Synaptic Cell Adhesion Molecule 3 (SynCAM3) Deletion Promotes Recovery from Spinal Cord Injury by Limiting Glial Scar Formation
Abstract
:1. Introduction
2. Results
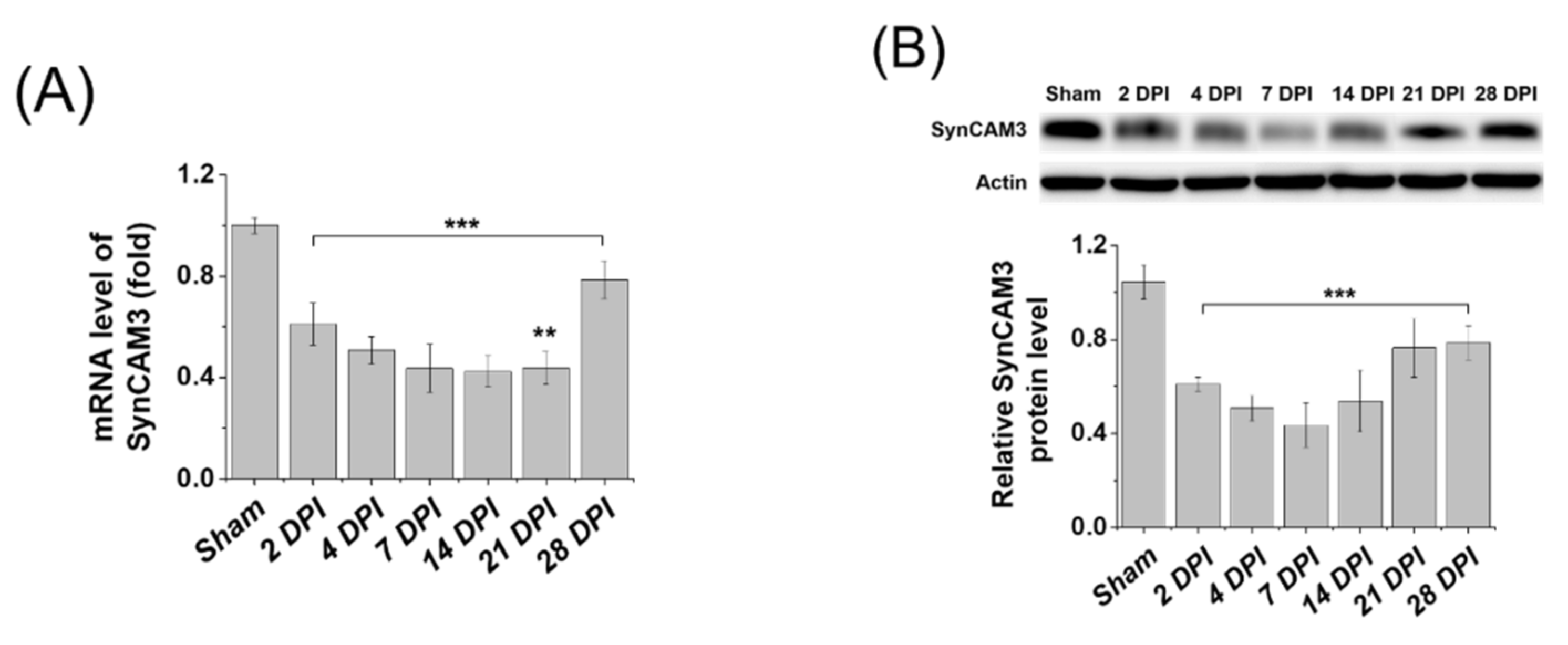
2.1. SynCAM3, Whose Expression Levels Are Restored in the Chronic Phase, May Affect ECM Repositioning
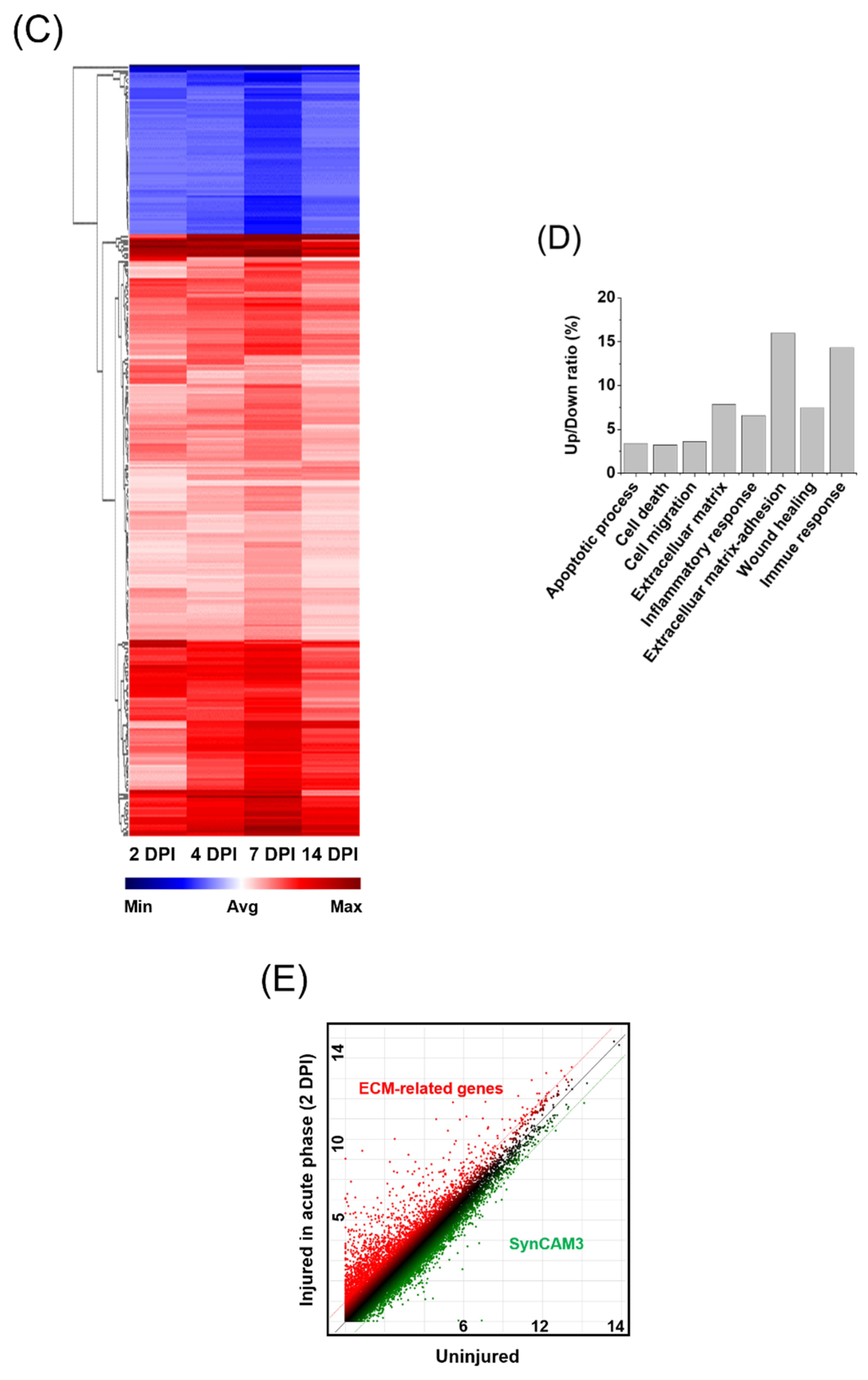
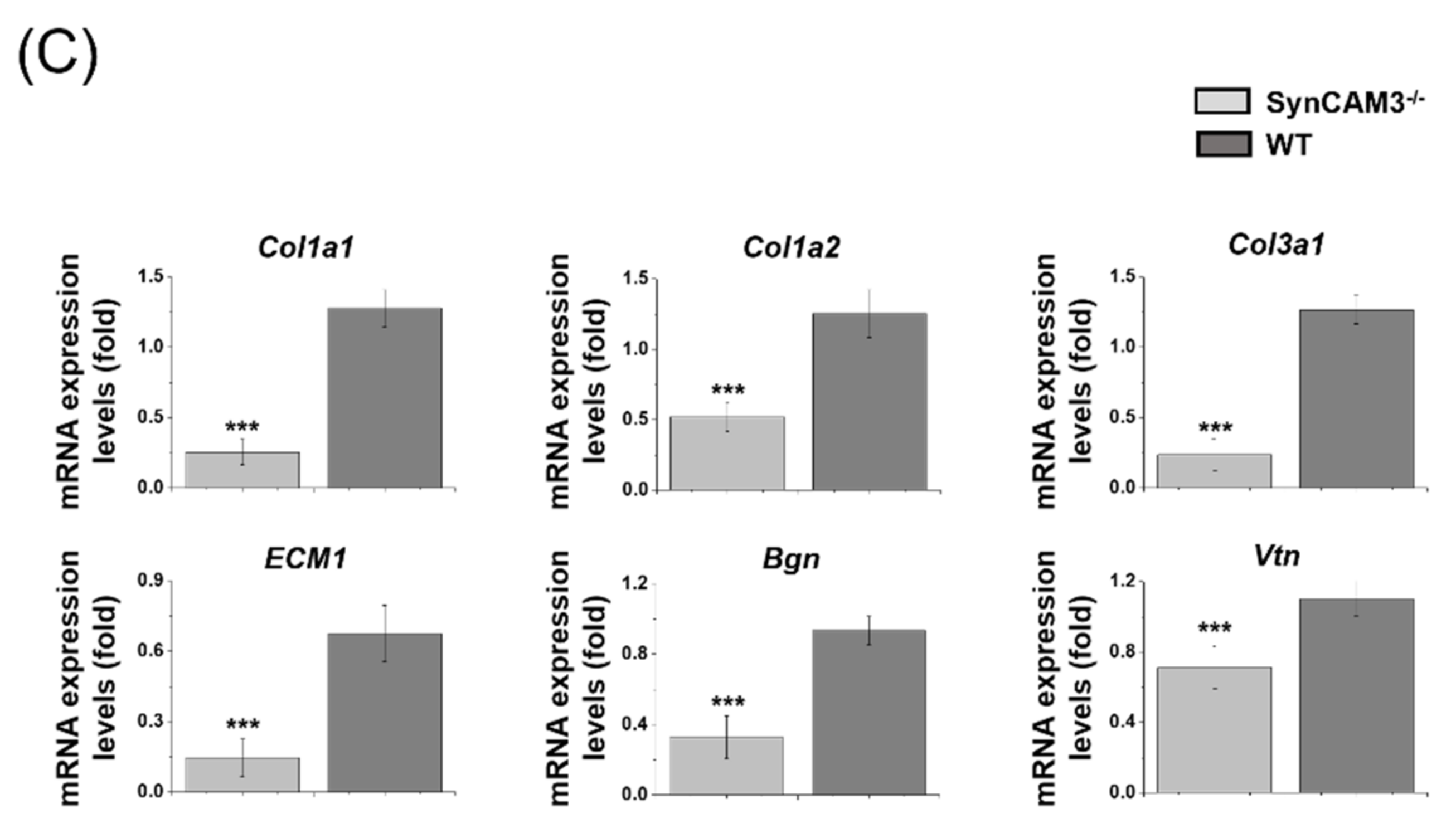
2.2. Downregulation of Gene Expression Is Associated with ECM in the Lesion Area after SCI in SynCAM3 KO Mice
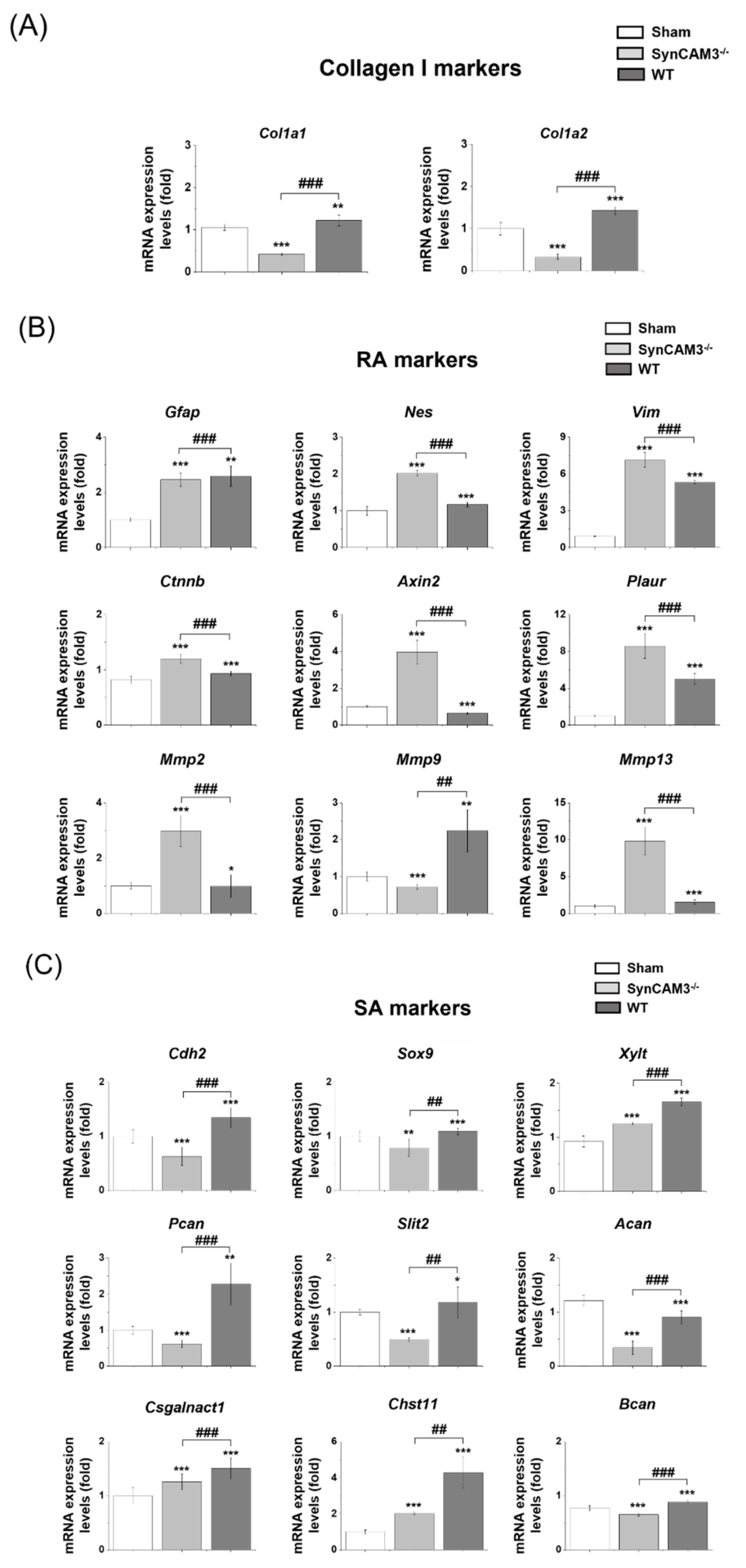
2.3. SynCAM3 Deletion Inhibits the Transformation of RAs into SAs through ECM-Adhesion Regulation in SCI
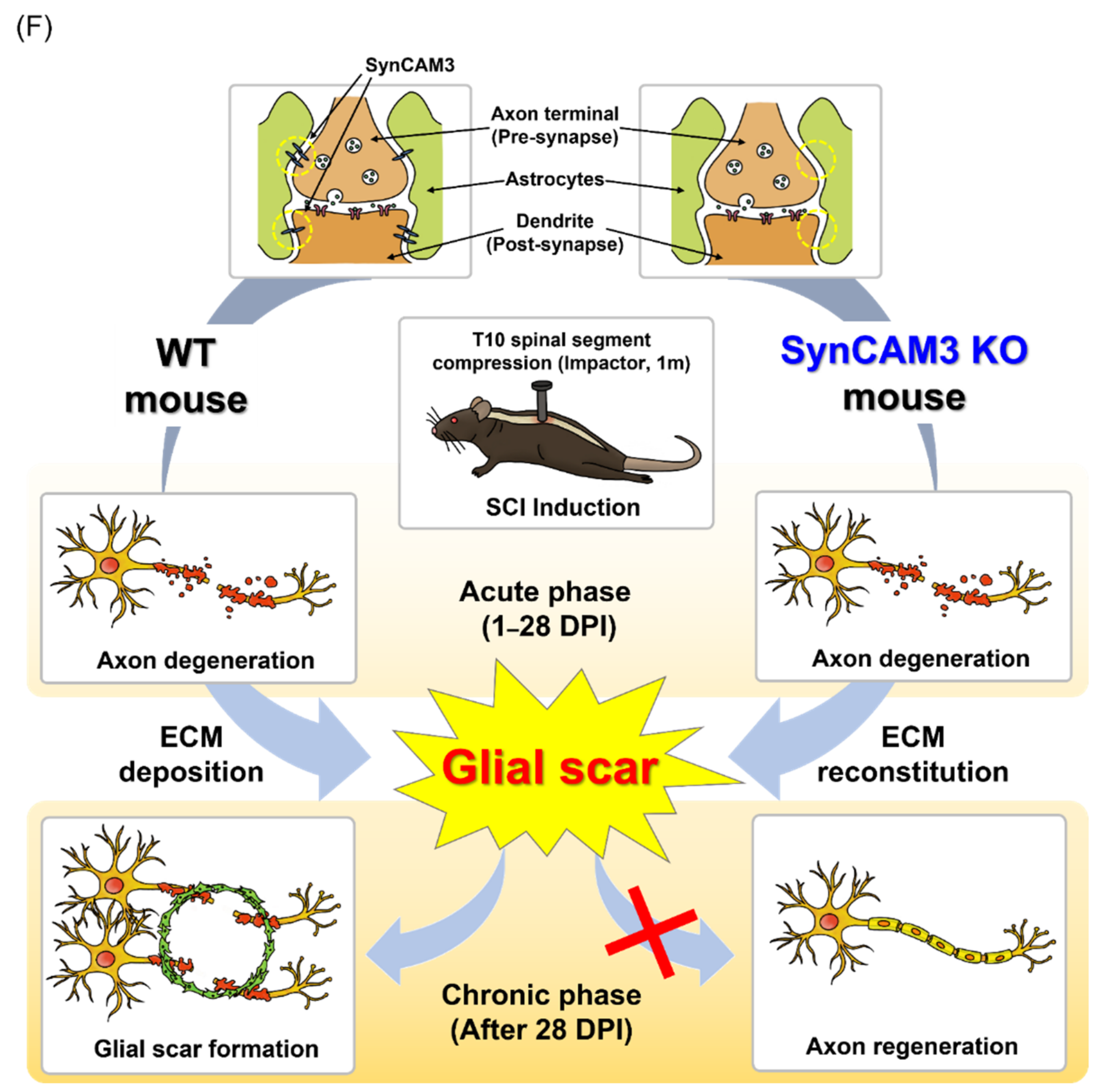
2.4. SynCAM3 Deletion Inhibits Glial Scar Formation
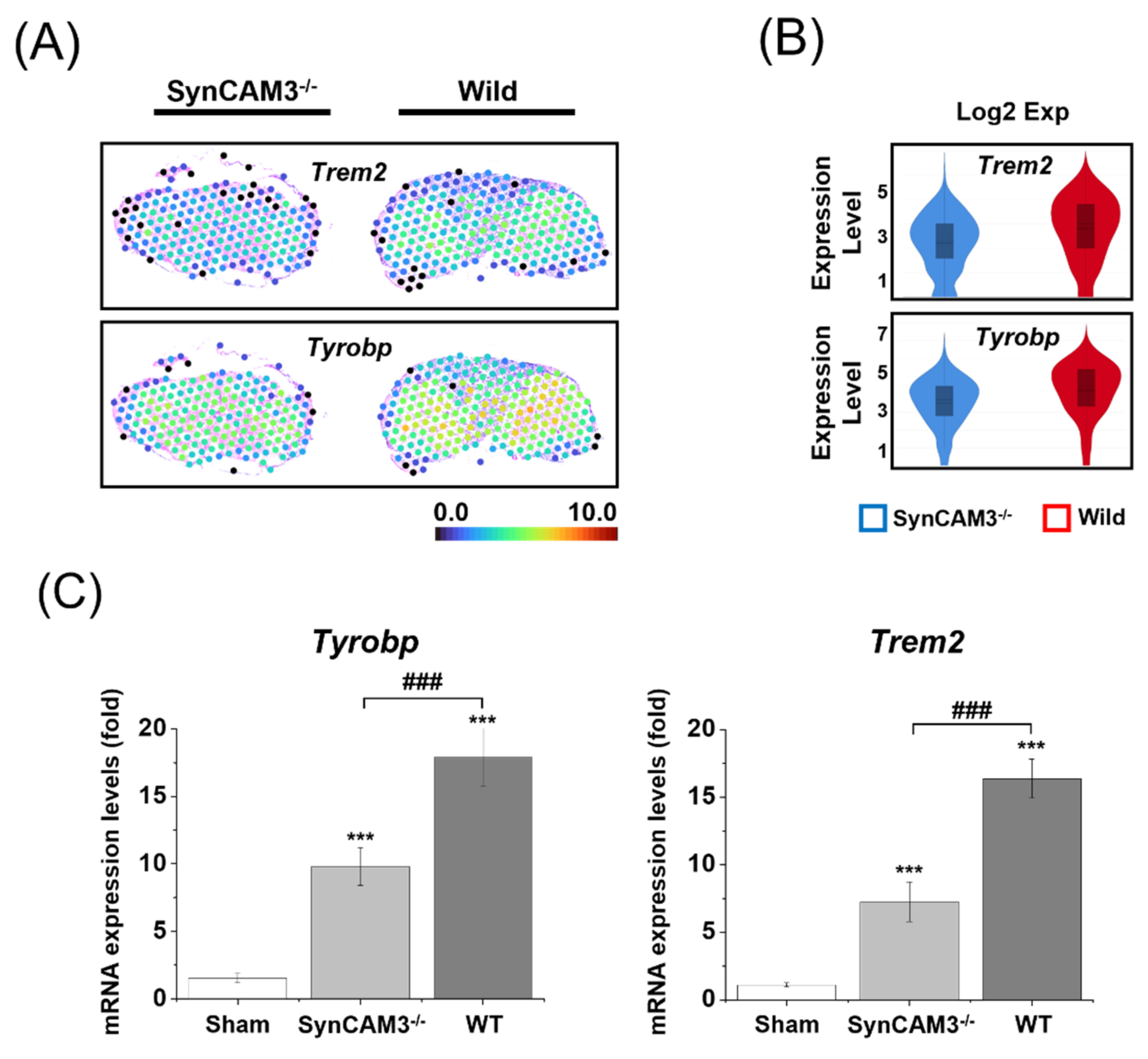
2.5. Reduction of Microglial Activation in SynCAM3 KO Mice
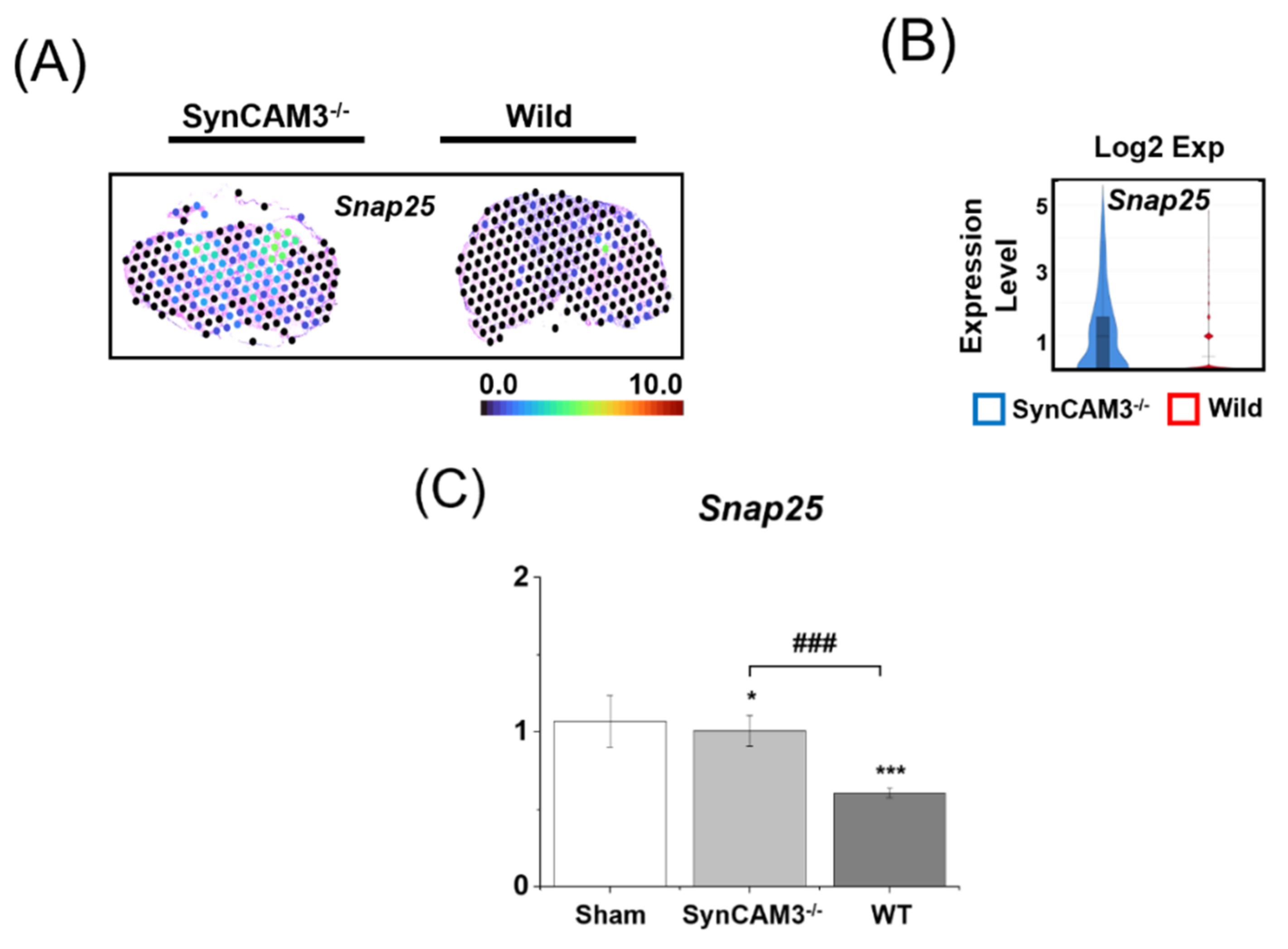
2.6. Appropriate Regulation of SNAP-25 Expression Induces Sensory and Locomotor Function in SCI
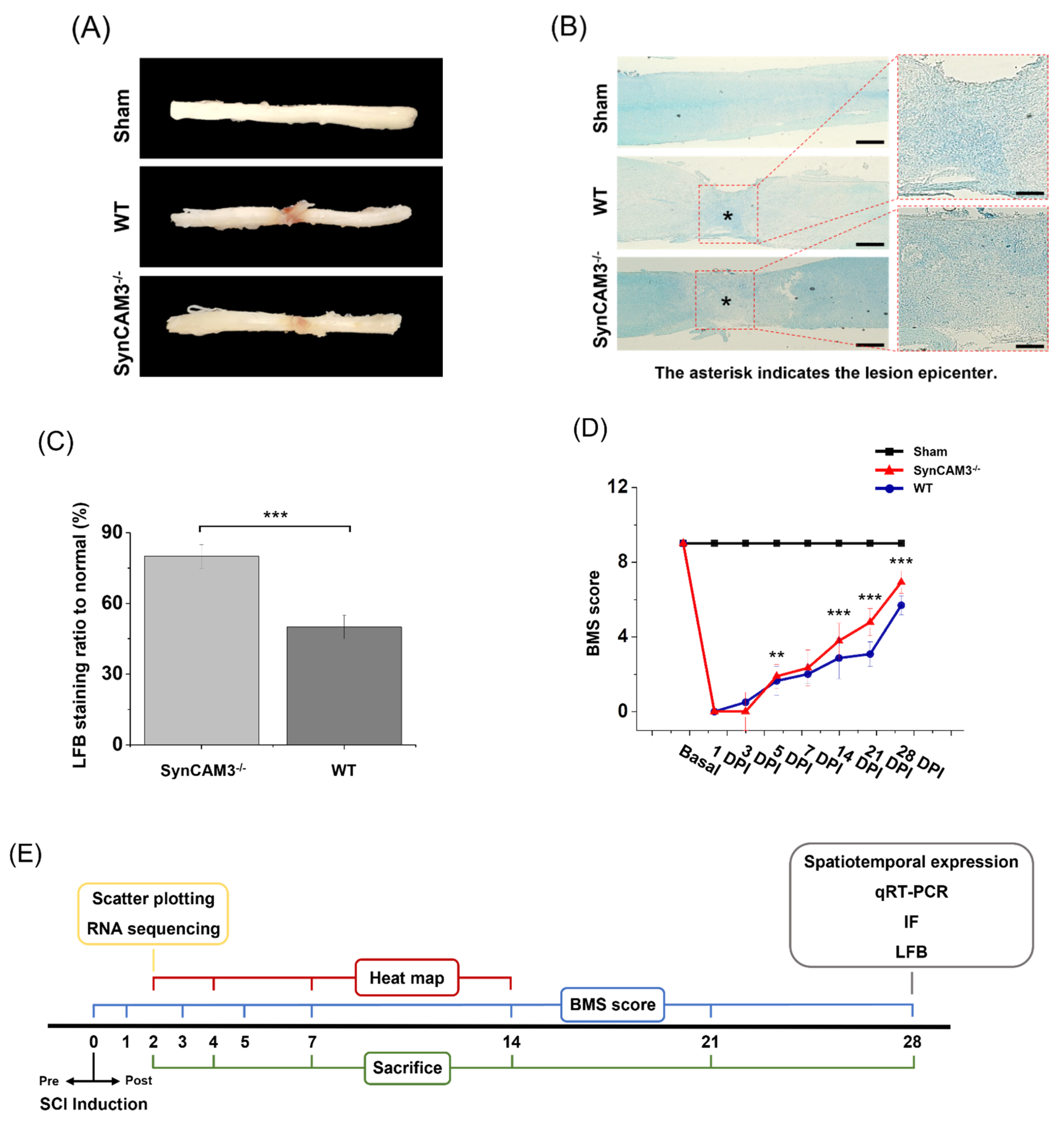
2.7. SynCAM3 Deletion Quickly Restores Myelination and Dysfunction of the Spinal Cord
3. Discussion
4. Materials and Methods
4.1. Subjects and Surgical Procedures
4.2. qRT-PCR
4.3. Western Blot Analysis
4.4. Behavioral Analyses
4.5. Histopathological Examination
4.6. LFB Staining
4.7. RNA Isolation
4.8. Library Preparation and Sequencing
4.9. Visium Experiment Methods
4.10. Library Kit
- -
- Visium Spatial Expression Slide & Reagent Kit, 16rxn PN-1000184
- -
- (-Spatial Gene Expression Slide Kit, -Spatial Gene Expression Reagent Kit, -Library Construction Kit)
- -
- Visium Gateway Package, 2 rxns PN-1000316
- -
- Visium Gateway Slide, 2 rxns PN-1000317
- -
- Visium Accessory Kit, PN-1000194
- -
- Dual Index Kit TT Seat A, 96 rxns PN-1000215
4.11. Visium BI Method
4.12. Data Analysis
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parthiban, J.; Zileli, M.; Sharif, S.Y. Outcomes of Spinal Cord Injury: WFNS Spine Committee Recommendations. Neurospine 2020, 17, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Peev, N.; Komarov, A.; Osorio-Fonseca, E.; Zileli, M. Rehabilitation of Spinal Cord Injury: WFNS Spine Committee Recommendations. Neurospine 2020, 17, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.C.; Tu, T.H.; Wu, J.C.; Huang, W.C.; Cheng, H. Acidic Fibroblast Growth Factor in Spinal Cord Injury. Neurospine 2019, 16, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sami, A.; Ghosh, B.; Urban, M.W.; Heinsinger, N.M.; Liang, S.S.; Smith, G.M.; Wright, M.C.; Li, S.; Lepore, A.C. LAR inhibitory peptide promotes recovery of diaphragm function and multiple forms of respiratory neural circuit plasticity after cervical spinal cord injury. Neurobiol. Dis. 2021, 147, 105153. [Google Scholar] [CrossRef]
- Mukherjee, N.; Ghosh, S. Myelin Associated Inhibitory Proteins as a Therapeutic Target for Healing of CNS Injury. ACS Chem. Neurosci. 2020, 11, 1699–1700. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- Boghdadi, A.G.; Teo, L.; Bourne, J.A. The Involvement of the Myelin-Associated Inhibitors and Their Receptors in CNS Plasticity and Injury. Mol. Neurobiol. 2018, 55, 1831–1846. [Google Scholar] [CrossRef]
- Fiani, B.; Kondilis, A.; Soula, M.; Tao, A.; Alvi, M.A. Novel Methods of Necroptosis Inhibition for Spinal Cord Injury Using Translational Research to Limit Secondary Injury and Enhance Endogenous Repair and Regeneration. Neurospine 2021, 18, 261–270. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success after Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Sami, A.; Selzer, M.E.; Li, S. Advances in the Signaling Pathways Downstream of Glial-Scar Axon Growth Inhibitors. Front. Cell. Neurosci. 2020, 14, 174. [Google Scholar] [CrossRef]
- Hira, K.; Ueno, Y.; Tanaka, R.; Miyamoto, N.; Yamashiro, K.; Inaba, T.; Urabe, T.; Okano, H.; Hattori, N. Astrocyte-Derived Exosomes Treated with a Semaphorin 3A Inhibitor Enhance Stroke Recovery via Prostaglandin D2 Synthase. Stroke 2018, 49, 2483–2494. [Google Scholar] [CrossRef]
- Thalhammer, A.; Cingolani, L.A. Cell adhesion and homeostatic synaptic plasticity. Neuropharmacology 2014, 78, 23–30. [Google Scholar] [CrossRef]
- Giza, J.I.; Jung, Y.; Jeffrey, R.A.; Neugebauer, N.M.; Picciotto, M.R.; Biederer, T. The synaptic adhesion molecule SynCAM 1 contributes to cocaine effects on synapse structure and psychostimulant behavior. Neuropsychopharmacology 2013, 38, 628–638. [Google Scholar] [CrossRef] [Green Version]
- Missler, M.; Sudhof, T.C.; Biederer, T. Synaptic cell adhesion. Cold Spring Harb. Perspect. Biol. 2012, 4, a005694. [Google Scholar] [CrossRef]
- Biederer, T. Hooking up new synapses. Nat. Neurosci. 2006, 9, 1203–1204. [Google Scholar] [CrossRef]
- Sukhanov, N.; Vainshtein, A.; Eshed-Eisenbach, Y.; Peles, E. Differential Contribution of Cadm1–Cadm3 Cell Adhesion Molecules to Peripheral Myelinated Axons. J. Neurosci. 2021, 41, 1393–1400. [Google Scholar] [CrossRef]
- Thomas, L.A.; Akins, M.R.; Biederer, T. Expression and adhesion profiles of SynCAM molecules indicate distinct neuronal functions. J. Comp. Neurol. 2008, 510, 47–67. [Google Scholar] [CrossRef] [Green Version]
- Togashi, H.; Sakisaka, T.; Takai, Y. Cell adhesion molecules in the central nervous system. Cell Adh. Migr. 2009, 3, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Rebelo, A.P.; Cortese, A.; Abraham, A.; Eshed-Eisenbach, Y.; Shner, G.; Vainshtein, A.; Buglo, E.; Camarena, V.; Gaidosh, G.; Shiekhattar, R.; et al. A CADM3 variant causes Charcot-Marie-Tooth disease with marked upper limb involvement. Brain 2021, 144, 1197–1213. [Google Scholar] [CrossRef]
- Park, J.; Liu, B.; Chen, T.; Li, H.; Hu, X.; Gao, J.; Zhu, Y.; Zhu, Q.; Qiang, B.; Yuan, J.; et al. Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J. Neurosci. 2008, 28, 12815–12819. [Google Scholar] [CrossRef]
- Chen, M.S.; Kim, H.; Jagot-Lacoussiere, L.; Maurel, P. Cadm3 (Necl-1) interferes with the activation of the PI3 kinase/Akt signaling cascade and inhibits Schwann cell myelination in vitro. Glia 2016, 64, 2247–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Chen, T.; Liu, J.; Liu, W.; Hu, G.; Guo, X.; Yin, B.; Gong, Y.; Zhao, J.; Qiang, B.; et al. Loss of NECL1, a novel tumor suppressor, can be restored in glioma by HDAC inhibitor—Trichostatin A through Sp1 binding site. Glia 2009, 57, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Mandai, K.; Rikitake, Y.; Mori, M.; Takai, Y. Nectins and nectin-like molecules in development and disease. Curr. Top. Dev. Biol. 2015, 112, 197–231. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.A.; An, J.; Hood, B.L.; Conrads, T.P.; Bowser, R.P. Label-Free LC-MS/MS Proteomic Analysis of Cerebrospinal Fluid Identifies Protein/Pathway Alterations and Candidate Biomarkers for Amyotrophic Lateral Sclerosis. J. Proteome Res. 2015, 14, 4486–4501. [Google Scholar] [CrossRef]
- Kakunaga, S.; Ikeda, W.; Itoh, S.; Deguchi-Tawarada, M.; Ohtsuka, T.; Mizoguchi, A.; Takai, Y. Nectin-like molecule-1/TSLL1/SynCAM3: A neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. J. Cell Sci. 2005, 118, 1267–1277. [Google Scholar] [CrossRef] [Green Version]
- Hillen, A.E.J.; Burbach, J.P.H.; Hol, E.M. Cell adhesion and matricellular support by astrocytes of the tripartite synapse. Prog. Neurobiol. 2018, 165–167, 66–86. [Google Scholar] [CrossRef]
- Durkee, C.A.; Araque, A. Diversity and Specificity of Astrocyte-neuron Communication. Neuroscience 2019, 396, 73–78. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, K.; Shan, L.; Kuang, F.; Chen, K.; Zhu, K.; Ma, H.; Ju, G.; Wang, Y.Z. Reactive astrocytes undergo M1 microglia/macrohpages-induced necroptosis in spinal cord injury. Mol. Neurodegener. 2016, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Saini, V.; Loers, G.; Kaur, G.; Schachner, M.; Jakovcevski, I. Impact of neural cell adhesion molecule deletion on regeneration after mouse spinal cord injury. Eur. J. Neurosci. 2016, 44, 1734–1746. [Google Scholar] [CrossRef]
- Xie, C.; Shen, X.; Xu, X.; Liu, H.; Li, F.; Lu, S.; Gao, Z.; Zhang, J.; Wu, Q.; Yang, D.; et al. Astrocytic YAP Promotes the Formation of Glia Scars and Neural Regeneration after Spinal Cord Injury. J. Neurosci. 2020, 40, 2644–2662. [Google Scholar] [CrossRef]
- Hara, M.; Kobayakawa, K.; Ohkawa, Y.; Kumamaru, H.; Yokota, K.; Saito, T.; Kijima, K.; Yoshizaki, S.; Harimaya, K.; Nakashima, Y.; et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med. 2017, 23, 818–828. [Google Scholar] [CrossRef]
- Joshi, H.P.; Kumar, H.; Choi, U.Y.; Lim, Y.C.; Choi, H.; Kim, J.; Kyung, J.W.; Sohn, S.; Kim, K.T.; Kim, J.K.; et al. CORM-2-Solid Lipid Nanoparticles Maintain Integrity of Blood-Spinal Cord Barrier after Spinal Cord Injury in Rats. Mol. Neurobiol. 2020, 57, 2671–2689. [Google Scholar] [CrossRef]
- Golan, N.; Kartvelishvily, E.; Spiegel, I.; Salomon, D.; Sabanay, H.; Rechav, K.; Vainshtein, A.; Frechter, S.; Maik-Rachline, G.; Eshed-Eisenbach, Y.; et al. Genetic deletion of Cadm4 results in myelin abnormalities resembling Charcot-Marie-Tooth neuropathy. J. Neurosci. 2013, 33, 10950–10961. [Google Scholar] [CrossRef] [Green Version]
- Bighinati, A.; Khalajzeyqami, Z.; Baldassarro, V.A.; Lorenzini, L.; Cescatti, M.; Moretti, M.; Giardino, L.; Calza, L. Time-Course Changes of Extracellular Matrix Encoding Genes Expression Level in the Spinal Cord Following Contusion Injury—A Data-Driven Approach. Int. J. Mol. Sci. 2021, 22, 1744. [Google Scholar] [CrossRef]
- Ma, S.; Wang, J.; Liu, L.; Xia, L.; Tao, R. Identification of temporal genes involved in the mechanisms of spinal cord injury. Spinal Cord 2017, 55, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Torres-Espin, A.; Hernandez, J.; Navarro, X. Gene expression changes in the injured spinal cord following transplantation of mesenchymal stem cells or olfactory ensheathing cells. PLoS ONE 2013, 8, e76141. [Google Scholar] [CrossRef] [Green Version]
- Maniatis, S.; Aijo, T.; Vickovic, S.; Braine, C.; Kang, K.; Mollbrink, A.; Fagegaltier, D.; Andrusivova, Z.; Saarenpaa, S.; Saiz-Castro, G.; et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 2019, 364, 89–93. [Google Scholar] [CrossRef]
- Burnside, E.R.; Bradbury, E.J. Manipulating the extracellular matrix and its role in brain and spinal cord plasticity and repair. Neuropathol. Appl. Neurobiol. 2014, 40, 26–59. [Google Scholar] [CrossRef]
- George, N.; Geller, H.M. Extracellular matrix and traumatic brain injury. J. Neurosci. Res. 2018, 96, 573–588. [Google Scholar] [CrossRef]
- Orr, M.B.; Gensel, J.C. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics 2018, 15, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Chen, X.; Zhang, C.; Zhang, Y.; Yao, W. An update on reactive astrocytes in chronic pain. J. Neuroinflamm. 2019, 16, 140. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Y.; Ning, B. Reactive Astrocytes in Central Nervous System Injury: Subgroup and Potential Therapy. Front. Cell. Neurosci. 2021, 15, 792764. [Google Scholar] [CrossRef]
- Fitch, M.T.; Silver, J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008, 209, 294–301. [Google Scholar] [CrossRef] [Green Version]
- Vicuna, L.; Strochlic, D.E.; Latremoliere, A.; Bali, K.K.; Simonetti, M.; Husainie, D.; Prokosch, S.; Riva, P.; Griffin, R.S.; Njoo, C.; et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat. Med. 2015, 21, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Konishi, H.; Sayo, A.; Takai, T.; Kiyama, H. TREM2/DAP12 Signal Elicits Proinflammatory Response in Microglia and Exacerbates Neuropathic Pain. J. Neurosci. 2016, 36, 11138–11150. [Google Scholar] [CrossRef]
- Zhong, Z.Q.; Xiang, Y.; Hu, X.; Wang, Y.C.; Zeng, X.; Wang, X.M.; Xia, Q.J.; Wang, T.H.; Zhang, X. Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion. Neural Regen. Res. 2017, 12, 969–976. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Kong, Y.; Huang, J.; Ding, Z.; Deng, M.; Guo, Q.; Zou, W. SNAP-25 Contributes to Neuropathic Pain by Regulation of VGLuT2 Expression in Rats. Neuroscience 2019, 423, 86–97. [Google Scholar] [CrossRef]
- Wang, W.; Wang, F.; Liu, J.; Zhao, W.; Zhao, Q.; He, M.; Qian, B.J.; Xu, Y.; Liu, R.; Liu, S.J.; et al. SNAP25 ameliorates sensory deficit in rats with spinal cord transection. Mol. Neurobiol. 2014, 50, 290–304. [Google Scholar] [CrossRef]
- Chen, G.; Fang, X.; Yu, M. Regulation of gene expression in rats with spinal cord injury based on microarray data. Mol. Med. Rep. 2015, 12, 2465–2472. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef] [PubMed]
- Cell Ranger hg38 Reference Genome “Refdata-Gex-GRCh38-2020-A”. Available online: https://cf.10xgenomics.com/supp/cell-exp/refdata-gex-GRCh38-2020-A.tar.gz (accessed on 11 April 2022).


| Primers | Directions | Sequence |
|---|---|---|
| Acan | Forward | 5′-TTTGATTTCCCACCGTGCCTTTCC-3′ |
| Reverse | 5′-TTCCTGGTCCTGTCTTCTTTCAGC-3′ | |
| Axin2 | Forward | 5′-GAGAAGTTTGGATTGCTGTCCACG-3′ |
| Reverse | 5′-ACCCATTTCTGCATGTGTCGATGG-3′ | |
| Bcan | Forward | 5′-AATTCTGCTGAAGGCTCAATGCCC-3′ |
| Reverse | 5′-CGGAAGTGACAGAATGGAAGATCC-3′ | |
| Cdh2 | Forward | 5′-TACGCAGCTGGTTGCAGATAAAGG-3′ |
| Reverse | 5′-TCTGCACTCCTCCATAGTCTATGC-3′ | |
| Chst11 | Forward | 5′-GTTCTGGTGAAGTCCACAAACTGC-3′ |
| Reverse | 5′-TGTCACCATGGGATTCTACACACG-3′ | |
| Cnpase | Forward | 5′-AAATGGCAGACCAGTATCAGTACC-3′ |
| Reverse | 5′-GTCTCAGAACTCTTTTTGGTCAGG-3′ | |
| Col1a1 | Forward | 5′-CATGGAGACAGGTCAGACCTGTGT-3′ |
| Reverse | 5′-GGACATTAGGCGCAGGAAGGTCAG-3′ | |
| Col1a2 | Forward | 5′-ATCCAAC TAAGTCTCCT CCCTTGG-3′ |
| Reverse | 5′-GGCTTCTGACTATCTTCCACAGAG-3′ | |
| Csgalnact1 | Forward | 5′-CTTGAGACAGTCTTGTCACAGAGC-3′ |
| Reverse | 5′-CAGTCCTTAGATCAGATCTCCAGG-3′ | |
| Ctnnb1 | Forward | 5′-GGGTGAATACTTTACTCTGCCTGC-3′ |
| Reverse | 5′-GTATAACGCTGCAAAAGCTGTGGC-3′ | |
| Gapdh | Forward | 5′-GACTTCAACAGCAACTCCCACTCT-3′ |
| Reverse | 5′-GGTTTCTTACTCCTTGGAGGCCAT-3′ | |
| Gfap | Forward | 5′-TGTACTAACAGAGCGAGCCTATGC-3′ |
| Reverse | 5′-GGGACTTGCTGCCTTTAACATTGG-3′ | |
| lba1 | Forward | 5′-CAAAGAACACAAGAGGCCAACTGG-3′ |
| Reverse | 5′-TTCCATGCTGCTGTCATCAGAAGC-3′ | |
| Mmp13 | Forward | 5′-GAGAGCTTAGTTCTGTGAACGAGC-3′ |
| Reverse | 5′-AAAGCAGATGGACCCCATGTTTGC-3′ | |
| Mmp2 | Forward | 5′-CTATCATCTTCATCGCTGCACACC-3′ |
| Reverse | 5′-GTACAGTCAGCACCTTTCTTTGGG-3′ | |
| Mmp9 | Forward | 5′-AAGGTATTCAGTTGCCCCTACTGG-3′ |
| Reverse | 5′-ACACGGAGAATCTCTGAGCAATCC-3′ | |
| Nefh | Forward | 5′-TAGCAAGAGAAGATAACCCTGAGC-3′ |
| Reverse | 5′-TCATCTGTCAGTTGGACATACAGG-3′ | |
| Nes | Forward | 5′-GTCAGCTGAGCCTATAGTTCAACG-3′ |
| Reverse | 5′-AGAGTCACTCATCATTGCTGCTCC-3′ | |
| Pcan | Forward | 5′-TAATGGTGCAGCTTTGCCTGATGG-3′ |
| Reverse | 5′-CCTGACAGTAACTCATTCTGCTGC-3′ | |
| Pdgfa | Forward | 5′-AGACAGATGTGAGGTGAGATGAGC-3′ |
| Reverse | 5′-ACGGAGGAGAACAAAGACCGCACG-3′ | |
| Pdgfb | Forward | 5′-TACCTCCACTCTGTGTCTTCTTCC-3′ |
| Reverse | 5′-CATCCCATTACAACCTTGCTCACC-3′ | |
| Plaur | Forward | 5′-TCTGGATCTTCAGAGCTTTCCACC-3′ |
| Reverse | 5′-CTTACGGTATAACTCCGGTTTCCC-3′ | |
| Slit2 | Forward | 5′-CGTCTCTAGAAGCTTCTAGCTTCG-3′ |
| Reverse | 5′-TGTAGGGGGAGCTTTAGTACAAGC-3′ | |
| Sox9 | Forward | 5′-GAAGGTAACGATTGCTGGGATTCC-3′ |
| Reverse | 5′-CGTCCTCCATGTTAACTCTGAAGG-3′ | |
| Tgfb1 | Forward | 5′-GTGACAGCAAAGATAACAAACTCC-3′ |
| Reverse | 5′-GAGCTGAAGCAATAGTTGGTATCC-3′ | |
| Tgfb2 | Forward | 5′-TCTGAGATTACAGCAACAACAACC-3′ |
| Reverse | 5′-CAATACGTACAACTCCACTGAACG-3′ | |
| Vim | Forward | 5′-TGCTAACTACCAGGACACTATTGG-3′ |
| Reverse | 5′-AGGTTAGTTTCTCTCAGGTTCAGG-3′ | |
| Xylt1 | Forward | 5′-CAGTGAAGATTCTCCATCACTGGG-3′ |
| Reverse | 5′-TCTGGAAACTCTGCTCCATGTAGG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.G.; Kwon, S.Y.; Kyung, J.W.; Roh, E.J.; Choi, H.; Lim, C.S.; An, S.B.; Sohn, S.; Han, I. Synaptic Cell Adhesion Molecule 3 (SynCAM3) Deletion Promotes Recovery from Spinal Cord Injury by Limiting Glial Scar Formation. Int. J. Mol. Sci. 2022, 23, 6218. https://doi.org/10.3390/ijms23116218
Song BG, Kwon SY, Kyung JW, Roh EJ, Choi H, Lim CS, An SB, Sohn S, Han I. Synaptic Cell Adhesion Molecule 3 (SynCAM3) Deletion Promotes Recovery from Spinal Cord Injury by Limiting Glial Scar Formation. International Journal of Molecular Sciences. 2022; 23(11):6218. https://doi.org/10.3390/ijms23116218
Chicago/Turabian StyleSong, Byeong Gwan, Su Yeon Kwon, Jae Won Kyung, Eun Ji Roh, Hyemin Choi, Chang Su Lim, Seong Bae An, Seil Sohn, and Inbo Han. 2022. "Synaptic Cell Adhesion Molecule 3 (SynCAM3) Deletion Promotes Recovery from Spinal Cord Injury by Limiting Glial Scar Formation" International Journal of Molecular Sciences 23, no. 11: 6218. https://doi.org/10.3390/ijms23116218
APA StyleSong, B. G., Kwon, S. Y., Kyung, J. W., Roh, E. J., Choi, H., Lim, C. S., An, S. B., Sohn, S., & Han, I. (2022). Synaptic Cell Adhesion Molecule 3 (SynCAM3) Deletion Promotes Recovery from Spinal Cord Injury by Limiting Glial Scar Formation. International Journal of Molecular Sciences, 23(11), 6218. https://doi.org/10.3390/ijms23116218







