Dielectric Study of Tetraalkylammonium and Tetraalkylphosphonium Levulinate Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
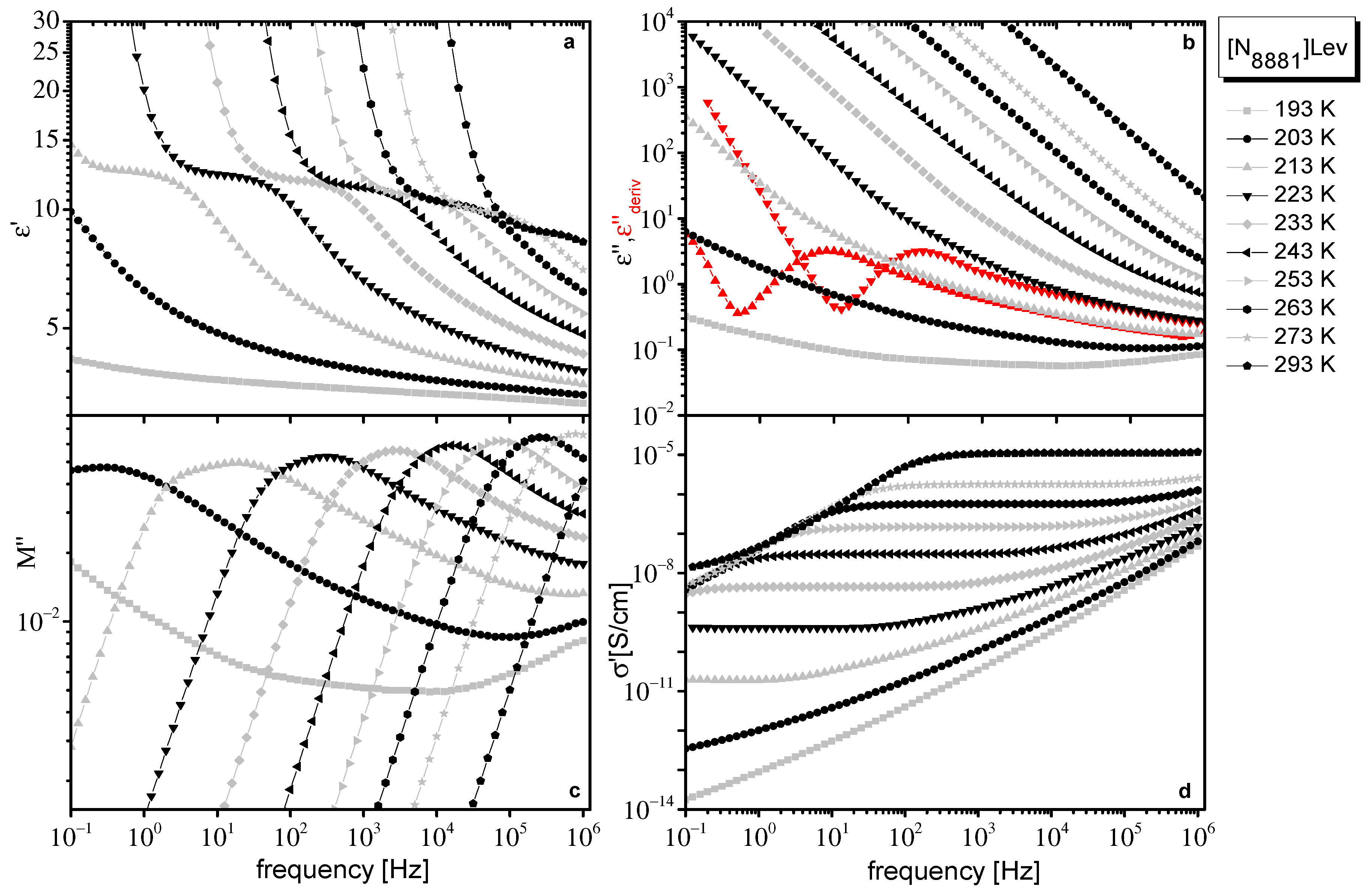
2.1. Dielectric Data
2.2. Dielectric Data Evaluation
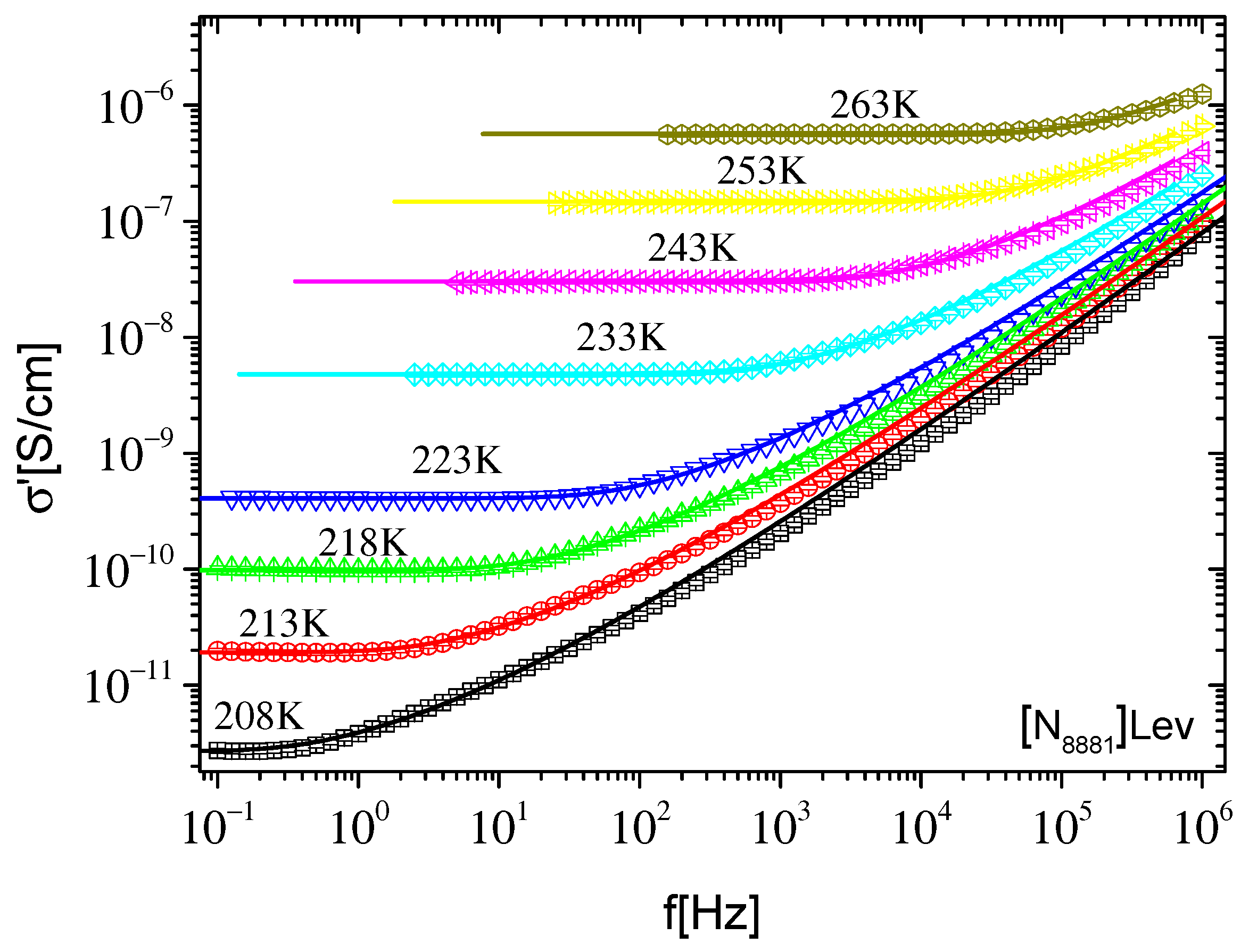
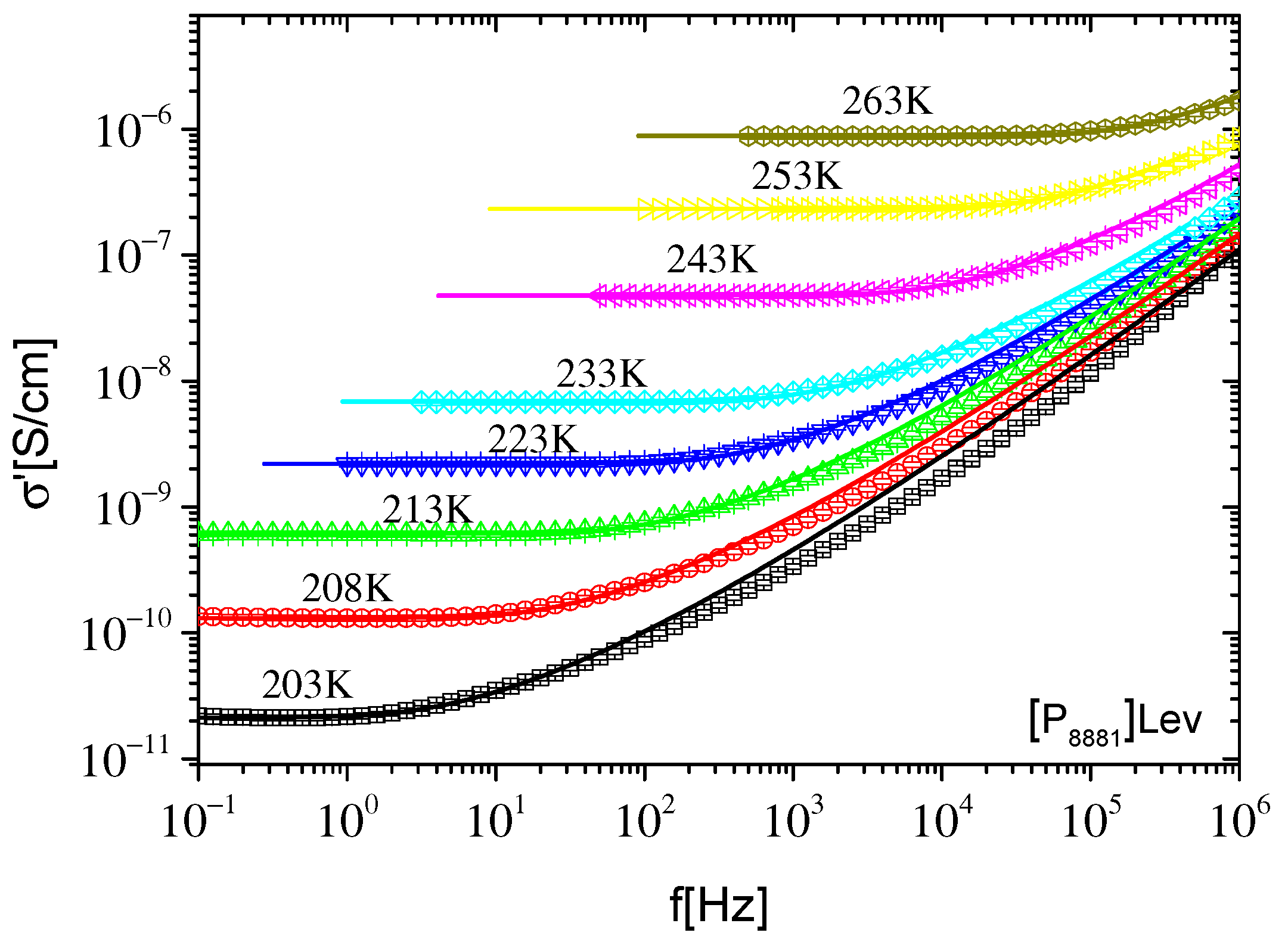
2.2.1. Ionic Conductivity
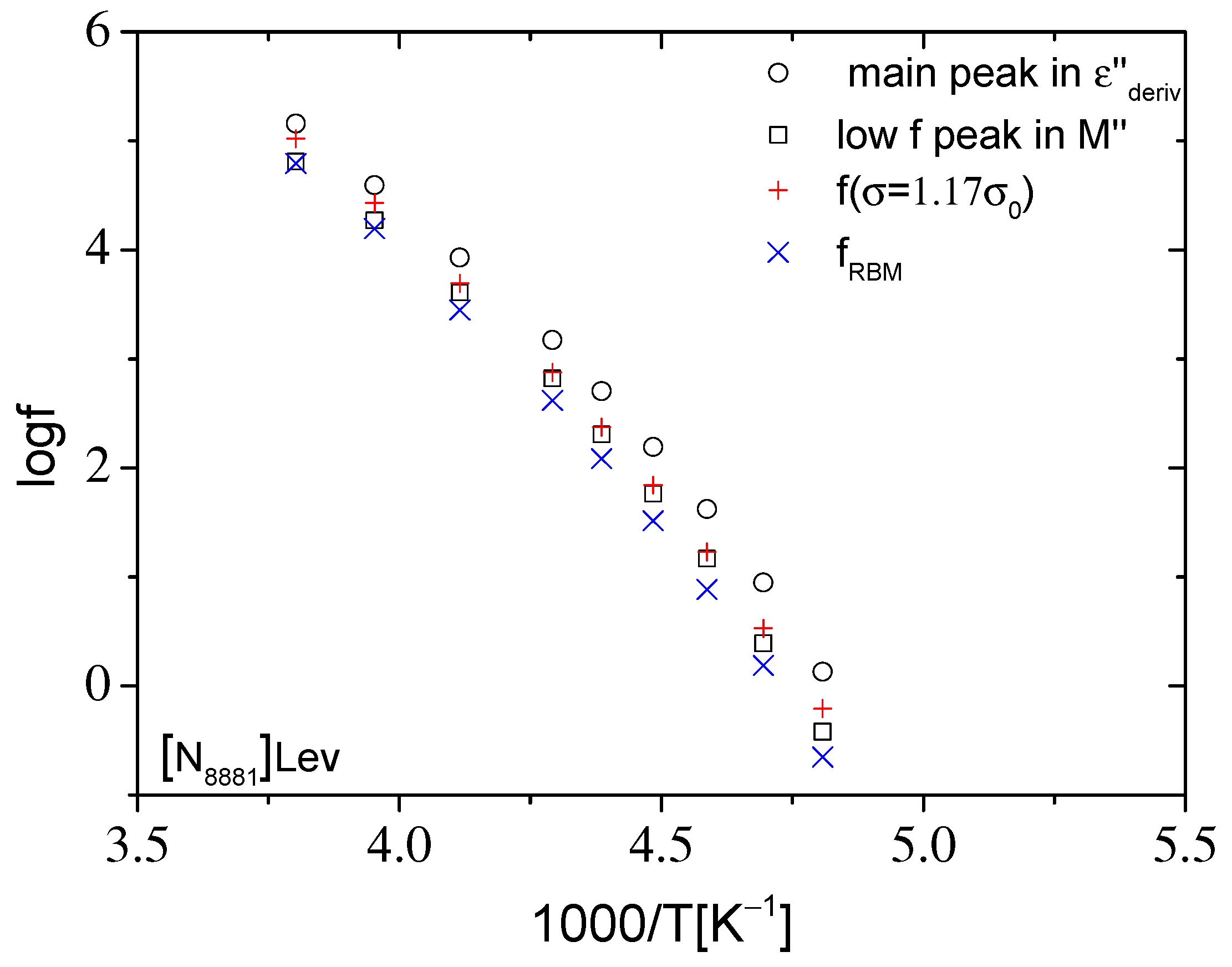
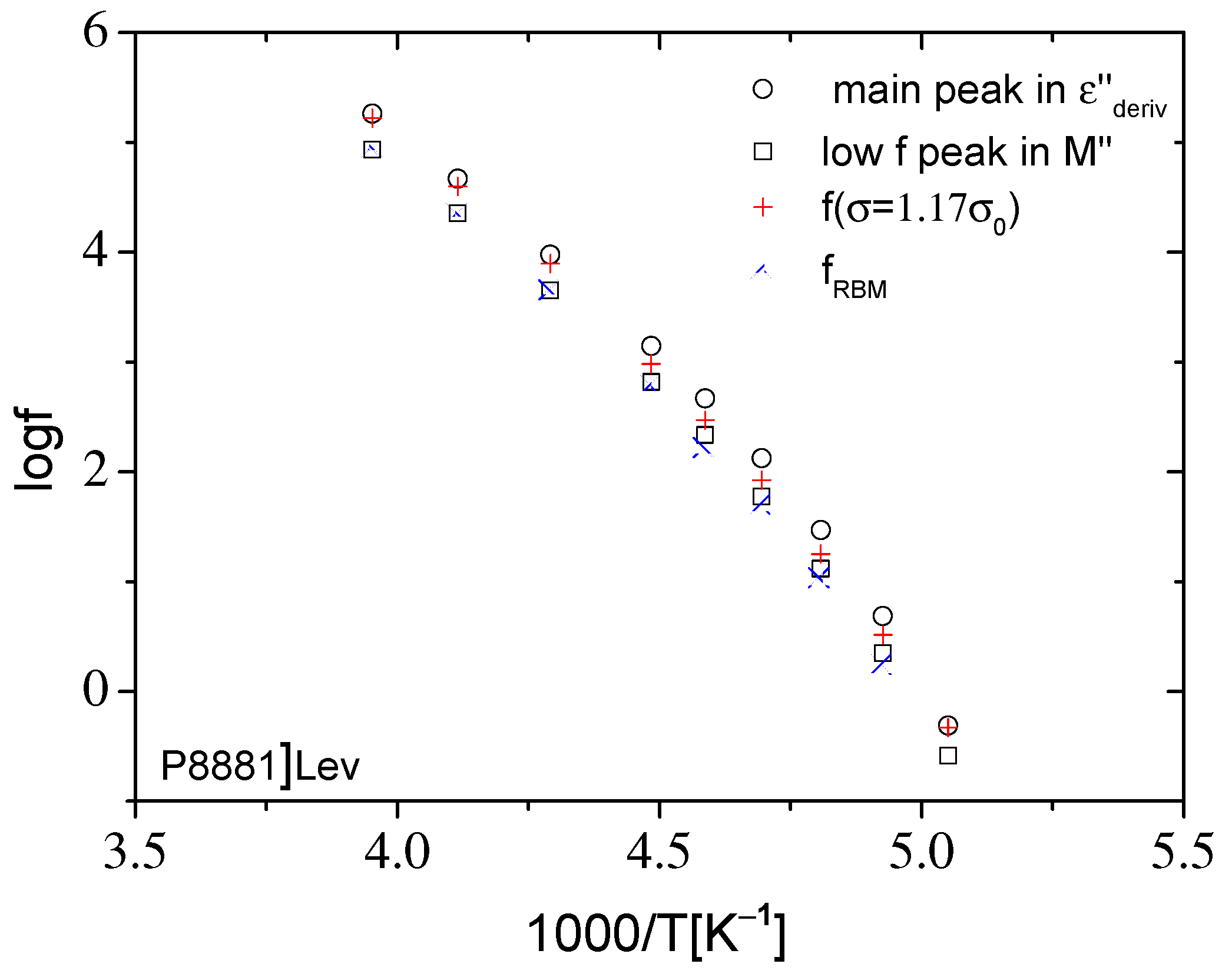
2.2.2. Dynamics
3. Materials and Methods
3.1. Materials
Synthesis of [N8881]Lev and [P8881]Lev
3.2. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsunashima, K.; Sugiya, M. Physical and electrochemical properties of low-viscosity phosphonium ionic liquids as potential electrolytes. Electrochem. Commun. 2007, 9, 2353–2358. [Google Scholar] [CrossRef]
- Seki, S.; Hayamizu, K.; Tsuzuki, S.; Fujii, K.; Umebayashi, Y.; Mitsugi, T.; Kobayashi, T.; Ohno, Y.; Kobayashi, Y.; Mita, Y.; et al. Relationships between center atom species (n, p) and ionic conductivity, viscosity, density, self-diffusion coefficient of quaternary cation room-temperature ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 3509. [Google Scholar] [CrossRef] [PubMed]
- Shirota, H.; Fukazawa, H.; Fujisawa, T.; Wishart, J. Heavy atom substitution effects in non-aromatic ionic liquids: Ultrafast dynamics and physical properties. J. Phys. Chem. B 2010, 114, 9400–9412. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.J.; Ventura, S.P.; Batista, M.L.; Schroeder, B.; Gonçalves, F.; Esperança, J.; Mutelet, F.; Coutinho, J.A. Understanding the impact of the central atom on the ionic liquid behavior: Phosphonium vs ammonium cations. J. Chem. Phys. 2014, 140, 064505. [Google Scholar] [CrossRef]
- Philippi, F.; Rauber, D.; Kuttich, B.; Kraus, T.; Kay, C.W.; Hempelmann, R.; Hunt, P.A.; Welton, T. Ether functionalisation, ion conformation and the optimisation of macroscopic properties in ionic liquids. Phys. Chem. Chem. Phys. 2020, 22, 23038–23056. [Google Scholar] [CrossRef]
- Hulsbosch, J.; Vos, D.E.; Binnemans, K.; Ameloot, R. Biobased ionic liquids: Solvents for a green processing industry? ACS Sustain. Chem. Eng. 2016, 4, 2917–2931. [Google Scholar] [CrossRef]
- Seitkalieva, M.M.; Vavina, A.V.; Posvyatenko, A.; Egorova, K.S.; Kashin, A.; Gordeev, E.; Strukova, E.N.; Romashov, L.V.; Ananikov, V. Biomass-derived ionic liquids based on a 5-HMF platform chemical: Synthesis, characterization, biological activity, and tunable interactions at the molecular level. ACS Sustain. Chem. Eng. 2021, 9, 3552–3570. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Y.; Wang, K.; Yu, H.; Yu, S.; Liu, S. High-efficient conversion of cellulose to levulinic acid catalyzed via functional brønstedlewis acidic ionic liquids. Catal. Lett. 2021, 152, 1064–1075. [Google Scholar] [CrossRef]
- Shahriari, S.; Tomé, L.; Araújo, J.M.; Rebelo, L.P.; Coutinho, J.A.; Marrucho, I.M.; Freire, M. Aqueous biphasic systems: A benign route using cholinium-based ionic liquids. RSC Adv. 2013, 3, 1835–1843. [Google Scholar] [CrossRef]
- Wagner, V.; Schulz, P.; Wasserscheid, P. Asymmetric hydrogenation catalysis via ion-pairing in chiral ionic liquids. J. Mol. Liq. 2014, 192, 177–184. [Google Scholar] [CrossRef]
- Stevanovic, S.; Podgorsek, A.; Moura, L.; Santini, C.C.; Padua, A.A.H.; Gomes, M.F. Absorption of carbon dioxide by ionic liquids with carboxylate anions. Int. J. Greenh. Gas Control 2013, 17, 78–88. [Google Scholar] [CrossRef]
- Avila, J.; Lepre, L.F.; Santini, C.C.; Tiano, M.; Denis-Quanquin, S.; Szeto, K.C.; Padua, A.A.H.; Gomes, M.C. High-performance porous ionic liquids for low-pressure CO2 capture. Angew. Chem. Int. Ed. 2021, 60, 12876–12882. [Google Scholar] [CrossRef] [PubMed]
- Boissou, F.T.; Muehlbauer, A.; Vigier, K.d.; Leclercq, L.; Kunz, W.; Marinkovic, S.; Estrine, B.; Nardello-Rataj, V.; Jérôme, F. Transition of cellulose crystalline structure in biodegradable mixtures of renewably-sourced levulinate alkyl ammonium ionic liquids, γ-valerolactone and water. Green Chem. 2014, 16, 2463–2471. [Google Scholar] [CrossRef]
- Becherini, S.; Mezzetta, A.; Chiappe, C.; Guazzelli, L. Levulinate amidinium protic ionic liquids (PILs) as suitable media for the dissolution and levulination of cellulose. New J. Chem. 2019, 43, 4554–4561. [Google Scholar] [CrossRef]
- Mezzetta, A.; Becherini, S.; Pretti, C.; Monni, G.; Casu, V.; Chiappe, C.; Guazzelli, L. Insights into the levulinate-based ionic liquid class: Synthesis, cellulose dissolution evaluation and ecotoxicity assessment. New J. Chem. 2019, 43, 13010–13019. [Google Scholar] [CrossRef]
- He, F.; Chen, J.; Gong, Z.; Xu, Q.; Yue, W.; Xie, H. Dissolution pretreatment of cellulose by using levulinic acid-based protic ionic liquids towards enhanced enzymatic hydrolysis. Carbohydr. Polym. 2021, 269, 118271. [Google Scholar] [CrossRef] [PubMed]
- Sangoro, J.R.; Serghei, A.; Naumov, S.; Galvosas, P.; Kaerger, J.; Wespe, C.; Bordusa, F.; Kremer, F. Charge transport and mass transport in imidazolium-based ionic liquids. Phys. Rev. E 2008, 77, 051202. [Google Scholar] [CrossRef]
- Sangoro, J.R.; Iacob, C.; Serghei, A.; Friedrich, C.; Kremer, F. Universal scaling of charge transport in glass-forming ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 913–916. [Google Scholar] [CrossRef]
- Krause, C.; Sangoro, J.R.; Iacob, C.; Kremer, F. Charge transport and dipolar relaxations in imidazolium-based ionic liquids. J. Phys. Chem. B 2009, 114, 382–386. [Google Scholar] [CrossRef]
- Sippel, P.; Krohns, S.; Reuter, D.; Lunkenheimer, P.; Loidl, A. Importance of reorientational dynamics for the charge transport in ionic liquids. Phys. Rev. E 2018, 98, 052605. [Google Scholar] [CrossRef] [Green Version]
- Thoms, E.; Sippel, P.; Reuter, D.; Weiß, M.; Loidl, A.; Krohns, S. Dielectric study on mixtures of ionic liquids. Sci. Rep. 2017, 7, 7463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabst, F.; Gabriel, J.; Weigl, P.; Blochowicz, T. Molecular dynamics of supercooled ionic liquids studied by light scattering and dielectric spectroscopy. Chem. Phys. 2017, 494, 103–110. [Google Scholar] [CrossRef]
- Ito, N.; Huang, W.; Richert, R. Dynamics of a supercooled ionic liquid studied by optical and dielectric spectroscopy. J. Phys. Chem. B 2006, 110, 4371–4377. [Google Scholar] [CrossRef] [PubMed]
- Griffin, P.J.; Agapov, A.L.; Kisliuk, A.; Sun, X.-G.; Dai, S.; Novikov, V.N.; Sokolov, A.P. Decoupling charge transport from the structural dynamics in room temperature ionic liquids. J. Chem. Phys. 2011, 135, 114509. [Google Scholar] [CrossRef]
- Griffin, P.J.; Holt, A.P.; Wang, Y.; Novikov, V.N.; Sangoro, J.R.; Kremer, F.; Sokolov, A.P. Interplay between hydrophobic aggregation and charge transport in the ionic liquid methyltrioctylammonium bis(trifluoromethylsulfonyl)imide. J. Phys. Chem. B 2014, 118, 783–790. [Google Scholar] [CrossRef]
- Griffin, P.J.; Holt, A.P.; Tsunashima, K.; Sangoro, J.R.; Kremer, F.; Sokolov, A.P. Ion transport and structural dynamics in homologous ammonium and phosphonium-based room temperature ionic liquids. J. Chem. Phys. 2015, 142, 084501. [Google Scholar] [CrossRef]
- Pabst, F.; Wojnarowska, Z.; Paluch, M.; Blochowicz, T. On the temperature and pressure dependence of dielectric relaxation processes in ionic liquids. Phys. Chem. Chem. Phys. 2021, 23, 14260–14275. [Google Scholar] [CrossRef]
- Cosby, T.; Sangoro, J.; Kremer, F. Rotational and Translational Diffusion in Ionic Liquids. In Dielectric Properties of Ionic Liquids; Paluch, M., Ed.; Advances in Dielectrics, Chapter 2; Springer: Cham, Switzerland, 2016; pp. 29–51. [Google Scholar]
- Griffin, P.J.; Agapov, A.L.; Sokolov, A.P. Translation-rotation decoupling and nonexponentiality in room temperature ionic liquids. Phys. Rev. E 2012, 86, 021508. [Google Scholar] [CrossRef] [Green Version]
- Dyre, J.C. A simple model of ac hopping conductivity in disordered solids. Phys. Lett. A 1985, 108, 457–461. [Google Scholar] [CrossRef] [Green Version]
- Schrøder, T.B.; Dyre, J.C. ac hopping conduction at extreme disorder takes place on the percolating cluster. Phys. Rev. Lett. 2008, 101, 025901. [Google Scholar] [CrossRef] [Green Version]
- Dyre, J.C. The random free-energy barrier model for ac conduction in disordered solids. J. Appl. Phys. 1988, 64, 2456–2468. [Google Scholar] [CrossRef] [Green Version]
- Gainaru, C.; Stacy, E.W.; Bocharova, V.; Gobet, M.; Holt, A.P.; Saito, T.; Greenbaum, S.; Sokolov, A.P. Mechanism of conductivity relaxation in liquid and polymeric electrolytes: Direct link between conductivity and diffusivity. J. Phys. Chem. B 2016, 120, 11074–11083. [Google Scholar] [CrossRef]
- Silva, W.; Zanatta, M.; Ferreira, A.S.; Corvo, M.C.; Cabrita, E.J. Revisiting ionic liquid structure-property relationship: A critical analysis. Int. J. Mol. Sci. 2020, 21, 7745. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cooper, E.I.; Angell, C.A. Ionic liquids: Ion mobilities, glass temperatures, and fragilities. J. Phys. Chem. B 2003, 107, 6170–6178. [Google Scholar] [CrossRef]
- Cosby, T.; Vicars, Z.; Mapesa, E.U.; Tsunashima, K.; Sangoro, J. Charge transport and dipolar relaxations in phosphonium-based ionic liquids. J. Chem. Phys. 2017, 147, 234504. [Google Scholar] [CrossRef] [PubMed]
- Cosby, T.; Vicars, Z.; Heres, M.; Tsunashima, K.; Sangoro, J. Dynamic and structural evidence of mesoscopic aggregation in phosphonium ionic liquids. J. Chem. Phys. 2018, 148, 193815. [Google Scholar] [CrossRef]
- Griffin, P.J.; Wang, Y.; Holt, A.P.; Sokolov, A.P. Communication: Influence of nanophase segregation on ion transport in room temperature ionic liquids. J. Chem. Phys. 2016, 144, 151104. [Google Scholar] [CrossRef] [Green Version]
- Wuebbenhorst, M.; van Turnhout, J. Analysis of complex dielectric spectra. i. one-dimensional derivative techniques and three-dimensional modelling. J. Non-Cryst. Solids 2002, 305, 40–49. [Google Scholar] [CrossRef]
- Macdonald, J.R. Comments on the electric modulus formalism model and superior alternatives to it for the analysis of the frequency response of ionic conductors. J. Phys. Chem. Solids 2009, 70, 546–554. [Google Scholar] [CrossRef]
- Lunkenheimer, P.; Bobnar, V.; Pronin, A.V.; Ritus, A.I.; Volkov, A.A.; Loidl, A. Origin of apparent colossal dielectric constants. Phys. Rev. B 2002, 66, 052105. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, J.R. Comparison and discussion of some theories of the equilibrium electrical double layer in liquid electrolytes. J. Electroanal. Chem. Interfacial Electrochem. 1987, 223, 1–23. [Google Scholar] [CrossRef]
- Serghei, A.; Tress, M.; Sangoro, J.R.; Kremer, F. Electrode polarization and charge transport at solid interfaces. Phys. Rev. B 2009, 80, 184301. [Google Scholar] [CrossRef]
- Emmert, S.; Wolf, M.; Gulich, R.; Krohns, S.; Kastner, S.; Lunkenheimer, P.; Loid, A.l. Electrode polarization effects in broadband dielectric spectroscopy. Eur. Phys. J. B 2011, 83, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Angell, C.A. Strong and fragile liquids. In Relaxations in Complex Systems; Ngai, K.L., Wright, G.B., Eds.; NRL: Washington, DC, USA, 1985; pp. 3–11. [Google Scholar]
- Boehmer, R.; Ngai, K.L.; Angell, C.A.; Plazek, D.J. Nonexponential relaxations in strong and fragile glass formers. J. Chem. Phys. 1993, 99, 4201–4209. [Google Scholar] [CrossRef]
- Sippel, P.; Lunkenheimer, P.; Krohns, S.; Thoms, E.; Loidl, A. Importance of liquid fragility for energy applications of ionic liquids. Sci. Rep. 2015, 5, 13922. [Google Scholar] [CrossRef] [Green Version]
- Mero, A.; Guglielmero, L.; Andrea, F.D.; Pomelli, C.S.; Guazzelli, L.; Koutsoumpos, S.; Tsonos, G.; Stavrakas, I.; Moutzouris, K.; Mezzetta, A. Influence of the cation partner on levulinate ionic liquids properties. J. Mol. Liq. 2022, 354, 118850. [Google Scholar] [CrossRef]
- Angell, C.A.; Byrne, N.; Belieres, J.-P. Parallel developments in aprotic and protic ionic liquids: Physical chemistry and applications. Acc. Chem. Res. 2007, 40, 1228–1236. [Google Scholar] [CrossRef]
- Fraser, K.J.; Izgorodina, E.I.; Forsyth, M.; Scott, J.L.; MacFarlane, D.R. Liquids intermediate between molecular and ionic liquids: Liquid ion pairs? Chem. Commun. 2007, 37, 3817. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Izgorodina, E.I.; Abbott, A.P.; Annat, G.; Fraser, K. On the concept of ionicity in ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 4962. [Google Scholar] [CrossRef]
- Musia, M.; Bair, S.; Cheng, S.; Wojnarowska, Z.; Paluch, M. Fractional walden rule for aprotic ionic liquids: Experimental verification over a wide range of temperatures and pressures. J. Mol. Liq. 2021, 331, 115772. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Rivera, A.; Roessler, E.A. Evidence of secondary relaxations in the dielectric spectra of ionic liquids. Phys. Rev. B 2006, 73, 212201. [Google Scholar] [CrossRef] [Green Version]
- Rivera, A.; Brodin, A.; Pugachev, A.; Roessler, E.A. Orientational and translational dynamics in room temperature ionic liquids. J. Chem. Phys. 2007, 126, 114503. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, H. Characterization of the diffusion process in oxide glasses based on the correlation between electric conduction and dielectric relaxation. J. Non-Cryst. Solids 1975, 18, 173–195. [Google Scholar] [CrossRef]
- Tsonos, C.; Kanapitsas, A.; Kechriniotis, A.; Petropoulos, N. AC and DC conductivity correlation: The coefficient of Barton-Nakajima-Namikawa relation. J. Non-Cryst. Solids 2012, 358, 1638–1643. [Google Scholar] [CrossRef] [Green Version]
- Stacy, E.W.; Gainaru, C.P.; Gobet, M.; Wojnarowska, Z.; Bocharova, V.; Greenbaum, S.G.; Sokolov, A.P. Fundamental limitations of ionic conductivity in polymerized ionic liquids. Macromolecules 2018, 51, 8637–8645. [Google Scholar] [CrossRef]
- Ausín, D.; Parajó, J.J.; Trenzado, J.L.; Varela, L.M.; Cabeza, O.; Segade, L. Influence of small quantities of water on the physical properties of alkylammonium nitrate ionic liquids. Int. J. Mol. Sci. 2021, 22, 7334. [Google Scholar] [CrossRef]

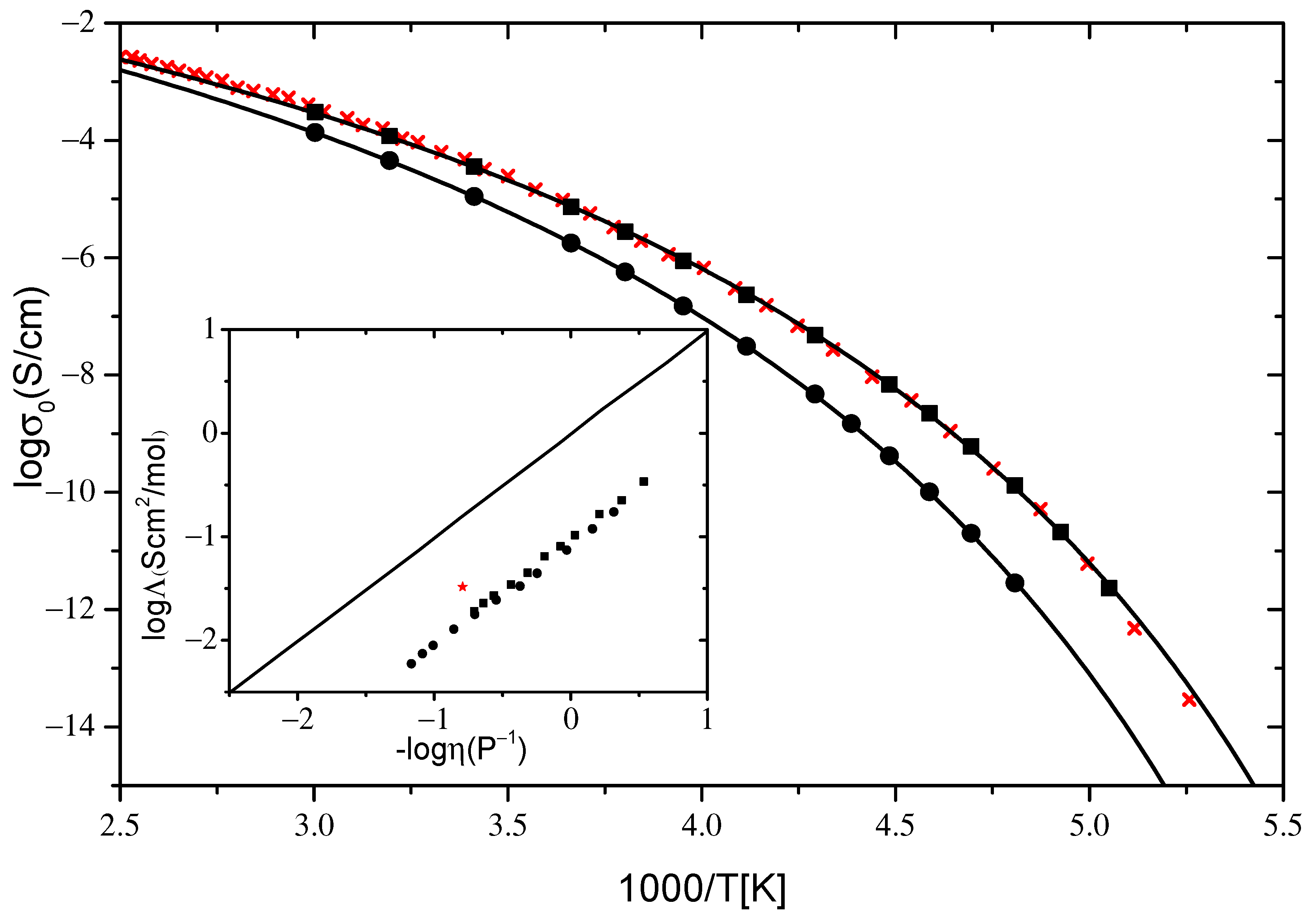


| VTF σ0 (T) | [P8881]Lev | [N8881]Lev |
|---|---|---|
| σ∞ [S/cm] | 1.11 | 1.92 |
| B | 1608 | 1845 |
| D | 11.7 | 13.2 |
| T0 [K] | 138 | 140 |
| m | 66 | 61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kripotou, S.; Tsonos, G.; Mezzetta, A.; Mero, A.; Guazzelli, L.; Moutzouris, K.; Stavrakas, I.; Tsonos, C. Dielectric Study of Tetraalkylammonium and Tetraalkylphosphonium Levulinate Ionic Liquids. Int. J. Mol. Sci. 2022, 23, 5642. https://doi.org/10.3390/ijms23105642
Kripotou S, Tsonos G, Mezzetta A, Mero A, Guazzelli L, Moutzouris K, Stavrakas I, Tsonos C. Dielectric Study of Tetraalkylammonium and Tetraalkylphosphonium Levulinate Ionic Liquids. International Journal of Molecular Sciences. 2022; 23(10):5642. https://doi.org/10.3390/ijms23105642
Chicago/Turabian StyleKripotou, Sotiria, Georgios Tsonos, Andrea Mezzetta, Angelica Mero, Lorenzo Guazzelli, Konstantinos Moutzouris, Ilias Stavrakas, and Christos Tsonos. 2022. "Dielectric Study of Tetraalkylammonium and Tetraalkylphosphonium Levulinate Ionic Liquids" International Journal of Molecular Sciences 23, no. 10: 5642. https://doi.org/10.3390/ijms23105642
APA StyleKripotou, S., Tsonos, G., Mezzetta, A., Mero, A., Guazzelli, L., Moutzouris, K., Stavrakas, I., & Tsonos, C. (2022). Dielectric Study of Tetraalkylammonium and Tetraalkylphosphonium Levulinate Ionic Liquids. International Journal of Molecular Sciences, 23(10), 5642. https://doi.org/10.3390/ijms23105642








