Association between Decreased ITGA7 Levels and Increased Muscle α-Synuclein in an MPTP-Induced Mouse Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
2.1. Decreased Expression of Tyrosine Hydroxylase (TH) in SN and ST in an MPTP-Induced PD Mouse Model
2.2. Behavior Tests in an MPTP-Induced PD Mouse Model
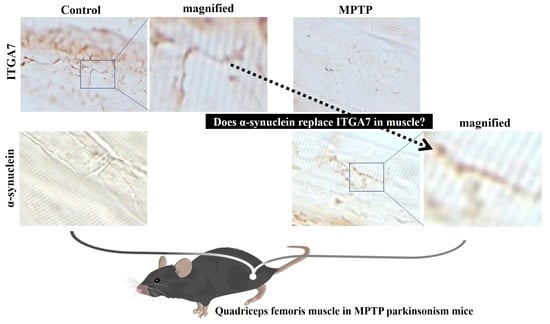
2.3. Reduced Muscle Expression of ITGA7 in an MPTP-Induced PD Mouse Model
2.4. Increased Muscle Expression of α-Syn in an MPTP-Induced PD Mouse Model
2.5. Immunofluorescence Analysis of ITGA7 Co-Localized with α-Syn in Skeletal Muscle
2.6. Western Blot Analysis of C2C12 Cells Transfected with ITGA7 siRNA
2.7. ITGA7 Co-Localized with α-Syn in C2C12 Cells
3. Discussion
4. Materials and Methods
4.1. MPTP Mouse Model
4.2. Behavior Tests
4.3. Cell Lines and Cultures
4.4. ITGA7 Small Interference RNA
4.5. MPP + Treatment
4.6. Western Blot Analysis
4.7. Immunohistochemistry
4.8. Immunofluorescence
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson's disease. J. Neural. Trans. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson's disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grünblatt, E. Commonalities in the genetics of Alzheimer'ss disease and Parkinson's disease. Expert Rev. Neurother. 2008, 8, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. α-Synuclein in Parkinson's disease. Cold Spring Harb Perspect Med. 2012, 2, a009399. [Google Scholar]
- Flintoff-Dye, N.L.; Welser, J.; Rooney, J.; Scowen, P.; Tamowski, S.; Hatton, W.; Burkin, D.J. Role for the alpha7beta1 integrin in vascular development and integrity. Dev. Dyn 2005, 234, 11–21. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, X.; Wang, Z. Integrin α7 knockdown suppresses cell proliferation, migration, invasion and EMT in hepatocellular carcinoma. Exp. Med. 2021, 21, 309. [Google Scholar] [CrossRef]
- Carrasco-Garcia, E.; Auzmendi-Iriarte, J.; Matheu, A. Integrin α7: A novel promising target in glioblastoma stem cells. Stem Cell Investig 2018, 5, 2. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Heller, K.N.; Montgomery, C.L.; Shontz, K.M.; Clark, K.R.; Mendell, J.R.; Rodino-Klapac, L.R. Human α7 Integrin Gene (ITGA7) Delivered by Adeno-Associated Virus Extends Survival of Severely Affected Dystrophin/Utrophin-Deficient Mice. Hum. Gene 2015, 26, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Mendell, J.R.; Boué, D.R.; Martin, P.T. The congenital muscular dystrophies: Recent advances and molecular insights. Pediatr Dev. Pathol. 2006, 9, 427–443. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, T.; Ye, J.; Yang, J.; Chen, C.; Cai, S.; Ma, J. Circ-ITGA7 sponges miR-3187-3p to upregulate ASXL1, suppressing colorectal cancer proliferation. Cancer Manag. Res. 2019, 11, 6499–6509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, A.; Xia, E.; Zhou, Y.; Guan, Y.; Xiang, J.; Kong, L.; Wang, Y.; Yang, F.; Wang, O.; Zhang, X. ITGA7 functions as a tumor suppressor and regulates migration and invasion in breast cancer. Cancer Manag. Res. 2018, 10, 969–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, S.; Chen, X.; Rizo, J.; Jahn, R.; Südhof, T.C. A Broken α-Helix in Folded α-Synuclein*. J. Biol. Chem. 2003, 278, 15313–15318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Seo, M.H.; Lim, S.; Yeo, S. Decrease in ITGA7 Levels Is Associated with an Increase in α-Synuclein Levels in an MPTP-Induced Parkinson’s Disease Mouse Model and SH-SY5Y Cells. Int. J. Mol. Sci. 2021, 22, 12616. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.E.; Gurpur, P.B.; Yablonka-Reuveni, Z.; Burkin, D.J. Laminin-111 restores regenerative capacity in a mouse model for alpha7 integrin congenital myopathy. Am. J. Pathol 2009, 174, 256–264. [Google Scholar] [CrossRef] [Green Version]
- Mayer, U.; Saher, G.; Fassler, R.; Bornemann, A.; Echtermeyer, F.; von der Mark, H.; Miosge, N.; Poschl, E.; von der Mark, K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat. Genet. 1997, 17, 318–323. [Google Scholar] [CrossRef]
- Dominici, C.; Richard, S. Muscle stem cell polarity requires QKI-mediated alternative splicing of Integrin Alpha-7 (Itga7). Life Sci. Alliance 2022, 5, e202101192. [Google Scholar] [CrossRef]
- Ito, A.; Yamamoto, M.; Ikeda, K.; Sato, M.; Kawabe, Y.; Kamihira, M. Effects of type IV collagen on myogenic characteristics of IGF-I gene-engineered myoblasts. J. Biosci. Bioeng. 2015, 119, 596–603. [Google Scholar] [CrossRef]
- Ding, R.; Horie, M.; Nagasaka, S.; Ohsumi, S.; Shimizu, K.; Honda, H.; Nagamori, E.; Fujita, H.; Kawamoto, T. Effect of cell-extracellular matrix interaction on myogenic characteristics and artificial skeletal muscle tissue. J. Biosci. Bioeng. 2020, 130, 98–105. [Google Scholar] [CrossRef]
- Recasens, A.; Dehay, B. Alpha-synuclein spreading in Parkinson's disease. Front. Neuroanat. 2014, 8, 159. [Google Scholar] [CrossRef] [Green Version]
- Pegoraro, E.; Cepollaro, F.; Prandini, P.; Marin, A.; Fanin, M.; Trevisan, C.P.; El-Messlemani, A.H.; Tarone, G.; Engvall, E.; Hoffman, E.P.; et al. Integrin alpha 7 beta 1 in muscular dystrophy/myopathy of unknown etiology. Am. J. Pathol 2002, 160, 2135–2143. [Google Scholar] [CrossRef]
- Surguchev, A.A.; Surguchov, A. Integrins—A missing link in synuclein’s pathogenic mechanism. J. Neurosci. Res. 2019, 97, 539–542. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Lim, S.; Yeo, S. Association between Decreased ITGA7 Levels and Increased Muscle α-Synuclein in an MPTP-Induced Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 5646. https://doi.org/10.3390/ijms23105646
Han S, Lim S, Yeo S. Association between Decreased ITGA7 Levels and Increased Muscle α-Synuclein in an MPTP-Induced Mouse Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2022; 23(10):5646. https://doi.org/10.3390/ijms23105646
Chicago/Turabian StyleHan, Sangeun, Sabina Lim, and Sujung Yeo. 2022. "Association between Decreased ITGA7 Levels and Increased Muscle α-Synuclein in an MPTP-Induced Mouse Model of Parkinson’s Disease" International Journal of Molecular Sciences 23, no. 10: 5646. https://doi.org/10.3390/ijms23105646