Abstract
In the context of the new life-threatening COVID-19 pandemic caused by the SARS-CoV-2 virus, finding new antiviral and antimicrobial compounds is a priority in current research. Pyridine is a privileged nucleus among heterocycles; its compounds have been noted for their therapeutic properties, such as antimicrobial, antiviral, antitumor, analgesic, anticonvulsant, anti-inflammatory, antioxidant, anti-Alzheimer’s, anti-ulcer or antidiabetic. It is known that a pyridine compound, which also contains a heterocycle, has improved therapeutic properties. The singular presence of the pyridine nucleus, or its one together with one or more heterocycles, as well as a simple hydrocarbon linker, or grafted with organic groups, gives the key molecule a certain geometry, which determines an interaction with a specific protein, and defines the antimicrobial and antiviral selectivity for the target molecule. Moreover, an important role of pyridine in medicinal chemistry is to improve water solubility due to its poor basicity. In this article, we aim to review the methods of synthesis of pyridine compounds, their antimicrobial and antiviral activities, the correlation of pharmaceutical properties with various groups present in molecules as well as the binding mode from Molecular Docking Studies.
1. Introduction
Pyridine (from the Greek pyr = fire and idine—which is used for aromatic bases) contains a single heteroaromatic ring, which comes from the replacement of a CH group in the benzene ring with the nitrogen atom. Ramsay (1877) synthesizes pyridine for the first time, by the reaction of acetylene with hydrogen cyanide in a red-hot iron tube furnace, this being the first synthesized heterocycle [1]. Arthur Hantzsch later synthesized (1881) pyridine compounds by the synthesis that bears his name, through a multicomponent reaction, starting from a β-ketoester, an aldehyde and ammonia [2,3]. An important role of pyridine is that it is used as an organic solvent or as ligand for coordination complexes [4,5,6,7,8]. The pyridine nucleus is found in many natural products, such as vitamins, alkaloids and coenzymes, as well as in many drugs (Figure 1) and pesticides [9,10]. The recent literature mentions various series of compounds containing the pyridine nucleus, as a unique heterocycle, and with other heterocycles or fused with other heterocycles, which have remarkable antibacterial, antifungal and antiviral properties [1,9,10,11,12,13]. The presence of an additional heterocycle compared to the pyridine one in the bioactive molecule proved to be beneficial in the sense that it intensified the therapeutic properties of the antimicrobial and antiviral ones, and also widened their range [3,4,5,6,7,8]. Moreover, the presence of certain organic groups, such as amino, hydroxy, methoxy, sulfamide, hydrazide, etc., increase the biological activities of the compounds, as noted in the literature. Moreover, current research has been stimulated by the unexpected evolution of the SARS-CoV-2 pandemic (COVID-19), which required new antimicrobial and antiviral agents to treat patients with moderate or severe cases of pneumonia [13].
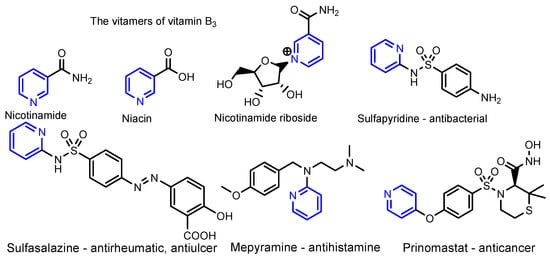
Figure 1.
Drugs containing the pyridine nucleus.
In the present paper, we aim to review antimicrobial and antiviral pyridine compounds, as well as their methods of synthesis. Their presentation will be based on the complexity of the molecules and their therapeutic properties.
2. Synthesis of Antimicrobial Compounds Containing Only Pyridine Ring
Sarova et al. [14] synthesized three dodecanoic acid derivatives 1−3 with yields of 59–61%, starting from dodecanoic acid in two steps, chlorination with thionyl chloride and reaction with the corresponding aminopyridine (Scheme 1). All compounds possessed good antibacterial activity against B. subtilis, S. aureus and E. coli and antifungal activity against A. niger and C. albicans.
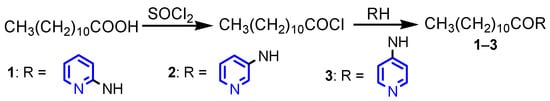
Scheme 1.
Synthesis of dodecanoic acid pyridines 1–3 [14].
Narang et al. [15] synthesized a series of 18 nicotinic acid benzylidene hydrazide derivatives in three steps with total yields of 60–80% (Scheme 2). The antimicrobial screening results indicated that compounds with nitro (4, 5 and 6) and dimethoxy (7) substituents were the most active ones against tested strains (S. aureus, B subtilis, E coli, C. albicans, and A. niger), some of them having an antimicrobial activity comparable to the standard drugs fluconazole and norfloxacin.
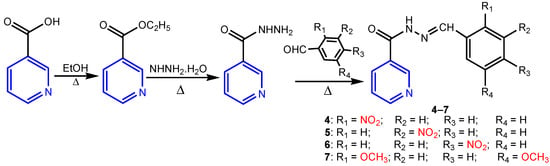
Scheme 2.
Synthesis of nicotinic acid benzylidene hydrazide derivatives 4–7 [15].
Malhotra et al. [16] synthesized ten substituted Mannich bases 8–17 using a classical Mannich reaction starting from 2-ethoxybenzylidene isonicotinohydrazide, formaldehyde and a secondary amine (Scheme 3). The highest antibacterial activity against Gram-positive species B. subtilis, S. aureus and Gram-negative species P. aeruginosa, E. coli were shown by compounds 12, 15, 16, and 17 (MIC = 6.25–12.5 μg mL−1) with Amoxicillin as standard. Furthermore, these compounds exhibited a high antifungal activity against C. albicans and C. gabrata species (MIC = 12.5 μg mL−1). Judge et al. [17] synthesized isonicotinic acid-1-(substituted phenyl)-ethylidene hydrazides 18–22 by refluxing isonicotinoyl hydrazide with various substituted acetophenones (Scheme 4). Compounds with Br, OCH3, and Cl groups were highly active antimicrobial agents, all of them exhibiting activities better than the standard compounds norfloxacin and fluconazole.
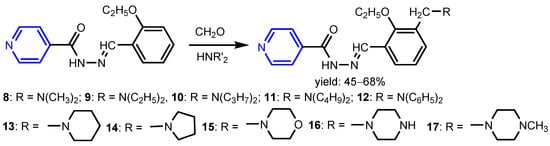
Scheme 3.
Synthesis of Mannich bases 8–17 [16].
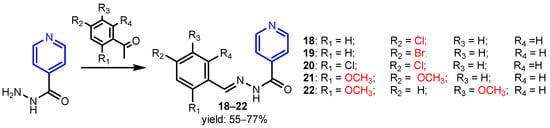
Scheme 4.
Synthesis of isonicotinic acid-1-(substituted phenyl)-ethylidene hydrazides 18–22 [17].
In a similar study, Judge et al. [18] synthesized a series of isonicotinic acid hydrazide derivatives by benzoilation of (E)-N’-benzylideneisonicotinohydrazide compounds (Scheme 5) and found that derivatives 23–27 were the most active antimicrobial agents against the tested strains S. aureus, B. subtilis, E. coli, C. albicans, and A. niger, with MIC between 2.18–3.08 μM mL−1. Eldeab [19] synthesized substituted pyridyl-4-chloro benzoates by microwave-assisted condensation of 2-oxo-1,2-dihydropyridine-3- carbonitriles with 4-chlorobenzoyl chloride under solvent-free conditions or by conventional heating in methylene chloride in the presence of triethylamine. The activity of 3-cyano-5-[(4-hydroxyphenyl)diazenyl]-4-methyl-6-phenylpyridin-2-yl-4-chloro benzoate 28 against B. subtilis and S. aureus was comparable to that of Amikacin, which is used as the standard antibiotic (Figure 2). Karunanidhi et al. [20] isolated 2-(methyldithio)pyridine-3-carbonitrile 29 from Persian Shallot (Allium stipitatum Regel.) and screened for its antimicrobial activity. The minimum inhibitory concentrations (MICs) were found to be in the range of 0.5 to 64 μg mL−1 for the tested bacterial strains (A. baumannii, A. iwoffii, Enterobacter sp., E. coli, S. aureus, P. aeruginosa, S. typhi, S. dysenteriae and S. maltophilia) and of 0.25 to 2 μg mL−1 for Candida species (Figure 2). Özgeriş [21] synthesized nicotinoyl compounds 30–34 by reaction of nicotinoyl chloride with potassium thiocyanate or ammonium thiocyanate to get nicotinoyl isothiocyanate, which refluxed with substituted phenethylamines in acetone to yield the nicotinoyl thioureas (Scheme 6). All compounds showed excellent antibacterial activity against the tested bacterial strains S. aureus, E. faecalis, E. coli, A. baumannii, and P. aeruginosa with MIC concentrations of 31.25 to 62.5 μg mL−1. Ragab et al. [22] reported the synthesis of pyridine compounds 36 (by ternary condensation of cyanoacetamide 35, aromatic aldehyde and malononitrile in an equimolar molar ratio 1:1:1 in basic ethanol) and 37 (by cyclo-condensation of cyanoacetamide 35 with acetylacetone in DMF) with very good antimicrobial activity against all tested strains B. subtilis, S. aureus, E. faecalis, E. coli, P. aeruginosa, S. typhi, C. albicans, and F. oxysporum (MIC = 18–31 μM) (Table 1).
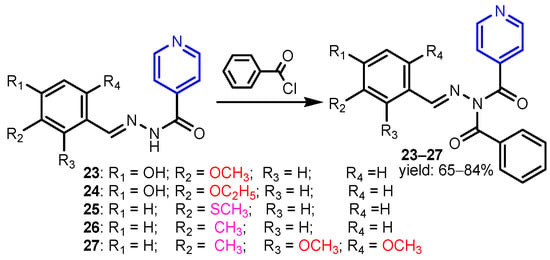
Scheme 5.
Synthesis of isonicotinic acid hydrazide benzoyl derivatives 23–27 [18].
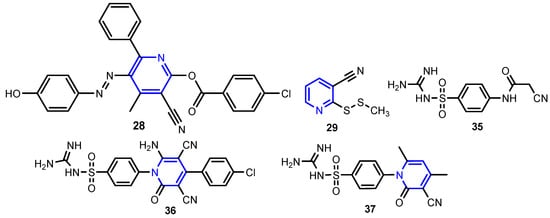
Figure 2.
Structure of pyridine compounds 28, 29, 36, 37, and intermediate 35 [19,20,22].
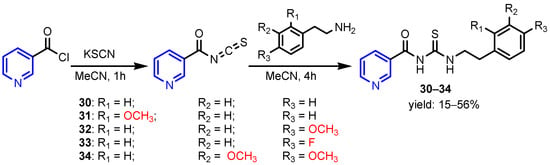
Scheme 6.
Synthesis of pyridine compounds 30–34 [21].

Table 1.
In vitro antibacterial activity (minimal inhibitory/bactericidal concentrations, MIC and MBC), expressed in μM, for sulfonamides 36 and 37 [22].
Marinescu et al. [23,24,25,26,27,28] synthesized a series of tetrasubstituted pyridine-N-oxides (octahydroacridines) 38–41, starting from the reaction of 2,2′-methylenedicyclohexanone with formamide at 167–170 °C and the separation of 1,2,3,4,5,6,7,8-octahydroacridine 36 from the reaction mixture (Scheme 7). Oxidation of compound 36 with hydrogen peroxide and acetic acid gave the key intermediate 37, sym-octahydroacridine-10-oxide, which, through reactions of nitration, reduction, alkylation, and hydrolysis, gave the compounds 38, 39, 40, and 41. It was found that antimicrobial activity against the tested pathogens S. epidermidis 1736, S. aureus ATCC 25923, E. faecalis ATCC 29212, B. subtilis 6688, P. stuartii 1116, P. aeruginosa 846, K. pneumoniae ESBL+, S. marcenscens 0804, C. freundii 1748, E. coli 1576, ESBL+, Salmonella sp. 9246, C. albicans ATCC 10231 increased in the order 40 < 39 < 38 < 41, simultaneously with the decrease of the hydration energy from 0.59, to 1.72, to 3.14 and respectively to 3.76 kcalMol−1.
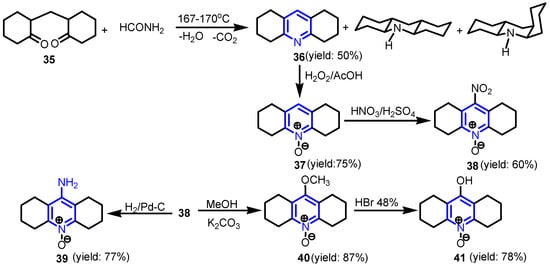
Scheme 7.
Synthesis of 1,2,3,4,5,6,7,8-octahydroacridine-10-oxides 38–41 [23,24,25,26,27,28].
3. Synthesis of Antimicrobial Pyridine Salts
Furdui et al. [29] reported efficient synthesis of symmetrical diquaternary salts by alkylation of 4-[2-(pyridin-4-yl)ethyl]pyridine or 4,4′-bipyridine, with various bromo- or chloro-acetophenone analogues (Scheme 8) and investigated their antimicrobial activity against nine different microorganisms: B. subtilis, B. cereus, S. lutea, R. glutinis, C. utilis, S. cerevisiae, A. niger, G. candidum and P. roqueforti. Compounds 42a–42d, 43a and 43d show efficient inhibitory properties at least against one bacterial strain. Marek et al. [30] synthesized a set of pyridine-4-aldoxime-based quaternary ammonium salts with differing lengths of alkyl side chains 44–50 (Scheme 9). The in vitro antibacterial activity of all compounds was tested on a panel of eight bacterial strains (S. aureus CCM 4516/08, S. aureus MRSA H 5996/08, S. epidermidis HK6966/08, Enterococcus sp. HK14365/08, E. coli CCM 4517, K. pneumoniae D 11750/08, K. pneumoniae J 14368/08, and P. aeruginosa CCM 1961), four yeasts (C. albicans ATCC 44859, C. krusei E28, C. tropicalis 156, C. glabrata 20/I) and four filamentous fungi (Trichosporon asahii 1188, Aspergillus fumigatus 231, Absidia corymbifera 272, Trichophyton mentagrophytes 445). The compounds with an alkyl chain of C12–C16 possessed the best antimicrobial activity of all.
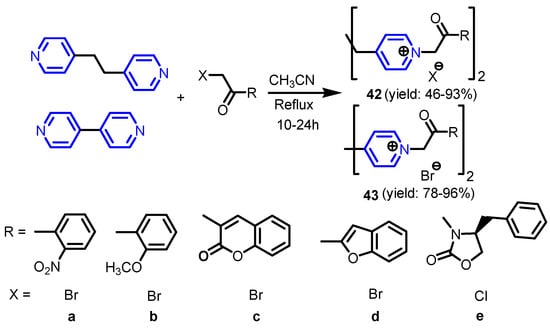
Scheme 8.
Synthesis of bis-pyridinium quaternary ammonium salts [29].
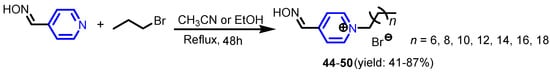
Scheme 9.
Synthesis of pyridine-4-aldoxime-based quaternary ammonium salts [30].
Brycki et al. [31] performed a Menshutkin reaction between 4-chloromethylpyridine and the N,N-dimethylalkylamines containing 8, 10, 12, 14, 16, 18 carbon atoms in an alkyl chain, respectively in acetonitrile to obtain compounds 51–56 in good yields at room temperature (Scheme 10). The minimal inhibitory concentration (MIC) values of pyridine salts 51–56 against the fungi A. niger, C. albicans, P. chrysogenum range from 0.1 to 12 mM and against bacteria S. aureus, B. subtilis, E. coli, P. aeruginosa range from 0.02 to 6 mM, as can be seen in Table 2.
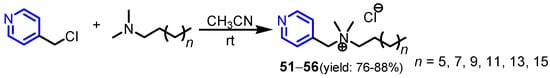
Scheme 10.
Synthesis of pyridine salts 51–56 [31].

Table 2.
Antimicrobial activities of compounds 51–56 expressed as MIC values (mM) [31].
Wang et al. [32] showed that pyridinium bromide salts 57–59 (Figure 3) exhibited excellent antibacterial activity against three Gram-negative bacteria Xanthomonas oryzae, Ralstonia solanacearum, and Xanthomonas axonopodis, because of the appropriate balance between hydrophobicity and hydrophilicity in these molecules.
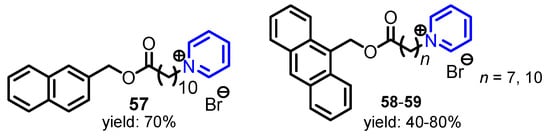
Figure 3.
Structure of pyridine salts 57–59 [32].
Ali et al. [33] reported synthesis of eight N-alkylated pyridine-based organic salts 60–67 (Scheme 11) and their antibacterial and antibiofilm activities. The best antibacterial activity was shown by compound 66 at the concentration of 100 μg mL−1 with MIC values of 56 ± 0.5% against S. aureus and 55 ± 0.5% against E. coli; followed by salt 65 (MIC = 55 ± 0.5% against E. coli) and 61 (MIC = 55 ± 0.5% against E. coli) at the same concentration. In the antibiofilm activity, salts 65, 60 and 67 inhibited 58 ± 0.4% S. aureus at a concentration of 75 μg mL−1, while 61 inhibited 55 ± 0.5% E. coli at concentration of 100 μg mL−1.
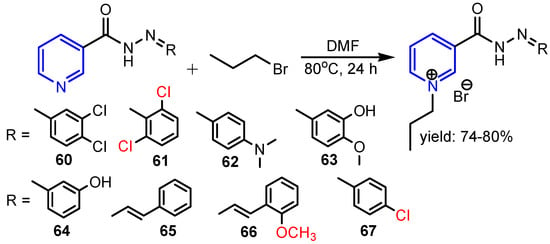
Scheme 11.
Synthesis of pyridine salts 60–67 [33].
Soukup et al. [34] synthesized five series of pyridinium salts 68a–68c, 69a–69c, 70a–70c, 71a–71c and 72a–72c via an SN2 type Menshutkin reaction (Figure 4) with yields of 81–98%. Compound 72c showed a rather versatile efficacy against the whole spectrum of microbes, three Gram-positive (S. aureus C1947, methicillin-resistant S. aureus MRSA C1926, vancomycin-resistant Enterococcus S2484), six Gram-negative strains (E. coli A1235, K. pneumoniae C1950, K. pneumoniae C1934, A. baumannii J3474, Stenotrophomonas maltophilia J3552, Yersinia bercovieri CNCTC 6230), and eight fungal strains, four yeasts (Candida parapsilosis sensu stricto EXF-8411, Rhodotorula mucilaginosa EXF-8417, Exophiala dermatitidis EXF-8470, Aureobasidium melanogenum EXF-8432) and four filamentous fungi (Bisifusarium dimerum EXF-8427, Penicillium chrysogenum EXF-1818, Aspergillus versicolor EXF-8692, and Aspergillus niger EXF-10185). Anwar et al. [35] reported synthesis of 4-(dimethylamino)pyridinepropylthioacetate 73 (Scheme 12) and effects of 73 coated with gold nanoparticles 73-AuNPs against E. coli (ATCC 8739). The MIC of the Pefloxacin + 73-AuNPs mixture was found to be 25 μg mL−1 as compared to Pefloxacin alone (50 μg mL−1), which clearly indicates that 73-AuNPs increased the potency of Pefloxacin.
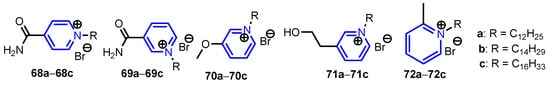
Figure 4.
Structure of pyridine salts 68–72 [34].
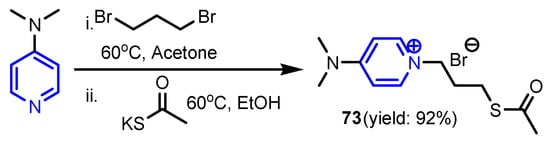
Scheme 12.
Synthesis of 4-(dimethylamino)pyridinepropylthioacetate 73 [35].
4. Synthesis of Antimicrobial Pyridine Compounds Containing a Five-Membered Ring with One or Two Heteroatoms
Tamilvendan et al. [36] synthesized two Mannich pyrol-pyridine bases 1-((pyridin-2-ylamino)methyl)pyrrolidine-2,5-dione 74 and 1-(phenyl(pyridin-2-yl amino)methyl) pyrrolidine-2,5-dione 75 using a classical Mannich reaction between succinimide, aniline, and formaldehyde or benzaldehyde (Figure 5) in good yields (78–80%). Both compounds showed moderate antimicrobial activity against the antibacterial panel (Escherichia coli, Salmonella typhi, and Bacillus subtilis) and antifungal agents (Aspergillus oryzae and Aspergillus fumigates), using Penicillin, Streptomycin, and Amphotericin B as standards.
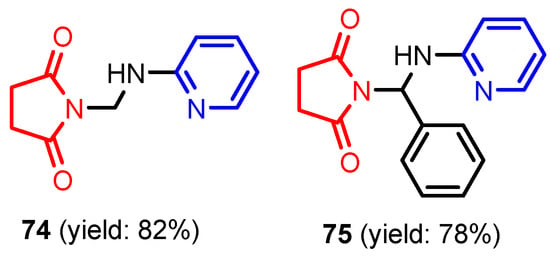
Figure 5.
Structure of Mannich pyrol-pyridine bases 74 and 75 [36].
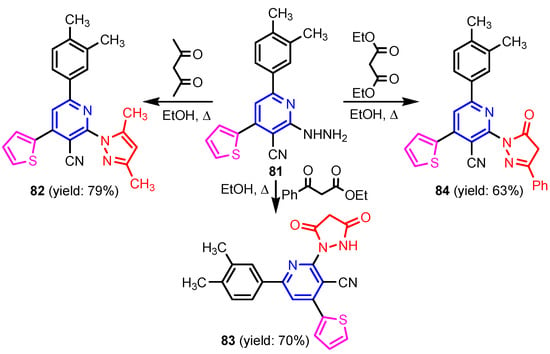
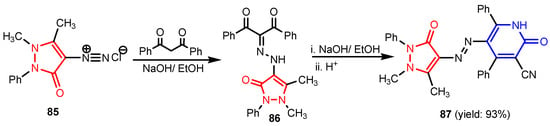
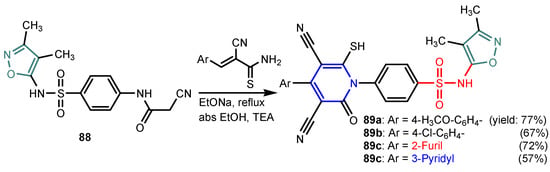
Angelova et al. [37] reported the synthesis of two pyridine-indole compounds 76a and 76b by condensation of nicotinohydrazide with 5-methoxy-1H-indole-3-carbaldehyde or 5-bromo-1H-indole-3-carbaldehyde in an ethanol solution, and evaluated their in vitro antibacterial activity against M. tuberculosis H37Rv (MIC = 1.6989 μM and 2.9139 μM respectively) using isoniazide and ethambutol as reference drugs (Scheme 13). Abo-Salem et al. [38] synthesized a new class of bis(indolyl)pyridines 77 analogues of the marine alkaloid nortopsentin in good yields (75–96%), using a one-pot four-component condensation of 3-cyanocarbomethylindole, various aldehydes, 3-acetylindole, and ammonium acetate in glacial acetic acid. Moreover, 2,6-bis(1H-indol-3-yl)-4-(benzofuran) pyridine-5-carbonitriles 78 were synthesized via a one-pot four-component condensation of 3-cyanocarbomethyl indole, 2-acetylbenzofuran, N-substituted-indole-3-aldehydes and ammonium acetate (Scheme 14). Four compounds—77b, 78a, 78c, and 78d—demonstrated good antimicrobial activity against S. aureus, E. coli., and C. albicans, as can be seen in Table 3. All antimicrobial active compounds studied (77b, 78a, 78c, and 78d) revealed favorable binding interactions and binding energy with the binding site of thymidylate kinase (ID: 4QGG) with values ranging from −15.52 to −25.36 kcal mol−1, which is comparable to the native ligand of −24.34 kcal mol−1. Figure 6 revealed the binding mode of the re-docked 78d within the binding pocket of TMPK (Thymidylate kinase, ID: 4QGG). The structures of Thymidylate kinase (TMPK), DNA gyrase subunit B, and DNA topoisomerase IV subunit B were retrieved from the protein data bank at http://www.rscb.org./pdb (accessed on 1 May 2022) using 4QGG, 6F86, 4HZ5 codes, respectively, and a Gaussian Contact surface around the binding sites were drawn using MOE 2008.10 (Molecular Operating Environment, http://www.chemcomp.com (accessed on 1 May 2022). Mabkhot et al. [39] reported synthesis of thiophene-pyridine compound 80 by reaction of the enaminone 79 with pentane-2,4-dione in glacial acetic acid in the presence of ammonium acetate (Scheme 15). The presence of the pyridine nucleus has been shown to be necessary for better antifungal activity than Amphotericin B (standard) against Aspergillus fumigates and Syncephalastrum racemosum. Moreover, compound 80 revealed a moderate activity towards Geotricum candidum and Candida albicans. Flefel et al. [40] reported the synthesis of thiophene-pyrazole-pyridine hybrids 82–84, by reaction between thiophene-pyridine 81 and carbonilic compounds (Scheme 16). All synthesized compounds possessed good antimicrobial activity against almost all tested strains, Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Salmonella typhimurium, Aspergillus flavus, and Candida albicans. Figure 7 shows the docked images of the drug candidates 82–84 on the target protein. The results of in silico studies revealed a moderate-to-good pattern of affinity and activity of these compounds on the target protein GlcN-6-P synthase, which is supported by in vitro antimicrobial screenings [40]. In this study, a Lamarckian genetic algorithm contained in AutoDock (version 4.0) was employed for in silico molecular docking studies. Alnassar et al. [41] synthesized phenyldiazenyl pyridone 87 starting from diazopyrazolone 85 in two steps: condensation with 1,3-diphenylpropane-1,3-dione in alkaline ethanole to give intermediate 86, and cyclisation of 86 in acidic medium (Scheme 17). The antibacterial activity revealed that compound 87 was active against two tested strains, Bacillus subtilis ATCC 6633 and Escherichia coli ATCC 25922. Nasr et al.; [42] synthesized izoxazole-pyridone derivatives by refluxing cyanothioacetamide 88 solution with the corresponding arylidene derivatives in absolute ethanol (Scheme 18). All compounds have good antimicrobial activity against all tested strains, S. pneumoniae, B. subtilis, S. epidermidis, E. coli, P. vulgaris, and K. pneumonia. The pyridonethiols 89b and 89c showed double potency of Ampicillin in inhibiting the growth of B. subtilis (MIC = 0.12 μg mL−1 vs 0.24 μg mL−1), while compound 89d was equipotent to Ampicillin against the same bacteria. Kamat et al. [43] reported a synthesis of thiazole-pyridine hybrids 94a–94c in four steps, starting from nicotinonitrile (Scheme 19). The in vitro antimicrobial assay showed that compounds 94 are effective against all tested strains S. aureus, B. subtilis, E. coli, P. aeruginosa, A. flavus, T. atroviridae, P. citranum, and C. albicans with safe pharmacokinetic properties. Patel et al. [44] synthesized two series of pyridine-benzothiazole hybrids 95 and 96, by reaction between 2-[N-(substitutedbenzo thiazolyl)amino]pyridine-3- carbonyl chlorides and 2-(piperazin-1-yl)ethanol or 2,3-dichloropiperazine, respectively with yields of 55–74% (Figure 8). It was found that all compounds have a poor to fair activity against all tested strains, P. aeruginosa, E. coli, S. aureus, B. subtilis, and C. albicans. Sedaghati et al. [45] synthesized novel pyrimidines 97, 98, and 99 by a classic Biginelli reaction, using FeCl3 as a catalyst (Scheme 20). Most of the compounds had better antifungal than antibacterial activity against the tested microorganisms (E. coli, S. enteritidis, P. aeruginosa, S. aureus, L. monocytogenes, B. subtilis, A. niger, C. albicans). Moderate antifungal agents, 97a, 98a, and 98b were determined among the studied compounds. Yadav et al. [46] reported benzimidazole-pyridine 100 synthesis with weak antimicrobial activity against tested strains, S. aureus, B. subtilis, E. coli, C. albicans, and A. niger, using Ciprofloxacin and Fluconazole as control drugs (Figure 9). Desai et al. [47] reported synthesis of benzimidazole-2-pyridone hybrids 104 using the reactions shown in Scheme 21. Condensation of 1-(1H-benzo[d]imidazol-2-yl)ethanone 101 with 2-hydrazinylacetyl cyanide in alcohol gave hydrazinylacetylcyanide 102, which is cyclized in the second step with 2-(2-hydroxy-benzylidene)malononitrile in the presence of a catalytic amount of piperidine to furnish dihydropyridine-3,5-dicarbonitrile 103. Finally, 103 is condensed with different aryl aldehydes to form the final compounds 104a–104h. Compounds bearing chloro and hydroxy groups exhibit very good to excellent antimicrobial activity against all tested strains, S. aureus, S. pyogenes, E. coli, P. aeruginosa, C. albicans, A. niger, and A. clavatus. A new series of benzimidazole derivatives bearing cyanopyridine and 4-thiazolidinone motifs were synthesized by Desai et al. [48] in four steps, starting by condensation of 1-(1H-benzo[d]imidazol-2-yl)ethanone with aromatic aldehydes to get compounds 105a–105e, which were treated with ammonium acetate and malononitrile to achieve derivatives 106a–106e, produced via a Knoevenagel condensation reaction (Scheme 22). Compounds 106 underwent simple condensation reactions with 3-nitrobenzaldehyde to provide Schiff bases 107a–107e, which in the last step generated final analogs 108a–108e using cyclization with thioglycolic acid. As can be seen in Table 4, the antimicrobial activities of the compounds 108a–108e were good to excellent, compound 108d having better antimicrobial activities than the standards used—Chloramphenicol and Ketoconazole—in most cases, followed by compound 108e, which proves that the presence of the methoxy group and the hydroxy group simultaneously, or, in the case of the methoxy group, leads to a better biological activity. Continuing the research, Desai et al. [49] reported the synthesis of 2-azetidinones 109 from the reaction of compounds 107 with chloroacetyl chloride in 1,4-dioxane. Compounds 109a–109d (Figure 10) showed excellent antimicrobial activity compared to the standards used—Chloramphenicol and Ketoconazole. Furthermore, Rani and Reddy [50] reported the synthesis and antimicrobial activity of pyridine containing substituted phenyl azetidine-2-ones 110. Compounds 3-chloro-1-(4-fluorophenyl)/(4-chlorophenyl)-4- (pyridine-3-yl) azetidine-2-one (110a and 110b) were found to be more potent against all tested strains, S. aureus, B. subtilis, E. coli, P. aeruginosa, A. niger, and P. rubrum. Sarojini et al. [51] reported synthesis of 4-(2,5-dichlorothiophen-3-yl)-2-(pyridin-3-yl) thiazole 112 by reaction of pyridine-4-carbothioamide 111 with 2-bromo-1-(2,5-dichlorothiophen -3-yl)ethanone in ethanolic KOH (Scheme 23). Compound 112 had a weak antimicrobial activity against the tested strains, S. aureus and P. aeruginosa (MIC = 100 μg mL−1), compared to the standard medicine Ampicillin (MIC< 6.5 mL−1). Desai et al. [52] reported the synthesis of pyrazole bearing pyridyl oxadiazole analogues 114a–114e starting from 3-(4-nitrophenyl)-1- phenyl-1H-pyrazole-4-carbaldehyde, in three steps: 1. condensation, with isonicotino hydrazide 113 with formation of hydrazide; 2. cyclization reaction with acetic anhydride under reflux condition, with the formation of the 1,3,4-oxadiazole; 3. Claisen-Schmidt condensation of 1,3,4-oxadiazole intermediate with aromatic aldehydes in ethanolic potassium hydroxide (Scheme 24). All compounds showed considerable inhibition against nearly all of the tested bacteria and fungi as compared to the reference drugs, Chloramphenicol and Griseofulvin, as can be observed in Table 5. Following the same synthetic steps, but starting from 3-(4-fluorophenyl)-1-phenyl-1H-pyrazole-4- carbaldehyde, Desai et al. [53] synthesized fluorinated pyrazole encompassing pyridyl 1,3,4-oxadiazole motifs 115a–115d (Figure 11). All compounds showed considerable inhibition against nearly all of the tested bacteria, and were proved to be potential antifungal agents, superior to the reference drug (Griseofulvin) at low level of cytotoxic concentrations.
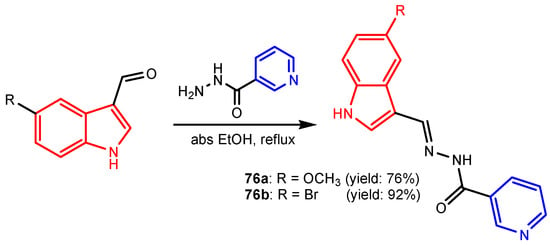
Scheme 13.
Synthesis of pyridine-indole compounds 76a and 76b [37].
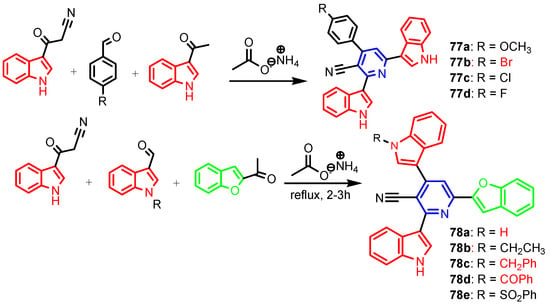
Scheme 14.
Synthesis of bis(indolyl)pyridines 77a–77d and 78a–78e [38].

Table 3.
Minimum inhibitory concentrations (MIC, μg mL−1) and minimum bactericidal concentrations (MBC, μg mL−1) of the most active bis(indolyl)pyridines [38].
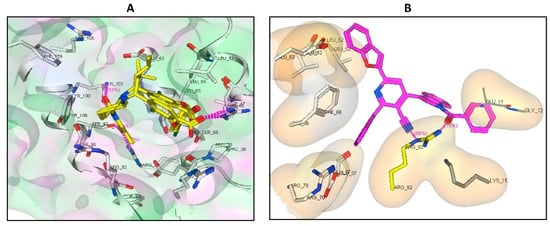
Figure 6.
(A) The binding mode of the re-docked ligand within the binding pocket of TMPK (ID: 4QGG). The re-docked ligand shows superimposed on the same position as the native ligand (yellow, stick) with the same orientation. The two ligands show the same H-bonds profile with Gln101, Arg48, Arg70, and Ser97 (white, stick). (B) The binding mode of compound 78d (pink, stick) within the binding pocket of TMPK (ID: 4QGG). Compound 78d shows π-cation interactions and two H-bound acceptors with the amine acid residue Arg92 (yellow, stick) [38].
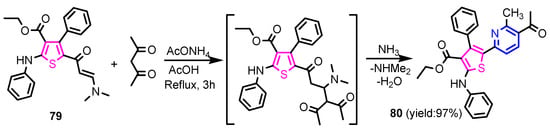
Scheme 15.
Synthesis of thiophene-pyridine compound 80 [39].
Scheme 16.
Synthesis of thiophene-pyrazole-pyridine compounds 82–84 [40].
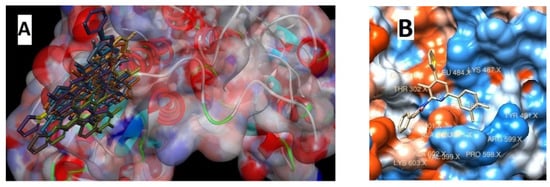
Figure 7.
Docking into the active site of glucosamine-6-phosphate (GlcN-6-P) synthase (PDB ID: 2VF5). (A) Docked poses of compounds 82–84 on the GlcN-6-P; (B) the interaction the compound 84 and GlcN-6-P [40].
Scheme 17.
Synthesis of phenyldiazenyl pyridone 87 [41].
Scheme 18.
Synthesis of isoxazol-pyridonethiols 89a–89d [42].
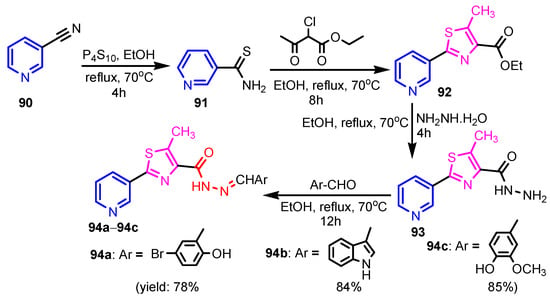
Scheme 19.
Synthesis of thiazole-pyridine hybrids 94a–94c [43].
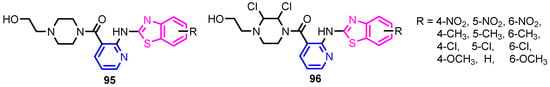
Figure 8.
Structures of pyridine-benzothiazole hybrids 95 and 96 [44].
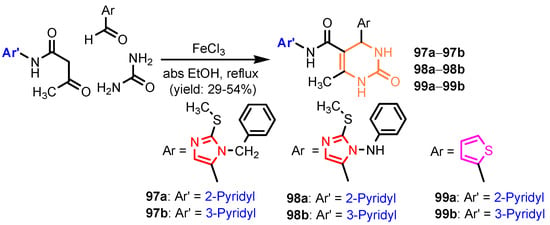
Scheme 20.
Synthesis of pyrimidines 97, 98 and 99 [45].
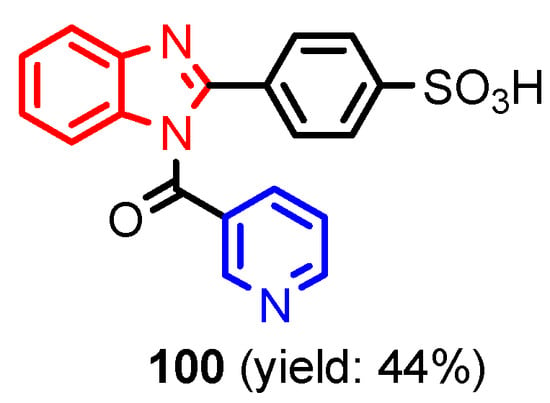
Figure 9.
Structure of benzimidazole-pyridine 100 [46].
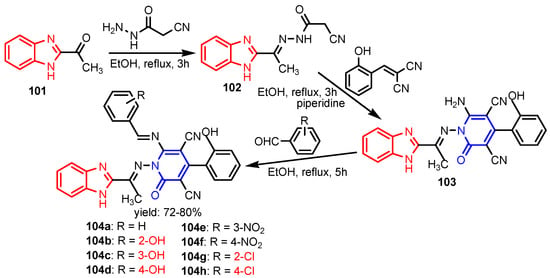
Scheme 21.
Synthesis of benzimidazole-2-pyridone hybrids 104a–104h [47].
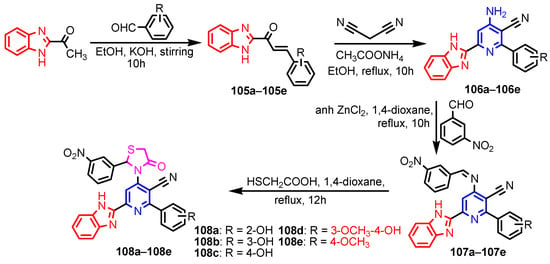
Scheme 22.
Synthesis of benzimidazole-cyanopyridine—thiazolidinones 108a–108e [48].

Table 4.
In vitro antimicrobial screening results of compounds 108a–108e.
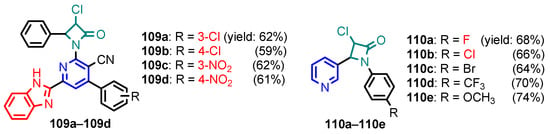
Figure 10.
Structure of benzimidazole-pyridine 109a–109d and 110a–110e [49,50].
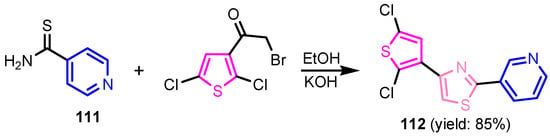
Scheme 23.
Synthesis of 4-(2,5-dichlorothiophen-3-yl)-2-(pyridin-3-yl)thiazole 112 [51].
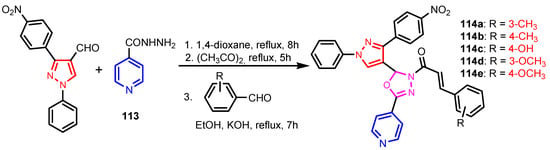
Scheme 24.
Synthesis of pyrazole bearing pyridyl oxadiazole analogues 114a–114e [52].

Table 5.
Antimicrobial activity (MIC values in μg mL−1) of analogues 114a–114e.
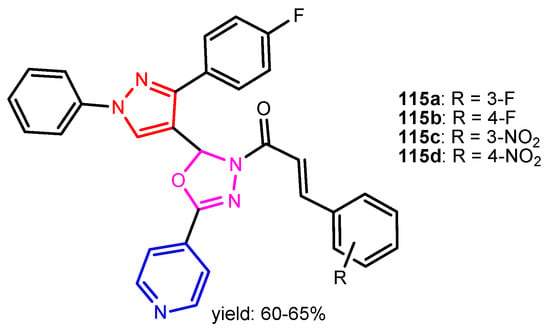
Figure 11.
Pyridyl-pyrazole-1,3,4-oxadiazoles 115a–115d [53].
5. Synthesis of Antimicrobial Pyridine Compounds Containing a Five-Membered Ring with Three Heteroatoms
Murty et al. [54] synthesized 1,3,4-oxadiazoles–pyridines 117a–117c through an oxidative C–O coupling by direct C–H bond activation of N-aroyl-N-arylidinehydrazines 116a–116c using a catalytic quantity of CuO nanoparticles (Scheme 25). Compound 117a showed broad-spectrum antibacterial activity for all tested strains, E. coli, B. subtilis, M. luteus, K. pnemoniae MIC 37.5 μg mL−1, and compounds 117a and 117b exerted antifungal activity better than standard drug, Cycloheximide.
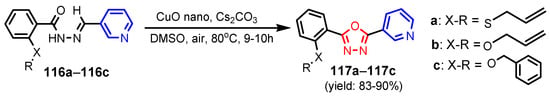
Scheme 25.
Synthesis of 1,3,4-oxadiazoles–pyridines 117a–117c [54].
Krishna et al. [55] synthesized compound 120 in two steps: alkylation of 4-hydroxy-2H-chromen-2-one 118 to give ester 119, which by condensation reaction with (Z)-N’-hydroxyisonicotinimidamide under reflux temperature for 24 h, gave 4-[(3-(4-pyridyl)-1,2,4-oxadiazol-5-yl)methoxy]-coumarin 120 (Scheme 26). Compounds 121 and 122 were synthesized by similar reactions (Figure 12) and showed good antibacterial activity against S. aureus and E. coli, and pyridines 120–122 presented better antifungal activity than that of standard drug, Clotrimazole. Bhardwaj et al. [56] synthesized pyridine imidazo [2,1b]-1,3,4-thiadiazoles 125a–125e in two steps, from pyridine-2,5-dicarboxylic acid 123, through intermediate 124 (Scheme 27). All compounds were found to be promising candidates against tested strains, B. pumillus, S. aureus, V. cholera, E. coli, P. mirabilis, P. aeruginosa and C. albicans. Panda and Jain [57] synthesized pyridine-triazoles 127a–127k by a condensation reaction between 3-hydrazinylpyridine 126, the corresponding aldehyde and ammonium acetate in acetic acid at 25 °C (Scheme 28). Minimum inhibitory concentrations (MIC, Table 6) showed excellent antimicrobial activity of compounds 127i–127k, substituted with trimethoxyphenyl, furanyl and thiophenyl, as well as good activity of compounds 127b, 127d, 127g and 127h. Table 3 marked green the values identical to those of the standard medicines used, Ciprofloxacin and Miconazole. Morsy et al. [58] synthesized 1,2,3-triazole nucleosides based on functionalized nicotinonitriles 131a–131b and 132, via [2 + 3]-dipolar-cycloaddition of terminal acetylenes 128a–128b, 129 and 1-azidoglucose 130 (Figure 13) in the presence of copper (I) ions generated from copper(II) sulfate and sodium L-(+)-ascorbate. Compounds 131a–131b and 132 showed good in vitro antibacterial activity against Staphylococcus aureus ATCC 6538, Micrococci luteus ATCC 10240, Pseudomonas aeruginosa ATCC 9027, Escherichia coli ATCC 10536, Candida albicans ATCC 10231, and Aspergillus niger ATCC 16404.
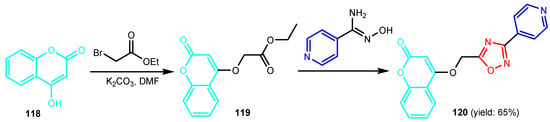
Scheme 26.
Synthesis of 4-[(3-(4-pyridyl)-1,2,4-oxadiazol-5-yl)methoxy]-coumarin 120 [55].
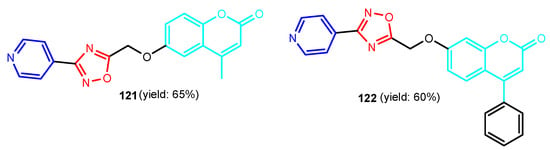
Figure 12.
Structure of 1,2,4-oxadiazol-coumarin-pyridines 121 and 122.
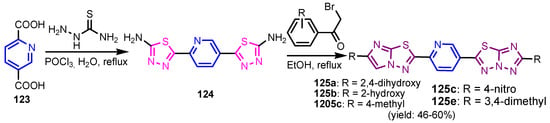
Scheme 27.
Synthesis of imidazo[2,1b]-1,3,4-thiadiazoles 120a–120e [56].
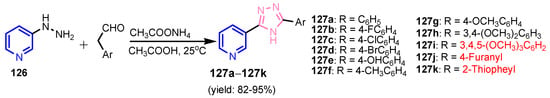
Scheme 28.
Synthesis of pyridine-triazoles 127a–127k [57].

Table 6.
Antimicrobial activity (MIC values in μg mL−1) of pyridine triazoles 122a−122k [57].
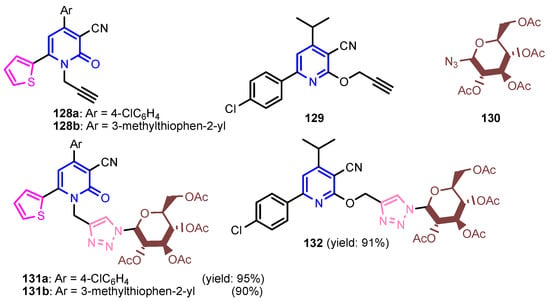
Figure 13.
Structures of compounds 128–132 [58].
6. Synthesis of Antimicrobial Pyridine Compounds Containing a Six-Membered Ring with One, Two, or Three Heteroatoms or Macrocycles
Deng et al. [59] synthesized (S)-2-(ethyl(2-oxo-1,2,3,4-tetrahydroquinolin-5-yl)amino) ethyl-2-(picolinamido)propanoate 134 in six steps, from 5-amino-3,4-dihydroquinolin- 2(1H)-one 133 (Scheme 29). Compound 134 showed moderated activity gainst all tested strains, S. aureus, MRSA, B. subtilis, B. proteus, E. coli, P. aeruginosa, C. albicans, C. mycoderma, S. cerevisiae, C. neofonmans, and A. flavus, considering Streptomycin and Fluconazole as standards. Ranjbar-Karimi and Poorfreidoni [60] reported the reaction of pentafluoropyridine 135a with 2-((7-chloroquinolin-4-yl) amino)ethan-1-ol 136 in the presence of potassium carbonate in CH3CN, to give 7-chloro-N-(2-((perfluoropyridin -4-yl)oxy)ethyl)-quinolin-4-amine 138a. Moreover, the reaction of N1-(7-chloroquinolin-4-yl) ethane-1,2-diamine 136 with 2,3,5,6-tetrafluoro-4-phenylsulphonyl pyridine 135b in the presence of potassium carbonate in CH3CN, gave N1-(7-chloroquinolin-4-yl)-N2- (3,5,6-trifluoro-4-(phenylsulfonyl)pyridin-2-yl)ethane-1,2-diamine 139a in a high yield. Similar reactions occurred to give products 138b, 139b, 140, and 141, respectively in good yields (75–80%) (Scheme 30). Compounds 138a, 138b, 139a, and 141 showed moderate antibacterial activity against Gram-positive bacterium, S. aureus. Myangar and Raval reported [61] synthesis of azetidinyl-3-quinazolin-4-one hybrids 143a–143h from N-(2-(chloromethyl)-4-oxoquinazolin-3(4H)-yl)isonicotinamide 142 in three steps: (1) synthesis of intermediate hydrazides by reflux with hydrazine in ethanol; (2) synthesis of Schiff bases by condensation of hydrazides with aldehydes in ethanol and acetic acid; and (3) synthesis of final azetidines 143 by reaction of Shiff bases with chloracetylchloride in dry benzene, in the presence of triethylamine (TEA) (Scheme 31). The antibacterial activity data indicate that the analogs with halogen and nitro substituents emerged as promising antimicrobials, showing better to moderate activity, while analogs bearing chloro and methoxy substituent showed better antifungal activities, see bold compounds (Table 7). Saleh et al. [62] synthesized pyridine-fluorophenyl[1,3,5]triazines 148a–148b starting from 2,4,6-trichloro -1,3,5-triazine 144, and pyridin-3-amines 145, via intermediates 146 and 147, and by using in the last step a Suzuki coupling reaction (Scheme 32). Compounds 148a and 148b showed excellent antibacterial activity against S. epidermidis, with MIC values of 16 μg mL−1 and 32 μg mL−1, respectively, taking Streptomycin sulfate as standard (MIC > 16 μg mL−1). Solankee and co-workers reported [63] that compound 149 (Figure 14) exhibited promising antibacterial activity against Micrococcus flavus with a zone of inhibition value (iv) of 7 mm, which was slightly less active than the standard Ampicillin (iv = 8mm) [54]. Rajavelu and Rajkumar reported [64,65] synthesis of triazino- oxacalixarenes 150 and 151 and their good antibacterial activity against E. coli (MIC = 50 μgmL−1). These oxacalixarenes were docked against the covalent acyl enzyme PDB: 1PW8, and the most potent compound 151 exhibited a good docking score, glide energy, a strong hydrogen bond and hydrophobic interactions with the amino acids in the active pocket. Moreover, oxacalixarene 151 showed strong hydrogen bond interactions with Ser 62, Asn 161, Thr301, and Tyr306 and hydrophobic interactions with Thr116, Phe 120, Thr123, Tyr159, Trp 233, Arg 285, Gln 303, and Asn 327, as seen in Figure 15. The computational work (molecular docking) for the oxacalixarenes used the Schrodinger software version 9.0 for the molecular docking.
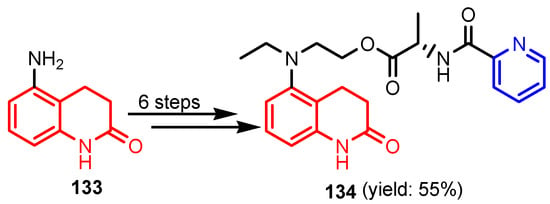
Scheme 29.
Synthesis of tetrahydroquinolin-pyridine 134 [59].
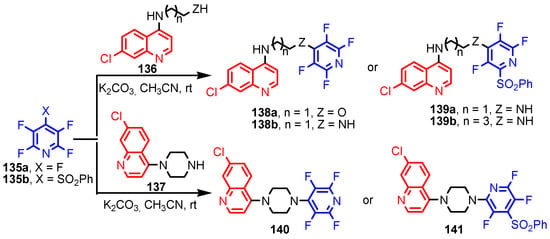
Scheme 30.
Synthesis of quinoline-pyridine compounds 136–141 [60].
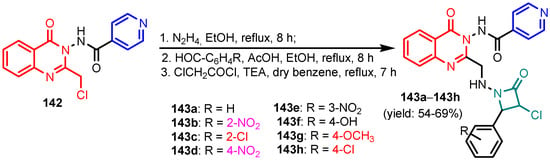
Scheme 31.
Synthesis of azetidinyl-3-quinazolin-4-one hybrids 143a–143h [61].

Table 7.
Minimal inhibition concentration (μg mL−1) of hybrids 143a–143h [61].
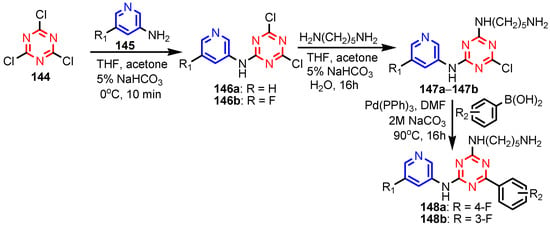
Scheme 32.
Synthesis of pyridine-fluorophenyl[1,3,5]triazines 143a–143b [62].
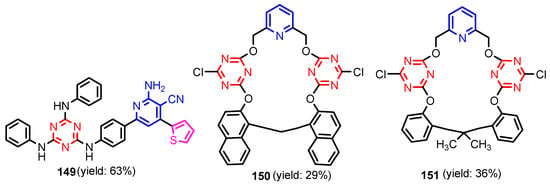
Figure 14.
Structures of compounds 144–146 [63,64,65].
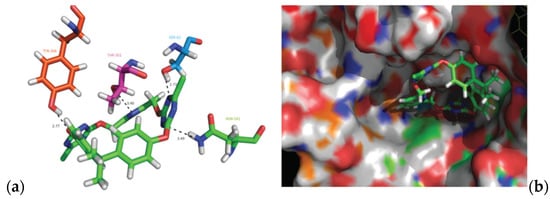
Figure 15.
(a) Binding of oxacalixarene 151 with the covalent acyl enzyme (PDB ID: 1PW8). (b) The oxacalixarene substrate 151 bound to the binding site of quadruple mutant, showing the fitting of the test oxacalixarene on the substrate surface.
7. Synthesis of Antimicrobial Macrocyclic Pyridine Compounds
Al-Salahi et al. [66] synthesized N2,N6-bis(1-hydrazinyl-1-oxopropan-2-yl)pyridine-2,6-dicarboxamide 153, in three steps from pyridine-2,6-dicarboxylic acid 152. Reaction of 153 with 1,2,4,5-benzenetetracarboxylic acid dianhydride or 1,4,5,8-naphthalene tetracarboxylic acid dianhydride in refluxing acetic acid afforded the corresponding macrocyclic octaamide-tetraimide chiral pyridine derivatives 154 and 155, respectively (Scheme 33). Table 8 shows that compounds 154 and 155 had significant antimicrobial activities against all tested strains, B. subtilis, S. aureus, E. coli, and C. albicans. Using a similar synthesis strategy, Abd El-Salam et al. [67] synthesized chiral macrocyclic polyamides 156, 157 and 158a–158f (Figure 16). As can be seen in Table 9, except for compounds 158b, 158d (against B. subtilis) and 158c and 158e (against S. aureus), the activities of the tested compounds against the Gram-positive bacteria are slightly higher than that of the reference drug Ampicillin. Compounds 156 and 158a also had better inhibitory activity than Ampicillin against E. coli. The antifungal activity of compounds 156–158 was moderate considering the standard drug Ketoconazole.
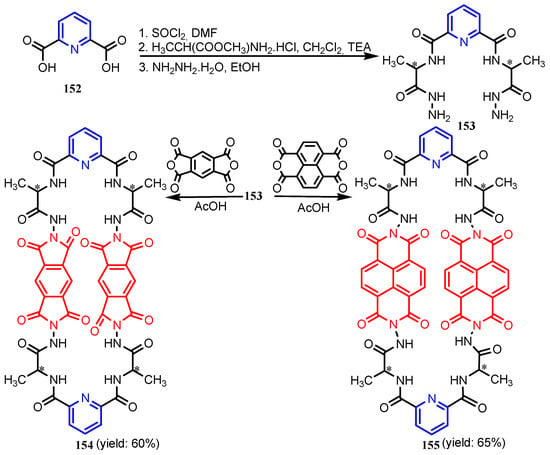
Scheme 33.
Syntheses of pyridine macrocyclic pyridine compounds 154 and 155 [66].

Table 8.
Antimicrobial activities of the compounds 154 and 155 [66].
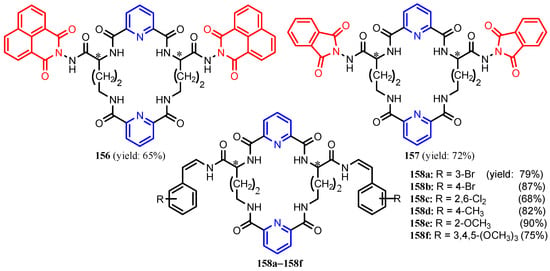
Figure 16.
Structures of compounds 151, 152 and 153a–153f [67].

Table 9.
Antimicrobial activities of the compounds 156–158 [67].
8. Synthesis of Fused Pyridines with Other Heterocycles with Antimicrobial Properties
Chandak et al. [68] synthesized pyrazolo[3,4-b]pyridines 159–161 by reaction between 3-substituted-(5-amino-1H-pyrazol-1-yl)benzenesulfonamide and appropriate trifluoro methyl-β-diketone (Scheme 34). Compound 159 was found to be the most potent analogue exhibiting a moderate antibacterial activity against Gram-positive bacteria, S. aureus and B. subtilis, while 160 and 161 exhibited moderate antifungal activity against S. cerevisiae and C. albicans, respectively (Table 10). Altalbawy [69] reported a reaction between compound 162 with 2-chloro-N-(4-bromophenyl) acetamide, in ethanolic sodium ethoxide solution at room temperature to give 4,4′-(5-methyl-1-phenyl-1H-pyrazole-3,4-diyl)bis-6-(2-furyl) thieno[2,3-b]pyridine-2-carboxamide 163 (Scheme 35). Compound 163 had moderate antimicrobial activity against E. coli and C. albicans strains. Jose et al. [70] synthesized imidazo[4,5-c] pyridines 166a–166c from substituted 3,4-diamino pyridine and carboxylic acids in the presence of DBU (1,8-Diazabicyclo(5.4.0)undec-7-ene) mediated by T3P (anhydride of propanephosphonic acid) (Scheme 36). All compounds displayed a promising antimicrobial activity compared to control antibiotic, Streptomycin and antifungal drug, Fluconazole. Poreba et al. [71] reported synthesis of new sulfonamide isoxazolo[5,4-b]pyridines 167a–167b by reaction between aryl sulfonic chlorides and amines (Figure 17). Both compounds showed moderate antibacterial activity against E. coli and P. aeruginosa. Reen et al. [72] prepared 2-(substituted-phenyl)oxazolo[4,5-b]pyridines 169a–169h using a one pot synthetic strategy in quantitative yield by reaction between 2-amino-3-hydroxy pyridine 168 and substituted benzoic acids in the presence of silica-supported perchloric acid (Scheme 37). As can be seen in Table 11, three compounds (169c, 169f, 169g) possessed strong antibacterial activity against all the four strains, with the best activity profile against S. aureus, and the other five compounds were found to be moderately active against all the tested strains of bacteria. Ali [73] reported synthesis of pyrazolo[3,4-b]pyridine 170 by treatment of 5-amino-1-(5,6-diphenyl-1,2,4-triazin-3-yl)-3-(methylthio)-1H-pyrazole-4-carbonitrile with benzoyl acetone in basic medium (Figure 16). Compound 170 showed moderate antimicrobial activity against tested strains, S. ureus, S. epidermidis, E. coli, and A. fumigatus. El-Borai et al. [74] reported that compound 5-(4-chlorobenzoyl)-1-phenyl-3-(pyridin-3-yl)-1H-pyrazolo[3,4-b]pyridin-6(7H)-one 171 exhibited strong activity against E. coli, E. cloaca, and Serratia. Appna et al. [75] synthesized pyrido[2,3-d]pyrimidines 173a–173c in four steps from ethyl 2-amino-6-(trifluoromethyl) nicotinate 172 (Scheme 38). All compounds exhibited significant antifungal activities against tested strains, C. albicans, C. parapsilosis, C. glabrata, C. krusei, and Issatchenkia hanoiensis. Patel et al. [76] reported synthesis of pyridoquinolones 175–180 from substituted aniline, substituted phenyl thioureas and 4-amino-N-(substitutedphenyl) benzenesulfonamide (Scheme 39). The structure–activity relationship study for pyridoquinolones demonstrated that both electron withdrawing as well as eletrodonating groups at the phenyl ring increase the antimicrobial activity. Furthermore, the presence of chloro and hydroxyl groups at the C–6 position showed a similar activity. Activities of amides increased in order: thioureido amides < sulfonamides < amides. Antibacterial activities increased in order: P. aeruginosa < S. aureus < B. substilis < E. coli.
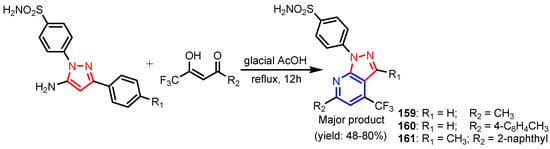
Scheme 34.
Synthesis of pyrazolo[3,4-b]pyridines 159–161 [68].

Table 10.
Minimum inhibitory concentration (MIC) in μg mL−1 of compounds 159–161.
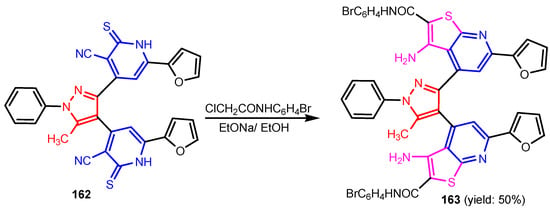
Scheme 35.
Synthesis of thieno[2,3-b]pyridine 163 [69].
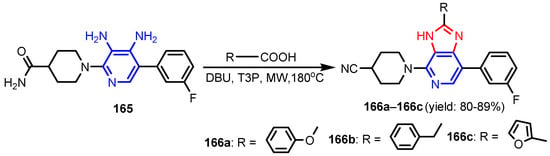
Scheme 36.
Synthesis of imidazo[4,5-c]pyridines 166a–166c [70].
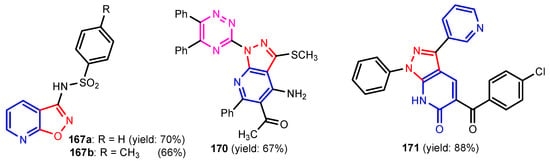
Figure 17.
Structures of compounds 167a, 167b, 170, and 171 [71,73,74].
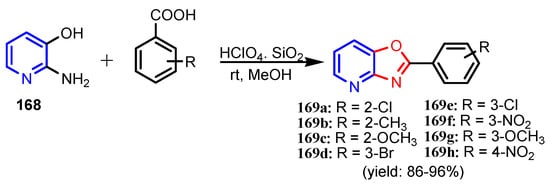
Scheme 37.
Synthesis of oxazolo[4,5-b]pyridines 169a–169h [72].

Table 11.
MIC values (in μg mL−1) of oxazolo[4,5-b]pyridine derivatives 169a–169h [72].
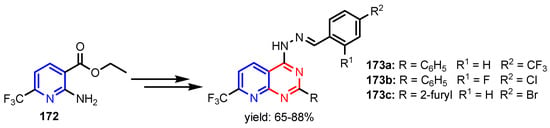
Scheme 38.
Synthesis of pyrido[2,3-d]pyrimidines 173a–173c [75].
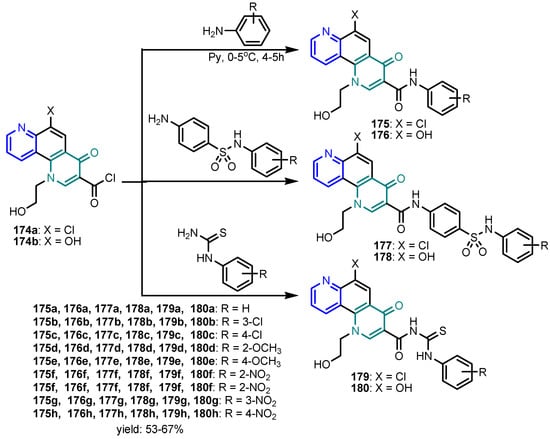
Scheme 39.
Synthesis of pyridoquinolone 175–180 [76].
Brahmbhatt et al. [77] synthesized coumarin-indenopyridine 183 using Krohnke’s conditions (Scheme 40), by the reaction of 3-coumarinylmethylpyridinium salts 181a–181b with appropriate 2-arylidene-1-indanones 182a–182b. Compounds 183a and 183b were the most potent against S. aureus and B. subtilis, respectively, while the rest of the compounds have moderate antibacterial and antifungal activity considering Ampicillin as standard. Salem et al. [78] reported the synthesis of ethyl 3-amino-4-(furan-2-yl)-6-(naphthalen-1-yl)furo[2,3-b]pyridine-2-carboxylate 184 using a one-pot four-component reaction. Compound 184 has a moderate antibacterial activity against E. coli and S. aureus considering Tetracycline as standard (Figure 18). Youssoufi et al. [79] reported the synthesis of 4H-pyrimido[2,1-b]benzothiazoles 185a–185h from curcumin and their antimicrobial activities against S. aureus, S. typhi, P. aeruginosa, E. coli, B. cereus, and P. rettgeri bacteria. All compounds showed moderate to high antibacterial activities (Table 12). Flefel et al. [80] reported the synthesis of compounds 187–192 in good yields (51–79%) from pyridine 186 using the pathways showed in Scheme 41. All synthesized compounds showed moderate to good antibacterial and antifungal activities against tested strains, S. aureus, B. subtilis, E. coli, S. typhimurium, A. flavus, and C. albicans, as compared to standard reference drugs, Gentamycin and Ketoconazole. El-Sayed et al. [81] reported that tetrazolo[1,5-a]pyridine derivative 193 and thienopyridine 194, synthesized from 6-ethoxy-2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitrile, have good antimicrobial activities against S. aureus, B. cereus, E. coli, P. aeruginosa, A. flavus, and A. niger as compared to standard drugs, Cefotaxime and Dermatin (Figure 19).
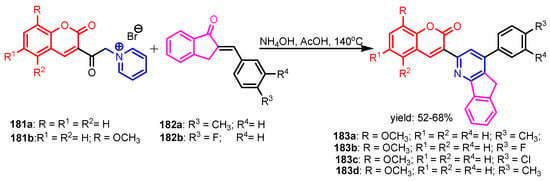
Scheme 40.
Synthesis of pyridoquinolone 183a–183b [77].
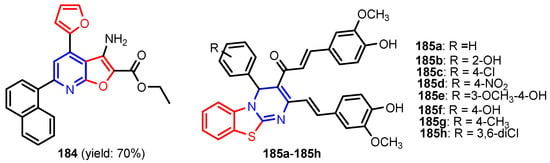
Figure 18.
Structures of compounds 184 and 185a–185h [78].

Table 12.
MIC values of 4H-pyrimido[2,1-b]benzothiazoles 185a–185h [79].
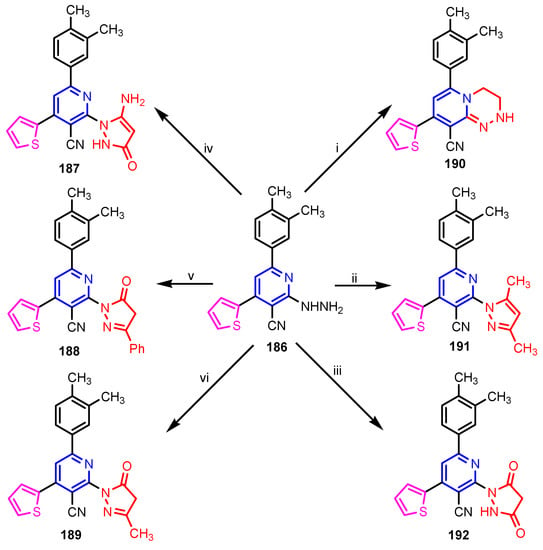
Scheme 41.
Synthesis of compounds 187–192; reagent and conditions: (i) ClCH2CH2Cl, DMF, heat; (ii) CH3COCH2COCH3, EtOH, heat; (iii) CH2(COOEt)2, EtOH, heat; (iv) NCCH2CO2Et, EtOH, heat; (v) PhCOCH2CO2Et, EtOH, heat; (vi) 1-CH3COCH2CO2Et, EtOH, heat; 2- EtONa, heat. [80].
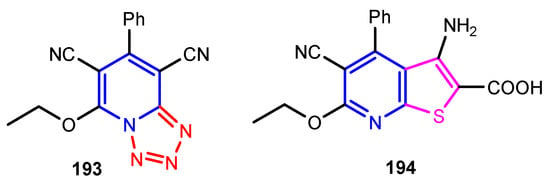
Figure 19.
Structures of compounds 193 and 194 [81].
El-Deen et al. [82] reported the synthesis of the pyridothienopyrimidine derivatives 196–198 in good yields (70–81%) from compounds 195a–195b, as can be seen in Scheme 42. The in vitro evaluation of these compounds against six strains of both Gram-positive (S. aureus, B. Subtilis, B. cereus) and Gram-negative (E. coli, S. typhimurium, P. aeruginosa) bacteria compared with Amoxicillin trihydrate as a reference drug showed a significant antibacterial activity for all compounds, especially against Gram-negative strains, with MIC values ranging from 7.81 to 62.5 μg mL−1
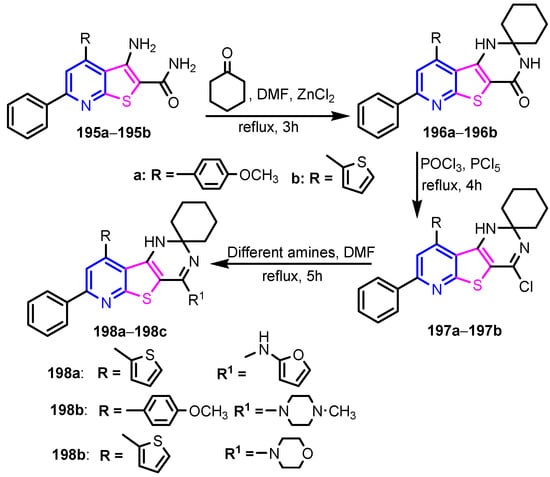
Scheme 42.
Synthesis of compounds 196–198 [82].
9. Synthesis of Pyridine Compounds Containing P, Se, and B with Antimicrobial Properties
Abdel-Megeed et al. [83] synthesized a series of diphenyl 1-(arylamino)-1-(pyridin-3-yl) ethyl phosphonates 200a–200e in high yields from reactions of 3-acetyl pyridine 199 with aromatic amines and triphenylphosphite in the presence of lithium perchlorate as a catalyst (Scheme 43). All compounds showed high antimicrobial activities against Escherichia coli NCIM2065, Bacillus subtilis PC1219, Staphylococcus aureus ATCC25292, Candida albicans, and Saccharomyces cerevisiae, at low concentrations (10–100 μg mL−1, Table 13).
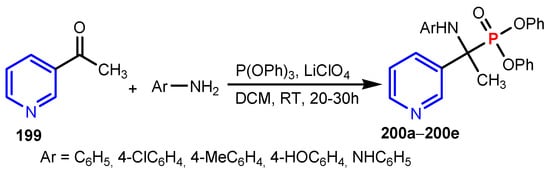
Scheme 43.
Synthesis of phosphorus containing pyridines 200a–200e [83].

Table 13.
Minimum inhibitory concentrations (MICs) of α-aminophosphonates 200a–200e.
Sekhar et al. [84] synthesized phosphorylated nucleosides 202a–202b from the reaction between compound 201 and the corresponding pyridines (Scheme 44). Both compounds 202a–202b exhibited better activity on Staphylococcus aureus when compared to standard, Gentamycine. Devineni et al. [85] synthesized compounds 205a–205d by reaction of 2-amino-2,3-dihydro-1H-2λ5-[1,3,2]diazaphospholo[4,5-b]pyridin-2-one 203 and compounds 204 (Scheme 45). Urea derivatives 205a, 205b, 205c, and 205d showed growth of inhibition against all the tested bacterial strains B. cereus ATCC 11778, S. faecalis MTCC 0459, E. coli ATCC 9637, and P. marginalis MTCC-2758.
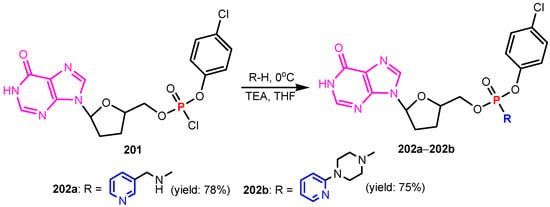
Scheme 44.
Synthesis of phosphorus containing pyridines 202a–202b [84].
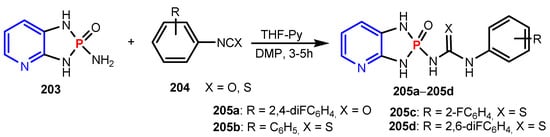
Scheme 45.
Synthesis of phosphorus containing pyridines 205a–205d [85].
Kumar et al. [86] synthesized organoselenium imidazo[1,2-a]pyridines compounds 207a–207c in four steps, starting with 2-aminopyridines 206a–206b (Scheme 46). Compounds 207a and 207c were observed to exhibit antibacterial activity with an MIC value of 2.48 μg mL−1 and 10.41 μg mL−1 respectively, against Gram-negative bacteria E. coli. These observed activities, in particular the one observed for compound 207a against E. coli, have been found to be more potent as compared to the few reports concerning antimicrobial activity of selenide-based compounds in the literature so far and quite similar to the Rifampicin (standard). Compound 207b was found to be exhibiting multi-spectrum activity against A. fumigates, C. krusei, A. niger, and C. parapsilosis with observed MIC values of 9.96, 9.96, 19.93, and 19.93 μg mL−1 respectively.
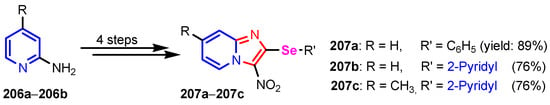
Scheme 46.
Synthesis of selenium containing pyridines 207a–207c [86].
Abdellattif et al. [87] synthesized organo-selenium compounds 210a–210c starting from 4,6-dimethyl-2-oxo-1,2-dihydropyridine-3-carbonitrile 208, in two steps (Scheme 47). Selanone 210c was found to be the most potent antibacterial agent against all tested strains, S. aureus, S. pyogenes, E. coli, and P. aeruginosa, as well as a good antifungal agent, against C. albicans, A. niger, and A. clavatus. A computational study of selenium compounds was performed for prediction of ADME properties by the QikProp3.2 tool available in Schrödinger 9.0 version (USA) and Molinspiration online property calculation toolkit to understand whether the compounds have the optimum pharmacokinetic properties to enter higher phases of the drug development process or not. It was found that compound 210c displayed the highest energy interaction (−8.48 KcalMol−1) within the binding pocket in the molecular docking study, as can be seen in Figure 20. Fontaine et al. [88] reported the synthesis of the boronic pyridines 212a and 212b (Scheme 48), which showed a 4-fold increase in activity compared to 6-(benzyloxy)pyridin-3-ylboronic acid. Furthermore, they emerged as potential Staphylococcus aureus NorA inhibitors as they potentiate the activity of Ciprofloxacin and Norfloxacin, have no significant intrinsic antibacterial activity, show a reduced cytotoxicity, and do not inhibit the mammalian P-gp efflux pump.
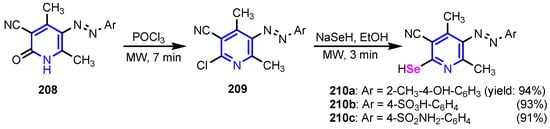
Scheme 47.
Synthesis of selenium-containing pyridines 210a–210c [87].
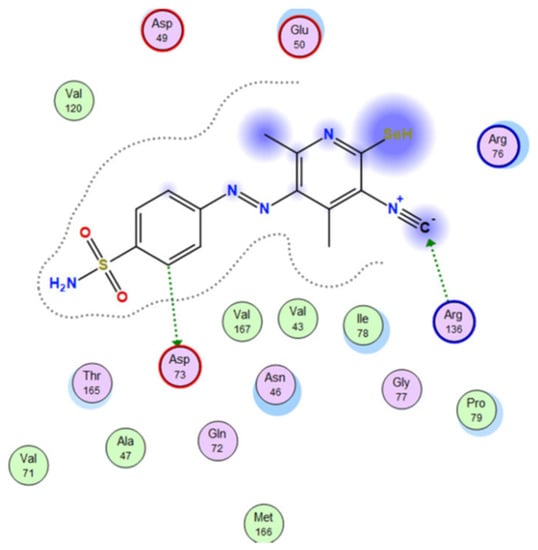
Figure 20.
3D interaction of selenium compound 210c with 1kzn protein [87].
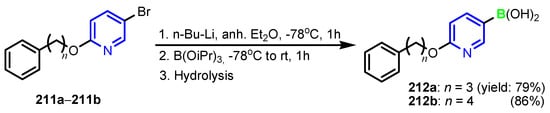
Scheme 48.
Synthesis of boron containing pyridines 210a–210c [88].
10. Synthesis of Antitubercular Pyridine Compounds
Tuberculosis (TB) remains a major threat to global public health, with at least 10 million new cases and 1.2 million deaths in 2019. The high incidence and mortality rates of TB can be attributed to the capacity of its etiological agent, intracellular bacterium Mycobacterium tuberculosis (MTb), to adapt to and survive in the aggressive physiological environment within the host [89]. The need for new tuberculostatic agents arises due to bacterial resistance to first-line drugs: Isoniazid (INH), Rifampicin (RIF), Ethambutol (ETH), Streptomycin (STR), and Pyrazinamide (PYR).
Navarrete-Vázquez et al. [90] synthesized 4-(5-substituted-1,3,4-oxadiazol-2-yl)pyridines 214a and 214b from INH and acid chlorides (Scheme 49). Compound 214a showed 10 and 20 times more potency than INH and STR, respectively, against the INH-resistant M. tuberculosis CIBIN 112 strain. Moreover, this compound was 27 times more active than ETH. Compound 214b showed the same behavior as 214a against this strain.
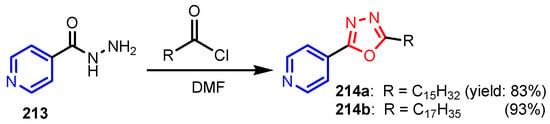
Scheme 49.
Synthesis of (1,3,4-oxadiazol-2-yl)pyridines 214a–214b [90].
Patel et al. [91] reported two series of compounds 215 and 216 with poor activity against M. tuberculosis when compared with Rifampicin (Figure 21). Furthermore, compounds showed a very good activity against C. albicans and possessed moderate to poor activity against A. niger and A. clavatus when compared to the standard, Griseofulvin. Sangani et al. [92] reported the synthesis of pyrido[1,2-a]benzimidazole derivatives 219a–219c, via a base-catalyzed microwave-assisted multicomponent cyclocondensation reaction (Scheme 50). Evaluation of antitubercular activity shows that compounds 219b and 219c were found to have better antitubercular activity when compared to standards Isoniazid and Rifampicin, and compound 219a appeared as the promising antimicrobial compound with a significant antitubercular activity.
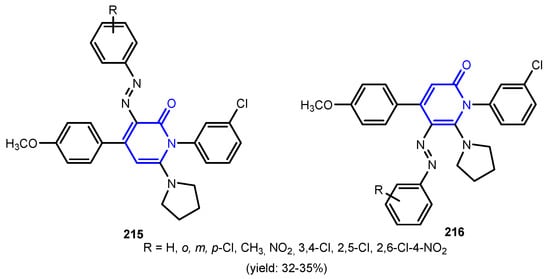
Figure 21.
Structures of compounds 215 and 216.
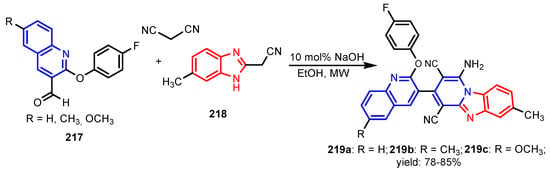
Scheme 50.
Synthesis of pyrido[1,2-a]benzimidazole derivatives 219a–219c [92].
Rathod et al. [93] synthesized a series of INH compounds 220a–220d and 221a–221d using multicomponent reactions in good yields (80–94%), as can be seen in Scheme 51. Electron-withdrawing groups were reported to increase the anti-TB activity [94,95]. Compound 220c (R1 = H) showed an MIC value of 3.12 μg mL−1, the minimum inhibitory concentration of 220a (R1 = Cl) was estimated at 6.25 μg mL−1, and the MIC value of 221a (R1 = Cl) was 12.5 μg mL−1. The other compounds were moderately active (MIC 50 μg mL−1) against M. tuberculosis H37Rv in comparison to the standard antitubercular drugs isoniazid (INH), pyrazinamide (PZA), streptomycin (STM), and ciprofloxacin (CPF), as can be seen in Table 14.
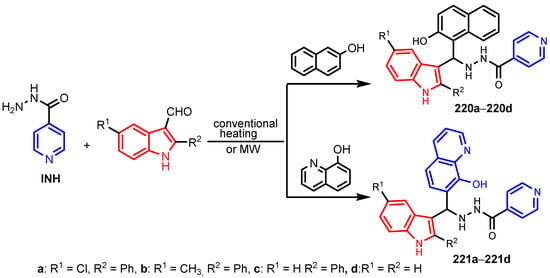
Scheme 51.
Synthesis of Isoniazid (INH) derivatives 220 and 221 as antitubercular agents [93].

Table 14.
In vitro antitubercular activity of compounds 220a and 221 in comparison to some reference drugs.
12. Conclusions
This review summarizes the syntheses of pyridine compounds with the antimicrobial and antiviral properties mentioned in the literature. Bioinformatics analysis plays a central role in finding new antimicrobial and antiviral structures, as is seen in the particular cases shown in the article. From the data presented, it can be concluded that the presence of another heterocycle (pyrazole, imidazole, thiazole, pyrimidine, benzimidazole, indole, etc.) on pyridine compounds improves antimicrobial or antiviral activities. In pyridinium salts it was found that an appropriate balance between hydrophobicity and hydrophilicity or an alkyl chain of C12–C16 improves the antimicrobial activity. Moreover, the presence of certain groups grafted on pyridine nuclei, such as -F, -Cl, -OH, -CF3, OCH3, -N(CH3)2, -NHCO, -COOCH3, -CHO, -NO2, and -CN, increases the antimicrobial and antiviral activity of the compounds. As is shown, the positions of the substituents on the pyridine molecule are also very important for the antimicrobial and antiviral activity of the compounds. Additionally, the binding linker between pyridine and another heterocycle is important for their antimicrobial and antiviral activity. Additionally, the antimicrobial activity is improved if the molecule contains linker groups such as carbonyl (CO), amide (NHCO), thioamide (NHCS), or other heteroatoms. It is also noted that pyridine compounds containing P, Se, and B, generally have an improved antimicrobial activity compared to those with only heterocyclic rings.
From the literature reports presented, we can highlight the following aspects related to the interaction of pyridine compounds, which determine the selectivity of antimicrobial action:
- -
- a favorable binding interaction with Thymidylate kinase (ID: 4QG), within the binding pocket of TMPK, determines a good antimicrobial activity of bis (1H-indol-3-yl)-pyridine 78d, against S. aureus, E. coli, and C. albicans strains;
- -
- affinity and activity of thiophene-pyridines 82–84 on the target protein GlcN-6-P synthase is supported by in vitro antimicrobial screenings against almost all tested strains, S. aureus, B. subtilis, E. coli, S. typhimurium, A. flavus, and C. albicans;
- -
- The good antimicrobial activity of triazino-oxacalixarenes 150 and 151 against E. coli is due to a good covalent interaction with acyl enzyme PDB: 1PW8;
- -
- 3D interaction of selenium compound 210c with 1kzn protein, the lower hydrophobicity, higher topological polar surface area, lower dipole moment, high H-bonding acceptor and donor, and in-silico absorption percentage of it explicates its highest antimicrobial activity against all tested strains, S. aureus, S. pyogenes, E. coli, P. aeruginosa, as well as a good antifungal agent, against C. albicans, A. niger, and A. clavatus;
- -
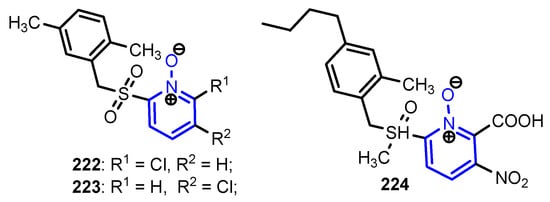
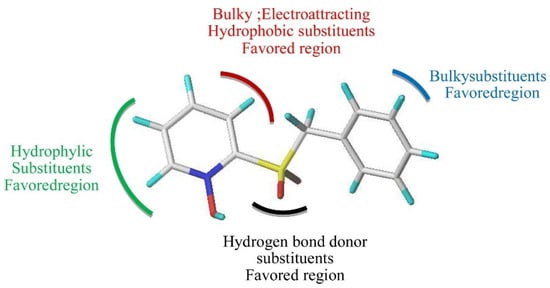
- 3D QSAR, molecular docking modeling showed that pyridine-N-oxide 223 shows an excellent stability to inhibit the main protease 3CLpro of CoV-2 and also has excellent bioavailability and no toxicity results;
- -
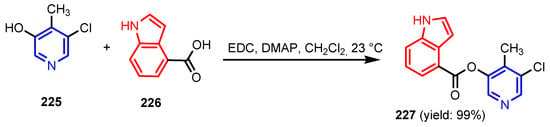
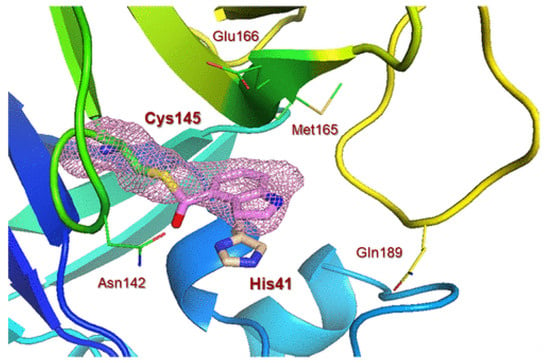
- the excellent antiviral activity of compound 227 (EC50∼2.2 μM) against SARS-CoV-2 3CLpro comparable to antiviral medicine Remdesivir is due to binding of the compound to SARS-CoV 3CLpro and SARS-CoV-2 3CLpro enzymes, which revealed that catalytic Cys145 formed a covalent bond with the indole carbonyl group, and also indole rings of inhibitor formed π−π stacking interactions with the imidazole ring of His41. It is once again confirmed that the cleavage of SARS-CoV-2 polyproteins by 3CLpro (3-Chymotrypsin-like protease) is facilitated by a Cys145–His41 catalytic dyad [105].
We hope that this review will be useful for the synthesis of other pyridine compounds with antimicrobial and antiviral properties, in order to help the current situation of the pandemic, with the infection with SARS-CoV-2.
Author Contributions
Conceptualization, M.M.; methodology, M.M.; software, M.M.; validation, M.M. and C.-V.P.; resources, C.-V.P.; data curation, M.M.; writing—review and editing original draft preparation M.M. and C.-V.P., visualization, M.M.; supervision, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Romanian Ministry of Education and Research, CCCDI-UEFISCDI, project number PN-III-P2-2.1-PED-2019-3009, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not available.
Acknowledgments
The author is thankful to Department of Organic Chemistry, Biochemistry and Catalysis, for providing necessary facilities to carry out this research work.
Conflicts of Interest
The author declares no conflict of interest.
References
- Altaf, A.A.; Shahza, A.; Gul, Z.; Rasool, N.; Badshah, A.; Lal, B.; Khan, E. A Review on the Medicinal Importance of Pyridine Derivatives. J. Drug Des. Med. Chem. 2015, 1, 1–11. [Google Scholar] [CrossRef]
- Hantzsch, A. Condensations produkte aus Aldehydammoniak und ketonartigen Verbindungen. Ber. Dtsch. Chem. Ges. 1881, 14, 1637–1638. [Google Scholar] [CrossRef] [Green Version]
- Marinescu, M. Biginelli Reaction Mediated Synthesis of Antimicrobial Pyrimidine Derivatives and Their Therapeutic Properties. Molecules 2021, 26, 6022. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Akman, F.; Vasilieva, N.Y.; Issaoui, N.; Malyar, Y.N.; Kondrasenko, A.A.; Borovkova, V.S.; Miroshnikova, A.V.; Kazachenko, A.S.; Al-Dossary, O.; et al. Catalytic Sulfation of Betulin with Sulfamic Acid: Experiment and DFT Calculation. Int. J. Mol. Sci. 2022, 23, 1602. [Google Scholar] [CrossRef]
- McCullough, J.P.; Douslin, D.R.; Messerly, J.F.; Hossenlopp, I.A.; Kincheloe, T.C.; Waddington, G. Pyridine: Experimental and Calculated Chemical Thermodynamic Properties between 0 and 1500°K.; a Revised Vibrational Assignment 1. J. Am. Chem. Soc. 1957, 79, 4289–4295. [Google Scholar] [CrossRef]
- Gero, A.; Markham, J.J. Studies on Pyridines: I. The Basicity of Pyridine Bases. J. Org. Chem. 1951, 16, 1835–1838. [Google Scholar] [CrossRef]
- Andreev, V.P.; Sobolev, P.S.; Zaitsev, D.O.; Timofeeva, S.M. Effect of the Solvent on the Coordination of Pyridine Derivatives with Zn Tetraphenylporphine. Russ. J. Gen. Chem. 2018, 88, 2108–2113. [Google Scholar] [CrossRef]
- Walczak, A.; Kurpik, G.; Stefankiewicz, A.R. Intrinsic Effect of Pyridine-N-Position on Structural Properties of Cu-Based Low-Dimensional Coordination Frameworks. Int. J. Mol. Sci. 2020, 21, 6171. [Google Scholar] [CrossRef]
- Hamada, Y. Role of Pyridines in Medicinal Chemistry and Design of BACE1 Inhibitors Possessing a Pyridine Scaffold. In Pyridine; Pandey, P.P., Ed.; IntechOpen: London, UK, 2018; pp. 10–29. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Hao, Z.-Y.; Liang, D.; Zhang, C.L.; Liu, Y.F.; Wang, Y. The Expanding Role of Pyridine and Dihydropyridine Scaffolds in Drug Design. Drug Des. Devel. Ther. 2021, 15, 4289–4338. [Google Scholar] [CrossRef]
- Zalaru, C.; Dumitrascu, F.; Draghici, C.; Tarcomnicu, I.; Tatia, R.; Moldovan, L.; Chifiriuc, M.C.; Lazar, V.; Marinescu, M.; Nitulescu, M.G.; et al. Synthesis, spectroscopic characterization, DFT study and antimicrobial activity of novel alkylaminopyrazole derivatives. J. Mol. Struct. 2018, 1156, 12–21. [Google Scholar] [CrossRef]
- Marinescu, M.; Cinteza, L.O.; Marton, G.I.; Chifiriuc, M.C.; Popa, M.; Stanculescu, I.; Zalaru, C.M.; Stavarache, C.E. Synthesis, density functional theory study and in vitro antimicrobial evaluation of new benzimidazole Mannich bases. BMC Chem. 2020, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M. Synthesis of Antimicrobial Benzimidazole–Pyrazole Compounds and Their Biological Activities. Antibiotics 2021, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Sarova, D.; Kapoor, A.; Narang, R.; Judge, V.; Narasimhan, B. Dodecanoic acid derivatives: Synthesis, antimicrobial evaluation and development of one-target and multi-target QSAR models. Med. Chem. Res. 2011, 20, 769–781. [Google Scholar] [CrossRef]
- Narang, R.; Narasimhan, B.; Sharma, S.; Sriram, D.; Yogeeswari, P.; De Clercq, E.; Pannecouque, C.; Balzarini, J. Synthesis, antimycobacterial, antiviral, antimicrobial activities, and QSAR studies of nicotinic acid benzylidene hydrazide derivatives. Med. Chem. Res. 2012, 21, 1557–1576. [Google Scholar] [CrossRef]
- Malhotra, M.; Sharma, S.; Deep, A. Synthesis, characterization and antimicrobial evaluation of novel derivatives of isoniazid. Med. Chem. Res. 2012, 21, 1237–1244. [Google Scholar] [CrossRef]
- Judge, V.; Narasimhan, B.; Ahuja, M.; Sriram, D.; Yogeeswari, P.; De Clercq, E.; Pannecouque, C.; Balzarini, J. Synthesis, antimycobacterial, antiviral, antimicrobial activities, and QSAR studies of isonicotinic acid-1-(substituted phenyl)-ethylidene/cycloheptylidene hydrazides. Med. Chem. Res. 2012, 21, 1935–1952. [Google Scholar] [CrossRef]
- Judge, V.; Narasimhan, B.; Ahuja, M.; Sriram, D.; Yogeeswari, P.; De Clercq, E.; Pannecouque, C.; Balzarini, J. Isonicotinic acid hydrazide derivatives: Synthesis, antimicrobial activity, and QSAR studies. Med. Chem. Res. 2012, 21, 1451–1470. [Google Scholar] [CrossRef]
- Eldeab, H.A. Green Synthetic Approach and Antimicrobial Evaluation for Some Novel Pyridyl Benzoate Derivatives. Russ. J. Org. Chem. 2019, 55, 1034–1040. [Google Scholar] [CrossRef]
- Karunanidhi, A.; Ghaznavi-Rad, E.; Jeevajothi Nathan, J.; Joseph, N.; Chigurupati, S.; Mohd Fauzi, F.; Pichika, M.R.; Hamat, R.A.; Lung, L.T.T.; van Belkum, A.; et al. Bioactive 2-(Methyldithio)Pyridine-3-Carbonitrile from Persian Shallot (Allium stipitatum Regel.) Exerts Broad-Spectrum Antimicrobial Activity. Molecules 2019, 24, 1003. [Google Scholar] [CrossRef] [Green Version]
- Özgeriş, B. Synthesis of Substituted Phenethylamine-Based Thioureas and Their Antimicrobial and Antioxidant Properties. Russ. J. Org. Chem. 2021, 57, 422–429. [Google Scholar] [CrossRef]
- Ragab, A.; Fouad, S.A.; Ali, O.A.A.; Ahmed, E.M.; Ali, A.M.; Askar, A.A.; Ammar, Y.A. Sulfaguanidine Hybrid with Some New Pyridine-2-One Derivatives: Design, Synthesis, and Antimicrobial Activity against Multidrug-Resistant Bacteria as Dual DNA Gyrase and DHFR Inhibitors. Antibiotics 2021, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Potmischil, F.; Deleanu, C.; Marinescu, M.; Gheorghiu, M.D. Hydroacridines: Part 23†. 1H and 13C NMR spectra of sym-octahydroacridine, its 9-(3-pyridyl) and 9-(4-pyridyl) derivatives and the corresponding N(10)-oxides. An experimental approach to the diamagnetic anisotropy of the pyridine nucleus. Magn. Reson. Chem. 2002, 40, 237–240. [Google Scholar] [CrossRef]
- Potmischil, F.; Marinescu, M.; Loloiu, T. Hydroacridines. XXVIII. Syntheses of New 9-Substituted 1,2,3,4,5,6,7,8-Octahydroacridine Derivatives and their N(10)-Oxides. Rev. Chim. 2007, 58, 795–798. (In Bucuresti) [Google Scholar]
- Potmischil, F.; Marinescu, M.; Nicolescu, A.; Deleanu, C.; Hillebrand, M. Hydroacridines: Part 29. 15N NMR chemical shifts of 9-substituted 1,2,3,4,5,6,7,8-octahydroacridines and their N-oxides—Taft, Swain–Lupton, and other types of linear correlations. N-oxides. Magn. Reson. Chem. 2008, 46, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Potmischil, F.; Marinescu, M.; Nicolescu, A.; Deleanu, C. Hydroacridines: Part 30. 1H and 13CNMR spectra of 9-substituted 1,2,3,4,5,6,7,8-octahydroacridines and of their N-oxides. Magn. Reson. Chem. 2009, 47, 1031–1035. [Google Scholar] [CrossRef]
- Marinescu, M.; Cinteza, L.O.; Marton, G.I.; Marutescu, L.G.; Chifiriuc, M.C.; Constantinescu, C. Density functional theory molecular modeling and antimicrobial behaviour of selected 1,2,3,4,5,6,7,8-octahydroacridine-N(10)-oxides. J. Mol. Struct. 2017, 1144, 14–23. [Google Scholar] [CrossRef]
- Ion, V.; Matei, A.; Constantinescu, C.; Mitu, B.; Ionita, I.; Marinescu, M.; Dinescu, M.; Emandi, A. Octahydroacridine thin films grown by matrix-assisted pulsed laser evaporation for non linear optical applications. Mater. Sci. Semicon. Process. 2015, 36, 78–83. [Google Scholar] [CrossRef]
- Furdui, B.; Parfene, G.; Ghinea, I.O.; Dinica, R.M.; Bahrim, G.; Demeunynck, M. Synthesis and in Vitro Antimicrobial Evaluation of New N-Heterocyclic Diquaternary Pyridinium Compounds. Molecules 2014, 19, 11572–11585. [Google Scholar] [CrossRef] [Green Version]
- Marek, J.; Malinak, D.; Dolezal, R.; Soukup, O.; Pasdiorova, M.; Dolezal, M.; Kuca, K. Synthesis and Disinfection Effect of the Pyridine-4-aldoxime Based Salts. Molecules 2015, 20, 3681–3696. [Google Scholar] [CrossRef]
- Brycki, B.; Małecka, I.; Koziróg, A.; Otlewska, A. Synthesis, Structure and Antimicrobial Properties of Novel Benzalkonium Chloride Analogues with Pyridine Rings. Molecules 2017, 22, 130. [Google Scholar] [CrossRef]
- Wang, P.; Gao, M.; Zhou, L.; Wu, Z.; Hu, D.; Hu, J.; Yang, S. Synthesis and antibacterial activity of pyridinium-tailored aromatic amphiphiles. Bioorg. Med. Chem. Lett. 2016, 26, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Burki, S.; El-Haj, B.M.; Shafiullah; Parveen, S.; Nadeem, H.S.; Nadeem, S.; Shah, M.R. Synthesis and characterization of pyridine-based organic salts: Their antibacterial, antibiofilm and wound healing activities. Bioorg. Chem. 2020, 100, 103937. [Google Scholar] [CrossRef] [PubMed]
- Soukup, O.; Benkova, M.; Dolezal, R.; Sleha, R.; Malinak, D.; Salajkova, S.; Markova, A.; Hympanova, M.; Prchal, L.; Ryskova, L.; et al. The wide-spectrum antimicrobial effect of novel N-alkyl monoquaternary ammonium salts and their mixtures; the QSAR study against bacteria. Eur. J. Med. Chem. 2020, 206, 112584. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Khalid, S.; Perveen, S.; Ahmed, S.; Siddiqui, R.; Khan, N.A.; Shah, M.R. Synthesis of 4-(dimethylamino)pyridine propylthioacetate coated gold nanoparticles and their antibacterial and photophysical activity. J. Nanobiotechnol. 2018, 16, 6. [Google Scholar] [CrossRef] [Green Version]
- Tamilvendan, D.; Rajeswari, S.; Ilavenil, S.; Chakkaravarthy, K.; Venkatesa Prabhu, G. Syntheses, spectral, crystallographic, antimicrobial, and antioxidant studies of few Mannich bases. Med. Chem. Res. 2012, 21, 4129–4138. [Google Scholar] [CrossRef]
- Angelova, V.; Pencheva, T.; Nikolay Vassilev, N.; Simeonova, R.; Momekov, G.; Valcheva, V. New indole and indazole derivatives as potential antimycobacterial agents. Med. Chem. Res. 2019, 28, 485–497. [Google Scholar] [CrossRef]
- Abo-Salem, H.M.; Abd El Salam, H.A.; Abdel-Aziem, A.M.; Abdel-Aziz, M.S.; El-Sawy, E.R. Synthesis, Molecular Docking, and Biofilm Formation Inhibitory Activity of Bis(Indolyl)Pyridines Analogues of the Marine Alkaloid Nortopsentin. Molecules 2021, 26, 4112. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Alatibi, F.; El-Sayed, N.N.E.; Kheder, N.A.; Al-Showiman, S.S. Synthesis and Structure-Activity Relationship of Some New Thiophene-Based Heterocycles as Potential Antimicrobial Agents. Molecules 2016, 21, 1036. [Google Scholar] [CrossRef] [Green Version]
- Flefel, E.M.; El-Sofany, W.I.; El-Shahat, M.; Naqvi, A.; Assirey, E. Synthesis, Molecular Docking and In Vitro Screening of Some Newly Synthesized Triazolopyridine, Pyridotriazine and Pyridine–Pyrazole Hybrid Derivatives. Molecules 2018, 23, 2548. [Google Scholar] [CrossRef] [Green Version]
- Alnassar, H.S.A.; Helal, M.H.E.; Askar, A.A.; Masoud, D.M.; Abdallah, E.M. Pyridine azo disperse dye derivatives and their selenium nanoparticles (SeNPs): Synthesis, fastness properties, and antimicrobial evaluations. Int. J. Nanomed. 2019, 14, 7903–7918. [Google Scholar] [CrossRef] [Green Version]
- Nasr, T.; Bondock, S.; Eid, S. Design, synthesis, antimicrobial evaluation and molecular docking studies of some new thiophene, pyrazole and pyridone derivatives bearing sulfisoxazole moiety. Eur. J. Med. Chem. 2014, 84, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Kamat, V.; Santosh, R.; Poojary, B.; Nayak, S.P.; Kumar, B.K.; Sankaranarayanan, M.; Faheem; Khanapure, S.; Barretto, D.A.; Vootla, S.K. Pyridine- and Thiazole-Based Hydrazides with Promising Antiinflammatory and Antimicrobial Activities along with Their In Silico Studies. ACS Omega 2020, 5, 25228–25239. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.B.; Agravat, S.N.; Shaikh, F.M. Synthesis and antimicrobial activity of new pyridine derivatives-I. Med. Chem. Res. 2011, 20, 1033–1041. [Google Scholar] [CrossRef]
- Sedaghati, B.; Fassihi, A.; Arbabi, S.; Ranjbar, M.; Memarian, H.R.; Saghaie, L.; Omidi, A.; Sardari, A.; Jalali, M.; Abedi, D. Synthesis and antimicrobial activity of novel derivatives of Biginelli pyrimidines. Med. Chem. Res. 2012, 21, 3973–3983. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, P.; De Clercq, E.; Balzarini, J.; Pannecouque, C.; Dewan, S.; Narasimhan, B. 4-[1-(Substituted aryl/alkyl carbonyl)-benzoimidazol-2-yl]-benzenesulfonic acids: Synthesis, antimicrobial activity, QSAR studies, and antiviral evaluation. Eur. J. Med. Chem. 2010, 45, 5985–5997. [Google Scholar] [CrossRef]
- Desai, N.C.; Dodiya, A.M.; Shihory, N.R. A search of novel antimicrobial based on benzimidazole and 2-pyridone heterocycles. Med. Chem. Res. 2012, 21, 2579–2586. [Google Scholar] [CrossRef]
- Desai, N.C.; Pandya, D.D.; Bhatt, K.A.; Kotadiya, G.M.; Desai, P. Synthesis, antimicrobial, and cytotoxic activities of novelbenzimidazole derivatives bearing cyanopyridine and 4-thiazolidinone motifs. Med. Chem. Res. 2014, 23, 3823–3835. [Google Scholar] [CrossRef]
- Desai, N.C.; Pandya, D.D.; Kotadiya, G.M.; Desai, P. Synthesis, antimicrobial and cytotoxic activity of 2-azetidinone derivatives of pyridyl benzimidazoles. Med Chem. Res. 2014, 23, 1725–1741. [Google Scholar] [CrossRef]
- Rani, V.E.; Reddy, P.R. Synthesis and Antimicrobial Activity of New Pyridine Containing Substituted Phenyl Azetidine-2-One Derivatives. Open J. Med. Chem. 2018, 8, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Sarojini, B.K.; Krishna, B.G.; Darshanraj, C.G.; Bharath, B.R.; Manjunatha, H. Synthesis, characterization, in vitro and molecular docking studies of new 2,5-dichloro thienyl substituted thiazole derivatives for antimicrobial properties. Eur. J. Med. Chem. 2010, 45, 3490–3496. [Google Scholar] [CrossRef]
- Desai, N.C.; Kotadiya, G.M.; Trivedi, A.R.; Khedkar, V.M.; Jha, P.C. Synthesis, biological valuation, and QSAR studies of novel pyrazole bearing pyridyl oxadiazole analogues as potential antimicrobial agents. Med. Chem. Res. 2016, 25, 712–727. [Google Scholar] [CrossRef]
- Desai, N.C.; Kotadiya, G.M.; Trivedi, A.R.; Khedkar, V.M.; Jha, P.C. Design, synthesis, and biological evaluation of novel fluorinated pyrazole encompassing pyridyl 1,3,4-oxadiazole motifs. Med. Chem. Res. 2016, 25, 2698–2717. [Google Scholar] [CrossRef]
- Murty, M.S.R.; Penthala, R.; Buddana, S.K.; Prakasham, R.S.; Das, P.; Polepalli, S.; Jain, N.; Bojja, S. Recyclable CuO nanoparticles-catalyzed synthesis of novel-2,5-disubstituted 1,3,4-oxadiazoles as antiproliferative, antibacterial, and antifungal agents. Med. Chem. Res. 2014, 23, 4579–4594. [Google Scholar] [CrossRef]
- Krishna, C.; Bhargavi, M.V.; Rao, C.P.; Krupadanam, G.L.D. Synthesis and antimicrobial assessment of novel coumarins featuring 1,2,4-oxadiazole. Med. Chem. Res. 2015, 24, 3743–3751. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Noolvi, M.N.; Jalhan, S.; Patel, H.M. Synthesis, and antimicrobial evaluation of new pyridine imidazo [2,1b]-1,3,4-thiadiazole derivatives. J. Saudi. Chem. Soc. 2016, 20, S406–S410. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.S.; Jain, S.C. Synthesis and QSAR studies of some novel disubstituted 1,2,4-triazoles as antimicrobial agents. Med. Chem. Res. 2014, 23, 848–861. [Google Scholar] [CrossRef]
- Morsy, H.A.; Mohammed, S.M.; Abdel Hamid, A.M.; Moustafa, A.H.; El-Sayed, H.A. Click Synthesis of 1,2,3-Triazole Nucleosides Based on Functionalized Nicotinonitriles. Russ. J. Org. Chem. 2020, 56, 143–147. [Google Scholar] [CrossRef]
- Deng, Q.; Ji, Q.-G.; Ge, Z.-Q.; Liu, X.F.; Yang, D.; Yuan, L.J. Synthesis and biological evaluation of novel quinolin-2(1H)-one derivatives as potential antimicrobial agents. Med. Chem. Res. 2014, 23, 5224–5236. [Google Scholar] [CrossRef]
- Ranjbar-Karimi, R.; Poorfreidoni, A. Incorporation of Fluorinated Pyridine in the Side Chain of 4-Aminoquinolines: Synthesis, Characterization and Antibacterial Activity. Drug. Res. 2018, 68, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Myangar, K.N.; Raval, J.P. Design, synthesis, and in vitro antimicrobial activities of novel azetidinyl-3-quinazolin-4-one hybrids. Med. Chem. Res. 2012, 21, 2762–2771. [Google Scholar] [CrossRef]
- Saleh, M.; Abbott, S.; Perron, V.; Lauzon, C.; Penney, C.; Zacharie, B. Synthesis and antimicrobial activity of 2-fluorophenyl-4,6-disubstituted [1,3,5]triazines. Bioorg. Med. Chem. Lett. 2010, 20, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Solankee, A.; Kapadia, K.; Ciric, A.; Sokovic, M.; Doytchinova, I.; Geronikaki, A. Synthesis of some new s-triazine based chalcones and their derivatives as potent antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Rajavelu, K.; Rajakumar, P. Synthesis and photophysical, electrochemical, antibacterial, and DNA binding studies of triazinocalix[2]arenes. J. Mater. Chem. B 2015, 3, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Long, S.; Rakesh, K.P.; Zha, G.F. Structure-activity relationships (SAR) of triazine derivatives: Promising antimicrobial agents. Eur. J. Med. Chem. 2020, 185, 111804. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.A.; Al-Omar, M.A.; Amr, A.E.-G.E. Synthesis of Chiral Macrocyclic or Linear Pyridine Carboxamides from Pyridine-2,6-dicarbonyl Dichloride as Antimicrobial Agents. Molecules 2010, 15, 6588–6597. [Google Scholar] [CrossRef] [Green Version]
- El-Salam, O.I.A.; Al-Omar, M.A.; Fayed, A.A.; Flefel, E.M.; Amr, A.E.-G.E. Synthesis of New Macrocyclic Polyamides as Antimicrobial Agent Candidates. Molecules 2012, 17, 14510–14521. [Google Scholar] [CrossRef]
- Chandak, N.; Kumar, S.; Kumar, P.; Sharma, C.; Aneja, K.R.; Sharma, P.K. Exploration of antimicrobial potential of pyrazolo [3,4-b]pyridine scaffold bearing benzenesulfonamide and trifluoromethyl moieties. Med. Chem. Res. 2013, 22, 5490–5503. [Google Scholar] [CrossRef]
- Altalbawy, F.M.A. Synthesis and Antimicrobial Evaluation of Some Novel Bis-α,β-Unsaturated Ketones, Nicotinonitrile, 1,2-Dihydropyridine-3-carbonitrile, Fused Thieno [2,3-b]pyridine and Pyrazolo [3,4-b]pyridine Derivatives. Int. J. Mol. Sci. 2013, 14, 2967–2979. [Google Scholar] [CrossRef]
- Jose, G.; Kumara, T.H.S.; Nagendrappa, G.; Sowmya, H.B.V.; Jasinski, J.P.; Millikan, S.P.; Chandrika, N.; More, S.S.; Harish, B.G. New polyfunctional imidazo [4,5-C]pyridine motifs: Synthesis, crystal studies, docking studies and antimicrobial evaluation. Eur. J. Med. Chem. 2014, 77, 288–297. [Google Scholar] [CrossRef]
- Poreba, K.; Pawlik, K.; Rembacz, K.; Kurowska, E.; Matuszyk, J.; Dlugosz, A. Synthesis and antibacterial activity of new sulfonamide S isoxazolo [5,4-b]pyridine derivatives. Acta Pol. Pharm. Drug Res. 2015, 72, 727–735. [Google Scholar]
- Reen, G.K.; Kumar, A.; Sharma, P. In vitro and in silico evaluation of 2-(substituted phenyl) oxazolo [4,5-b]pyridine derivatives as potential antibacterial agents. Med. Chem. Res. 2017, 26, 3336–3344. [Google Scholar] [CrossRef]
- Ali, T.E.S. Synthesis of some novel pyrazolo [3,4-b]pyridine and pyrazolo [3,4-d]pyrimidine derivatives bearing 5,6-diphenyl-1,2,4-triazine moiety as potential antimicrobial agents. Eur. J. Med. Chem. 2009, 44, 4385–4392. [Google Scholar] [CrossRef]
- El-Borai, M.A.; Rizk, H.F.; Beltagy, D.M.; El-Deeb, I.Y. Microwave-assisted synthesis of some new pyrazolopyridines and their antioxidant, antitumor and antimicrobial activities. Eur. J. Med. Chem. 2013, 66, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Appna, N.R.; Nagiri, R.K.; Korupolu, R.B.; Kanugala, S.; Chityal, G.K.; Thipparapu, G.; Banda, N. Design and synthesis of novel 4-hydrazone functionalized/1,2,4-triazole fused pyrido [2,3-d]pyrimidine derivatives, their evaluation for antifungal activity and docking studies. Med. Chem. Res. 2019, 28, 1509–1528. [Google Scholar] [CrossRef]
- Patel, N.B.; Patel, S.D.; Chauhan, H.I. Synthesis and in vitro microbial activities of amides of pyridoquinolone. Med. Chem. Res. 2011, 20, 1054–1067. [Google Scholar] [CrossRef]
- Brahmbhatt, D.I.; Patel, C.V.; Bhila, V.G.; Patel, N.H.; Patel, A.A. An efficient synthesis and antimicrobial screening of new hybrid molecules containing coumarin and indenopyridine moiety. Med. Chem. Res. 2015, 24, 1596–1604. [Google Scholar] [CrossRef]
- Salem, M.S.; Sakr, S.I.; El-Senousy, W.M.; Madkour, H.M.F. Synthesis, Antibacterial, and Antiviral Evaluation of New Heterocycles Containing the Pyridine Moiety. Arch. Pharm. Chem. Life Sci. 2013, 346, 766–773. [Google Scholar] [CrossRef]
- Youssoufi, M.H.; Sahu, P.K.; Sahu, P.K.; Agarwal, D.D.; Ahmad, M.; Messali, M.; Lahsasni, S.; Hadda, T.B. POM analyses of anti icrobial activity of 4H-pyrimido [2,1-b]benzothiazole, pyrazole, and benzylidene derivatives of curcumin. Med. Chem. Res. 2015, 24, 2381–2392. [Google Scholar] [CrossRef]
- Flefel, E.M.; Alsafi, M.A.; Alahmadi, S.M.; Amr, A.E.G.E.; Fayed, A.A. Synthesis, antibacterial, and antiviral evaluation of new heterocycles containing the pyridine moiety. Biomed. Res. 2018, 29, 1407–1413. [Google Scholar]
- El-Sayed, H.A.; Moustafa, A.H.; El-Torky, A.E.; Abd El-Salam, E.A. A Series of Pyridines and Pyridine Based Sulfa-Drugs as Antimicrobial Agents: Design, Synthesis and Antimicrobial Activity. Russ. J. Gen. Chem. 2017, 87, 2401–2408. [Google Scholar] [CrossRef]
- Mohi El-Deen, E.M.; Abd El-Meguid, E.A.; Karam, E.A.; Nossier, E.S.; Ahmed, M.F. Synthesis and Biological Evaluation of New Pyridothienopyrimidine Derivatives as Antibacterial Agents and Escherichia coli Topoisomerase II Inhibitors. Antibiotics 2020, 9, 695. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Megeed, M.F.; Badr, B.E.; Azaam, M.M.; El-Hiti, G.A. Synthesis, antimicrobial and anticancer activities of a novel series of diphenyl 1-(pyridin-3-yl)ethylphosphonates. Bioorg. Med. Chem. 2012, 20, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.C.; Basha, S.K.T.; Bhuvaneswar, C.; Bhaskar, B.V.; Rajendra, W.; Raju, C.N.; Ghosh, S.K. Didanosine phosphoramidates: Synthesis, docking to viral NA, antibacterial and antiviral activity. Med. Chem. Res. 2015, 24, 209–219. [Google Scholar] [CrossRef]
- Devineni, S.R.; Goll, M.; Chamarthi, N.R.; Meriga, B.; Saddal, M.S.; Asupathri, U.R. 2-Amino-2,3-dihydro-1H-2λ5-[1,3,2]diazaphospholo[4,5-b]pyridin-2-one-based urea and thiourea derivatives: Synthesis, molecular docking study and evaluation of anti-inflammatory and antimicrobial activities. Med. Chem. Res. 2016, 25, 751–768. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, N.; Maurya, I.K.; Bhasin, A.K.K.; Wangoo, N.; Brandao, P.; Felix, V.; Bhasin, K.K.; Sharma, R.K. Facile synthesis, structural evaluation, antimicrobial activity and synergistic effects of novel imidazo [1,2-a]pyridine based organoselenium compounds. Eur. J. Med. Chem. 2016, 123, 916–924. [Google Scholar] [CrossRef]
- Abdellattif, M.H.; Abdel-Rahman, A.A.H.; Helmy Arief, M.M.; Mouneir, S.M.; Ali, A.; Hussien, M.A.; Okasha, R.M.; Afifi, T.H.; Hagar, M. Novel 2-Hydroselenonicotinonitriles and Selenopheno [2,3-b]pyridines: Efficient Synthesis, Molecular Docking-DFT Modeling, and Antimicrobial Assessment. Front. Chem. 2021, 9, 672503. [Google Scholar] [CrossRef]
- Fontaine, F.; Hequet, A.; Voisin-Chiret, A.-S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. Boronic species as promising inhibitors of the Staphylococcus aureus NorA efflux pump: Study of 6-substituted pyridine-3-boronic acid derivatives. Eur. J. Med. Chem. 2015, 95, 185–198. [Google Scholar] [CrossRef]
- Bychenko, O.; Skvortsova, Y.; Ziganshin, R.; Grigorov, A.; Aseev, L.; Ostrik, A.; Kaprelyants, A.; Salina, E.G.; Azhikina, T. Mycobacterium tuberculosis Small RNA MTS1338 Confers Pathogenic Properties to Non-Pathogenic Mycobacterium smegmatis. Microorganisms 2021, 9, 414. [Google Scholar] [CrossRef]
- Navarrete-Vazquez, G.; Molina-Salinas, G.M.; Duarte-Fajardo, Z.V.; Vargas-Villarreal, J.; Estrada-Soto, S.; Gonzalez-Salazar, F.; Hernandez-Nunez, E.; Said-Fernandez, S. Synthesis and antimycobacterial activity of 4-(5-substituted-1,3,4-oxadiazol-2-yl)pyridines. Bioorg. Med. Chem. 2007, 15, 5502–5508. [Google Scholar] [CrossRef]
- Patel, N.B.; Sharma, R.D.; Rajani, S.D. In vitro antimicrobial and antitubercular studies of novel 6-substituted (pyrrolidin-1-yl)-2(1H)-pyridinones. Med. Chem. Res. 2012, 21, 4108–4119. [Google Scholar] [CrossRef]
- Sangani, C.B.; Jardosh, H.H.; Patel, M.P.; Patel, R.G. Microwave-assisted synthesis of pyrido [1,2-a]benzimidazole derivatives of b-aryloxyquinoline and their antimicrobial nd antituberculosis activities. Med. Chem. Res. 2013, 22, 3035–3047. [Google Scholar] [CrossRef]
- Rathod, A.S.; Godipurge, S.S.; Biradar, J.S. Microwave Assisted, Solvent-Free, “Green” Synthesis of Novel Indole Analogs as Potent Antitubercular and Antimicrobial Agents and Their Molecular Docking Studies. Russ. J. Gen. Chem. 2018, 88, 1238–1246. [Google Scholar] [CrossRef]
- Rathod, A.S.; Godipurge, S.S.; Biradar, J.S. Symthesis of indole, coumarinyl and pyridinyl derivatives of isoniazid as potent antitubercular and animicrobial agents and their molecular docking studies. Int. J. Pharm. Pharm. Sci. 2017, 9, 233–240. [Google Scholar] [CrossRef]
- Rathod, A.S.; Reddy, P.V.; Biradar, J.S. Microwave-Assisted Synthesis of Some Indole and Isoniazid Derivatives as Antitubercular Agents and Molecular Docking Study. Russ. J. Org. Chem. 2020, 56, 662–670. [Google Scholar] [CrossRef]
- De, A.; Sarkar, S.; Majee, A. Recent advances on heterocyclic compounds with antiviral properties. Chem. Het. Comp. 2021, 57, 410–416. [Google Scholar] [CrossRef]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19). A Review. Clin. Rev. Educ. 2020, 3213, 1824–1836. [Google Scholar] [CrossRef]
- Balzarini, J.; Stevens, M.; Andrei, G.; Snoeck, R.; Strunk, R.; Pierce, J.B.; Lacadie, J.A.; De Clercq, E.; Pannecouque, C. Pridine Oxide Derivatives: Structure-Activity Relationship for Inhibition of Human Immunodeficiency Virus and Cytomegalovirus Replication in Cell Culture. Helv. Chim. Acta. 2002, 85, 2961–2974. [Google Scholar] [CrossRef]
- Balzarini, J.; Keyaert, E.; Vijgen, L.; Vandermeer, F.; Stevens, M.; De Clercq, E.; Egberink, H.; Van Ranst, M. Pyridine N-oxide derivatives are inhibitory to the human SARS and feline infectious peritonitis coronavirus in cell culture. J. Antimicrob. Chemother. 2006, 57, 472–481. [Google Scholar] [CrossRef] [Green Version]
- Ghaleb, A.; Aouidate, A.; El Ayouchia, H.B.; Aarjane, M.; Anane, H.; Stiriba, S.E. In silico molecular investigations of pyridine N-Oxide compounds as potential inhibitors of SARS-CoV-2: 3D QSAR, molecular docking modeling, and ADMET screening. J. Biomol. Struct. Dyn. 2020, 40, 143–153. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Raghavaiah, J.; Shahabi, D.; Yadav, M.; Anson, B.J.; Lendy, E.K.; Hattori, S.-i.; Higashi-Kuwata, N.; Mitsuya, H.; Mesecar, A.D. Indole Chloropyridinyl Ester-Derived SARS-CoV-2 3CLpro Inhibitors: Enzyme Inhibition, Antiviral Efficacy, Structure−Activity Relationship, and X-ray Structural Studies. J. Med. Chem. 2021, 64, 14702–14714. [Google Scholar] [CrossRef]
- Starčević, K.; Kralj, M.; Ester, K.; Sabol, I.; Grce, M.; Pavelić, K.; Karminski-Zamola, G. Synthesis, antiviral and antitumor activity of 2-substituted-5-amidino-benzimidazoles. Bioorg. Med. Chem. 2007, 15, 4419–4426. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.S.; Marzouk, M.I.; Ali, S.N.; Madkour, H.M.F. Synthesis, structure characterization and biological evaluation of new 6,8-dichloro-2-methyl-4H-chromen-4-one derivatives. Eur. J. Chem. 2012, 3, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Abu-Hashem, A.A.; Gouda, M.A.; Badria, F.A. Design, synthesis and identification of novel substituted isothiochromene analogs as potential antiviral and cytotoxic agents. Med. Chem. Res. 2018, 27, 2297–2311. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Fadl, S.; Villanueva, A.J.; Rabeh, W.M. Catalytic Dyad Residues His41 and Cys145 Impact the Catalytic Activity and Overall Conformational Fold of the Main SARS-CoV-2 Protease 3-Chymotrypsin-Like Protease. Front. Chem. 2021, 9, 692168. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).