A MZB Cell Activation Profile Present in the Lacrimal Glands of Sjögren’s Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mice Defined by Global RNA Transcriptomic Analyses
Abstract
:1. Introduction
2. Results
2.1. Early and Temporal Transcriptome Expressions in Lacrimal Glands of C57BL/6.NOD-Aec1Aec2 Mice Identify MZB Cell Involvement in SS Pathology but with Potentially Unique BCRs
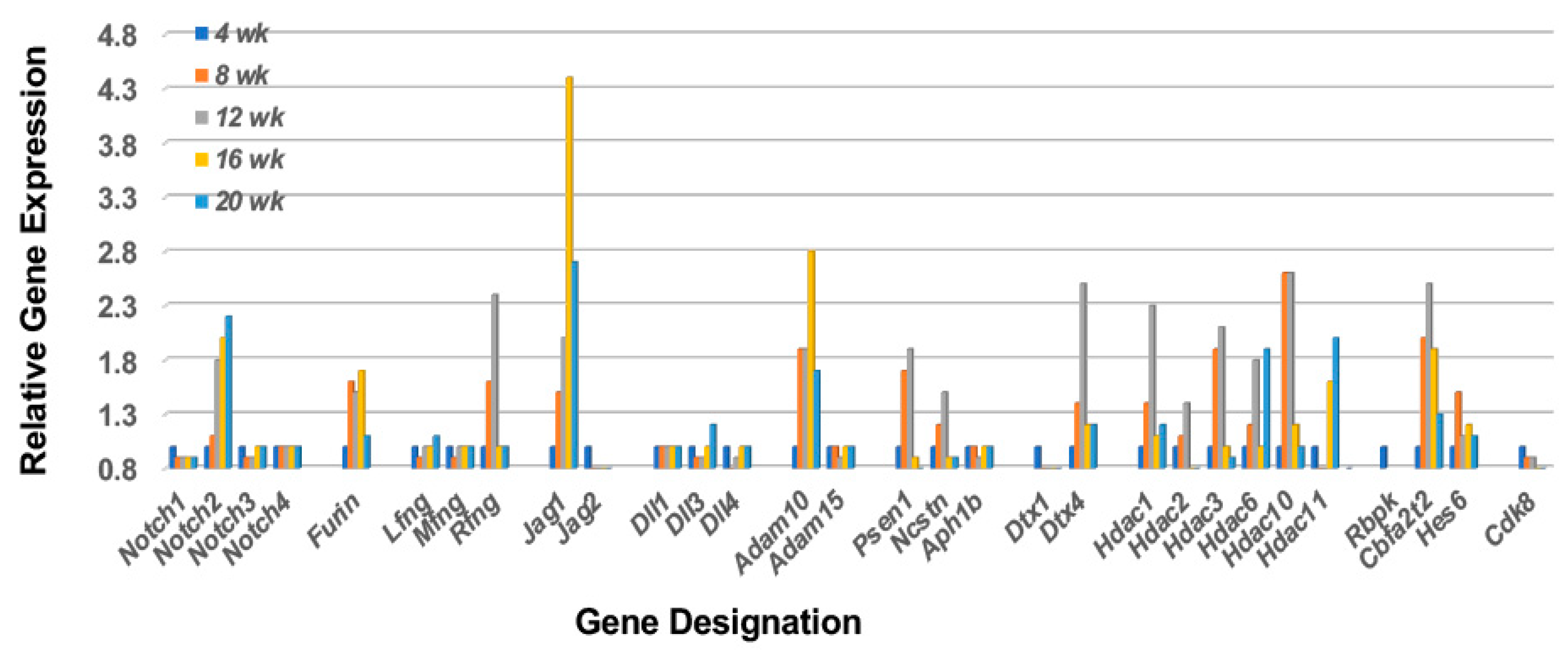
2.2. The Notch2 Signaling Pathway Predicts Activation of Mitogen-Activated Protein Kinase 14 (p38Mapk14α)
2.3. Limited Receptor Gene Expressions That Can Activate Early-Phase Immune Functions of MZB
2.4. Receptor-Ligand Dependent Emigration of Immune Cells to the Lacrimal Glands
2.5. Identification of Cell Emigration to the Lacrimal Glands in C57BL/6.NOD-Aec1Aec2 Mice
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. RNA Preparations
4.3. Microarray Data Analyses
5. Conclusions: Summary and Contribution to the Field
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting Cell |
| APRIL | Tnfsf13 (Tumor necrosis factor superfamily, member 13 |
| ARHGAP | Rho GTPase activating protein |
| ARHGEF | Rho GTPase exchange factor |
| BAFF | Tnfsf13b |
| BCR | B cell receptor |
| B220 | CD45-receptor |
| CCR | Chemokine (C-C motif) receptor |
| CCL | Chemokine (C-C motif) ligand |
| CD | Cell differentiation antigen |
| CDC42 | Cell division cycle 42 |
| CXCL | Chemokine (C-X-C motif) ligand |
| CXCR | Chemokine (C-X-C motif) receptor |
| DC | Dendritic cell |
| DOCK | Dedicator of cytokinesis |
| FACS | Fluoresec-activated cell sorting |
| GC | Granulocyte |
| GCRMA | Guanine-cytosine robust multi-array average |
| GTP-GAP | G protein bound-guanine nucleotide activating protein |
| GTP-GEF | G protein bound-guanine nucleotide exchange factor |
| HDAC | Histone deacetylase complex |
| ICAM1 | Intercellular adhesion molecule 1 |
| IFN | Interferon |
| IL | Interleukin |
| KO | Knock-out (gene) |
| LIMMA | Linear models for microarray analysis |
| LF | Lymphocytic foci |
| LFA1 | Lymphocyte function-association antigen 1 |
| LTI | Lymphoid tissue inducerr cell |
| MADCAM1 | Mucosal cell adhesion molecule-1 |
| MZ | Marginal zone |
| Mapk | Mitogen-activated protein kinase |
| MRC | Marginal reticular cell |
| MZB | Marginal zone B cell |
| NF-kb | Nuclear factor-kb |
| NK | Natural killer (cell) |
| NOTCH | Notch gene homolog |
| PALS | Periarteriolar lymphoid sheaths |
| PTK2 | Protein tyrosine kinase |
| RAC | Ras-related C3 botulinum toxin substrate |
| RANKL | Receptor activator of NF-kb |
| rt-PCR | Real-time polymerase chain reaction |
| RA | Rheumatoid arthritis |
| RHO-GTP | Ras homolog member |
| RNA | Ribonucleic acid |
| RND | Rho-family GTPase |
| RSF | Remodeling and spacing factor |
| SLE | Systemic lupus erythematosus |
| SS | Sjögren’s syndrome |
| S1P | Sphingosin-1-phosphate |
| S1PR | Sphingosine-1-phosphate receptor |
| TG | Transgenic |
| TLR | Toll-like receptor |
| VCAM1 | Vascular cell adhesion molecule 1 |
| VLA4 | Very late antigen 4 |
References
- Vivino, F.B.; Bunya, V.Y.; Massaro-Giordano, G.; Johr, C.R.; Giattino, S.L.; Schorpion, A.; Shafer, B.; Peck, A.; Sivils, K.; Rasmussen, A.; et al. Sjogren’s syndrome: An update on disease pathogenesis, clinical manifestations and treatment. Clin. Immunol. 2019, 203, 81–121. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.E.; Grimbacher, B.; Witte, T. Autoimmunity and primary immunodeficiency: Two sides of the same coin? Nat. Rev. Rheumatol. 2018, 14, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, Y.; Zhu, H.; Zhao, M.; Lu, Q. The Pathogenic Role of Dysregulated Epigenetic Modifications in Autoimmune Diseases. Front. Immunol. 2019, 10, 2305. [Google Scholar] [CrossRef] [Green Version]
- Slight-Webb, S.; Bourn, R.L.; Holers, V.M.; James, J.A. Shared and unique immune alterations in pre-clinical autoimmunity. Curr. Opin. Immunol. 2019, 61, 60–68. [Google Scholar] [CrossRef]
- Teng, X.; Cornaby, C.; Li, W.; Morel, L. Metabolic regulation of pathogenic autoimmunity: Therapeutic targeting. Curr. Opin. Immunol. 2019, 61, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Marketos, N.; Cinoku, I.; Rapti, A.; Mavragani, C.P. Type I interferon signature in Sjogren’s syndrome: Pathophysiological and clinical implications. Clin. Exp. Rheumatol. 2019, 37, S185–S191. [Google Scholar]
- Brito-Zeron, P.; Theander, E.; Baldini, C.; Seror, R.; Retamozo, S.; Quartuccio, L.; Bootsma, H.; Bowman, S.J.; Dorner, T.; Gottenberg, J.E.; et al. Early diagnosis of primary Sjogren’s syndrome: EULAR-SS task force clinical recommendations. Expert Rev. Clin. Immunol. 2016, 12, 137–156. [Google Scholar] [CrossRef]
- Fisher, B.A.; Jonsson, R.; Daniels, T.; Bombardieri, M.; Brown, R.M.; Morgan, P.; Bombardieri, S.; Ng, W.F.; Tzioufas, A.G.; Vitali, C.; et al. Sjogren’s histopathology workshop group from, E. Standardisation of labial salivary gland histopathology in clinical trials in primary Sjogren’s syndrome. Ann. Rheum. Dis. 2017, 76, 1161–1168. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Wu, H.; Chen, Y.; Zhang, J.; Zheng, M.; Chen, G.; Li, L.; Lu, Q. The Therapeutic and Pathogenic Role of Autophagy in Autoimmune Diseases. Front. Immunol. 2018, 9, 1512. [Google Scholar] [CrossRef] [Green Version]
- Stojanov, S.; Kastner, D.L. Familial autoinflammatory diseases: Genetics, pathogenesis and treatment. Curr. Opin. Rheumatol. 2005, 17, 586–599. [Google Scholar] [CrossRef]
- Dorner, T. Crossroads of B cell activation in autoimmunity: Rationale of targeting B cells. J. Rheumatol. Suppl. 2006, 77, 3–11. [Google Scholar] [PubMed]
- Hofmann, K.; Clauder, A.K.; Manz, R.A. Targeting B Cells and Plasma Cells in Autoimmune Diseases. Front. Immunol. 2018, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, L.I.; Daoussis, D.; Mavropoulos, A.; Liossis, S.N.; Bogdanos, D.P. Regulatory B cells: New players in inflammatory and autoimmune rheumatic diseases. Semin. Arthritis Rheum. 2019, 48, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Kroese, F.G.M.; Abdulahad, W.H.; Haacke, E.; Bos, N.A.; Vissink, A.; Bootsma, H. B-cell hyperactivity in primary Sjogren’s syndrome. Expert Rev. Clin. Immunol. 2014, 10, 483–499. [Google Scholar] [CrossRef]
- Nocturne, G.; Mariette, X. B cells in the pathogenesis of primary Sjogren syndrome. Nat. Rev. Rheumatol. 2018, 14, 133–145. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Cha, S.R.; Peck, A.B. Sjogren’s syndrome (SjS)-like disease of mice: The importance of B lymphocytes and autoantibodies. Front. Biosci. 2007, 12, 1767–1789. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Gauna, A.E.; Pauley, K.M.; Park, Y.J.; Cha, S. Animal Models in Autoimmune Diseases: Lessons Learned from Mouse Models for Sjogren’s Syndrome. Clin. Rev. Allergy Immunol. 2012, 42, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Delaleu, N.; Nguyen, C.Q.; Peck, A.B.; Jonsson, R. Sjogren’s syndrome: Studying the disease in mice. Arthritis Res. Ther. 2011, 13, 217. [Google Scholar] [CrossRef] [Green Version]
- Cha, S.; Peck, A.B.; Humphreys-Beher, M.G. Progress in understanding autoimmune exocrinopathy using the non-obese diabetic mouse: An update. Crit. Rev. Oral Biol. Med. 2002, 13, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.Q.; Kim, H.; Cornelius, J.G.; Peck, A.B. Development of Sjogren’s syndrome in nonobese diabetic-derived autoimmune-prone C57BL/6.NOD-Aec1Aec2 mice is dependent on complement component-3. J. Immunol. 2007, 179, 2318–2329. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.P.; Yamachika, S.; Bounous, D.I.; Brayer, J.; Jonsson, R.; Holmdahl, R.; Peck, A.B.; Humphreys-Beher, M.G. A novel NOD-derived murine model of primary Sjogren’s syndrome. Arthritis Rheum. 1998, 41, 150–156. [Google Scholar] [CrossRef]
- Kong, L.; Robinson, C.P.; Peck, A.B.; Vela-Roch, N.; Sakata, K.M.; Dang, H.; Talal, N.; Humphreys-Beher, M.G. Inappropriate apoptosis of salivary and lacrimal gland epithelium of immunodeficient NOD-scid mice. Clin. Exp. Rheumatol. 1998, 16, 675–681. [Google Scholar] [PubMed]
- Cha, S.; Brayer, J.; Gao, J.; Brown, V.; Killedar, S.; Yasunari, U.; Peck, A.B. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand. J. Immunol. 2004, 60, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; Killedar, S.; Cornelius, J.G.; Nguyen, C.; Cha, S.H.; Peck, A.B. Sjogren’s syndrome in the NOD mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J. Autoimmun. 2006, 26, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.Q.; Gao, J.H.; Kim, H.; Saban, D.R.; Cornelius, J.G.; Peck, A.B. IL-4-STAT6 signal transduction-dependent induction of the clinical phase of Sjogren’s Syndrome-like disease of the nonobese diabetic mouse. J. Immunol. 2007, 179, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.; Cornelius, J.; Singson, E.; Killedar, S.; Cha, S.H.; Peck, A.B. Role of complement and B lymphocytes in Sjogren’s syndrome-like autoimmune exocrinopathy of NOD.B10-H2(B) mice. Mol. Immunol. 2006, 43, 1332–1339. [Google Scholar] [CrossRef]
- Nguyen, C. Pathogenic effect of interleukin-17A in induction of Sjogren syndrome-like disease using adenovirus-mediated gene transfer. Arthritis Res. Ther. 2010, 12, R220. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Zhang, C.; Wang, T.; Brooks, S.; Ford, R.J.; Lin-Lee, Y.C.; Kasianowicz, A.; Kumar, V.; Martin, L.; Liang, P.; et al. Development of autoimmunity in IL-14α-transgenic mice. J. Immunol. 2006, 177, 5676–5686. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Suresh, L.; Li, H.; Zhang, C.J.; Kumar, V.; Pankewycz, O.; Ambrus, J.L. IL-14 alpha, the nexus for primary Sjogren’s disease in mice and humans. Clin. Immunol. 2009, 130, 304–312. [Google Scholar] [CrossRef]
- Cha, S.; Nagashima, H.; Brown, V.B.; Peck, A.B.; Humphreys-Beher, M.G. Two NOD Idd-associated intervals contribute synergistically to the development of autoimmune exocrinopathy (Sjogren’s syndrome) on a healthy murine background. Arthritis Rheum. 2002, 46, 1390–1398. [Google Scholar] [CrossRef]
- Delaleu, N.; Nguyen, C.Q.; Tekle, K.M.; Jonsson, R.; Peck, A.B. Transcriptional landscapes of emerging autoimmunity: Transient aberrations in the targeted tissue’s extracellular milieu precede immune responses in Sjogren’s syndrome. Arthritis Res. Ther. 2013, 15, R174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, F.B.; Saulep-Easton, D.; Figgett, W.A.; Fairfax, K.A.; Mackay, F. The BAFF/APRIL system: Emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 2013, 24, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, W.; Haskett, S.; Pellerin, A.; Xu, S.Q.; Petersen, B.; Jandreski, L.; Hamann, S.; Reynolds, T.L.; Zheng, T.S.; et al. BAFF overexpression increases lymphocytic infiltration in Sjogren’s target tissue, but only inefficiently promotes ectopic B-cell differentiation. Clin. Immunol. 2016, 169, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kiripolsky, J.; Klimatcheva, E.; Howell, A.; Fereidouni, F.; Levenson, R.; Rothstein, T.L.; Kramer, J.M. Early BAFF receptor blockade mitigates murine Sjogren’s syndrome: Concomitant targeting of CXCL13 and the BAFF receptor prevents salivary hypofunction. Clin. Immunol. 2016, 164, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Suresh, L.; Wu, J.; Xuan, J.; Li, H.; Zhang, C.; Pankewycz, O.; Ambrus, J.L. A Role for Lymphotoxin in Primary Sjogren’s Disease. J. Immunol. 2010, 185, 6355–6363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, A.B.; Nguyen, C.Q.; Ambrus, J. Early Covert Appearance of Marginal Zone B Cells in Salivary Glands of Sjogren’s Syndrome-Susceptible Mice: Initiators of Subsequent Overt Clinical Disease. Int. J. Mol. Sci. 2021, 22, 1919. [Google Scholar] [CrossRef] [PubMed]
- Peck, A.B.; Nguyen, C.Q.; Ambrus, J.L. Upregulated Chemokine and Rho-GTPase Genes Define Immune Cell Emigration into Salivary Glands of Sjogren’s Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mice. Int. J. Mol. Sci. 2021, 22, 16. [Google Scholar] [CrossRef]
- Doyle, M.E.; Boggs, L.; Attia, R.; Cooper, L.R.; Saban, D.R.; Nguyen, C.Q.; Peck, A.B. Autoimmune dacryoadenitis of NOD/Lt mice and its subsequent effects on tear protein composition. Am. J. Pathol. 2007, 171, 1224–1236. [Google Scholar] [CrossRef] [Green Version]
- Hammad, H.; Vanderkerken, M.; Pouliot, P.; Deswarte, K.; Toussaint, W.; Vergote, K.; Vandersarren, L.; Janssens, S.; Ramou, I.; Savvides, S.N.; et al. Transitional B cells commit to marginal zone B cell fate by Taok3-mediated surface expression of ADAM10. Nat. Immunol. 2017, 18, 313–320. [Google Scholar] [CrossRef]
- Arruga, F.; Vaisitti, T.; Deaglio, S. The NOTCH Pathway and Its Mutations in Mature B Cell Malignancies. Front. Oncol. 2018, 8, 550. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.; Cariappa, A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009, 9, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Kearney, J.F. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity 2000, 12, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Lopes-Carvalho, T.; Foote, J.; Kearney, J.F. Marginal zone B cells in lymphocyte activation and regulation. Curr. Opin. Immunol. 2005, 17, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.D.; Harwood, N.E. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 2009, 9, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A.; Cols, M.; Puga, I. Marginal zone B cells: Virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013, 13, 118–132. [Google Scholar] [CrossRef] [Green Version]
- Ciccia, F.; Guggino, G.; Rizzo, A.; Bombardieri, M.; Raimondo, S.; Carubbi, F.; Cannizzaro, A.; Sireci, G.; Dieli, F.; Campisi, G.; et al. Interleukin (IL)-22 receptoris over-expressed in primary Sjogren’s syndrome and Sjogren-associated non-Hodgkin lymphomas and is regulated by IL-18. Clin. Exp. Immunol. 2015, 181, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Pikkarainen, T.; Elomaa, O.; Soininen, R.; Kodama, T.; Kraal, G.; Tryggvason, K. Defective microarchitecture of the spleen marginal zone and impaired response to a thymus-independent type 2 antigen in mice lacking scavenger receptors MARCO and SR-A. J. Immunol. 2005, 175, 8173–8180. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.T.; Cyster, J.G. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 2002, 297, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Peck, A.B.; Saylor, B.T.; Nguyen, L.; Sharma, A.; She, J.-X.; Nguyen, C.Q.; McIndoe, R.A. Gene expression profiling of early-phase Sjogren’s syndrome in C57BL/6.NOD-Aec1Aec2 mice identifies focal adhesion maturation associated with infiltrating leukocytes. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5647–5655. [Google Scholar] [CrossRef] [Green Version]
- Tybulewicz, V.L.; Henderson, R.B. Rho family GTPases and their regulators in lymphocytes. Nat. Rev. Immunol. 2009, 9, 630–644. [Google Scholar] [CrossRef]
- Namekata, K.; Kimura, A.; Kawamura, K.; Harada, C.; Harada, T. Dock GEFs and their therapeutic potential: Neuroprotection and axon regeneration. Prog. Retin. Eye Res. 2014, 43, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gadea, G.; Blangy, A. Dock-family exchange factors in cell migration and disease. Eur. J. Cell Biol. 2014, 93, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.D.; Ridley, A.J. Rho GTPase signaling complexes in cell migration and invasion. J. Cell Biol. 2018, 217, 447–457. [Google Scholar] [CrossRef]
- Gotoh, K.; Tanaka, Y.; Nishikimi, A.; Inayoshi, A.; Enjoji, M.; Takayanagi, R.; Sasazuki, T.; Fukui, Y. Differential requirement for DOCK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood 2008, 111, 2973–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, C.P.; Yamamoto, H.; Peck, A.B.; Humphreys-Beher, M.G. Genetically programmed development of salivary gland abnormalities in the NOD (nonobese diabetic)-scid mouse in the absence of detectable lymphocytic infiltration: A potential trigger for sialoadenitis of NOD mice. Clin. Immunol. Immunopathol. 1996, 79, 50–59. [Google Scholar] [CrossRef]
- Schenke-Layland, K.; Xie, J.; Magnusson, M.; Angelis, E.; Li, X.; Wu, K.; Reinhardt, D.P.; MacLellan, W.R.; Hamm-Alvarez, S.F. Lymphocytic infiltration leads to degradation of lacrimal gland extracellular matrix structures in NOD mice exhibiting a Sjogren’s syndrome exocrinopathy. Exp. Eye Res. 2010, 90, 223–237. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.P.; Brayer, J.; Yamachika, S.; Esch, T.R.; Peck, A.B.; Stewart, C.A.; Peen, E.; Jonsson, R.; Humphreys-Beher, M.G. Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjogren’s syndrome. Proc. Natl. Acad. Sci. USA 1998, 95, 7538–7543. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.B.; Xu, K.; Cretegny, K.; Visan, I.; Yuan, J.S.; Egan, S.E.; Guidos, C.J. Lunatic and Manic Fringe Cooperatively Marginal Zone B Cell Precursor Competition for Delta-like 1 in Splenic Endothelial Niches. Immunity 2009, 30, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Baker, N.; Ehrenstein, M.R. Cutting edge: Selection of B lymphocyte subsets is regulated by natural IgM. J. Immunol. 2002, 169, 6686–6690. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Gao, C.; Suresh, L.; Xian, Z.; Song, N.; Chaves, L.D.; Yu, M.; Ambrus, J.L., Jr. Central role for marginal zone B cells in an animal model of Sjogren’s syndrome. Clin. Immunol. 2016, 168, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Karlsen, M.; Jakobsen, K.; Jonsson, R.; Hammenfors, D.; Hansen, T.; Appel, S. Expression of Toll-Like Receptors in Peripheral Blood Mononuclear Cells of Patients with Primary Sjogren’s Syndrome. Scand. J. Immunol. 2017, 85, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Hillion, S.; Arleevskaya, M.I.; Blanco, P.; Bordron, A.; Brooks, W.H.; Cesbron, J.Y.; Bordron, A.; Vivier, E.; Brooks, W. The Innate Part of the Adaptive Immune System. Clin. Rev. Allergy Immunol. 2020, 58, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.C.; Guinamard, R.; Bolland, S.; Sankala, M.; Steinman, R.M.; Ravetch, J.V. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J. Exp. Med. 2003, 198, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Cinamon, G.; Matloubian, M.; Lesneski, M.J.; Xu, Y.; Low, C.; Lu, T.; Proia, R.L.; Cyster, J.G. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 2004, 5, 713–720. [Google Scholar] [CrossRef]
- Cinamon, G.; Zachariah, M.A.; Lam, O.M.; Foss, F.W., Jr.; Cyster, J.G. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat. Immunol. 2008, 9, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girkontaite, L.; Missy, K.; Sakk, V.; Harenberg, A.; Tedford, K.; Potzel, T.; Pfeffer, K.; Fischer, H.D. Lsc is required for marginal zone B cells, regulation of lymphocyte motility and immune responses. Nat. Immunol. 2001, 2, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I.; Tsubota, K.; Satake, Y.; Kita, Y.; Matsumura, R.; Murata, H.; Namekawa, T.; Nishioka, K.; Iwamoto, I.; Saitoh, Y.; et al. Common T cell receptor clonotype in lacrimal glands and labial salivary glands from patients with Sjogren’s syndrome. J. Clin. Investig. 1996, 97, 1969–1977. [Google Scholar] [CrossRef] [Green Version]
- Katsifis, G.E.; Moutsopoulos, N.M.; Wahl, S.M. T lymphocytes in Sjogren’s syndrome: Contributors to and regulators of pathophysiology. Clin. Rev. Allergy Immunol. 2007, 32, 252–264. [Google Scholar] [CrossRef]
- Fujihara, T.; Fujita, H.; Tsubota, K.; Saito, K.; Tsuzaka, K.; Abe, T.; Takeuchi, T. Preferential localization of CD8+ αEβ7+ T cells around acinar epithelial cells with apoptosis in patients with Sjogren’s syndrome. J. Immunol. 1999, 163, 2226–2235. [Google Scholar]
- Hayashi, T.; Shimoyama, N.; Mizuno, T. Destruction of Salivary and Lacrimal Glands by Th1-Polarized Reaction in a Model of Secondary Sjogren’s Syndrome in Lupus-Prone Female NZB × NZWF1 Mice. Inflammation 2012, 35, 638–646. [Google Scholar] [CrossRef]
- Tedford, K.; Steiner, M.; Koshutin, S.; Richter, K.; Tech, L.; Eggers, Y.; Jansing, I.; Schilling, K.; Hauser, A.E.; Korthals, M.; et al. The opposing forces of shear flow and sphingosine-1-phosphate control marginal zone B cell shuttling. Nat. Commun. 2017, 8, 2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Carvalho, T.; Kearney, J.F. Development and selection of marginal zone B cells. Immunol. Rev. 2004, 197, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.; Anders, H.J. The role of innate immunity in autoimmune tissue injury. Curr. Opin. Rheumatol. 2008, 20, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Theofilopoulos, A.N.; Kono, D.H.; Baccala, R. The multiple pathways to autoimmunity. Nat. Immunol. 2017, 18, 716–724. [Google Scholar] [CrossRef]
- Weidenbusch, M.; Kulkarni, O.P.; Anders, H.J. The innate immune system in human systemic lupus erythematosus. Clin. Sci. 2017, 131, 625–634. [Google Scholar] [CrossRef]
- Oliver, A.M.; Martin, F.; Gartland, G.L.; Carter, R.H.; Kearney, J.F. Marginal Zone B Cells Exhibit Unique Activation, Proliferative and Immunoglobulin Secretory Responses. Eur. J. Immunol. 1997, 27, 2366–2374. [Google Scholar] [CrossRef]
- Colonna, M. Innate Lymphoid Cells: Diversity, Plasticity, and Unique Functions in Immunity. Immunity 2018, 48, 1104–1117. [Google Scholar] [CrossRef] [Green Version]
- Magri, G.; Miyajima, M.; Bascones, S.; Mortha, A.; Puga, I.; Cassis, L.; Barra, C.M.; Comerma, L.; Chudnovskiy, A.; Gentile, M.; et al. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat. Immunol. 2014, 15, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Sozzani, S.; Bosisio, D.; Scarsi, M.; Tincani, A. Type I interferons in systemic autoimmunity. Autoimmunity 2010, 43, 196–203. [Google Scholar] [CrossRef]
- Cui, J.; Li, Y.; Zhu, L.; Liu, D.; Zhou, S.; Wang, H.Y.; Wang, R.-F. NLRP4 negatively regulates type I interferon signaling by targeting the kinase TBK1 for degradation via the ubiquitin ligase DTX4. Nat. Immunol. 2012, 13, 387–395. [Google Scholar] [CrossRef]
- He, B.; Santamaria, R.; Xu, W.; Cols, M.; Chen, K.; Puga, I.; Shan, M.; Xiong, H.; Bussel, J.B.; Chiu, A.; et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat. Immunol. 2010, 11, 836–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigl, M.; Brenner, M.B. How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin. Immunol. 2010, 22, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Marino, E.; Batten, M.; Groom, J.; Walters, S.; Liuwantara, D.; Mackay, F.; Grey, S.T. Marginal-zone B-Cells of Nonobese diabetic mice expand with diabetes onset, invade the pancreatic lymph nodes, and present autoantigen to diabetogenic T-Cells. Diabetes 2008, 57, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubtsov, A.; Strauch, P.; DiGiacomo, A.; Hu, J.; Pelanda, R.; Torres, R.M. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity 2005, 23, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.Q.; Cornelius, J.G.; Cooper, L.; Neff, J.; Tao, J.; Lee, B.H.; Peck, A.B. Identification of possible candidate genes regulating Sjogren’s syndrome-associated autoimmunity: A potential role for TNFSF4 in autoimmune exocrinopathy. Arthritis Res. Ther. 2008, 10, R137. [Google Scholar] [CrossRef] [Green Version]
- Tedford, K.; Nitschke, L.; Girkontaite, I.; Charlesworth, A.; Chan, G.; Sakk, V.; Barbacid, M.; Fischer, K.D. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat. Immunol. 2001, 2, 548–555. [Google Scholar] [CrossRef]
- Wang, J.; Lin, F.; Wan, Z.; Sun, X.; Lu, Y.; Huang, J.; Wang, F.; Zeng, Y.; Chen, Y.-H.; Shi, Y.; et al. Profiling the origin, dynamics, and function of traction force in B cell activation. Sci. Signal. 2018, 11, 542. [Google Scholar] [CrossRef] [Green Version]
- Matsuzawa, K.; Akita, H.; Watanabe, T.; Kakeno, M.; Matsui, T.; Wang, S.; Kaibuchi, K. PAR3-aPKC regulates Tiam1 by modulating suppressive internal interactions. Mol. Biol. Cell 2016, 27, 1511–1523. [Google Scholar] [CrossRef]
- Garcia-Serna, A.M.; Alcaraz-Garcia, M.J.; Ruiz-Lafuente, N.; Sebastian-Ruiz, S.; Martinez, C.M.; Moya-Quiles, M.R.; Minguela, A.; Garcia-Alonso, A.M.; Martin-Orozco, E.; Parrado, A. Dock10 regulates CD23 expression and sustains B-cell lymphopoiesis in secondary lymphoid tissue. Immunobiology 2016, 221, 1343–1350. [Google Scholar] [CrossRef]
- Nombela-Arrieta, C.; Lacalle, R.A.; Montoya, M.C.; Kunisaki, Y.; Megias, D.; Marques, M.; Carrera, A.C.; Manes, S.; Fukui, Y.; Martinez-A, C.; et al. Differential requirements for DOCK2 and phosphoinositide-3-kinase γ during T and B lymphocyte homing. Immunity 2004, 21, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Ushijima, M.; Uruno, T.; Nishikimi, A.; Sanematsu, F.; Kamikaseda, Y.; Kunimura, K.; Sakata, D.; Okada, T.; Fukui, Y. The Rac Activator DOCK2 Mediates Plasma Cell Differentiation and IgG Antibody Production. Front. Immunol. 2018, 9, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.Q.; Sharma, A.; She, J.X.; McIndoe, R.A.; Peck, A.B. Differential gene expressions in the lacrimal gland during development and onset of keratoconjunctivitis sicca in Sjogren’s syndrome (SJS)-like disease of the C57BL/6.NOD-Aec1Aec2 mouse. Exp. Eye Res. 2009, 88, 398–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.Q.; Sharma, A.; Lee, B.H.; She, J.X.; McIndoe, R.A.; Peck, A.B. Differential gene expression in the salivary gland during development and onset of xerostomia in Sjogren’s syndrome-like disease of the C57BL/6.NOD-Aec1Aec2 mouse. Arthritis Res. Ther. 2009, 11, R56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.; Brill-Dashoff, J.; Shinton, S.A.; Asano, M.; Hardy, R.R.; Hayakawa, K. Evidence of marginal-zone B cell-positive selection in spleen. Immunity 2005, 23, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Benjanini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peck, A.B.; Nguyen, C.Q.; Ambrus, J.L., Jr. A MZB Cell Activation Profile Present in the Lacrimal Glands of Sjögren’s Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mice Defined by Global RNA Transcriptomic Analyses. Int. J. Mol. Sci. 2022, 23, 6106. https://doi.org/10.3390/ijms23116106
Peck AB, Nguyen CQ, Ambrus JL Jr. A MZB Cell Activation Profile Present in the Lacrimal Glands of Sjögren’s Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mice Defined by Global RNA Transcriptomic Analyses. International Journal of Molecular Sciences. 2022; 23(11):6106. https://doi.org/10.3390/ijms23116106
Chicago/Turabian StylePeck, Ammon B., Cuong Q. Nguyen, and Julian L. Ambrus, Jr. 2022. "A MZB Cell Activation Profile Present in the Lacrimal Glands of Sjögren’s Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mice Defined by Global RNA Transcriptomic Analyses" International Journal of Molecular Sciences 23, no. 11: 6106. https://doi.org/10.3390/ijms23116106
APA StylePeck, A. B., Nguyen, C. Q., & Ambrus, J. L., Jr. (2022). A MZB Cell Activation Profile Present in the Lacrimal Glands of Sjögren’s Syndrome-Susceptible C57BL/6.NOD-Aec1Aec2 Mice Defined by Global RNA Transcriptomic Analyses. International Journal of Molecular Sciences, 23(11), 6106. https://doi.org/10.3390/ijms23116106





