Food Additive Zinc Oxide Nanoparticles: Dissolution, Interaction, Fate, Cytotoxicity, and Oral Toxicity
Abstract
:1. Introduction
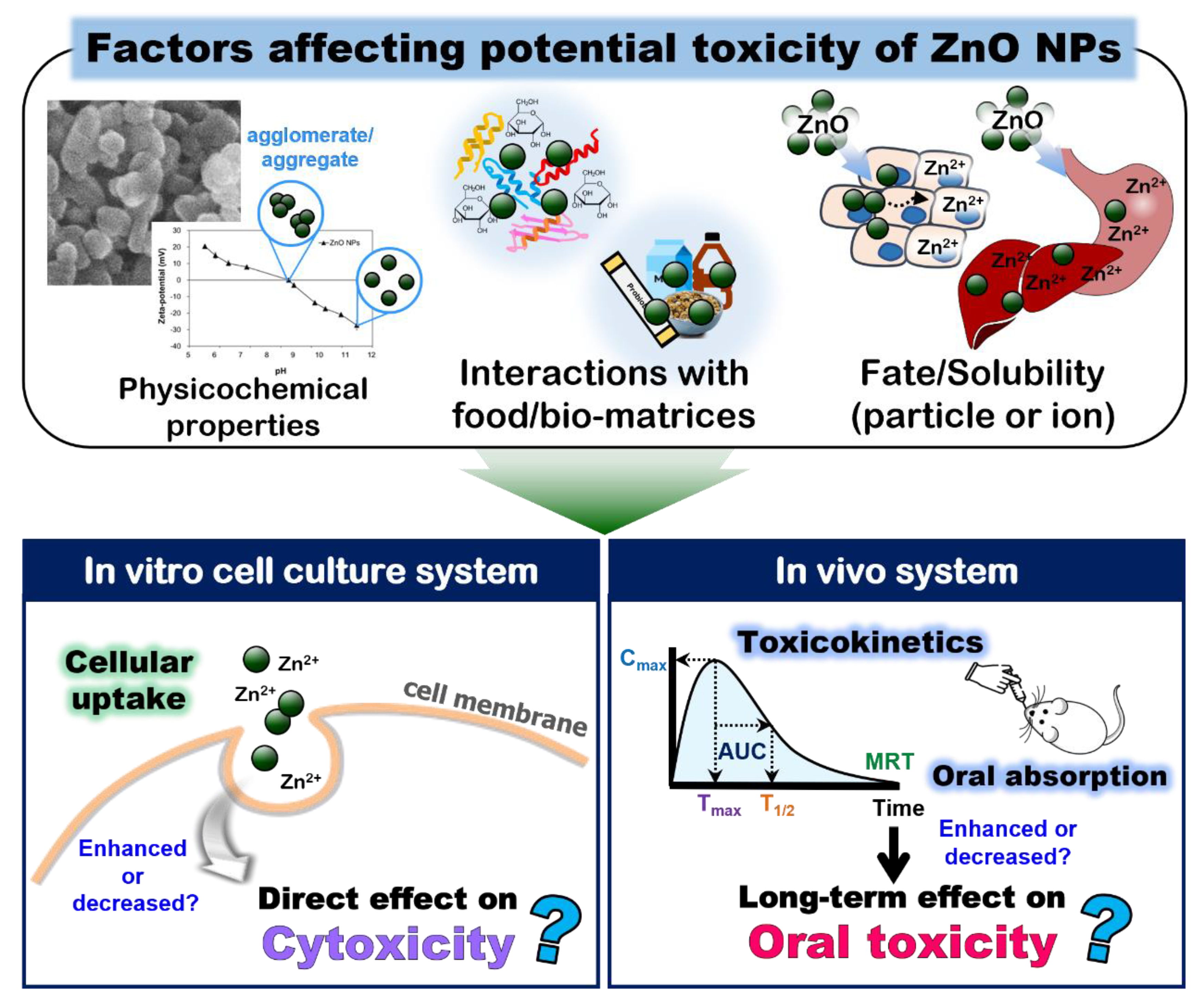
2. Dissolution Properties of ZnO NPs
2.1. pH Environments
2.2. In Vitro Digestion Systems
2.3. Interaction Effects
3. Interactions between ZnO NPs and Bio- or Food-Matrices
3.1. NP Interactions with Bio-Matrices
3.2. NP Interactions with Food-Matrices
4. Fates of ZnO NPs in Biological- and Food-Matrices
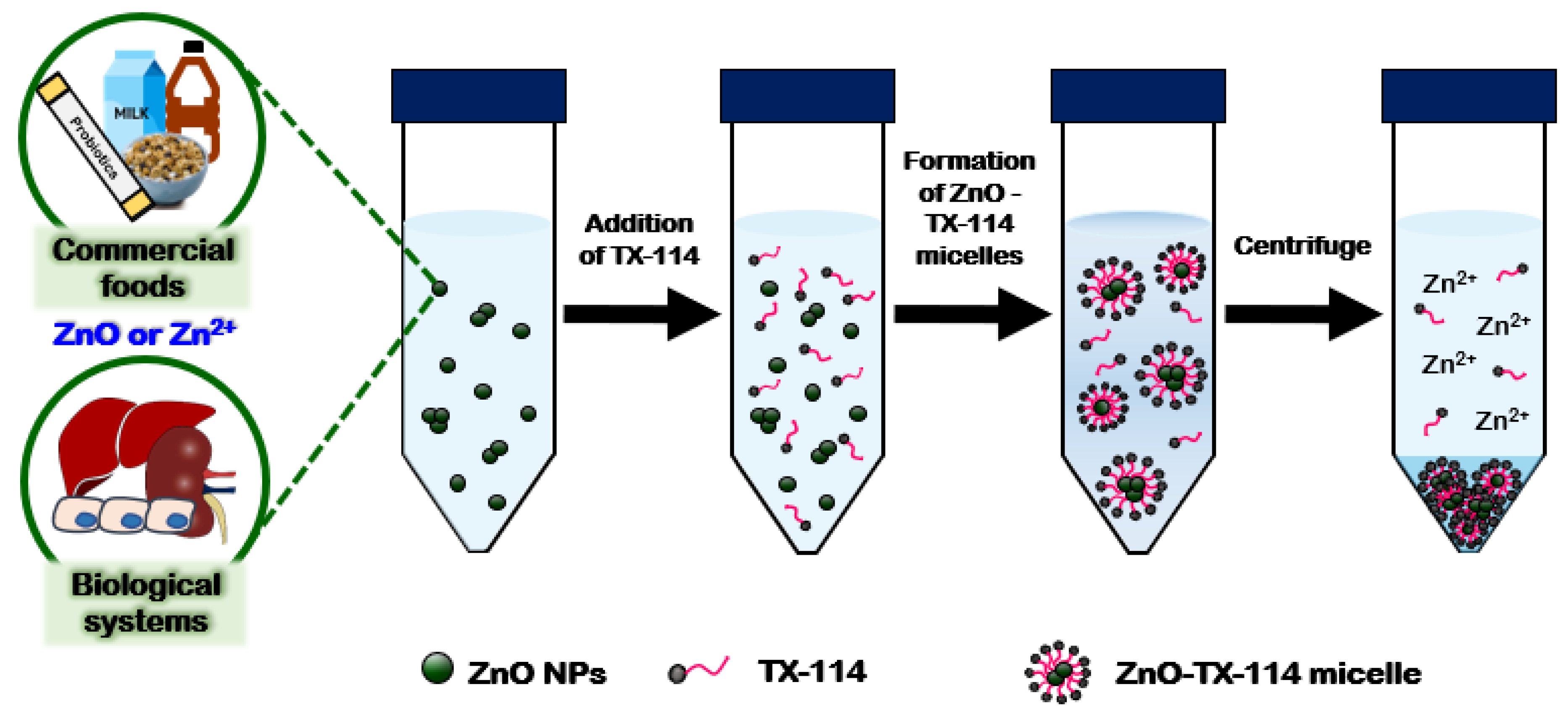
4.1. Methodological Approaches for Fate Determination of ZnO NPs
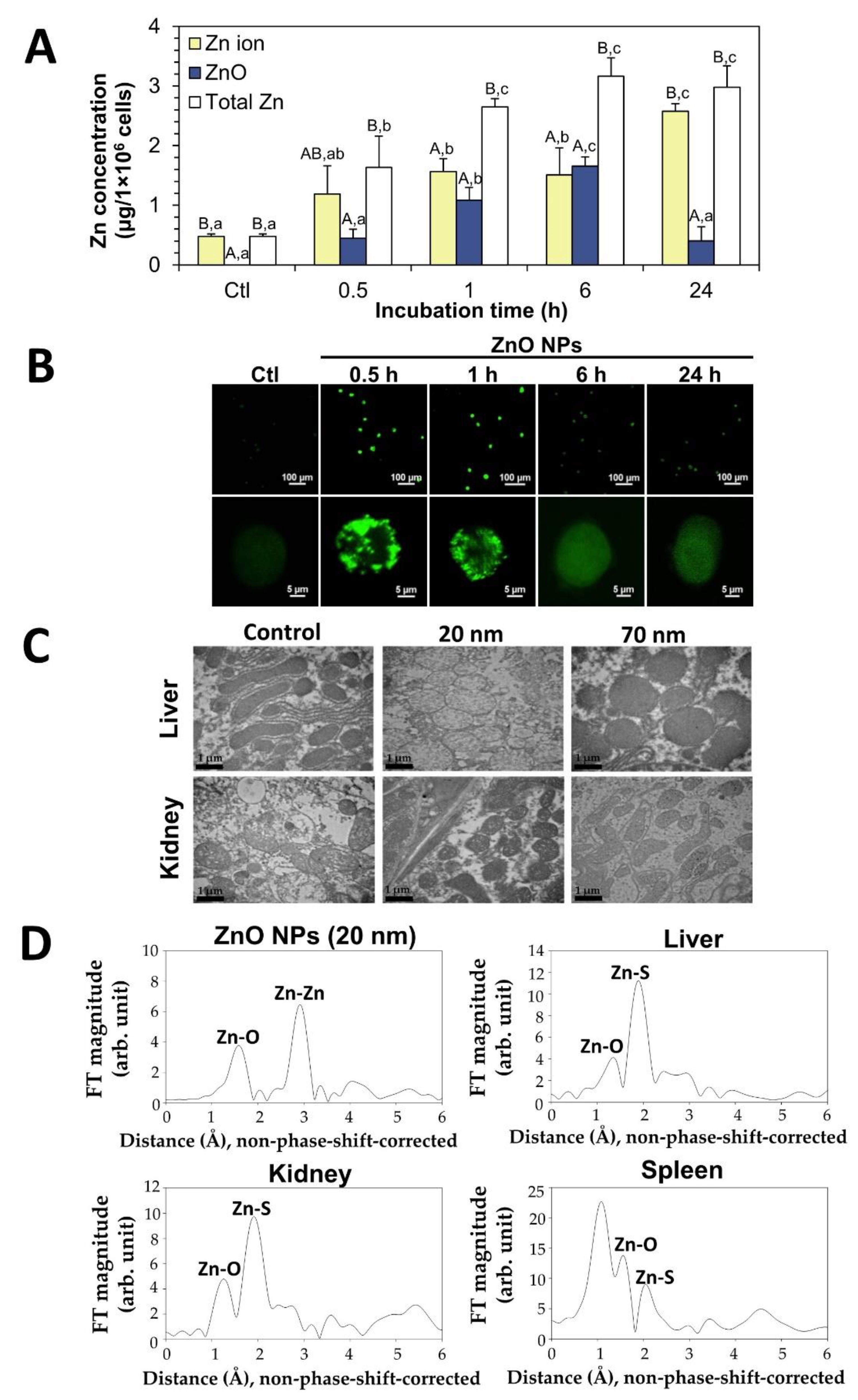
4.2. Fates of ZnO NPs in Biological Systems and Processed Foods
5. Cytotoxicity and Oral Toxicity of ZnO NPs Interacted with Bio- or Food-Matrices
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, S.; Yadav, S. Bioaccumulation of ZnO-NPs in earthworm Eisenia fetida (Savigny). J. Bioremediation Biodegrad. 2014, 5, 250–256. [Google Scholar]
- National Institutes of Health (NIH). Zinc: Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/ (accessed on 7 December 2021).
- Agostoni, C.; Canani, R.B.; Fairweather-Tait, S.; Heinonen, M.; Korhonen, H.; La Vielle, S.; Marchelli, R.; Martin, A.; Naska, A.; Neuhäuser-Berthold, M.; et al. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar]
- Guillem, A.; Alegría, A.; Barberá, R.; Farré, R.; Lagarda, M.J.; Clemente, G. In vitro dialyzability of Zinc from different salts used in the supplementation of infant formulas. Biol. Trace Elem. Res. 2000, 75, 11–19. [Google Scholar] [CrossRef]
- Wegmuller, R.; Tay, F.; Zeder, C.; Brnic, M.; Hurrell, R.F. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J. Nutr. 2014, 144, 132–136. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration (FDA). Select Committee on GRAS Substances Database. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=SCOGS (accessed on 1 March 2022).
- Yang, Y.; Zhang, C.; Hu, Z. Impact of metallic and metal oxide nanoparticles on wastewater treatment and anaerobic digestion. Environ. Sci. Process. Impacts 2013, 15, 39–48. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Shahin, K.; Zaglool, A.W.; Roushdy, E.M.; Ahmed, S.A.A. Efficacy of nano zinc oxide dietary supplements on growth performance, immunomodulation and disease resistance of African catfish Clarias gariepinus. Dis. Aquat. Organ. 2020, 142, 147–160. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tan, S.X.; Xiao, X.Y.; Qiu, X.S.; Pan, J.Q.; Tang, Z.X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 2014, 160, 361–367. [Google Scholar] [CrossRef]
- Pei, X.; Xiao, Z.; Liu, L.; Wang, G.; Tao, W.; Wang, M.; Zou, J.; Leng, D. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J. Sci. Food Agric. 2019, 99, 1366–1374. [Google Scholar] [CrossRef]
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Dominic, G.; Selvaraju, S. Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Anim. Nutr. 2016, 2, 134–141. [Google Scholar] [CrossRef]
- Wang, B.; Feng, W.Y.; Wang, T.C.; Jia, G.; Wang, M.; Shi, J.W.; Zhang, F.; Zhao, Y.L.; Chai, Z.F. Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicol. Lett. 2006, 161, 115–123. [Google Scholar] [CrossRef]
- Porea, T.J.; Belmont, J.W.; Mahoney, D.H., Jr. Zinc-induced anemia and neutropenia in an adolescent. J. Pediatr. 2000, 136, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Huang, C.W.; Liao, V.H. Early-life long-term exposure to ZnO nanoparticles suppresses innate immunity regulated by SKN-1/Nrf and the p38 MAPK signaling pathway in Caenorhabditis elegans. Environ. Pollut. 2020, 256, 113382. [Google Scholar] [CrossRef] [PubMed]
- Hoffman II, H.N.; Phyliky, R.L.; Fleming, C.R. Zinc-induced copper deficiency. Gastroenterology 1988, 94, 508–512. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Food Additive Status List. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (accessed on 26 August 2021).
- Grasso, A.; Ferrante, M.; Moreda-Pineiro, A.; Arena, G.; Magarini, R.; Oliveri Conti, G.; Cristaldi, A.; Copat, C. Dietary exposure of zinc oxide nanoparticles (ZnO-NPs) from canned seafood by single particle ICP-MS: Balancing of risks and benefits for human health. Ecotoxicol. Environ. Saf. 2022, 231, 113217. [Google Scholar] [CrossRef]
- Choi, S.J.; Choy, J.H. Biokinetics of zinc oxide nanoparticles: Toxicokinetics, biological fates, and protein interaction. Int. J. Nanomed. 2014, 9, 261–269. [Google Scholar]
- Zheng, X.; Wu, R.; Chen, Y. Effects of ZnO nanoparticles on wastewater biological nitrogen and phosphorus removal. Environ. Sci. Technol. 2011, 45, 2826–2832. [Google Scholar] [CrossRef]
- Saliani, M.; Jalal, R.; Kafshdare, G.E. Effects of pH and temperature on antibacterial activity of Zinc Oxide nanofluid against Escherichia coli O157: H7 and Staphylococcus aureus. Jundishapur. J. Microbiol. 2015, 8, e17115. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Rong, R.; Zhu, S.; Guo, M.; Gao, S.; Wang, S.; Xu, X. Effects of ZnO nanoparticles on dimethoate-induced toxicity in mice. J. Agric. Food Chem. 2015, 63, 8292–8298. [Google Scholar] [CrossRef]
- Cardoso, D.; Narcy, A.; Durosoy, S.; Bordes, C.; Chevalier, Y. Dissolution kinetics of zinc oxide and its relationship with physicochemical characteristics. Powder Technol. 2021, 378, 746–759. [Google Scholar] [CrossRef]
- Meulenkamp, E.A. Size dependence of the dissolution of ZnO nanoparticles. J. Phys. Chem. B 1998, 102, 7764–7769. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Rupasinghe, T.; Wu, C.M.; Grassian, V.H. Dissolution of ZnO nanoparticles at circumneutral pH: A study of size effects in the presence and absence of citric acid. Langmuir 2012, 28, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Avramescu, M.L.; Rasmussen, P.E.; Chenier, M.; Gardner, H.D. Influence of pH, particle size and crystal form on dissolution behaviour of engineered nanomaterials. Environ. Sci. Pollut. Res. 2017, 24, 1553–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Kim, H.J.; Go, M.R.; Bae, S.H.; Choi, S.J. ZnO interactions with biomatrices: Effect of particle size on ZnO-protein corona. Nanomaterials 2017, 7, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Choi, S.J. Particle size and biological fate of ZnO do not cause acute toxicity, but affect toxicokinetics and gene expression profiles in the rat livers after oral administration. Int. J. Mol. Sci. 2021, 22, 1698. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef]
- Jung, E.B.; Yu, J.; Choi, S.J. Interaction between ZnO nanoparticles and albumin and its effect on cytotoxicity, cellular uptake, intestinal transport, toxicokinetics, and acute oral toxicity. Nanomaterials 2021, 11, 2922. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, L.; Yan, F.; Liu, Z.; Bao, H.; Liu, T. Solubility of ZnO nanoparticles in food media: An analysis using a novel semiclosed dynamic system. J. Agric. Food Chem. 2021, 69, 11065–11073. [Google Scholar] [CrossRef]
- Voss, L.; Saloga, P.E.J.; Stock, V.; Böhmert, L.; Braeuning, A.; Thünemann, A.F.; Lampen, A.; Sieg, H. Environmental impact of ZnO nanoparticles evaluated by in vitro simulated digestion. ACS Appl. Nano Mater. 2020, 3, 724–733. [Google Scholar] [CrossRef]
- Du, L.J.; Xiang, K.; Liu, J.H.; Song, Z.M.; Liu, Y.; Cao, A.; Wang, H. Intestinal injury alters tissue distribution and toxicity of ZnO nanoparticles in mice. Toxicol. Lett. 2018, 295, 74–85. [Google Scholar] [CrossRef]
- Gomez, B.G.; Perez-Corona, M.T.; Madrid, Y. Availability of zinc from infant formula by in vitro methods (solubility and dialyzability) and size-exclusion chromatography coupled to inductively coupled plasma-mass spectrometry. J. Dairy Sci. 2016, 99, 9405–9414. [Google Scholar] [CrossRef] [Green Version]
- Turney, T.W.; Duriska, M.B.; Jayaratne, V.; Elbaz, A.; O’Keefe, S.J.; Hastings, A.S.; Piva, T.J.; Wright, P.F.; Feltis, B.N. Formation of zinc-containing nanoparticles from Zn2+ ions in cell culture media: Implications for the nanotoxicology of ZnO. Chem. Res. Toxicol. 2012, 25, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.B.; Ladner, D.A.; Higgins, C.P.; Westerhoff, P.; Ranville, J.F. Solubility of nano-zinc oxide in environmentally and biologically important matrices. Environ. Toxicol. Chem. 2012, 31, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.R.; Yu, J.; Choi, S.J. Fate determination of ZnO in commercial foods and human intestinal cells. Int. J. Mol. Sci. 2020, 21, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limmer, S.J.; Kulp, E.A.; Switzer, J.A. Epitaxial electrodeposition of ZnO on Au(111) from alkaline solution: Exploiting amphoterism in Zn(II). Langmuir 2006, 22, 10535–10539. [Google Scholar] [CrossRef]
- Go, M.R.; Yu, J.; Bae, S.H.; Kim, H.J.; Choi, S.J. Effects of interactions between ZnO Nanoparticles and saccharides on biological responses. Int. J. Mol. Sci. 2018, 19, 486. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Shen, C.; Feltis, B.N.; Martin, L.L.; Hughes, A.E.; Wright, P.F.; Turney, T.W. Reducing ZnO nanoparticle cytotoxicity by surface modification. Nanoscale 2014, 6, 5791–5798. [Google Scholar] [CrossRef]
- Deng, Z.J.; Mortimer, G.; Schiller, T.; Musumeci, A.; Martin, D.; Minchin, R.F. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology 2009, 20, 455101–455110. [Google Scholar] [CrossRef]
- Bhogale, A.; Patel, N.; Sarpotdar, P.; Mariam, J.; Dongre, P.M.; Miotello, A.; Kothari, D.C. Systematic investigation on the interaction of bovine serum albumin with ZnO nanoparticles using fluorescence spectroscopy. Colloids Surf. B Biointerfaces 2013, 102, 257–264. [Google Scholar] [CrossRef]
- Bae, S.H.; Yu, J.; Lee, T.G.; Choi, S.J. Protein food matrix-ZnO nanoparticle interactions affect protein conformation, but may not be biological responses. Int. J. Mol. Sci. 2018, 19, 3926. [Google Scholar] [CrossRef] [Green Version]
- Shim, K.H.; Hulme, J.; Maeng, E.H.; Kim, M.K.; An, S.S.A. Analysis of zinc oxide nanoparticles binding proteins in rat blood and brain homogenate. Int. J. Nanomed. 2014, 9, 217–224. [Google Scholar]
- Kathiravan, A.; Paramaguru, G.; Renganathan, R. Study on the binding of colloidal zinc oxide nanoparticles with bovine serum albumin. J. Mol. Struct. 2009, 934, 129–137. [Google Scholar] [CrossRef]
- Bhunia, A.K.; Samanta, P.K.; Saha, S.; Kamilya, T. ZnO nanoparticle-protein interaction: Corona formation with associated unfolding. Appl. Phys. Lett. 2013, 103, 143701. [Google Scholar] [CrossRef]
- Bukackova, M.; Marsalek, R. Interaction of BSA with ZnO, TiO2, and CeO2 nanoparticles. Biophys. Chem. 2020, 267, 106475. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, N.P.; Chandran, P.; Sudheer Khan, S. Interaction of colloidal zinc oxide nanoparticles with bovine serum albumin and its adsorption isotherms and kinetics. Colloids Surf. B Biointerfaces 2013, 102, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Saifi, Z.; Kumar, M.; Reimers, A.; Krishnananda, S.D.; Adelung, R.; Baum, M. Role of structural specificity of ZnO particles in preserving functionality of proteins in their corona. Sci. Rep. 2021, 11, 15945. [Google Scholar] [CrossRef]
- Giannousi, K.; Geromichalos, G.; Kakolyri, D.; Mourdikoudis, S.; Dendrinou-Samara, C. Interaction of ZnO nanostructures with proteins: In vitro fibrillation/antifibrillation studies and in silico molecular docking simulations. ACS Chem. Neurosci. 2020, 11, 436–444. [Google Scholar] [CrossRef]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Jo, M.-R.; Chung, H.-E.; Kim, H.-J.; Bae, S.-H.; Go, M.-R.; Yu, J.; Choi, S.-J. Effects of zinc oxide nanoparticle dispersants on cytotoxicity and cellular uptake. Mol. Cell. Toxicol. 2016, 12, 281–288. [Google Scholar] [CrossRef]
- Tantra, R.; Tompkins, J.; Quincey, P. Characterisation of the de-agglomeration effects of bovine serum albumin on nanoparticles in aqueous suspension. Colloids Surf. B Biointerfaces 2010, 75, 275–281. [Google Scholar] [CrossRef]
- Meißner, T.; Oelschlägel, K.; Potthoff, A. Implications of the stability behavior of zinc oxide nanoparticles for toxicological studies. Int. Nano Lett. 2014, 4, 1–13. [Google Scholar] [CrossRef]
- García-López, J.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.; Díaz Barriga-Castro, E.; Ruiz-Torres, N.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Gu, T.; Yao, C.; Zhang, K.; Li, C.; Ding, L.; Huang, Y.; Wu, M.; Wang, Y. Toxic effects of zinc oxide nanoparticles combined with vitamin C and casein phosphopeptides on gastric epithelium cells and the intestinal absorption of mice. RSC Adv. 2018, 8, 26078–26088. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernandez-Jerez, A.; Hougaard, B.S.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: Human and animal health. EFSA J. 2021, 19, e06768. [Google Scholar] [PubMed]
- Tada-Oikawa, S.; Ichihara, G.; Suzuki, Y.; Izuoka, K.; Wu, W.; Yamada, Y.; Mishima, T.; Ichihara, S. Zn(II) released from zinc oxide nano/micro particles suppresses vasculogenesis in human endothelial colony-forming cells. Toxicol. Rep. 2015, 2, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Frechétte-Viens, L.; Hadioui, M.; Wilkinson, K.J. Quantification of ZnO nanoparticles and other Zn containing colloids in natural waters using a high sensitivity single particle ICP-MS. Talanta 2019, 200, 156–162. [Google Scholar] [CrossRef]
- Hadioui, M.; Merdzan, V.; Wilkinson, K.J. Detection and characterization of ZnO nanoparticles in surface and waste waters using single particle ICPMS. Environ. Sci. Technol. 2015, 49, 6141–6148. [Google Scholar] [CrossRef]
- Donovan, A.R.; Adams, C.D.; Ma, Y.; Stephan, C.; Eichholz, T.; Shi, H. Detection of zinc oxide and cerium dioxide nanoparticles during drinking water treatment by rapid single particle ICP-MS methods. Anal. Bioanal. Chem. 2016, 408, 5137–5145. [Google Scholar] [CrossRef]
- He, X.; Zhang, H.; Shi, H.; Liu, W.; Sahle-Demessie, E. Fates of Au, Ag, ZnO, and CeO2 nanoparticles in simulated gastric fluid studied using single-particle-inductively coupled plasma-mass spectrometry. J. Am. Soc. Mass Spectr. 2020, 31, 2180–2190. [Google Scholar] [CrossRef]
- Majedi, S.M.; Lee, H.K.; Kelly, B.C. Chemometric analytical approach for the cloud point extraction and inductively coupled plasma mass spectrometric determination of zinc oxide nanoparticles in water samples. Anal. Chem. 2012, 84, 6546–6552. [Google Scholar] [CrossRef]
- Mohd, A.N.A.; Rahim, N.Y.; Raoov, M.; Asman, S. Optimisation and evaluation of Zinc in food samples by cloud point extraction and spectrophotometric detection. Sci. Res. J. 2019, 16, 41–59. [Google Scholar] [CrossRef]
- Tabrizi, A.B. Cloud point extraction and spectrofluorimetric determination of aluminium and zinc in foodstuffs and water samples. Food Chem. 2007, 100, 1698–1703. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Mao, Z.; Yu, D.; Gao, C. Toxicity of ZnO nanoparticles to macrophages due to cell uptake and intracellular release of zinc ions. J. Nanosci. Nanotechnol. 2014, 14, 5688–5696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condello, M.; De Berardis, B.; Ammendolia, M.G.; Barone, F.; Condello, G.; Degan, P.; Meschini, S. ZnO nanoparticle tracking from uptake to genotoxic damage in human colon carcinoma cells. Toxicol. In Vitro 2016, 35, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Parashar, V.; Chauhan, L.K.; Shanker, R.; Das, M.; Tripathi, A.; Dwivedi, P.D. Mechanism of uptake of ZnO nanoparticles and inflammatory responses in macrophages require PI3K mediated MAPKs signaling. Toxicol. In Vitro 2014, 28, 457–467. [Google Scholar] [CrossRef]
- Gilbert, B.; Fakra, S.C.; Xia, T.; Pokhrel, S.; Madler, L.; Nel, A.E. The fate of ZnO nanoparticles administered to human bronchial epithelial cells. ACS Nano 2012, 6, 4921–4930. [Google Scholar] [CrossRef] [Green Version]
- Baek, M.; Chung, H.E.; Yu, J.; Lee, J.A.; Kim, T.H.; Oh, J.M.; Lee, W.J.; Paek, S.M.; Lee, J.K.; Jeong, J.; et al. Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. Int. J. Nanomed. 2012, 7, 3081–3097. [Google Scholar]
- Bell, S.G.; Vallee, B.L. The metallothionein/thionein system: An oxidoreductive metabolic zinc link. Chembiochem 2009, 10, 55–62. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Mao, L.; Dong, X.; Peng, Q.; Chen, J.; Tan, C.; Hu, R. Effect of ZnO nanoparticle on cell viability, zinc uptake efficiency, and zinc transporters gene expression: A comparison with ZnO and ZnSO4. Czech. J. Anim. Sci. 2017, 62, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Yin, H.; Casey, P.S. Effects of iron or manganese doping of ZnO nanoparticles on their dissolution, ROS generation and cytotoxicity. RSC Adv. 2014, 4, 26149–26157. [Google Scholar] [CrossRef]
- Fukui, H.; Horie, M.; Endoh, S.; Kato, H.; Fujita, K.; Nishio, K.; Komaba, L.K.; Maru, J.; Miyauhi, A.; Nakamura, A.; et al. Association of zinc ion release and oxidative stress induced by intratracheal instillation of ZnO nanoparticles to rat lung. Chem.-Biol. Interact. 2012, 198, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Canta, M.; Cauda, V. The investigation of the parameters affecting the ZnO nanoparticle cytotoxicity behavior: A tutorial review. Biomater. Sci. 2020, 22, 6157–6174. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Guan, R.; Chen, X.; Song, Y.; Jiang, H.; Zhao, J. In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells. Nanoscale Res. Lett. 2013, 8, 496. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Wang, H.; He, M.; Chen, B.; Yang, B.; Hu, B. Size-dependent cytotoxicity study of ZnO nanoparticles in HepG2 cells. Ecotoxicol. Environ. Saf. 2019, 171, 337–346. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M. Preferential cytotoxicity of ZnO nanoparticle towards cervical cancer cells induced by ROS-mediated apoptosis and cell cycle arrest for cancer therapy. J. Nanopart. Res. 2016, 18, 219. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Leong, D.T. Mechanistic Investigation of the Biological Effects of SiO2, TiO2, and ZnO nanoparticles on intestinal cells. Small 2015, 11, 3458–3468. [Google Scholar] [CrossRef]
- Anders, C.B.; Chess, J.J.; Wingett, D.G.; Punnoose, A. Serum proteins enhance dispersion stability and influence the cytotoxicity and dosimetry of ZnO nanoparticles in suspension and adherent cancer cell models. Nanoscale Res. Lett. 2015, 10, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Janani, B.; Raju, L.L.; Thomas, A.M.; Alyemeni, M.N.; Dudin, G.A.; Wijaya, L.; Alsahli, A.A.; Ahmad, P.; Khan, S.S. Impact of bovine serum albumin—A protein corona on toxicity of ZnO NPs in environmental model systems of plant, bacteria, algae and crustaceans. Chemosphere 2021, 270, 128629. [Google Scholar] [CrossRef]
- Precupas, A.; Gheorghe, D.; Botea-Petcu, A.; Leonties, A.R.; Sandu, R.; Popa, V.T.; Mariussen, E.; Naouale, E.Y.; Runden-Pran, E.; Dumit, V.; et al. Thermodynamic parameters at bio-nano interface and nanomaterial toxicity: A case study on BSA interaction with ZnO, SiO2, and TiO2. Chem. Res. Toxicol. 2020, 33, 2054–2071. [Google Scholar] [CrossRef]
- Da Silva, E.; Kembouche, Y.; Tegner, U.; Baun, A.; Jensen, K.A. Interaction of biologically relevant proteins with ZnO nanomaterials: A confounding factor for in vitro toxicity endpoints. Toxicol. In Vitro 2019, 56, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Roursgaard, M.; Kermanizadeh, A.; Loft, S.; Moller, P. Synergistic effects of zinc oxide nanoparticles and fatty acids on toxicity to caco-2 cells. Int. J. Toxicol. 2015, 34, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, L.; Yao, C.; Ding, L.; Li, C.; Fang, J.; Sui, K.; Liu, Y.; Wu, M. A combined toxicity study of zinc oxide nanoparticles and vitamin C in food additives. Nanoscale 2014, 6, 15333–15342. [Google Scholar] [CrossRef] [PubMed]

| Physicochemical Properties | Conditions | Concentrations | Solubilities | Reference |
|---|---|---|---|---|
| 28 nm 1, 1976 nm 2 290 nm 1, 3453 nm 2 | Simulated gastric (pH 1.5) fluid | 5 mg/mL | 24.5% | [26] |
| Simulated intestinal (pH 6.8) fluid | 0.2% | |||
| Simulated plasma fluid | 2.8% | |||
| Rat-extracted gastric fluid | 5 mg/mL | ~12% | ||
| Rat-extracted intestinal fluid | ~9% | |||
| Rat-extracted plasma fluid | ~2% | |||
| 86 nm 1, 401 nm 2 268 nm 1, 604 nm 2 | In vivo rat gastric fluid (oral administration) | 100 mg/kg | ~12% | [27] |
| <50 nm 1 <100 nm 1 | Neutral pH (7.0) | 0.5 mg/mL | 1.87–2.13% | [25] |
| Low pH (1.5) | 93.6–97.0% | |||
| 78 nm 1, 375 nm 2 | DW | 5 mg/mL | 0.1% | [29] |
| Cell culture MEM | 0.5–0.7% | |||
| Simulated saliva | 5 mg/mL | ~0.1% | ||
| Simulated gastric fluid | ~96% | |||
| Simulated intestinal fluid | ~4% | |||
| Simulated saliva + gastric fluid | 5 mg/mL | 95% | ||
| Simulated saliva + gastric + intestinal fluids | 25% | |||
| 15–70 nm 1, 180 nm 2 20–350 nm 1, 245 nm 2 | Simulated saliva | 209–8338 μg/mL ~93–3706 μg/mL 31–1250 μg/mL | <5% | [31] |
| Simulated saliva + gastric fluid | ~100% | |||
| Simulated saliva + gastric + intestinal fluid | 13–34% | |||
| 20 nm × 100 nm 1 (rod type), 1636 nm 2 ~200 nm 1, 1107 nm 2 | Simulated gastric fluid | 30 μg/mL ~11 μg/mL | 10.6–14.2% | [32] |
| Simulated gastric + intestinal fluid | 1.72–1.89% | |||
| 40 nm 1 | Water | 100 μg/mL | 2.2% | [39] |
| Cell culture RPMI 1640 | 2% | |||
| Cell culture RPMI 1640 + FBS | 1% | |||
| Artificial lysosomal fluid | 98.1% | |||
| 61 nm 1, 261 nm 2 | DW | 50 μg/mL | 1.2% | [37] |
| Coffee mix solution | 39.4% | |||
| Skim milk solution | 30.1% | |||
| Milk | 49.2% | |||
| Sports drink | 90.9% | |||
| Cell culture MEM | 18.0–24.8% | |||
| 25 nm 1, 1999 nm 2 | DW | 5 mg/mL | 0.2% | [38] |
| 10% honey | 0.7% | |||
| 5% sugar mixture | 0.2% |
| Interaction Matrices | Results | Reference | |
|---|---|---|---|
| ZnO | Matrix Types | ||
| Bulk ZnO (290 nm 1) ZnO NPs (28 nm 1) | Simulated gastric fluid Simulated intestinal fluid Simulated plasma fluid | Hydrodynamic diameters, zeta potentials, and fluorescence quenching ratios of proteins changed. | [26] |
| Rat plasma proteins | Serum albumin and fibrinogen strongly interacted with both the bulk ZnO and ZnO NPs, but complement C was only adsorbed onto the ZnO NPs. | ||
| ZnO NPs (7.5 nm 1) | BSA | ZnO NPs formed ground state complex with BSA. | [41] |
| +, −-charged ZnO NPs (20 nm 1, 100 nm 1) | Rat brain proteins Rat plasma proteins | Size or surface change of ZnO NPs did not affect the number of proteins adsorbed. | [43] |
| Colloidal ZnO NPs (2.5 nm 1) | BSA | Interaction between ZnO NPs and BSA led to conformational change of BSA. | [44] |
| ZnO NPs (15–20 nm 1) | BSA | Formation of a stable BSA–ZnO NP corona was associated with conformational change/unfolding of BSA. | [45] |
| ZnO NPs (68.1 nm 1, 78.8 nm 1) | BSA | BSA adsorbed onto ZnO NPs showed α-helical structural change. | [46] |
| Colloidal ZnO NPs (65 nm 2) | BSA | Electrostatic force of attraction was involved in BSA adsorption onto ZnO NPs. | [47] |
| Tetrapodal ZnO (15 μm 1)Spherical ZnO NPs (100 nm 1) | Insulin | Tetrapodal ZnO preserved the polarity and surface charge distribution of insulin. | [48] |
| ZnO nanoflower (168 nm 1) ZnO@PEG NPs (40 nm 1) | BSA Human insulin | ZnO nanoflower showed higher amyloid degradation rate in both proteins. | [49] |
| ZnO NPs (25 nm 1) | 10% honey 5% sugar mixtures 5% monosaccharide solutions | ZnO NPs actively interacted with glucose in the honey and sugar mixtures, but they most strongly interacted with fructose among the monosaccharide solutions. | [38] |
| ZnO NPs (25 nm 1, 1957 nm 2) | Skim milk Casein | The hydrodynamic diameters, zeta potentials, fluorescence quenching ratios of protein, and α-helical protein structure changed, but not the digestion efficacy. | [42] |
| ZnO NPs (78 nm 1, 375 nm 2) | Albumin Casein Zein | Primary structural stability or digestion efficacy of proteins were not affected by the interactions. | [29] |
| ZnO NPs (234 nm 2) | Lecithin BSA/serum | Adsorption of proteins on the surface of ZnO NPs prevented agglomeration. | [53] |
| Interaction Matrices | Models | Results | Reference | |
|---|---|---|---|---|
| ZnO | Matrix Types | |||
| ZnO NPs (70 nm 1) | BSA FBS | A549 cell | ZnO NPs dispersed in BSA and FBS showed a high cell proliferation inhibition associated with enhanced cellular uptake. | [51] |
| ZnO NPs (10 nm 1) | FBS | Jurkat T cell Hut-78 T cell T-47D cell LNCaP cell | Interactions between ZnO NPs and FBS increased or decreased the cytotoxicity depending on cell lines. | [81] |
| ZnO NPs (65 nm 2) | BSA | p. aeruginosa/S. aureus (bacteria) C. pyrenoidsa (algae) Daphnia sp. (crustacean) A. Cepa root cells (plant) | ZnO that interacted with BSA reduced the ROS generation, lipid peroxidation, and chromosomal aberrations. | [82] |
| ZnO NPs (158 nm 1) | BSA | A549 cell | BSA adsorption onto the ZnO NPs was spontaneous and enthalpy-controlled, decreasing the structural stability of BSA and causing biological alterations. | [83] |
| ZnO NPs (106 nm 1, 101 nm 1) | LDH | Complete Ham’s F12 medium | ZnO NPs decreased the LDH activity due to LDH adsorption onto the ZnO and interaction with dissolved Zn ions | [84] |
| ZnO NPs (78 nm 1, 375 nm 2) | Albumin Casein Zein Glucose | Caco-2 cell SD rat |
| [29] |
| ZnO NPs (25 nm 1, 1957 nm 2) | Skim milk Casein | Caco-2 cell | ZnO NPs that interacted with casein did not increase the cytotoxicity. | [42] |
| ZnO NPs (25 nm 1, 1999 nm 2) | 10% honey 5% sugar mixtures Monosaccharide solutions | Caco-2 cell SD rat | Cytotoxicity of the ZnO NPs was not affected by the interactions with saccharides, but their toxicokinetics and oral absorption increased. | [38] |
| ZnO NPs (150 nm 2) | Palmitic acid Free fatty acids mixture | Caco-2 cell | ZnO NP interaction with palmitic acid, but not with free fatty acid, increased the cytotoxicity related to ROS generation. | [85] |
| ZnO NPs (25 nm 1) | Vitamin C | GES-1 cell Kunming mice |
| [86] |
| Bulk ZnO (268 nm 1) ZnO NPs (86 nm 1) | - | SD rat | Gene expression profiles in the livers were influenced by the fate and particle size of the ZnO. | [27] |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, S.-M.; Choi, S.-J. Food Additive Zinc Oxide Nanoparticles: Dissolution, Interaction, Fate, Cytotoxicity, and Oral Toxicity. Int. J. Mol. Sci. 2022, 23, 6074. https://doi.org/10.3390/ijms23116074
Youn S-M, Choi S-J. Food Additive Zinc Oxide Nanoparticles: Dissolution, Interaction, Fate, Cytotoxicity, and Oral Toxicity. International Journal of Molecular Sciences. 2022; 23(11):6074. https://doi.org/10.3390/ijms23116074
Chicago/Turabian StyleYoun, Su-Min, and Soo-Jin Choi. 2022. "Food Additive Zinc Oxide Nanoparticles: Dissolution, Interaction, Fate, Cytotoxicity, and Oral Toxicity" International Journal of Molecular Sciences 23, no. 11: 6074. https://doi.org/10.3390/ijms23116074
APA StyleYoun, S.-M., & Choi, S.-J. (2022). Food Additive Zinc Oxide Nanoparticles: Dissolution, Interaction, Fate, Cytotoxicity, and Oral Toxicity. International Journal of Molecular Sciences, 23(11), 6074. https://doi.org/10.3390/ijms23116074







