Edible Bioactive Film with Curcumin: A Potential “Functional” Packaging?
Abstract
:1. Introduction
2. Functional-Edible-Films: A New Concept in Food Packaging
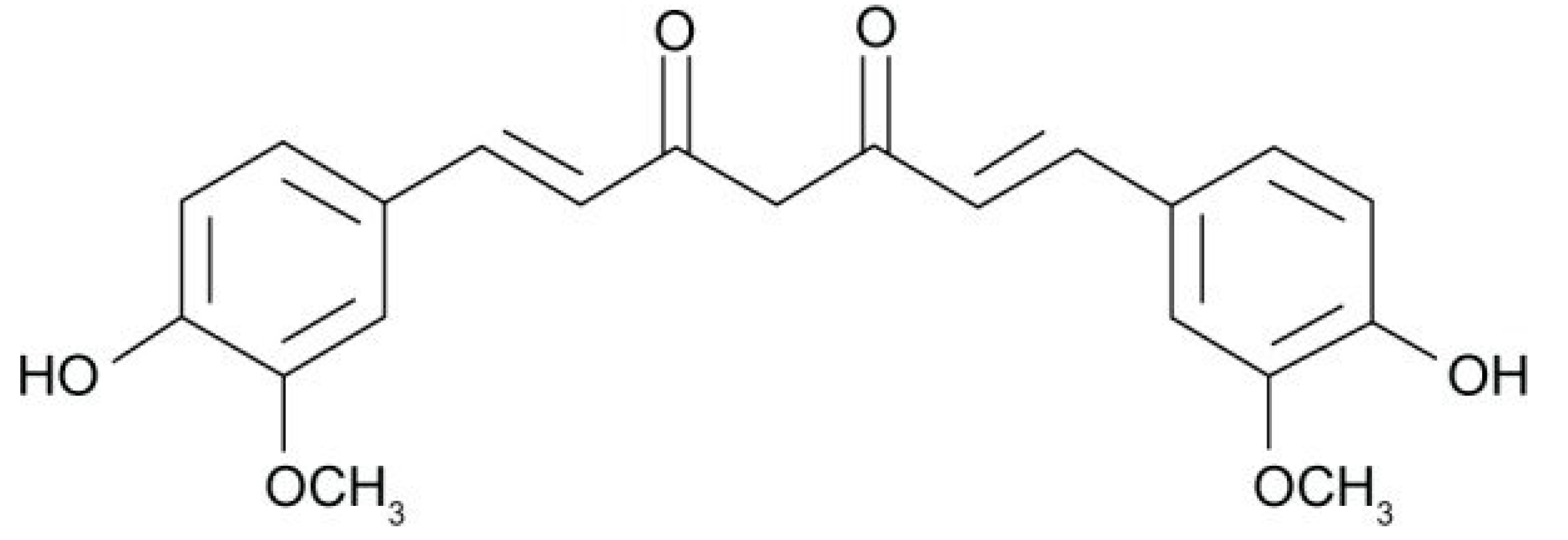
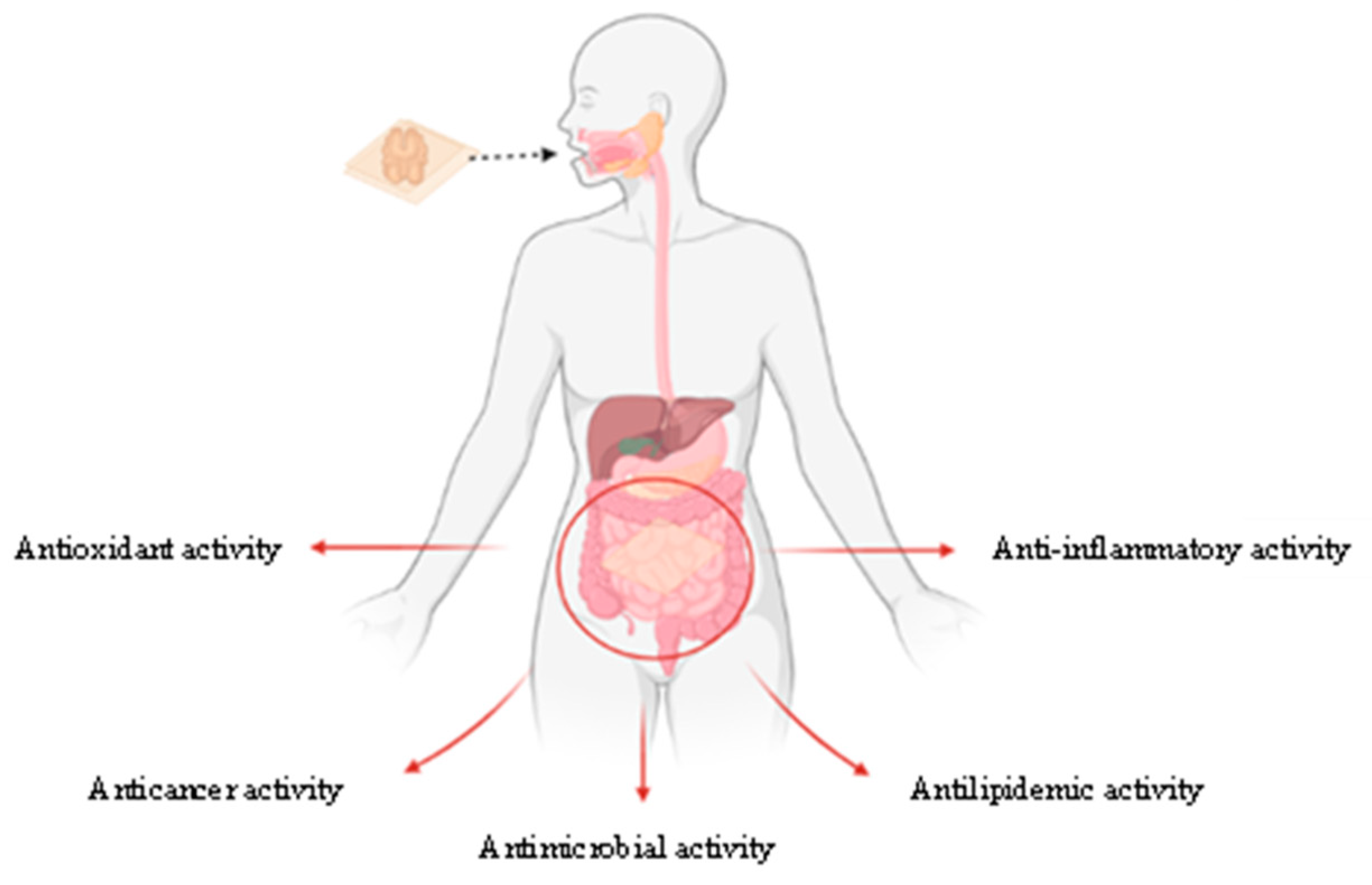
3. Curcumin: A Bioactive Molecule with Potential for Edible Bioactive Film Production
4. Encapsulation of Curcumin for Application as a Bioactive Agent
5. Functional-Edible-Films Containing Curcumin
| Matrix | Effects/Results | Reference |
|---|---|---|
| Poly (lactic acid) | Positively affected: mechanical and UV-barrier properties and antioxidant activity.Negatively affected: contact angle and water vapor permeability. | Akhtar et al. [98] |
| Soy protein isolate | Positively affected: color and antioxidant activity. | Xiao et al. [88] |
| Carboxymethyl cellulose | Positively affected: UV-barrier properties and antioxidant activity.Negatively affected: mechanical proprieties and transparency | Roy and Rhim et al. [93] |
| Poly (lactic acid)/sodium carboxymethyl cellulose | Positively affected: solubility and elongation at breakNegatively affected: tensile strength | Gunathilake et al. [92] |
| Alginate or carrageenan | Lipid digestion and curcumin release was retarded upon encapsulation. | Zhang et al. [93] |
| Banana starch | Positively affected: water vapor permeability, and elongation at break. | Sanchez et al. [99] |
| Gelatin | Positively affected: antioxidant activity. | Musso, Salgado and Mauri [15] |
| Mucilage of Melissa officinalis seed/montmorillonite | Positively affected: antimicrobial activity. | Rostami and Esfahani [95] |
| Mucilage of Lallemantia iberica seed | Positively affected: water vapor permeability, antioxidant, and antimicrobial activities. | Taghinia, Abdolshahi, Sedaghati and Shokrollahi [96] |
| Carboxymethylated guar gum grafted gelatin | Positively affected: antimicrobial activity. | Manna, Mitra, Pramanik, Kavitha, Gnanamani, and Kundu [97] |
| Chitosan | Positively affected: yellowness, light barriers, moisture content, water solubility, water vapor permeability, and antioxidant activity. | Rachtanapun et al. [100] |
| Chitosan nanoparticles | Positively affected: antioxidant activity and inhibit lipid oxidation of fresh pork. | Shen et al. [101] |
| Cellulose nanofibers/chitosan | Positively affected: edible coating materials were effective in reducing mass loss, firmness loss, respiration rate, and microbial count of the kiwifruits during storage life. | Ghosh et al. [102] |
| Carboxymethylcellulose | Negatively affected: water vapor permeability and tensile strength. | Bourbon et al. [103] |
| Alginate | Positively affected: antioxidant activity and inhibit lipid oxidation of fresh pork, beef, and chicken. | Bojorges, Ríos-Corripio, Hernández-Cázares, Hidalgo-Contreras, and Contreras-Oliva [26] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliveira Filho, J.D.; Albiero, B.; Cipriano, L.; Bezerra, C.D.O.; Oldoni, F.; Egea, M.; de Azeredo, H.; Ferreira, M. Arrowroot starch-based films incorporated with a carnauba wax nanoemulsion, cellulose nanocrystals, and essential oils: A new functional material for food packaging applications. Cellulose 2021, 28, 6499–6511. [Google Scholar] [CrossRef]
- Ncube, L.; Ude, A.; Ogunmuyiwa, E.; Na, C.-S.; Zulkifli, R.; Beas, I. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Filho, J.; Rodrigues, J.; Valadares, A.; Almeida, A.; Lima, T.; Takeuchi, K.; Alves, C.; Sousa, H.; Silva, E.; Dyszy, F.; et al. Active food packaging: Alginate films with cottonseed protein hydrolysates. Food Hydrocoll. 2019, 92, 267–275. [Google Scholar] [CrossRef]
- Maan, A.A.; Ahmed, Z.F.R.; Khan, M.K.I.; Riaz, A.; Nazir, A. Aloe vera gel, an excellent base material for edible films and coatings. Trends Food Sci. Technol. 2021, 116, 329–341. [Google Scholar] [CrossRef]
- Viana, R.M.; Sá, N.M.; Barros, M.O.; de Fátima Borges, M.; Azeredo, H.M. Nanofibrillated bacterial cellulose and pectin edible films added with fruit purees. Carbohydr. Polym. 2018, 196, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Espitia, P.J.; Batista, R.A.; Azeredo, H.M.; Otoni, C.G. Probiotics and their potential applications in active edible films and coatings. Food Res. Int. 2016, 90, 42–52. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Filho, J.G.; de Almeida, M.J.; Sousa, T.; de Santos, D.; Egea, M. Bioactive Compounds of Turmeric (Curcuma longa L.). Bioact. Compd. Underutilized Veg. Legumes 2021, 297–318. [Google Scholar]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT 2022, 153, 112527. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusi, F.; Trezza, A.; Tramaglino, M.; Sgaragli, G.; Saponara, S.; Spiga, O. The beneficial health effects of flavonoids on the cardiovascular system: Focus on K+ channels. Pharmacol. Res. 2020, 152, 104625. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [Green Version]
- Khamrai, M.; Banerjee, S.; Paul, S.; Samanta, S.; Kundu, P. Curcumin entrapped gelatin/ionically modified bacterial cellulose based self-healable hydrogel film: An eco-friendly sustainable synthesis method of wound healing patch. Int. J. Biol. Macromol. 2019, 122, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Musso, Y.; Salgado, P.; Mauri, A. Smart edible films based on gelatin and curcumin. Food Hydrocoll. 2017, 66, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Ren, Y.; Wang, L. Investigation of antioxidant activity and release kinetics of curcumin from tara gum/polyvinyl alcohol active film. Food Hydrocoll. 2017, 70, 286–292. [Google Scholar] [CrossRef]
- Wang, L.; Mu, R.-J.; Li, Y.; Lin, L.; Lin, Z.; Pang, J. Characterization and antibacterial activity evaluation of curcumin loaded konjac glucomannan and zein nanofibril films. LWT 2019, 113, 108293. [Google Scholar] [CrossRef]
- Kunwar, A.; Priyadarsini, K. Curcumin and its role in chronic diseases. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Gupta, S., Prasad, S., Aggarwal, B., Eds.; Springer: Cham, Germany, 2016; Volume 928, pp. 1–25. [Google Scholar]
- Munekata, P.; Pateiro, M.; Zhang, W.; Dominguez, R.; Xing, L.; Fierro, E.; Lorenzo, J. Health benefits, extraction and development of functional foods with curcuminoids. J. Funct. Foods 2021, 79, 104392. [Google Scholar] [CrossRef]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Wang, L.; Xue, J.; Zhang, Y. Preparation and characterization of curcumin loaded caseinate/zein nanocomposite film using pH-driven method. Ind. Crops Prod. 2019, 130, 71–80. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Chang, Q.; Zhang, Y.; Zheng, B.; Zeng, H. Structural and physicochemical properties of ginger (Rhizoma curcumae longae) starch and resistant starch: A comparative study. Int. J. Biol. Macromol. 2020, 144, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Curcumin and its uses in active and smart food packaging applications-A comprehensive review. Food Chem. 2021, 375, 131885. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Filho, J.; Bertolo, M.; Rodrigues, M.; Marangon, C.; da Cruz Silva, G.; Odoni, F.; Egea, M. Curcumin: A multifunctional molecule for the development of smart and active biodegradable polymer-based films. Trends Food Sci. Technol. 2021, 118, 840–849. [Google Scholar] [CrossRef]
- Tambawala, H.; Batra, S.; Shirapure, Y.; More, A.P. Curcumin-A Bio-based Precursor for Smart and Active Food Packaging Systems: A Review. J. Polym. Environ. 2022, 30, 2177–2208. [Google Scholar] [CrossRef]
- Bojorges, H.; Ríos-Corripio, M.; Hernández-Cázares, A.S.; Hidalgo-Contreras, J.V.; Contreras-Oliva, A. Effect of the application of an edible film with turmeric (Curcuma longa L.) on the oxidative stability of meat. Food Sci. Nutr. 2020, 8, 4308–4319. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Debeaufort, F. Bioactive edible films for food applications: Influence of the bioactive compounds on film structure and properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 1137–1153. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Immobilization of heteroatom-doped carbon dots onto nonpolar plastics for antifogging, antioxidant, and food monitoring applications. Langmuir 2021, 37, 3508–3520. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 116178. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Otoni, C.; Avena-Bustillos, R.; Azeredo, H.; Lorevice, M.; Moura, M.; Mattoso, L.; McHugh, T. Recent advances on edible films based on fruits and vegetables—A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, B.; Li, C.; Xu, Y.; Luo, Y.; Liang, D.; Huang, C. Comprehensive Review of Polysaccharide-Based Materials in Edible Packaging: A Sustainable Approach. Foods 2021, 10, 1845. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Filho, J.; Bezerra, C.; Albiero, B.; Oldoni, F.; Miranda, M.; Egea, M.; Azeredo, H.; Ferreira, M. New approach in the development of edible films: The use of carnauba wax micro- or nanoemulsions in arrowroot starch-based films. Food Packag. Shelf Life 2020, 26, 100589. [Google Scholar] [CrossRef]
- Ricaurte, L.; Santagapita, P.R.; Díaz, L.E.; Quintanilla-Carvajal, M.X. Edible gelatin-based nanofibres loaded with oil encapsulating high-oleic palm oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124673. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.; Mor, R.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of bioactive functional poly (lactic acid)/curcumin composite film for food packaging application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rubio, A.; Gavara, R.; Lagaron, J. Bioactive packaging: Turning foods into healthier foods through biomaterials. Trends Food Sci. Technol. 2006, 17, 567–575. [Google Scholar] [CrossRef]
- Arroyo, B.; Santos, A.; de Melo, E.D.A.; Campos, A.; Lins, L.; Boyano-Orozco, L. Bioactive compounds and their potential use as ingredients for food and its application in food packaging. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–156. [Google Scholar]
- Majid, I.; Nayik, G.A.; Dar, S.M.; Nanda, V. Novel food packaging technologies: Innovations and future prospective. J. Saudi Soc. Agric. Sci. 2018, 17, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Pavli, F.; Tassou, C.; Nychas, G.-J.; Chorianopoulos, N. Probiotic incorporation in edible films and coatings: Bioactive solution for functional foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Filho, J.; Braga, A.; de Oliveira, B.; Gomes, F.; Moreira, V.; Pereira, V.; Egea, M. The potential of anthocyanins in smart, active, and bioactive eco-friendly polymer-based films: A review. Food Res. Int. 2021, 142, 110202. [Google Scholar] [CrossRef]
- Zabihollahi, N.; Alizadeh, A.; Almasi, H.; Hanifian, S.; Hamishekar, H. Development and characterization of carboxymethyl cellulose based probiotic nanocomposite film containing cellulose nanofiber and inulin for chicken fillet shelf life extension. Int. J. Biol. Macromol. 2020, 160, 409–417. [Google Scholar] [CrossRef]
- Oliveira-Alcântara, A.V.; Abreu, A.A.S.; Gonçalves, C.; Fuciños, P.; Cerqueira, M.A.; Gama, F.M.; Pastrana, L.M.; Rodrigues, S.; Azeredo, H.M. Bacterial cellulose/cashew gum films as probiotic carriers. LWT 2020, 130, 109699. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Antioxidant and antimicrobial poly (vinyl alcohol)-based films incorporated with grapefruit seed extract and curcumin. J. Environ. Chem. Eng. 2021, 9, 104694. [Google Scholar] [CrossRef]
- Narasagoudr, S.S.; Hegde, V.G.; Chougale, R.B.; Masti, S.P.; Vootla, S.; Malabadi, R.B. Physico-chemical and functional properties of rutin induced chitosan/poly (vinyl alcohol) bioactive films for food packaging applications. Food Hydrocoll. 2020, 109, 106096. [Google Scholar] [CrossRef]
- Ounkaew, A.; Janaum, N.; Kasemsiri, P.; Okhawilai, M.; Hiziroglu, S.; Chindaprasirt, P. Synergistic effect of starch/polyvinyl alcohol/citric acid films decorated with in-situ green-synthesized nano silver on bioactive packaging films. J. Environ. Chem. Eng. 2021, 9, 106793. [Google Scholar] [CrossRef]
- Hiremani, V.D.; Khanapure, S.; Gasti, T.; Goudar, N.; Vootla, S.K.; Masti, S.P.; Malabadi, R.B.; Mudigoudra, B.S.; Chougale, R.B. Preparation and physicochemical assessment of bioactive films based on chitosan and starchy powder of white turmeric rhizomes (Curcuma Zedoaria) for green packaging applications. Int. J. Biol. Macromol. 2021, 193, 2192–2201. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Dong, M.; Zhang, H.; Li, L.; Wang, L. Food spoilage, bioactive food fresh-keeping films and functional edible coatings: Research status, existing problems and development trend. Trends Food Sci. Technol. 2022, 119, 122–132. [Google Scholar] [CrossRef]
- Galanakis, C. Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques; Academic Press: London, UK, 2016. [Google Scholar]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives–A review. J. Tradit. Complementary Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [Green Version]
- Elahi, M.; Heli, H.; Bathaie, S.; Mousavi, M. Electrocatalytic oxidation of glucose at a Ni-curcumin modified glassy carbon electrode. J. Solid State Electrochem. 2007, 11, 273–282. [Google Scholar] [CrossRef]
- Halevas, E.; Arvanitidou, M.; Mavroidi, B.; Hatzidimitriou, A.; Politopoulos, K.; Alexandratou, E.; Pelecanou, M.; Sagnou, M. A novel curcumin gallium complex as photosensitizer in photodynamic therapy: Synthesis, structural and physicochemical characterization, photophysical properties and in vitro studies against breast cancer cells. J. Mol. Struct. 2021, 1240, 130485. [Google Scholar] [CrossRef]
- Li, H.; Sureda, A.; Devkota, H.; Pittalà, V.; Barreca, D.; Silva, A.; Tewari, D.; Xu, S.; Nabavi, S. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef]
- Malik, P.; Mukherjee, T. Structure-function elucidation of antioxidative and prooxidative activities of the polyphenolic compound curcumin. Chin. J. Biol. 2014, 2014, 396708. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsini, K.; Maity, D.; Naik, G.; Kumar, M.; Unnikrishnan, M.; Satav, J.; Mohan, H. Role of phenolic OH and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free. Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.; Thomas, C.; Ristow, M. Interrelation between ROS and Ca2+ in aging and age-related diseases. Redox Biol. 2020, 36, 101678. [Google Scholar] [CrossRef]
- Calabrese, E.; Kozumbo, W. The hormetic dose-response mechanism: Nrf2 activation. Pharmacol. Res. 2021, 167, 105526. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Rizvi, S. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Wlaschek, M.; Scharffetter-Kochanek, K. Pathogenesis of wound healing disorders in the elderly. JDDG J. Dtsch. Dermatol. Ges. 2017, 15, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Dairaku, I.; Han, Y.; Yanaka, N.; Kato, N. Inhibitory effect of curcumin on IMP dehydrogenase, the target for anticancer and antiviral chemotherapy agents. Biosci. Biotechnol. Biochem. 2010, 74, 185–187. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.; Gupta, S.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [Green Version]
- WHO. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 20 January 2022).
- Pourbagher-Shahri, A.; Farkhondeh, T.; Ashrafizadeh, M.; Talebi, M.; Samargahndian, S. Curcumin and cardiovascular diseases: Focus on cellular targets and cascades. Biomed. Pharmacother. 2021, 136, 111214. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Anaparti, V.; El-Gabalawy, H.; Mookherjee, N. A bioavailable form of curcumin, in combination with vitamin-D-and omega-3-enriched diet, modifies disease onset and outcomes in a murine model of collagen-induced arthritis. Arthritis Res. Ther. 2021, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Lankarani, K.; Tabrizi, R.; Ghayour-Mobarhan, M.; Peymani, P.; Ferns, G.; Ghaderi, A.; Asemi, Z. The effects of curcumin on weight loss among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2019, 10, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safarian, H.; Parizadeh, S.; Saberi-Karimain, M.; Darroudi, S.; Javandoost, A.; Mohammadi, F.; Moammeri, M.; Ferns, G.; Ghayour-Mobarhan, M.; Mohebati, M. The effect of curcumin on serum copper and zinc and Zn/Cu ratio in individuals with metabolic syndrome: A double-blind clinical trial. J. Diet. Suppl. 2019, 16, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Cheng, J.; Zheng, S.; Feng, Q.; Xiao, X. Curcumin, a polyphenolic curcuminoid with its protective effects and molecular mechanisms in diabetes and diabetic cardiomyopathy. Front. Pharmacol. 2018, 9, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assis, R.; Arcaro, C.; Gutierres, V.; Oliveira, J.; Costa, P.; Baviera, A.; Brunetti, I. Combined effects of curcumin and lycopene or bixin in yoghurt on inhibition of LDL oxidation and increases in HDL and paraoxonase levels in streptozotocin-diabetic rats. Int. J. Mol. Sci. 2017, 18, 332. [Google Scholar] [CrossRef] [Green Version]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Aghadavod, E.; Shafabakhsh, R.; Hoseini, A.; Asemi, Z. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2020, 36, 128–133. [Google Scholar] [CrossRef]
- Tomeh, M.; Hadianamrei, R.; Zhao, X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [Green Version]
- CDC. Mental and Chronic Diseases. Available online: https://www.cdc.gov/chronicdisease/index.htm (accessed on 20 January 2022).
- Ramaholimihaso, T.; Bouazzaoui, F.; Kaladjian, A. Curcumin in Depression: Potential Mechanisms of Action and Current Evidence—A Narrative Review. Front. Psychiatry 2020, 11, 572533. [Google Scholar] [CrossRef]
- Vareed, S.; Kakarala, M.; Ruffin, M.; Crowell, J.; Normolle, D.; Djuric, Z.; Brenner, D. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Prev. Biomark. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [Green Version]
- Dei Cas, M.; Ghidoni, R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanidad, K.; Sukamtoh, E.; Xiao, H.; McClements, D.; Zhang, G. Curcumin: Recent advances in the development of strategies to improve oral bioavailability. Annu. Rev. Food Sci. Technol. 2019, 10, 597–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- D’Angelo, N.A.; Noronha, M.A.; Kurnik, I.S.; Câmara, M.C.; Vieira, J.M.; Abrunhosa, L.; Martins, J.T.; Alves, T.F.; Tundisi, L.L.; Ataide, J.A. Curcumin encapsulation in nanostructures for cancer therapy: A 10-year overview. Int. J. Pharm. 2021, 604, 120534. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A promising candidate for therapeutic applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Valencia, L.; Nomena, E.; Mathew, A.; Velikov, K. Biobased cellulose nanofibril–oil composite films for active edible barriers. ACS Appl. Mater. Interfaces 2019, 11, 16040–16047. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, X.; Peng, S.; McClements, D. Impact of curcumin delivery system format on bioaccessibility: Nanocrystals, nanoemulsion droplets, and natural oil bodies. Food Funct. 2019, 10, 4339–4349. [Google Scholar] [CrossRef]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D. Enhancement of curcumin bioavailability by encapsulation in sophorolipid-coated nanoparticles: An in vitro and in vivo study. J. Agric. Food Chem. 2018, 66, 1488–1497. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.; Ji, N.; Dai, L.; Xiong, L.; Sun, Q. Acetylated debranched starch micelles as a promising nanocarrier for curcumin. Food Hydrocoll. 2021, 111, 106253. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Sipoli, C.C.; de La Torre, L.G.; López-Rubio, A. Microencapsulation structures based on protein-coated liposomes obtained through electrospraying for the stabilization and improved bioaccessibility of curcumin. Food Chem. 2017, 233, 343–350. [Google Scholar] [CrossRef]
- Aditya, N.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D. Impact of delivery system type on curcumin bioaccessibility: Comparison of curcumin-loaded nanoemulsions with commercial curcumin supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion-and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Shah, B.R.; Zhang, C.; Li, Y.; Li, B. Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and Pickering emulsion based on chitosan-tripolyphosphate nanoparticles. Food Res. Int. 2016, 89, 399–407. [Google Scholar] [CrossRef]
- Pan, K.; Luo, Y.; Gan, Y.; Baek, S.; Zhong, Q. pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity. Soft Matter 2014, 10, 6820–6830. [Google Scholar] [CrossRef]
- de Campos, A.; Claro, P.C.; Luchesi, B.R.; Miranda, M.; Souza, F.V.; Ferreira, M.D.; Marconcini, J.M. Curaua cellulose sheets dip coated with micro and nano carnauba wax emulsions. Cellulose 2019, 26, 7983–7993. [Google Scholar] [CrossRef]
- Kevij, H.; Salami, M.; Mohammadian, M.; Khodadadi, M. Fabrication and investigation of physicochemical, food simulant release, and antioxidant properties of whey protein isolate-based films activated by loading with curcumin through the pH-driven method. Food Hydrocoll. 2020, 108, 106026. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Cui, M.; Xu, H. Development of pH-responsive antioxidant soy protein isolate films incorporated with cellulose nanocrystals and curcumin nanocapsules to monitor shrimp freshness. Food Hydrocoll. 2021, 120, 106893. [Google Scholar] [CrossRef]
- Rostami, H.; Esfahani, A.A. Development a smart edible nanocomposite based on mucilage of Melissa officinalis seed/montmorillonite (MMT)/curcumin. Int. J. Biol. Macromol. 2019, 141, 171–177. [Google Scholar] [CrossRef]
- Taghinia, P.; Abdolshahi, A.; Sedaghati, S.; Shokrollahi, B. Smart edible films based on mucilage of lallemantia iberica seed incorporated with curcumin for freshness monitoring. Food Sci. Nutr. 2021, 9, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.J.; Mitra, T.; Pramanik, N.; Kavitha, V.; Gnanamani, A.; Kundu, P.P. Potential use of curcumin loaded carboxymethylated guar gum grafted gelatin film for biomedical applications. Int. J. Biol. Macromol. 2015, 75, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Jacquot, M.; Jasniewski, J.; Jacquot, C.; Imran, M.; Jamshidian, M.; Paris, C.; Desobry, S. Antioxidant capacity and light-aging study of HPMC films functionalized with natural plant extract. Carbohydr. Polym. 2012, 89, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of active edible films made from banana starch and curcumin-loaded nanoemulsions. Food Chem. 2022, 371, 131121. [Google Scholar] [CrossRef] [PubMed]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S. Characterization of chitosan film incorporated with curcumin extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Yan, M.; Wu, S.; Ge, X.; Liu, S.; Du, Y.; Zheng, Y.; Wu, L.; Zhang, Y.; Mao, Y. Chitosan nanoparticles embedded with curcumin and its application in pork antioxidant edible coating. Int. J. Biol. Macromol. 2022, 204, 410–418. [Google Scholar] [CrossRef]
- Ghosh, T.; Nakano, K.; Katiyar, V. Curcumin doped functionalized cellulose nanofibers based edible chitosan coating on kiwifruits. Int. J. Biol. Macromol. 2021, 184, 936–945. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Costa, M.J.; Maciel, L.C.; Pastrana, L.; Vicente, A.A.; Cerqueira, M.A. Active carboxymethylcellulose-based edible films: Influence of free and encapsulated curcumin on films’ properties. Foods 2021, 10, 1512. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [Green Version]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunathilake, T.; Ching, Y.; Chuah, C.; Abd Rahman, N.; Nai-Shang, L. pH-responsive poly (lactic acid)/sodium carboxymethyl cellulose film for enhanced delivery of curcumin in vitro. J. Drug Deliv. Sci. Technol. 2020, 58, 101787. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Zou, L.; Chen, L.; Ahmed, Y.; Al Bishri, W.; Balamash, K.; McClements, D. Encapsulation of curcumin in polysaccharide-based hydrogel beads: Impact of bead type on lipid digestion and curcumin bioaccessibility. Food Hydrocoll. 2016, 58, 160–170. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira Filho, J.G.; Egea, M.B. Edible Bioactive Film with Curcumin: A Potential “Functional” Packaging? Int. J. Mol. Sci. 2022, 23, 5638. https://doi.org/10.3390/ijms23105638
Oliveira Filho JG, Egea MB. Edible Bioactive Film with Curcumin: A Potential “Functional” Packaging? International Journal of Molecular Sciences. 2022; 23(10):5638. https://doi.org/10.3390/ijms23105638
Chicago/Turabian StyleOliveira Filho, Josemar Gonçalves, and Mariana Buranelo Egea. 2022. "Edible Bioactive Film with Curcumin: A Potential “Functional” Packaging?" International Journal of Molecular Sciences 23, no. 10: 5638. https://doi.org/10.3390/ijms23105638
APA StyleOliveira Filho, J. G., & Egea, M. B. (2022). Edible Bioactive Film with Curcumin: A Potential “Functional” Packaging? International Journal of Molecular Sciences, 23(10), 5638. https://doi.org/10.3390/ijms23105638







