Kub3 Deficiency Causes Aberrant Late Embryonic Lung Development in Mice by the FGF Signaling Pathway
Abstract
1. Introduction
2. Results
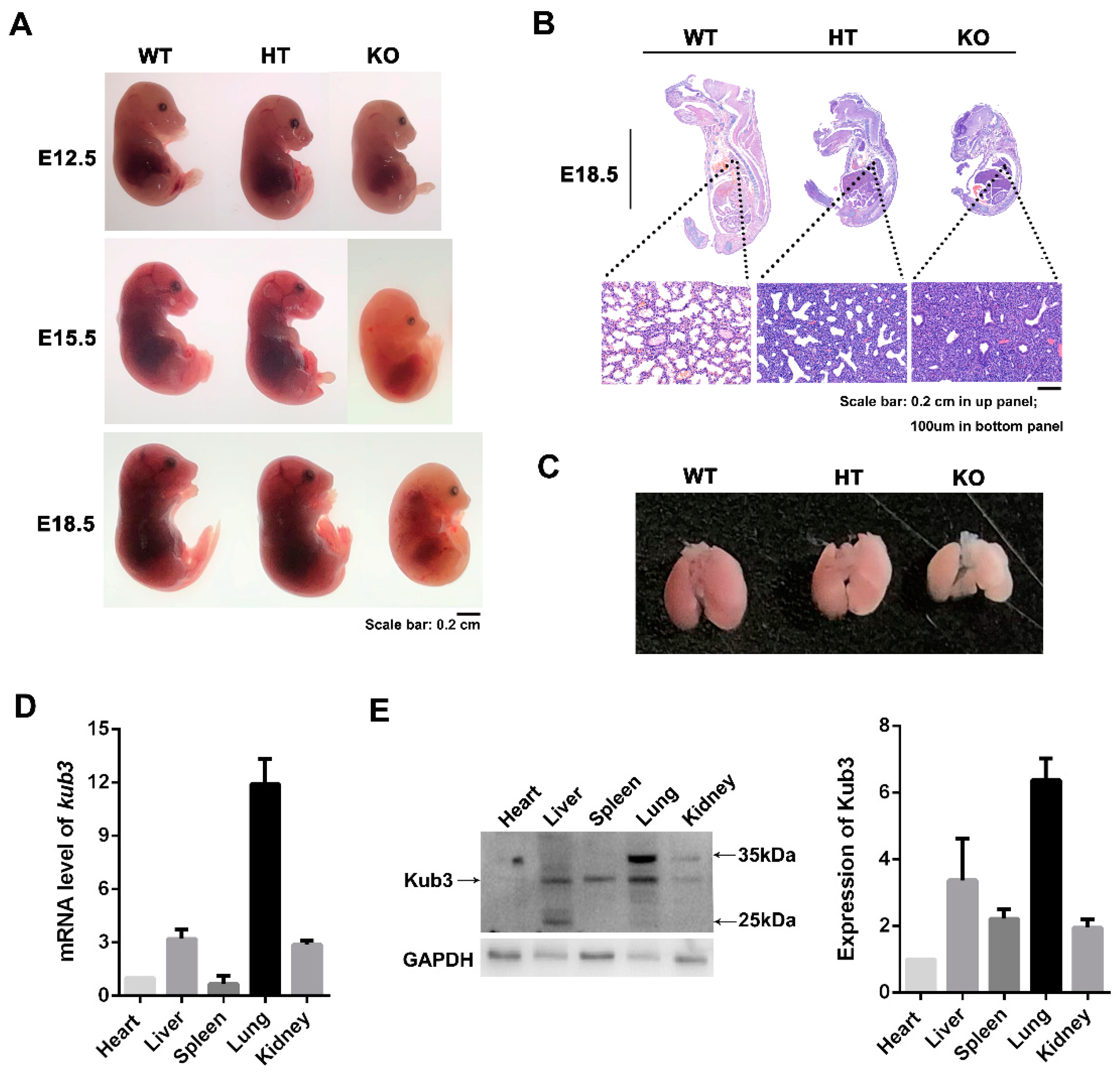
2.1. Kub3 Gene Knockout in Mice Results in Perinatal or Neonatal Lethality
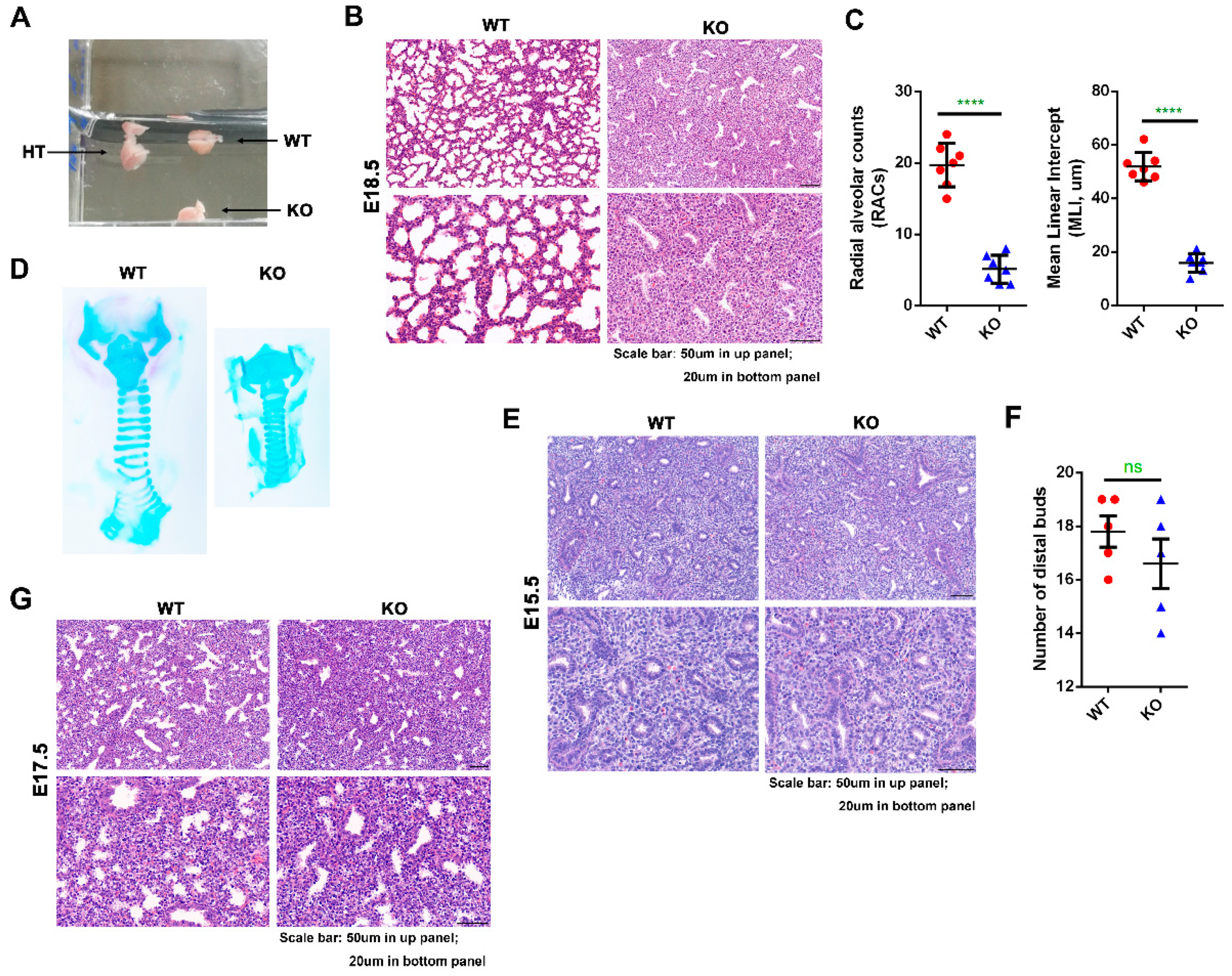
2.2. Kub3 Deletion Results in Abnormal Lung Morphogenesis
2.3. Kub3 Deletion Results in Pulmonary Atelectasis in E18.5 Lungs
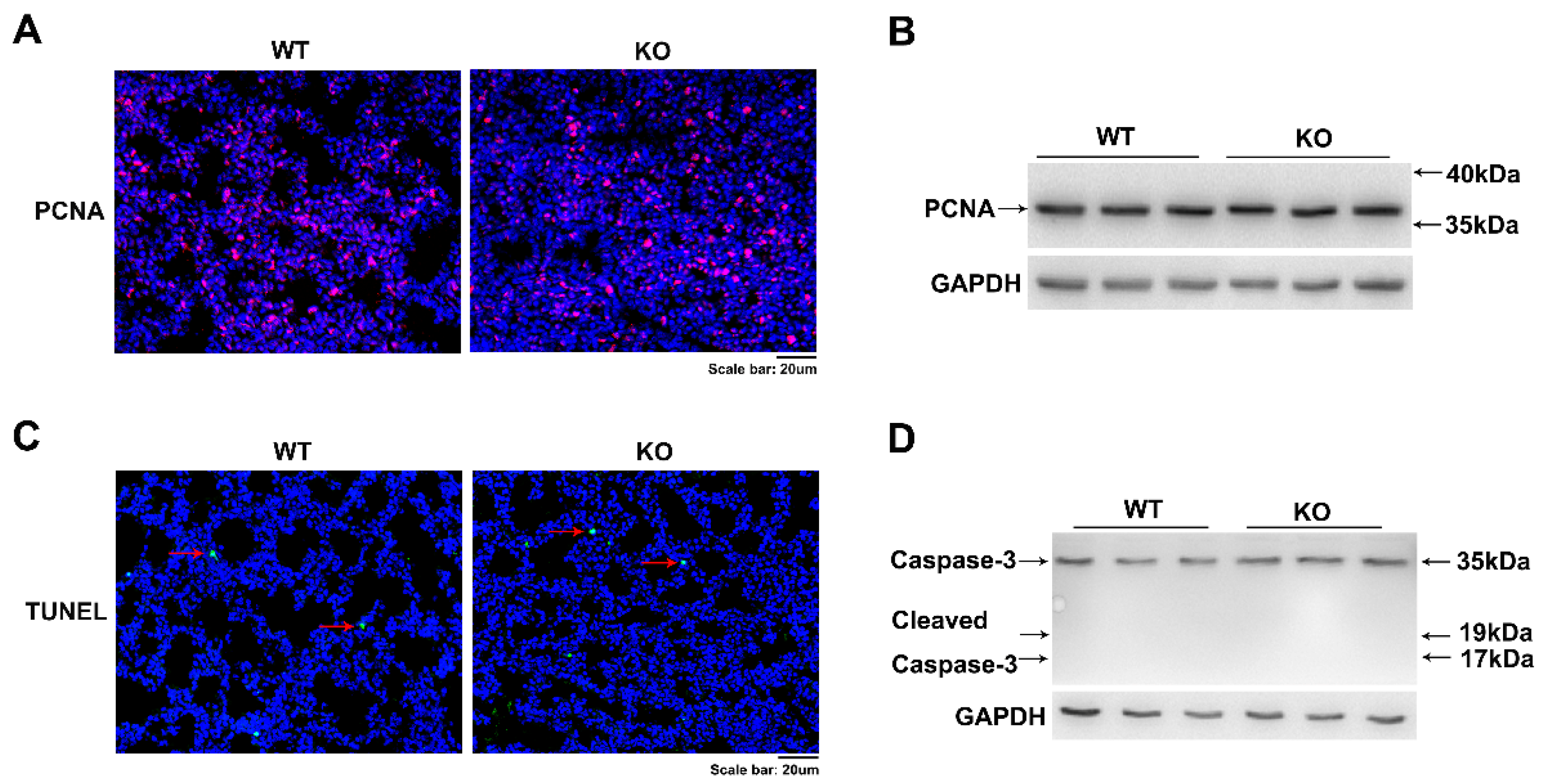
2.4. Kub3 Deletion Has No Effect on Lung Cell Proliferation and Cell Apoptosis
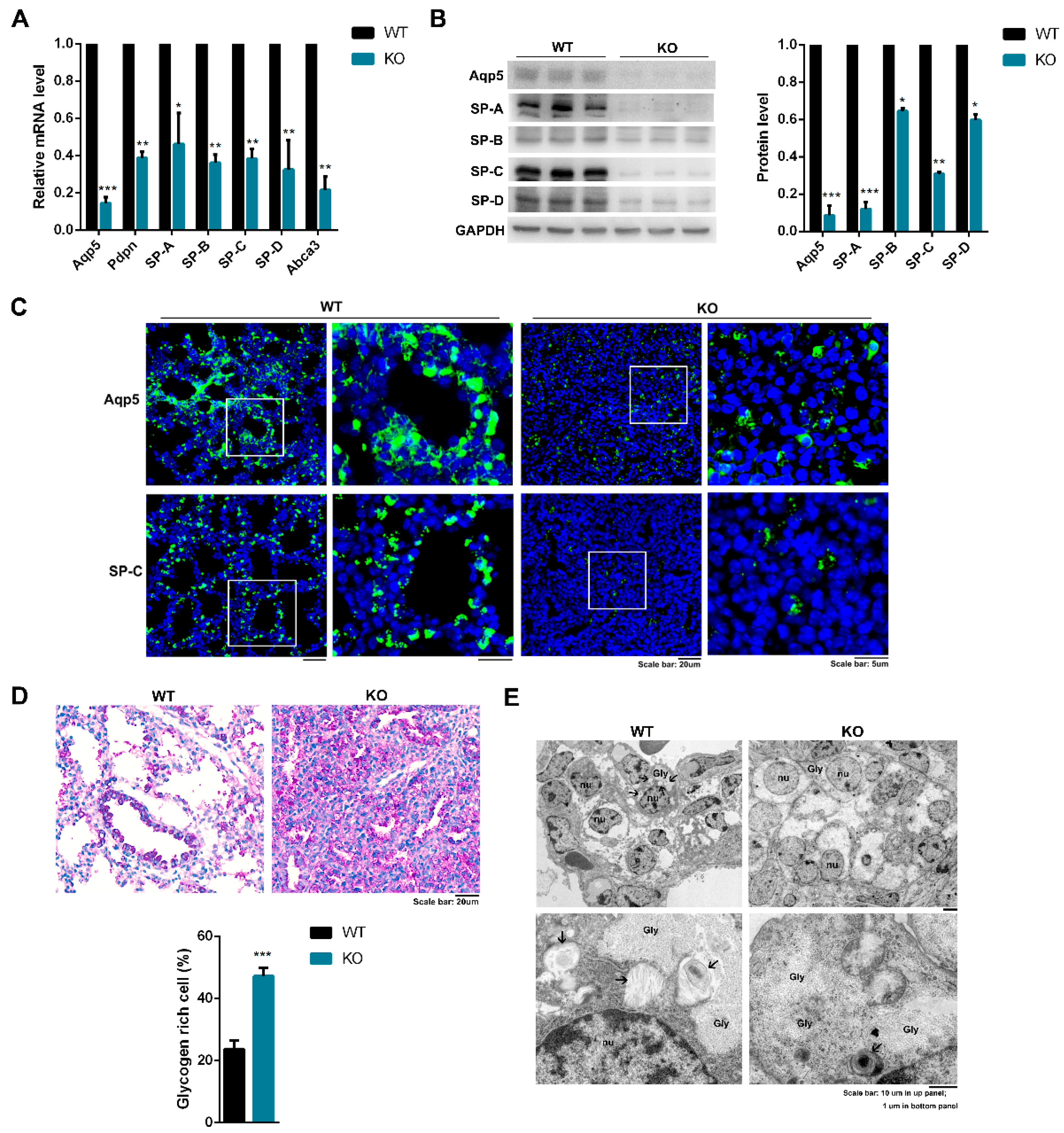
2.5. Kub3 Deletion Impairs the Differentiation of Alveolar Epithelial Cells and the Maturation of AECIIs
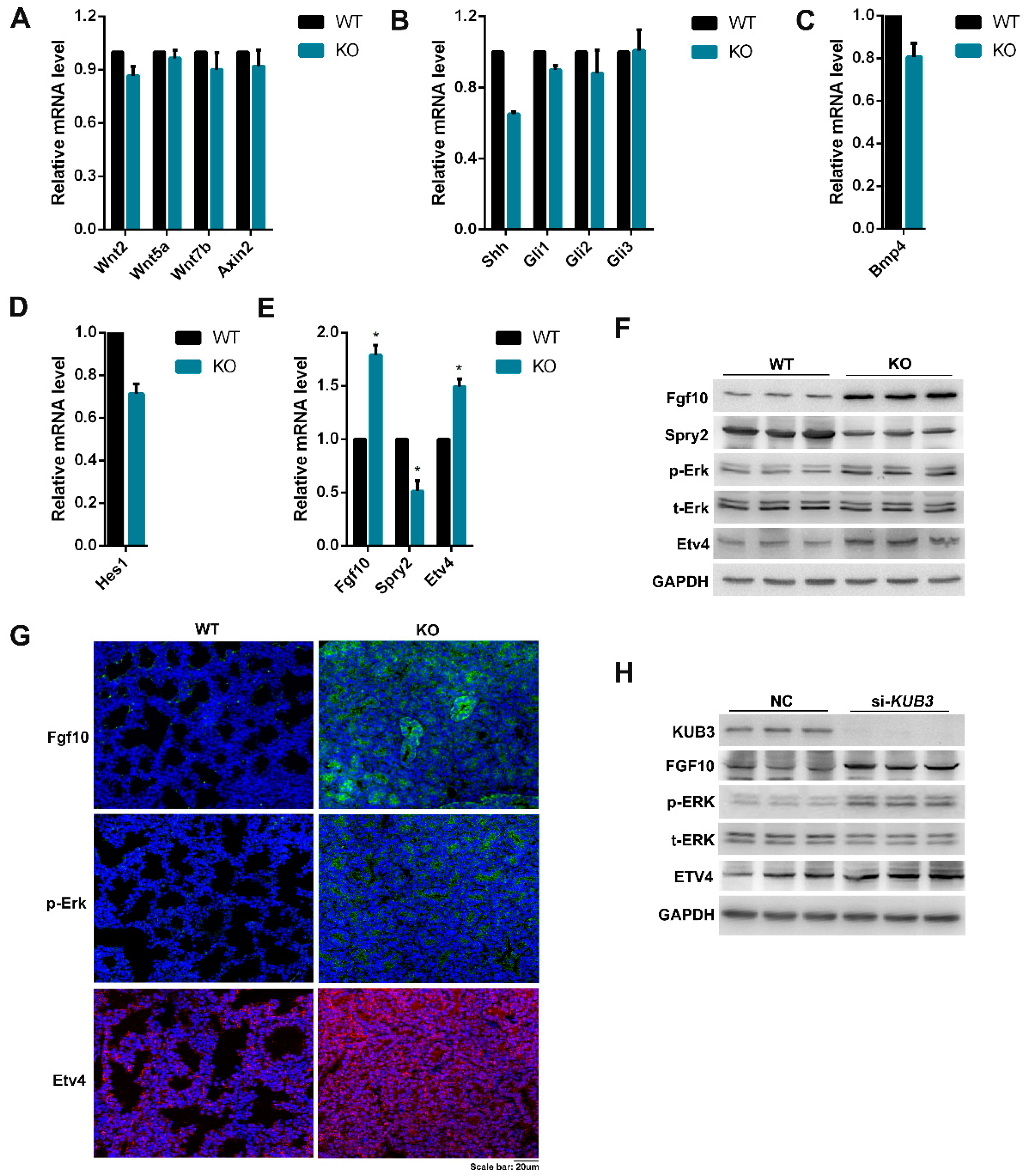
2.6. Kub3 Deletion Results in the Abnormal FGF Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Generation and Genotyping of the Kub3 Knockout Mice
4.3. Histological Analyses
4.4. In Situ Hybridization
4.5. Immunofluorescence
4.6. Transmission Electron Microscopy
4.7. Hydrostatic Lung Test and Trachea Alcian Blue–Alizarin Staining
4.8. Cell Culture and Transfection
4.9. Tissue Total RNA Isolation and Quantitative Real-Time RT-PCR
4.10. Western-Blot Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrisey, E.E.; Hogan, B.L. Preparing for the first breath: Genetic and cellular mechanisms in lung development. Dev. Cell. 2010, 18, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Loering, S.; Cameron, G.J.M.; Starkey, M.R.; Hansbro, P.M. Lung development and emerging roles for type 2 immunity. J. Pathol. 2019, 247, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, B.A.; Barakat, A.Z.; Held, T.; Elkenani, M.; Mühlfeld, C.; Männer, J.; Adham, I.M. Respiratory distress and early neonatal lethality in Hspa4l/Hspa4 double-mutant mice. Am. J. Respir. Cell Mol. Biol. 2014, 50, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Rabata, A.; Fedr, R.; Soucek, K.; Hampl, A.; Koledova, Z. 3D cell culture models demonstrate a role for FGF and WNT signaling in regulation of lung epithelial cell fate and morphogenesis. Front. Cell Dev. Biol. 2020, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Danilenko, D.M.; Scully, S.A.; Bolon, B.; Ring, B.D.; Tarpley, J.E.; DeRose, M.; Simonet, W.S. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998, 12, 3156–3161. [Google Scholar] [CrossRef]
- Ohuchi, H.; Hori, Y.; Yamasaki, M.; Harada, H.; Sekine, K.; Kato, S.; Itoh, N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem. Biophys. Res. Commun. 2000, 277, 643–649. [Google Scholar] [CrossRef]
- Cardoso, W.V.; Lü, J. Regulation of early lung morphogenesis: Questions, facts and controversies. Development 2006, 133, 1611–1624. [Google Scholar] [CrossRef]
- De Moerlooze, L.; Spencer-Dene, B.; Revest, J.M.; Hajihosseini, M.; Rosewell, I.; Dickson, C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 2000, 127, 483–492. [Google Scholar] [CrossRef]
- Dailey, L.; Ambrosetti, D.; Mansukhani, A.; Basilico, C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005, 16, 233–247. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef]
- Lv, Y.Q.; Dhlamini, Q.; Chen, C.; Li, X.; Bellusci, S.; Zhang, J.S. FGF10 and lipofibroblasts in lung homeostasis and disease: Insights gained from the adipocytes. Front. Cell Dev. Biol. 2021, 9, 645400. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ornitz, D.M. FGF9 and FGF10 activate distinct signaling pathways to direct lung epithelial specification and branching. Sci. Signal. 2020, 13, eaay4353. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Mitsuyasu, T.; Kawano, S.; Nakamura, N.; Kanda, S.; Nakamura, S. Fibroblast growth factors 7 and 10 are involved in ameloblastoma proliferation via the mitogen-activated protein kinase pathway. Int. J. Oncol. 2013, 43, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.; Jones, M.R.; Olmer, R.; Ulrich, S.; Danopoulos, S.; Shen, C.; Chen, C.; Wilhelm, J.; Martin, U.; Chen, C.; et al. Fgf10 Ssignaling-based evidence for the existence of an embryonic stage distinct from the pseudoglandular dtage during mouse lung development. Front. Cell Dev. Biol. 2020, 8, 576604. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.L. Morphogenesis. Cell 1999, 96, 225–233. [Google Scholar] [CrossRef]
- Itoh, N. FGF10: A multifunctional mesenchymal–epithelial signaling growth factor in development, health, and disease. Cytokine Growth Factor Rev. 2016, 28, 63–69. [Google Scholar] [CrossRef]
- Sekine, K.; Ohuchi, H.; Fujiwara, M.; Yamasaki, M.; Yoshizawa, T.; Sato, T.; Yagishita, N.; Matsui, D.; Koga, Y.; Itoh, N.; et al. Fgf10 is essential for limb and lung formation. Nat. Genet. 1999, 21, 138–141. [Google Scholar] [CrossRef]
- Yuan, T.; Volckaert, T.; Chanda, D.; Thannickal, V.J.; De Langhe, S.P. Fgf10 Signaling in lung development, homeostasis, disease, and repair after injury. Front. Genet. 2018, 9, 418. [Google Scholar] [CrossRef]
- Wu, J.; Chu, X.; Chen, C.; Bellusci, S. Role of fibroblast growth factor 10 in mesenchymal cell differentiation during lung development and disease. Front. Genet. 2018, 9, 545. [Google Scholar] [CrossRef]
- Al Alam, D.; El Agha, E.; Sakurai, R.; Kheirollahi, V.; Moiseenko, A.; Danopoulos, S.; Shrestha, A.; Schmoldt, C.; Quantius, J.; Herold, S.; et al. Evidence for the involvement of fibroblast growth factor 10 in lipofibroblast formation during embryonic lung development. Development 2015, 142, 4139–4150. [Google Scholar] [CrossRef]
- Hogan, B.L.; Grindley, J.; Bellusci, S.; Dunn, N.R.; Emoto, H.; Itoh, N. Branching morphogenesis of the lung: New models for a classical problem. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997; Volume 62, pp. 249–256. [Google Scholar]
- El Agha, E.; Bellusci, S. Walking along the fibroblast growth factor 10 route: A key pathway to understand the control and regulation of epithelial and mesenchymal cell-lineage formation during lung development and repair after injury. Scientifica 2014, 2014, 538379. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.M.; Yahya, F.; Moiseenko, A.; Tiozzo, C.; Shrestha, A.; Ahmadvand, N.; El Agha, E.; Quantius, J.; Dilai, S.; Kheirollahi, V.; et al. Fgf10 deficiency is causative for lethality in a mouse model of bronchopulmonary dysplasia. J. Pathol. 2017, 241, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Nyeng, P.; Norgaard, G.A.; Kobberup, S.; Jensen, J. FGF10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev. Biol. 2008, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.R.; Martinez Alanis, D.; Miller, R.K.; Ji, H.; Akiyama, H.; McCrea, P.D.; Chen, J. Lung epithelial branching program antagonizes alveolar differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, 18042–18051. [Google Scholar] [CrossRef]
- Volckaert, T.; Campbell, A.; Dill, E.; Li, C.; Minoo, P.; De Langhe, S. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development 2013, 140, 3731–3742. [Google Scholar] [CrossRef]
- Volckaert, T.; Yuan, T.; Yuan, J.; Boateng, E.; Hopkins, S.; Zhang, J.S.; Thannickal, V.J.; Fässler, R.; De Langhe, S.P. Hippo signaling promotes lung epithelial lineage commitment by curbing Fgf10 and β-catenin signaling. Development 2019, 146, dev166454. [Google Scholar] [CrossRef]
- Yang, C.R.; Yeh, S.; Leskov, K.; Odegaard, E.; Hsu, H.L.; Chang, C.; Kinsella, T.J.; Chen, D.J.; Boothman, D.A. Isolation of Ku70-binding proteins (KUBs). Nucleic Acids Res. 1999, 27, 2165–2174. [Google Scholar] [CrossRef]
- Fischer, U.; Hemmer, D.; Heckel, D.; Michel, A.; Feiden, W.; Steudel, W.I.; Hulsebos, T.; Meese, E. KUB3 amplification and overexpression in human gliomas. Glia 2001, 36, 1–10. [Google Scholar] [CrossRef]
- Fischer, U.; Rheinheimer, S.; Krempler, A.; Löbrich, M.; Meese, E. Glioma-amplified sequence KUB3 influences double-strand break repair after ionizing radiation. Int. J. Oncol. 2013, 43, 50–56. [Google Scholar] [CrossRef]
- Jin, J.; Li, Y.; Ren, J.; Man Lam, S.; Zhang, Y.; Hou, Y.; Zhang, X.; Xu, R.; Shui, G.; Ma, R.Z. Neonatal respiratory failure with retarded perinatal lung maturation in mice caused by reticulocalbin 3 disruption. Am. J. Respir. Cell Mol. Biol. 2016, 54, 410–423. [Google Scholar] [CrossRef]
- Zeng, X.; Neupert, W.; Tzagoloff, A. The metalloprotease encoded by ATP23 has a dual function in processing and assembly of subunit 6 of mitochondrial ATPase. Mol. Biol. Cell 2007, 18, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Antony, N.; McDougall, A.R.; Mantamadiotis, T.; Cole, T.J.; Bird, A.D. Creb1 regulates late stage mammalian lung development via respiratory epithelial and mesenchymal-independent mechanisms. Sci. Rep. 2016, 6, 25569. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.J.; Brownfield, D.G.; Krasnow, M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014, 507, 190–194. [Google Scholar] [CrossRef]
- Cardoso, W.V.; Whitsett, J.A. Resident cellular components of the lung: Developmental aspects. Proc. Am. Thorac. Soc. 2008, 5, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, S.; Shiosaki, J.; Al Alam, D. FGF signaling in lung development and disease: Human Versus Mouse. Front. Genet. 2019, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.K.; Mailleux, A.A.; Gupte, V.V.; Mata, F.; Sala, F.G.; Veltmaat, J.M.; Del Moral, P.M.; De Langhe, S.; Parsa, S.; Kelly, L.K.; et al. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev. Biol. 2007, 307, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama, J.; Yamagishi, A.; Kuroiwa, A. Tbx4-Fgf10 system controls lung bud formation during chicken embryonic development. Development 2003, 130, 1225–1234. [Google Scholar] [CrossRef]
- Tian, X.L.; Yong, S.L.; Wan, X.; Wu, L.; Chung, M.K.; Tchou, P.J.; Rosenbaum, D.S.; Van Wagoner, D.R.; Kirsch, G.E.; Wang, Q. Mechanisms by which SCN5A mutation N(1325)S causes cardiac arrhythmias and sudden death in vivo. Cardiovasc. Res. 2004, 61, 256–267. [Google Scholar] [CrossRef]
- Zhang, T.; Yong, S.L.; Tian, X.L.; Wang, Q.K. Cardiac-specific overexpression of SCN5A gene leads to shorter P wave duration and PR interval in transgenic mice. Biochem. Biophys. Res. Commun. 2007, 355, 444–450. [Google Scholar] [CrossRef][Green Version]
- Reginensi, A.; Enderle, L.; Gregorieff, A.; Johnson, R.L.; Wrana, J.L.; McNeill, H. A critical role for NF2 and the Hippo pathway in branching morphogenesis. Nat. Commun. 2016, 7, 12309. [Google Scholar] [CrossRef]
- Camolotto, S.A.; Pattabiraman, S.; Mosbruger, T.L.; Jones, A.; Belova, V.K.; Orstad, G.; Streiff, M.; Salmond, L.; Stubben, C.; Kaestner, K.H.; et al. FoxA1 and FoxA2 drive gastric differentiation and suppress squamous identity in NKX2-1-negative lung cancer. eLife 2018, 7, e38579. [Google Scholar] [CrossRef]
- Sah, R.K.; Ma, J.; Bah, F.B.; Xing, Z.; Adlat, S.; Oo, Z.M.; Wang, Y.; Bahadar, N.; Bohio, A.A.; Nagi, F.H.; et al. Targeted disruption of mouse Dip2B leads to abnormal lung development and prenatal lethality. Int. J. Mol. Sci. 2020, 21, 8223. [Google Scholar] [CrossRef]
- Yang, G.; Ding, Y.; Shang, X.; Zhao, T.; Lu, S.; Tian, J.; Weng, J.; Zeng, X. Atp23p and Atp10p coordinate to regulate the assembly of yeast mitochondrial ATP synthase. FASEB J. 2021, 35, e21538. [Google Scholar] [CrossRef]

| (A) Postnatal Offspring of HT Intercrosses | |||||
| WT | HT | KO | Total | ||
| P(>1 d) | Actual | 28 | 29 | 0 | 57 |
| Expected | 28 | 56 | 28 | 112 | |
| P1(<1 d) | Actual | 8 | 14 | 9 | 31 |
| Expected | 8 | 16 | 8 | 32 | |
| (B) Embryos of HT Intercrosses | |||||
| WT | HT | KO | Total | ||
| E15.5 | Actual | 11 | 25 | 8 | 44 |
| Expected | 11 | 22 | 11 | 44 | |
| E18.5 | Actual | 11 | 21 | 13 | 45 |
| Expected | 11 | 22 | 11 | 44 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Lu, S.; Jiang, J.; Weng, J.; Zeng, X. Kub3 Deficiency Causes Aberrant Late Embryonic Lung Development in Mice by the FGF Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 6014. https://doi.org/10.3390/ijms23116014
Yang G, Lu S, Jiang J, Weng J, Zeng X. Kub3 Deficiency Causes Aberrant Late Embryonic Lung Development in Mice by the FGF Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(11):6014. https://doi.org/10.3390/ijms23116014
Chicago/Turabian StyleYang, Guangying, Shan Lu, Jia Jiang, Jun Weng, and Xiaomei Zeng. 2022. "Kub3 Deficiency Causes Aberrant Late Embryonic Lung Development in Mice by the FGF Signaling Pathway" International Journal of Molecular Sciences 23, no. 11: 6014. https://doi.org/10.3390/ijms23116014
APA StyleYang, G., Lu, S., Jiang, J., Weng, J., & Zeng, X. (2022). Kub3 Deficiency Causes Aberrant Late Embryonic Lung Development in Mice by the FGF Signaling Pathway. International Journal of Molecular Sciences, 23(11), 6014. https://doi.org/10.3390/ijms23116014





