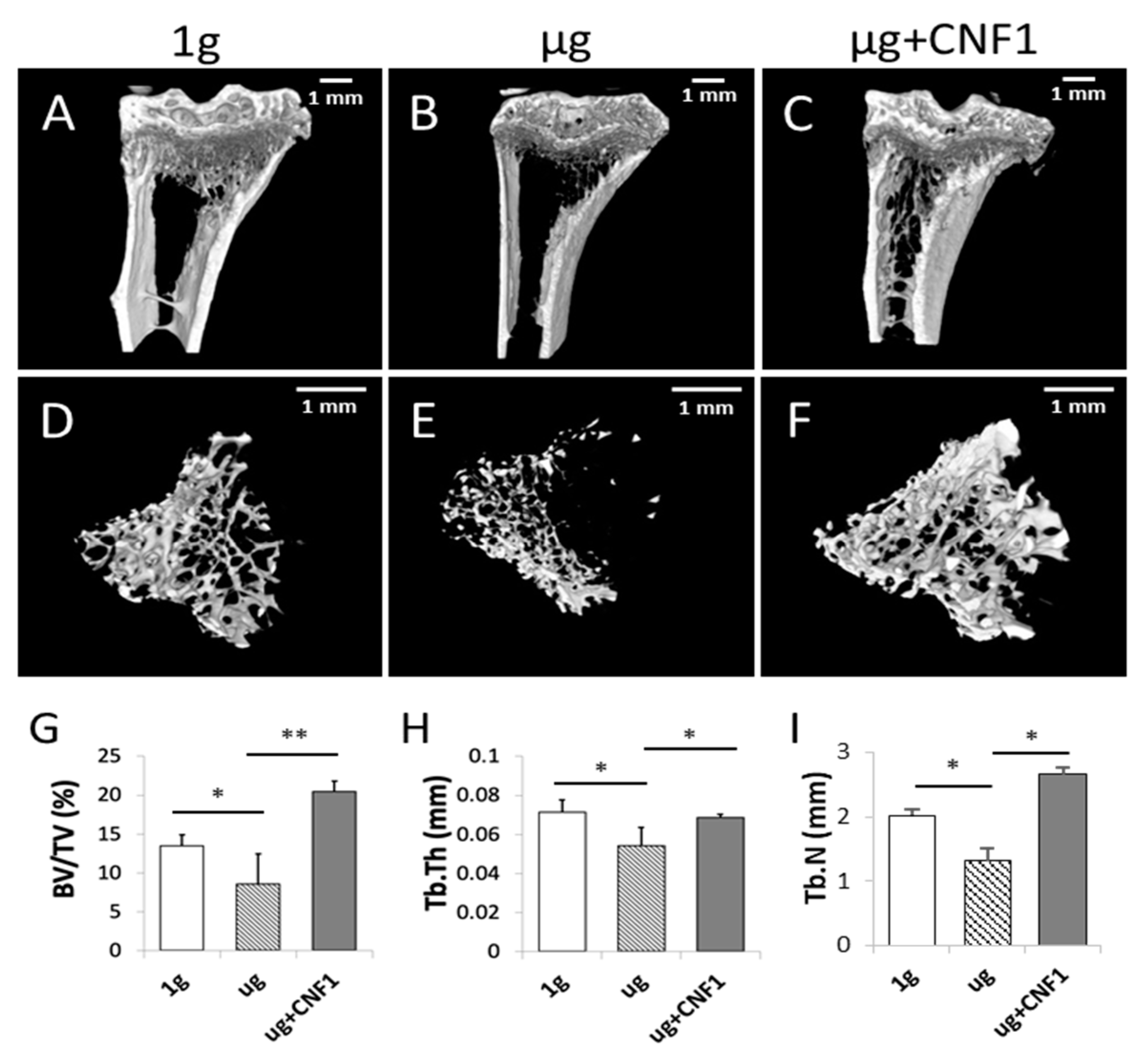
2.6. HU Induces Bone Density Reduction and Bone Loss in HU-Treated Mice
2.7. CNF1 Rescues HU-Induced Bone Density Reduction and Bone Loss
3. Discussion
4. Materials and Methods
4.1. Cells, Antibodies and Reagents
4.2. Clinostat of Simulated Microgravity and Cell Culture
4.3. Fluorescent Microscopy
4.4. Western Blotting Analysis
4.5. Confocal Microscopy
4.6. ALP Activity Assay
4.7. Mineralization
4.8. Hindlimb Unloading
4.9. Micro-CT Imaging
4.10. Histology
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| AMG | aerospace microgravity |
| AMPK, AMP | activated protein kinase |
| BMP2 | bone morphogenic protein-2 |
| BSA | bovine serum albumin |
| CNF1 | cytotoxic necrotizing factor-1 |
| COL1 | type-1 collagen |
| ERK1/2 | extracellular signal-regulated kinase-1/2 |
| FAK | focal adhesion kinase |
| FA | focal adhesion |
| FACs | focal adhesion complex |
| FITC | fluorescein isothiocyanate |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GSK3β | glycogen synthase kinase-3β |
| HU | hindlimb unloading |
| IAP | intracellular adaptor protein |
| IGF1 | insulin-like growth factor-1 |
| IGF1R, IGF1 | receptor |
| LEF | lymphoid enhancer binding factor |
| Micro-CT | micro-computed tomography |
| mTORC1 | mammalian target of rapamycin complex-1 |
| OBD | osteoblast differentiation |
| pFAK | phosphor FAK |
| PVDF | polyninylidene fluoride |
| SMG | simulated microgravity |
| TCF1 | T cell factor-1 |
References
- Williams, D.; Kuipers, A.; Mukai, C.; Thirsk, R. Acclimation during space flight: Effects on human physiology. CMAJ 2009, 180, 1317–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juhl, O.J.t.; Buettmann, E.G.; Friedman, M.A.; DeNapoli, R.C.; Hoppock, G.A.; Donahue, H.J. Update on the effects of microgravity on the musculoskeletal system. NPJ Microgravity 2021, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt pathway in bone repair and regeneration-what do we know so far. Front. Cell Dev. Biol. 2018, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef]
- Enzo, M.V.; Rastrelli, M.; Rossi, C.R.; Hladnik, U.; Segat, D. The Wnt/beta-catenin pathway in human fibrotic-like diseases and its eligibility as a therapeutic target. Mol. Cell Ther. 2015, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Xu, Z.; Duan, C.; Liu, W.; Sun, J.; Han, B. Role of TCF/LEF transcription factors in bone development and osteogenesis. Int. J. Med. Sci. 2018, 15, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Gioia, M.; Michaletti, A.; Scimeca, M.; Marini, M.; Tarantino, U.; Zolla, L.; Coletta, M. Simulated microgravity induces a cellular regression of the mature phenotype in human primary osteoblasts. Cell Death Discov. 2018, 4, 59. [Google Scholar] [CrossRef] [Green Version]
- Wickstead, B.; Gull, K. The evolution of the cytoskeleton. J. Cell Biol. 2011, 194, 513–525. [Google Scholar] [CrossRef]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef]
- Legerstee, K.; Houtsmuller, A.B. A Layered View on Focal Adhesions. Biology 2021, 10, 1189. [Google Scholar] [CrossRef]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, R.; Luo, M.; Mo, X.; Lu, J.; Yeo, S.K.; Guan, J.L. FAK activates AKT-mTOR signaling to promote the growth and progression of MMTV-Wnt1-driven basal-like mammary tumors. Breast Cancer Res. 2020, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Khoe, M.; Befekadu, M.; Chung, S.; Takata, Y.; Ilic, D.; Bryer-Ash, M. Focal adhesion kinase mediates cell survival via NF-kappaB and ERK signaling pathways. Am. J. Physiol. Cell Physiol. 2007, 292, C1339–C1352. [Google Scholar] [CrossRef]
- Deng, B.; Liu, R.; Tian, X.; Han, Z.; Chen, J. Simulated microgravity inhibits the viability and migration of glioma via FAK/RhoA/Rock and FAK/Nek2 signaling. Vitr. Cell. Dev. Biol. Anim. 2019, 55, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.B.; Souza, A.T.P.; Freitas, G.P.; Elias, C.N.; Rosa, A.L.; Beloti, M.M. Effect of focal adhesion kinase inhibition on osteoblastic cells grown on titanium with different topographies. J. Appl. Oral Sci. 2020, 28, e20190156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Xu, A.; Zhao, T.; Zhao, Q.; Zhang, J.; Fan, C.; Deng, Y.; Freywald, A.; Genth, H.; Xiang, J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via FAK/RhoA-regulated mTORC1 and AMPK pathways. Sci. Rep. 2018, 8, 3769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.; Li, R.; Tan, X.; Zhang, J.; Fan, C.; Zhao, Q.; Deng, Y.; Xu, A.; Lukong, K.E.; Genth, H.; et al. Simulated microgravity reduces focal adhesions and alters cytoskeleton and nuclear positioning leading to enhanced apoptosis via suppressing FAK/RhoA-mediated mTORC1/NF-κB and ERK1/2 pathways. Int. J. Mol. Sci. 2018, 19, 1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.-G.; Lukong, K.E.; Xiang, J. A critical role of FAK/Rho signaling in simulated microgravity-altered cell apoptosis, rroliferation and metastasis. J. Cell Signal. 2018, 3, 3. [Google Scholar] [CrossRef]
- Shi, W.; Xie, Y.; He, J.; Zhou, J.; Gao, Y.; Wei, W.; Ding, N.; Ma, H.; Xian, C.J.; Chen, K.; et al. Microgravity induces inhibition of osteoblastic differentiation and mineralization through abrogating primary cilia. Sci. Rep. 2017, 7, 1866. [Google Scholar] [CrossRef]
- Xu, A.; Leary, S.C.; Islam, M.F.; Wu, Z.; Bhanumathy, K.K.; Ara, A.; Chibbar, R.; Fleywald, A.; Ahmed, K.A.; Xiang, J. Prosurvival IL-7-stimulated weak strength of mTORC1-S6K controls T cell memory via transcriptional FOXO1-TCF1-Id3 and metabolic AMPKalpha1-ULK1-ATG7 pathways. J. Immunol. 2022, 208, 155–168. [Google Scholar] [CrossRef]
- Ara, A.; Xu, A.; Ahmed, K.A.; Leary, S.C.; Islam, M.F.; Wu, Z.; Chibbar, R.; Xiang, J. The energy sensor AMPKα1 is critical in rapamycin-inhibition of mTORC1-S6K-induced T-cell memory. Int. J. Mol. Sci. 2022, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Suzuki, K.; Koshikawa, M.; Ando, M.; Iida, J. Necessity of enzymatic activity of alkaline phosphatase for mineralization of osteoblastic cells. Jpn. J. Pharmacol. 2002, 88, 262–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travaglione, S.; Loizzo, S.; Ballan, G.; Fiorentini, C.; Fabbri, A. The E. coli CNF1 as a pioneering therapy for the central nervus system diseases. Toxin 2014, 6, 270–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre, J.I.; Plotkin, L.I.; Stewart, S.A.; Weinstein, R.S.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 2006, 21, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Zhang, Y.; Wayne, J.S.; Farber, C.R.; Donahue, H.J. Single limb immobilization model for bone loss from unloading. J. Biomech. 2019, 83, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bullock, W.A.; Hoggatt, A.M.; Horan, D.J.; Lewis, K.J.; Yokota, H.; Hann, S.; Warman, M.L.; Sebastian, A.; Loots, G.G.; Pavalko, F.M.; et al. Expression of a degradation-resistant beta-Catenin mutant in osteocytes protects the skeleton from mechanodeprivation-induced bone wasting. J. Bone Miner. Res. 2019, 34, 1964–1975. [Google Scholar] [CrossRef]
- Fabbri, A.; Travaglione, S.; Fiorentini, C. Escherichia coli cytotoxic necrotizing factor 1 (CNF1): Toxin biology, in vivo applications and therapeutic potential. Toxins 2010, 2, 283–296. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Hiraiwa, M.; Ozaki, K.; Yamada, T.; Iezaki, T.; Park, G.; Fukasawa, K.; Horie, T.; Kamada, H.; Tokumura, K.; Motono, M.; et al. mTORC1 activation in osteoclasts prevents bone loss in a mouse model of osteoporosis. Front. Pharmacol. 2019, 10, 684. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, B.M.; Alzahrani, A.M.; Kassem, M. Secreted Clusterin protein inhibits osteoblast differentiation of bone marrow mesenchymal stem cells by suppressing ERK1/2 signaling pathway. Bone 2018, 110, 221–229. [Google Scholar] [CrossRef]
- Li, Y.; Su, J.; Sun, W.; Cai, L.; Deng, Z. AMP-activated protein kinase stimulates osteoblast differentiation and mineralization through autophagy induction. Int. J. Mol. Med. 2018, 41, 2535–2544. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, K.; Luo, M.; Wang, X.; Zhu, X.; Liu, X.; Jiang, T.; Zhang, Q.; Wang, S.; Pang, Z. Size dependency of circulation and biodistribution of biomimetic nanoparticles: Red blood cell membrane-coated nanoparticles. Cells 2019, 8, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Liu, X.; Wang, J.; Chen, X.; Zhang, H.; Kim, S.H.; Cui, J.; Li, R.; Zhang, W.; Kong, Y.; et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther. Adv. Musculoskelet Dis. 2013, 5, 13–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. The skeletal-protecting action and mechanisms of action for mood-stabilizing drug lithium chloride: Current evidence and future potential research areas. Front. Pharmacol. 2020, 11, 430. [Google Scholar] [CrossRef]
- Rybakowski, J.K. Challenging the negative perception of lithium and optimizing its long-term administration. Front. Mol. Neurosci. 2018, 11, 349. [Google Scholar] [CrossRef]
- Kawai, M.; Rosen, C.J. Insulin-like growth factor-I and bone: Lessons from mice and men. Pediatr. Nephrol. 2009, 24, 1277–1285. [Google Scholar] [CrossRef]
- Govoni, K.E. Insulin-like growth factor-I molecular pathways in osteoblasts: Potential targets for pharmacological manipulation. Curr. Mol. Pharmacol. 2012, 5, 143–152. [Google Scholar] [CrossRef]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Wang, Y.; Bikle, D.D. IGF-1 signaling mediated cell-specific skeletal mechano-transduction. J. Orthop. Res. 2018, 36, 576–583. [Google Scholar] [CrossRef]
- Bateman, T.A.; Zimmerman, R.J.; Ayers, R.A.; Ferguson, V.L.; Chapes, S.K.; Simske, S.J. Histomorphometric, physical, and mechanical effects of spaceflight and insulin-like growth factor-I on rat long bones. Bone 1998, 23, 527–535. [Google Scholar] [CrossRef]
- Misra, M.; McGrane, J.; Miller, K.K.; Goldstein, M.A.; Ebrahimi, S.; Weigel, T.; Klibanski, A. Effects of rhIGF-1 administration on surrogate markers of bone turnover in adolescents with anorexia nervosa. Bone 2009, 45, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backeljauw, P. Therapy with recombinant human IGF-1 for children with primary insulin-like growth factor-I deficiency. Growth Horm IGF Res. 2020, 51, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Teruel, T.; Valverde, A.M.; Benito, M.; Lorenzo, M. Insulin-like growth factor I and insulin induce adipogenic-related gene expression in fetal brown adipocyte primary cultures. Biochem. J. 1996, 319 Pt 2, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Friedlander, A.L.; Butterfield, G.E.; Moynihan, S.; Grillo, J.; Pollack, M.; Holloway, L.; Friedman, L.; Yesavage, J.; Matthias, D.; Lee, S.; et al. One year of insulin-like growth factor I treatment does not affect bone density, body composition, or psychological measures in postmenopausal women. J. Clin. Endocrinol. Metab. 2001, 86, 1496–1503. [Google Scholar]
- Yao, S.; Zhu, Y.; Chen, L. Advances in targeting cell surface signaling molecules for immune modulation. Nat. Rev. Drug Discov. 2013, 12, 130–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, J.L.; Souza, G.R. Using space-based investigations to inform cancer research on Earth. Nat. Rev. Cancer 2013, 13, 315–327. [Google Scholar] [CrossRef]
- Tantillo, E.; Colistra, A.; Vannini, E.; Cerri, C.; Pancrazi, L.; Baroncelli, E.; Costa, M.; Caleo, M. Bacterial toxins and targeted brain therapy: New insights from cytotoxic necrotizing factor 1 (CNF1). Int. J. Mol. Sci. 2018, 19, 1632. [Google Scholar] [CrossRef] [Green Version]
- May, M.; Kolbe, T.; Wang, T.; Schmidt, G.; Genth, H. Increased cell-matrix adhesion upon constitutive activation of Rho proteins by cytotoxic necrotizing factors from E. Coli and Y. Pseudotuberculosis. J. Signal. Transduct. 2012, 2012, 570183. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Cheng, Y.; Wang, J.; Ding, Z.; Halim, A.; Luo, Q.; Song, G. Simulated microgravity suppresses osteogenic differentiation of mesenchymal stem cells by inhibiting oxidative phosphorylation. Int. J. Mol. Sci. 2020, 21, 9747. [Google Scholar] [CrossRef]
- Moon, J.; Ko, H.; Park, J.; Kim, J.; Kim, S.; Kim, M. Inhibition of human mesenchymal stem cell proliferation via Wnt signaling activation. J. Cell Biochem. 2018, 119, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Meng, Z. Insulin growth factor-1 promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells through the Wnt/β-catenin pathway. Exp. Med. 2021, 22, 891. [Google Scholar] [CrossRef] [PubMed]
- Bradamante, S.; Rivero, D.; Barenghi, L.; Balsamo, M.; Milardi, S.P.; Vitali, F.; Cavaliri, D. SCD-stem cell differentiation toward osteoblast on board the international space station. Microgravity Sci. Technol. 2018, 30, 713–729. [Google Scholar] [CrossRef] [Green Version]
- Carlini, F.; Maroccia, Z.; Fiorentini, C.; Travaglione, S.; Fabbri, A. Effects if the Escherichia coli bacterial toxin cytotoxic necrotizing factor-1 on different human and animal cells. A systemic review. Int. J. Mol. Sci. 2021, 22, 12610. [Google Scholar] [CrossRef]
- Fiorentini, C.; Fabbri, A.; Flatau, G.; Donelli, G.; Matarrese, P.; Lemichez, E.; Falzano, L.; Hoquet, P. Escherichia coli cytotoxic necrotizing factor-1 (CNF1), a toxin that activates the Rho GTPase. J. Biol. Chem. 1997, 272, 19532–19537. [Google Scholar] [CrossRef] [Green Version]
- Gall-Mass, L.; Fabbri, A.; Namini, M.; Givskov, M.; Fiorentini, C.; Krejsgaard, T. The bacterial toxin CNF1 induces activation and maturation of human monocyte-derived dendritic cells. Int. J. Mol. Sci. 2018, 19, 1408. [Google Scholar] [CrossRef] [Green Version]
- Munro, P.; Flatau, G.; Lemichez, E. Intranasal immunization with tetanus toxoid and CNF1 as a new mucosal adjuvant protects BALB/c mice against lethal challenge. Vaccine 2007, 25, 8702–8706. [Google Scholar] [CrossRef]
- Nazemi, S.M.; Cooper, D.M.; Johnston, J.D. Quantifying trabecular bone material anisotropy and orientation using low resolution clinical CT images: A feasibility study. Med. Eng. Phys. 2016, 38, 978. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, C.; Wu, Z.; Cooper, D.M.L.; Magnus, A.; Harrison, K.; Eames, B.F.; Chibbar, R.; Groot, G.; Huang, J.; Genth, H.; et al. Activation of Focal Adhesion Kinase Restores Simulated Microgravity-Induced Inhibition of Osteoblast Differentiation via Wnt/Β-Catenin Pathway. Int. J. Mol. Sci. 2022, 23, 5593. https://doi.org/10.3390/ijms23105593
Fan C, Wu Z, Cooper DML, Magnus A, Harrison K, Eames BF, Chibbar R, Groot G, Huang J, Genth H, et al. Activation of Focal Adhesion Kinase Restores Simulated Microgravity-Induced Inhibition of Osteoblast Differentiation via Wnt/Β-Catenin Pathway. International Journal of Molecular Sciences. 2022; 23(10):5593. https://doi.org/10.3390/ijms23105593
Chicago/Turabian StyleFan, Cuihong, Zhaojia Wu, David M. L. Cooper, Adam Magnus, Kim Harrison, B. Frank Eames, Rajni Chibbar, Gary Groot, Junqiong Huang, Harald Genth, and et al. 2022. "Activation of Focal Adhesion Kinase Restores Simulated Microgravity-Induced Inhibition of Osteoblast Differentiation via Wnt/Β-Catenin Pathway" International Journal of Molecular Sciences 23, no. 10: 5593. https://doi.org/10.3390/ijms23105593
APA StyleFan, C., Wu, Z., Cooper, D. M. L., Magnus, A., Harrison, K., Eames, B. F., Chibbar, R., Groot, G., Huang, J., Genth, H., Zhang, J., Tan, X., Deng, Y., & Xiang, J. (2022). Activation of Focal Adhesion Kinase Restores Simulated Microgravity-Induced Inhibition of Osteoblast Differentiation via Wnt/Β-Catenin Pathway. International Journal of Molecular Sciences, 23(10), 5593. https://doi.org/10.3390/ijms23105593





