Inhibitors of Mitochondrial Human Carbonic Anhydrases VA and VB as a Therapeutic Strategy against Paclitaxel-Induced Neuropathic Pain in Mice
Abstract
:1. Introduction
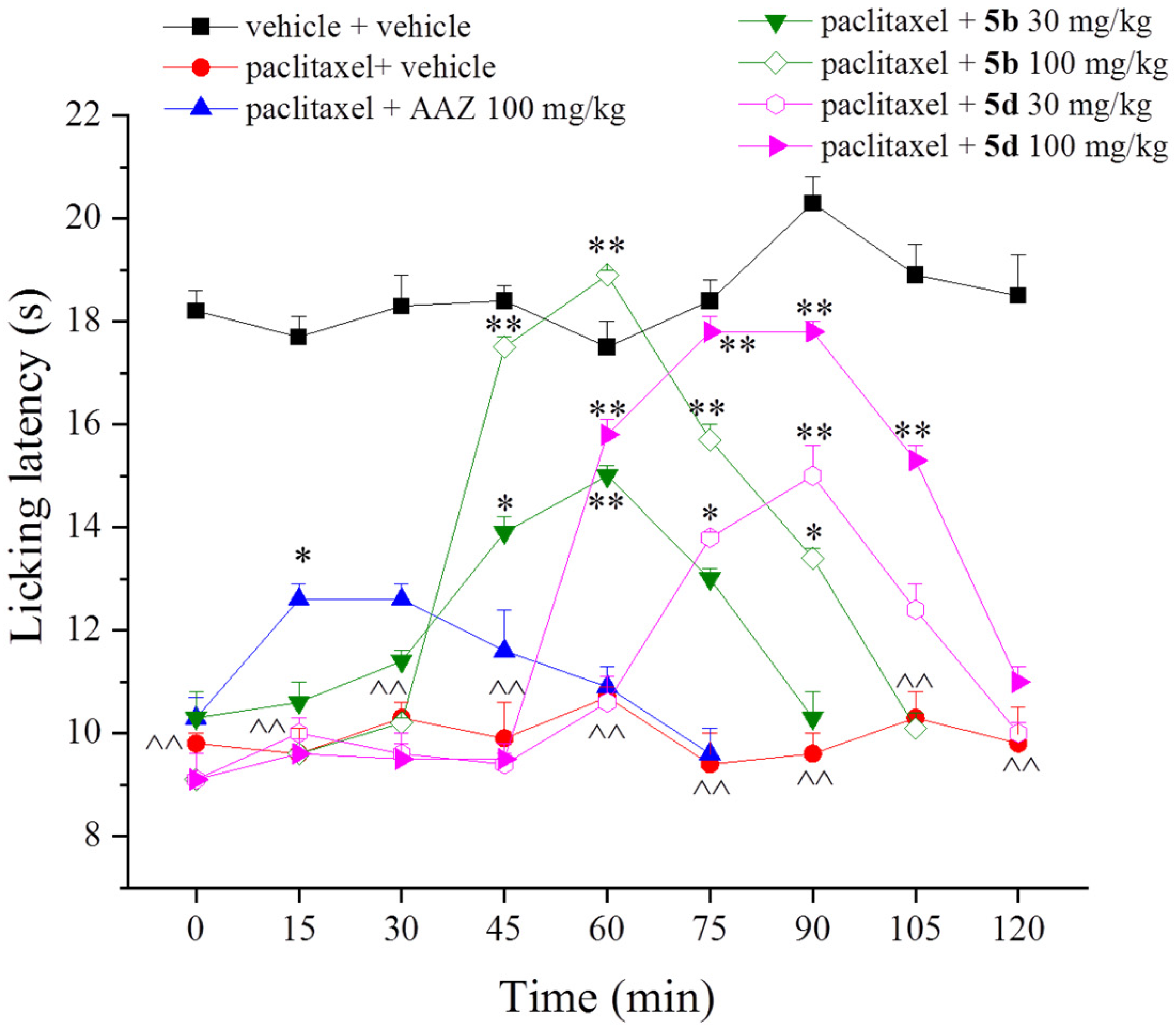
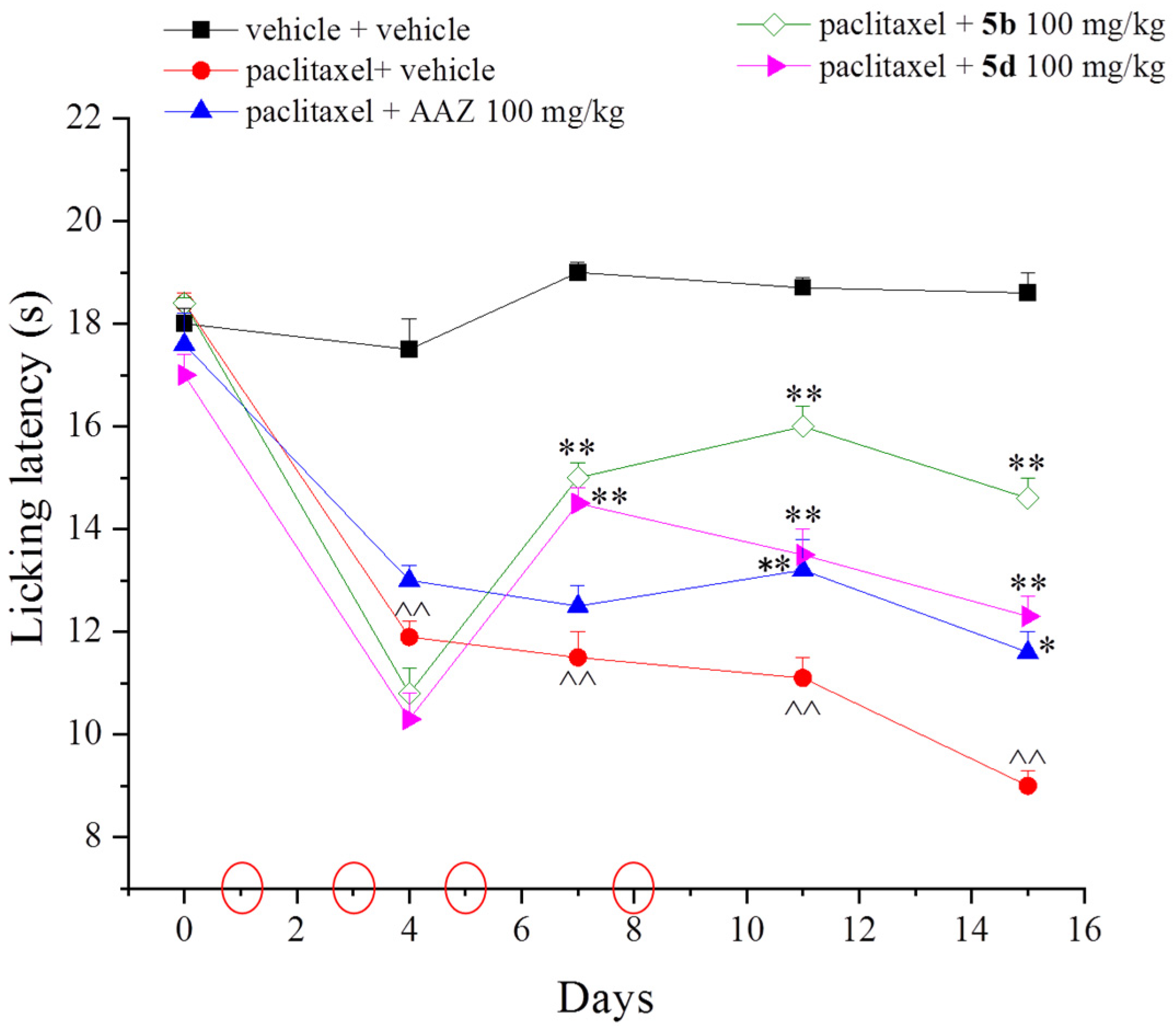
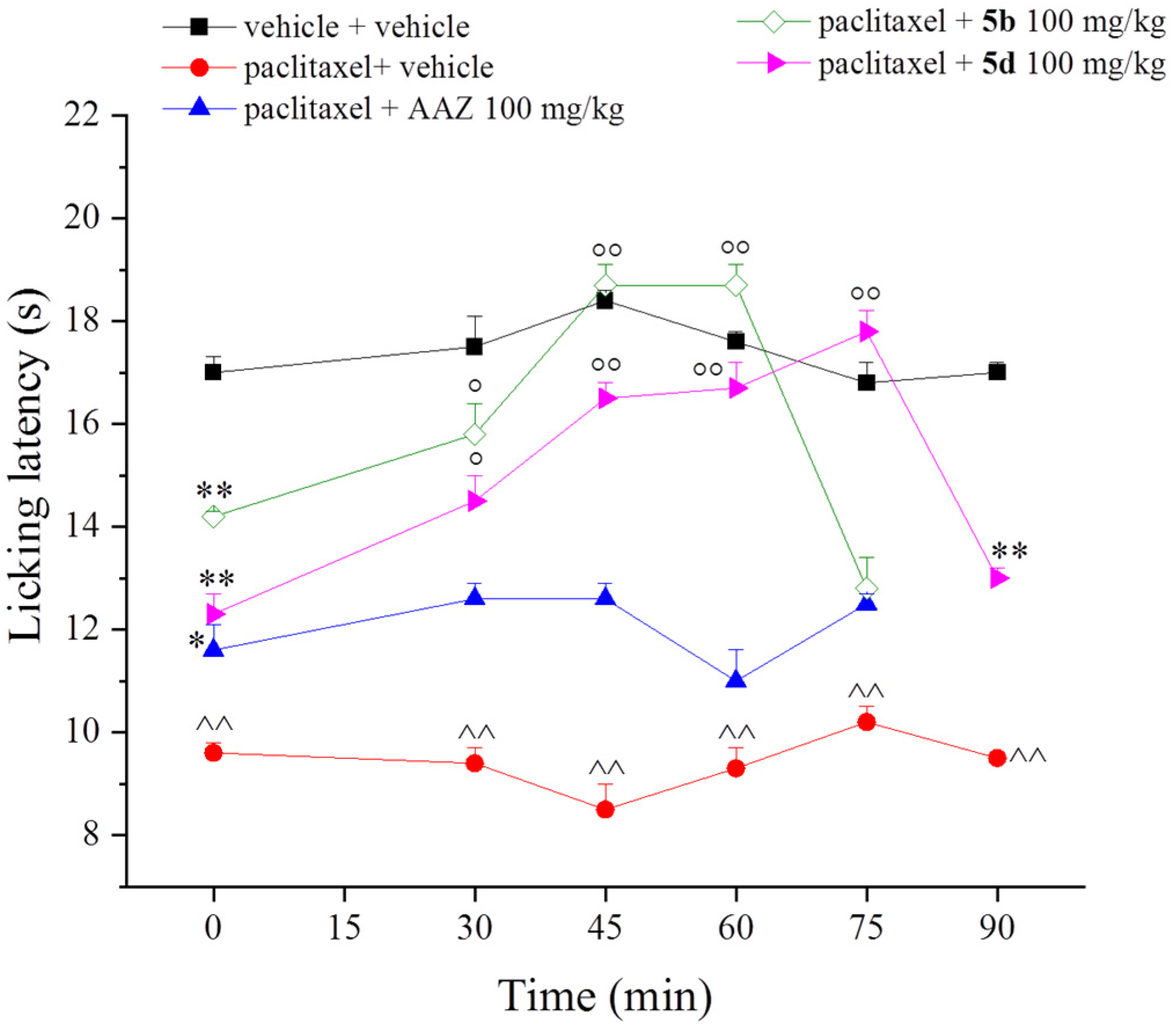
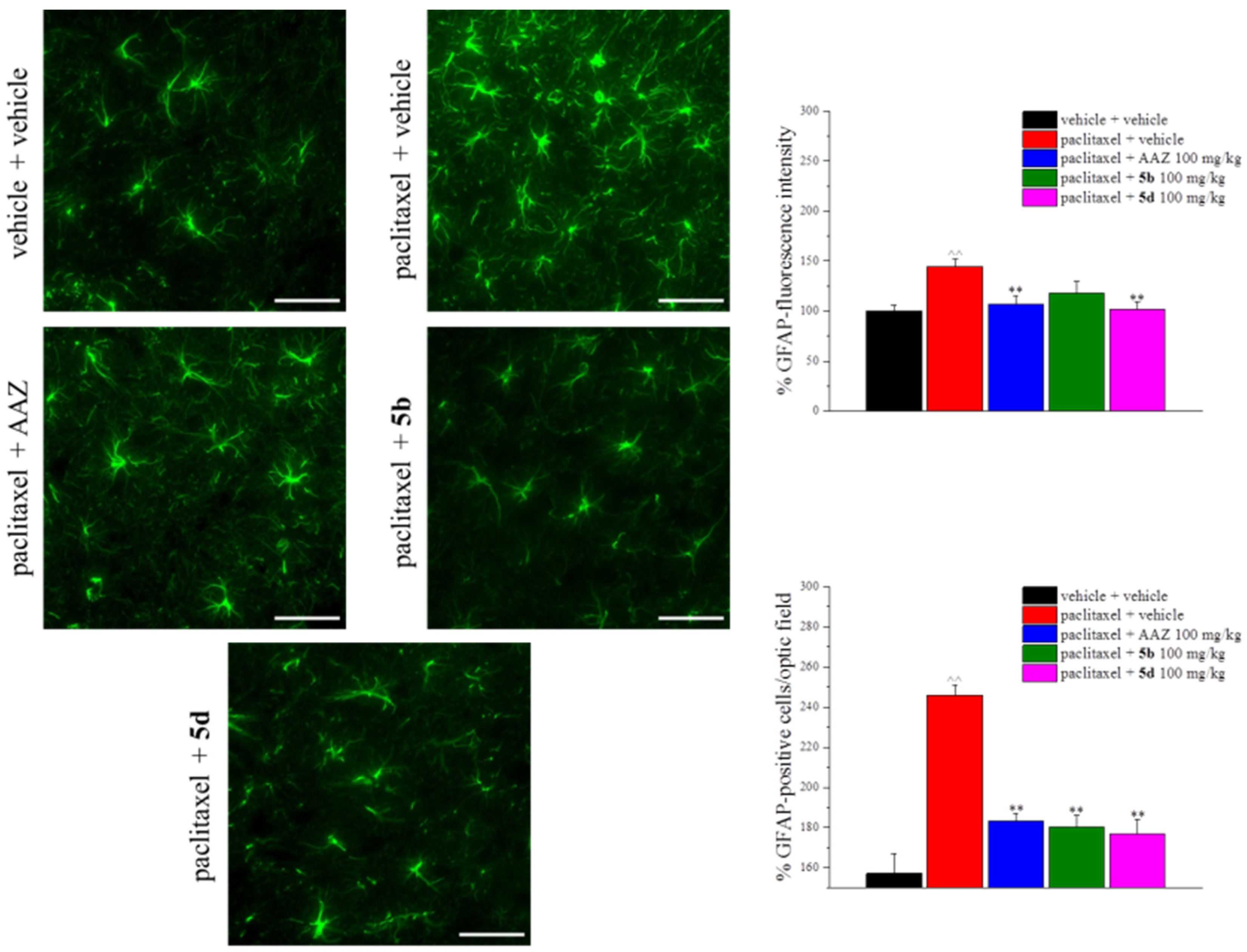
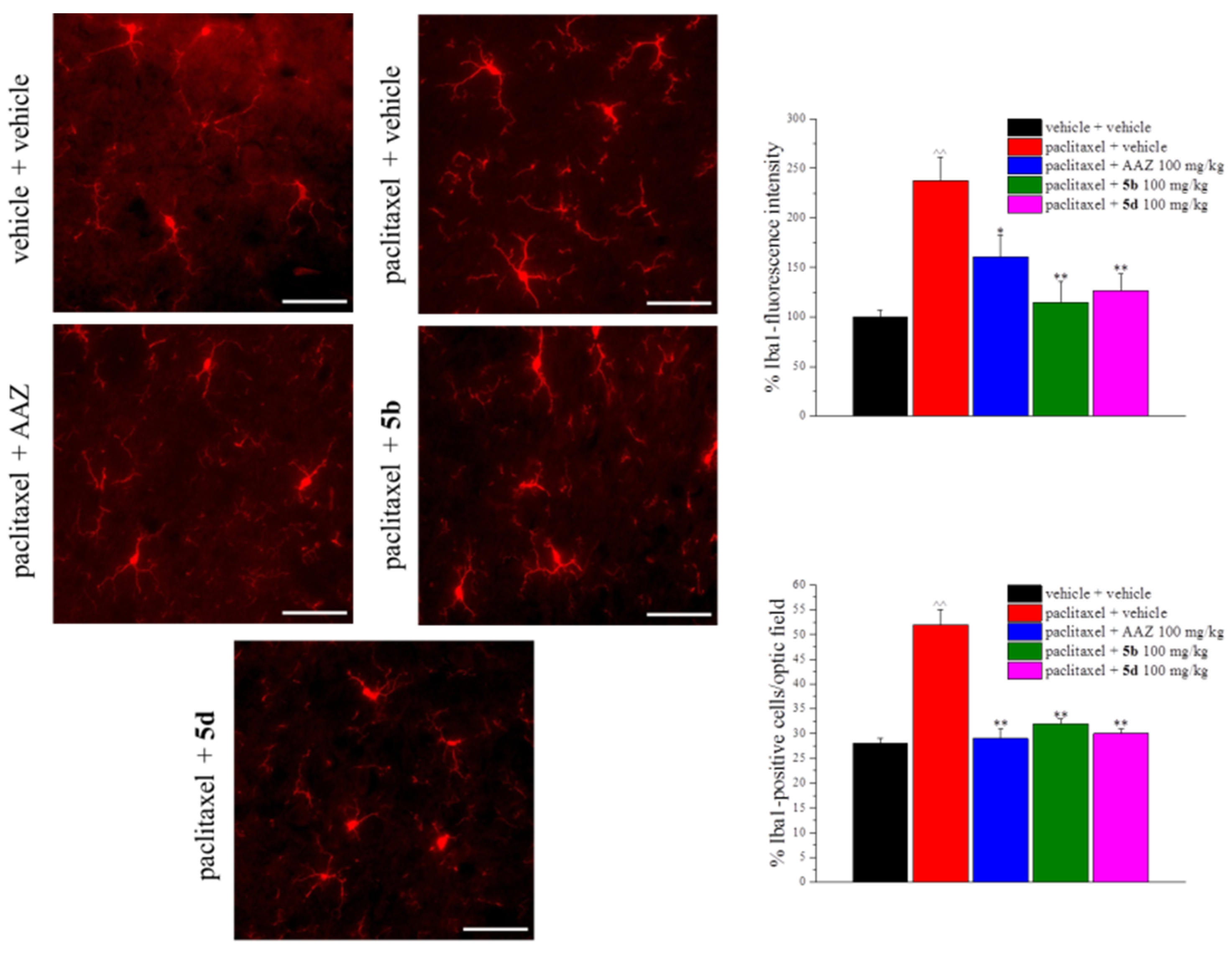
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.2. Cell Culture and Treatment
3.3. Cell Viability Assay
3.4. Animals
3.5. Paclitaxel Mouse Model of Neuropathy
3.6. Treatments
3.7. Cold Plate
3.8. Immunohistochemistry
3.9. Statistical Analysis
3.10. Citrate Synthase (CS) Activity
3.11. Mitochondrial Membrane Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowinsky, E.K.; Donehower, R.C. Paclitaxel (Taxol). N. Engl. J. Med. 1995, 332, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, A.; Nisticó, C.; Cuppone, F.; Bria, E.; Galié, E.; Graziano, G.; Natoli, G.; Sperduti, I.; Jandolo, B.; Calabretta, F.; et al. Peripheral Neurotoxicity of Weekly Paclitaxel Chemotherapy: A Schedule or a Dose Issue? Clin. Breast Cancer 2007, 7, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Swain, S.M. Peripheral Neuropathy Induced by Microtubule-Stabilizing Agents. J. Clin. Oncol. 2006, 24, 1633–1642. [Google Scholar] [CrossRef] [Green Version]
- Boyette-Davis, J.A.; Cata, J.P.; Driver, L.C.; Novy, D.M.; Bruel, B.M.; Mooring, D.L.; Wendelschafer-Crabb, G.; Kennedy, W.R.; Dougherty, P.M. Persistent chemoneuropathy in patients receiving the plant alkaloids paclitaxel and vincristine. Cancer Chemother. Pharmacol. 2013, 71, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, P.M.; Cata, J.P.; Cordella, J.V.; Burton, A.; Weng, H.-R. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain 2004, 109, 132–142. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Supuran, C.T. Carbonic anhydrase inhibition and the management of neuropathic pain. Expert Rev. Neurother. 2016, 16, 961–968. [Google Scholar] [CrossRef]
- Bua, S.; Lucarini, L.; Micheli, L.; Menicatti, M.; Bartolucci, G.; Selleri, S.; Di Cesare Mannelli, L.; Ghelardini, C.; Masini, E.; Carta, F.; et al. Bioisosteric Development of Multitarget Nonsteroidal Anti-Inflammatory Drug–Carbonic Anhydrases Inhibitor Hybrids for the Management of Rheumatoid Arthritis. J. Med. Chem. 2020, 63, 2325–2342. [Google Scholar] [CrossRef]
- Berrino, E.; Milazzo, L.; Micheli, L.; Vullo, D.; Angeli, A.; Bozdag, M.; Nocentini, A.; Menicatti, M.; Bartolucci, G.; Di Cesare Mannelli, L.; et al. Synthesis and Evaluation of Carbonic Anhydrase Inhibitors with Carbon Monoxide Releasing Properties for the Management of Rheumatoid Arthritis. J. Med. Chem. 2019, 62, 7233–7249. [Google Scholar] [CrossRef]
- Micheli, L.; Bozdag, M.; Akgul, O.; Carta, F.; Guccione, C.; Bergonzi, M.C.; Bilia, A.R.; Cinci, L.; Lucarini, E.; Parisio, C.; et al. Pain Relieving Effect of-NSAIDs-CAIs Hybrid Molecules: Systemic and Intra-Articular Treatments against Rheumatoid Arthritis. Int. J. Mol. Sci. 2019, 20, 1923. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Nocentini, A.; Bonardi, A.; Chiaramonte, N.; Parisio, C.; Micheli, L.; Toti, A.; Ferrara, V.; Carrino, D.; Pacini, A.; et al. Carbonic Anhydrase IV Selective Inhibitors Counteract the Development of Colitis-Associated Visceral Pain in Rats. Cells 2021, 10, 2540. [Google Scholar] [CrossRef] [PubMed]
- Tanini, D.; Carradori, S.; Capperucci, A.; Lupori, L.; Zara, S.; Ferraroni, M.; Ghelardini, C.; Di Cesare Mannelli, L.; Micheli, L.; Lucarini, E.; et al. Chalcogenides-incorporating carbonic anhydrase inhibitors concomitantly reverted oxaliplatin-induced neuropathy and enhanced antiproliferative action. Eur. J. Med. Chem. 2021, 225, 113793. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Alterio, V.; Bua, S.; Micheli, L.; Esposito, D.; Buonanno, M.; Bartolucci, G.; Osman, S.M.; ALOthman, Z.A.; Cirilli, R.; et al. Phenyl(thio)phosphon(amid)ate Benzenesulfonamides as Potent and Selective Inhibitors of Human Carbonic Anhydrases II and VII Counteract Allodynia in a Mouse Model of Oxaliplatin-Induced Neuropathy. J. Med. Chem. 2020, 63, 5185–5200. [Google Scholar] [CrossRef]
- Nagelhus, E.A.; Mathiisen, T.M.; Bateman, A.C.; Haug, F.-M.; Ottersen, O.P.; Grubb, J.H.; Waheed, A.; Sly, W.S. Carbonic anhydrase {XIV} is enriched in specific membrane domains of retinal pigment epithelium, Müller cells, and astrocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 8030–8035. [Google Scholar] [CrossRef] [Green Version]
- Supuran, C.T. Carbonic anhydrase inhibitors in the treatment and prophylaxis of obesity. Expert Opin. Ther. Pat. 2003, 13, 1545–1550. [Google Scholar] [CrossRef]
- Kivelä, A.; Parkkila, S.; Saarnio, J.; Karttunen, T.J.; Kivelä, J.; Parkkila, A.-K.; Waheed, A.; Sly, W.S.; Grubb, J.H.; Shah, G.; et al. Expression of a Novel Transmembrane Carbonic Anhydrase Isozyme XII in Normal Human Gut and Colorectal Tumors. Am. J. Pathol. 2000, 156, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Kivelä, A.J. Carbonic anhydrases in normal gastrointestinal tract and gastrointestinal tumours. World J. Gastroenterol. 2005, 11, 155. [Google Scholar] [CrossRef]
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The Role of Carbonic Anhydrase 9 in Regulating Extracellular and Intracellular pH in Three-dimensional Tumor Cell Growths. J. Biol. Chem. 2009, 284, 20299–20310. [Google Scholar] [CrossRef] [Green Version]
- Thiry, A.; Dogné, J.-M.; Supuran, C.T.; Masereel, B. Carbonic anhydrase inhibitors as anticonvulsant agents. Curr. Top. Med. Chem. 2007, 7, 855–864. [Google Scholar] [CrossRef]
- Shah, G.N.; Hewett-Emmett, D.; Grubb, J.H.; Migas, M.C.; Fleming, R.E.; Waheed, A.; Sly, W.S. Mitochondrial carbonic anhydrase CA VB: Differences in tissue distribution and pattern of evolution from those of CA VA suggest distinct physiological roles. Proc. Natl. Acad. Sci. USA 2000, 97, 1677–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, T.M.; Salvemini, D. Mini-Review: Mitochondrial dysfunction and chemotherapy-induced neuropathic pain. Neurosci. Lett. 2021, 760, 136087. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cesare Mannelli, L.; Zanardelli, M.; Failli, P.; Ghelardini, C. Oxaliplatin-Induced Neuropathy: Oxidative Stress as Pathological Mechanism. Protective Effect of Silibinin. J. Pain 2012, 13, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Muthuraman, A.; Jaggi, A.S.; Singh, N.; Singh, D. Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. Eur. J. Pharmacol. 2008, 587, 104–111. [Google Scholar] [CrossRef]
- Sharawy, N.; Rashed, L.; Youakim, M.F. Evaluation of multi-neuroprotective effects of erythropoietin using cisplatin induced peripheral neurotoxicity model. Exp. Toxicol. Pathol. 2015, 67, 315–322. [Google Scholar] [CrossRef]
- Trecarichi, A.; Duggett, N.A.; Granat, L.; Lo, S.; Malik, A.N.; Zuliani-Álvarez, L.; Flatters, S.J.L. Preclinical evidence for mitochondrial DNA as a potential blood biomarker for chemotherapy-induced peripheral neuropathy. PLoS ONE 2022, 17, e0262544. [Google Scholar] [CrossRef]
- Ghandour, M.S.; Parkkila, A.-K.; Parkkila, S.; Waheed, A.; Sly, W.S. Mitochondrial Carbonic Anhydrase in the Nervous System: Expression in Neuronal and Glial Cells. J. Neurochem. 2002, 75, 2212–2220. [Google Scholar] [CrossRef]
- Shah, G.N.; Price, T.O.; Banks, W.A.; Morofuji, Y.; Kovac, A.; Ercal, N.; Sorenson, C.M.; Shin, E.S.; Sheibani, N. Pharmacological Inhibition of Mitochondrial Carbonic Anhydrases Protects Mouse Cerebral Pericytes from High Glucose-Induced Oxidative Stress and Apoptosis. J. Pharmacol. Exp. Ther. 2013, 344, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.A.; Derry, S.; Aldington, D.; Cole, P.; Wiffen, P.J. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015, CD008242. [Google Scholar] [CrossRef]
- Kautio, A.-L.; Haanpää, M.; Saarto, T.; Kalso, E. Amitriptyline in the Treatment of Chemotherapy-Induced Neuropathic Symptoms. J. Pain Symptom Manag. 2008, 35, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Akgül, Ö.; Lucarini, E.; Di Cesare Mannelli, L.; Ghelardini, C.; D’Ambrosio, K.; Buonanno, M.; Monti, S.M.; De Simone, G.; Angeli, A.; Supuran, C.T.; et al. Sultam based Carbonic Anhydrase VII inhibitors for the management of neuropathic pain. Eur. J. Med. Chem. 2022, 227, 113956. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Di Cesare Mannelli, L.; Trallori, E.; Peat, T.S.; Ghelardini, C.; Carta, F.; Supuran, C.T. Design, Synthesis, and X-ray of Selenides as New Class of Agents for Prevention of Diabetic Cerebrovascular Pathology. ACS Med. Chem. Lett. 2018, 9, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Angeli, A.; Tanini, D.; Viglianisi, C.; Panzella, L.; Capperucci, A.; Menichetti, S.; Supuran, C.T. Evaluation of selenide, diselenide and selenoheterocycle derivatives as carbonic anhydrase I, II, IV, VII and IX inhibitors. Bioorg. Med. Chem. 2017, 25, 2518–2523. [Google Scholar] [CrossRef]
- Angeli, A.; Tanini, D.; Peat, T.S.; Di Cesare Mannelli, L.; Bartolucci, G.; Capperucci, A.; Ghelardini, C.; Supuran, C.T.; Carta, F. Discovery of New Selenoureido Analogues of 4-(4-Fluorophenylureido)benzenesulfonamide as Carbonic Anhydrase Inhibitors. ACS Med. Chem. Lett. 2017, 8, 963–968. [Google Scholar] [CrossRef]
- Duggett, N.A.; Griffiths, L.A.; McKenna, O.E.; de Santis, V.; Yongsanguanchai, N.; Mokori, E.B.; Flatters, S.J.L. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 2016, 333, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Ishii, N.; Tsubouchi, H.; Miura, A.; Yanagi, S.; Ueno, H.; Shiomi, K.; Nakazato, M. Ghrelin alleviates paclitaxel-induced peripheral neuropathy by reducing oxidative stress and enhancing mitochondrial anti-oxidant functions in mice. Eur. J. Pharmacol. 2018, 819, 35–42. [Google Scholar] [CrossRef]
- Micheli, L.; Di Cesare Mannelli, L.; Guerrini, R.; Trapella, C.; Zanardelli, M.; Ciccocioppo, R.; Rizzi, A.; Ghelardini, C.; Calò, G. Acute and subchronic antinociceptive effects of nociceptin/orphanin FQ receptor agonists infused by intrathecal route in rats. Eur. J. Pharmacol. 2015, 754, 73–81. [Google Scholar] [CrossRef]
- Micheli, L.; Lucarini, E.; Toti, A.; Ferrara, V.; Ciampi, C.; Parisio, C.; Bartolucci, G.; Di Cesare Mannelli, L.; Ghelardini, C. Effects of Ultramicronized N-Palmitoylethanolamine Supplementation on Tramadol and Oxycodone Analgesia and Tolerance Prevention. Pharmaceutics 2022, 14, 403. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Micheli, L.; Lucarini, E.; Ghelardini, C. Ultramicronized N-Palmitoylethanolamine Supplementation for Long-Lasting, Low-Dosed Morphine Antinociception. Front. Pharmacol. 2018, 9, 473. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Dhopeshwarkar, A.S.; Huibregtse, M.; Mackie, K.; Hohmann, A.G. Slowly Signaling G Protein–Biased CB2 Cannabinoid Receptor Agonist LY2828360 Suppresses Neuropathic Pain with Sustained Efficacy and Attenuates Morphine Tolerance and Dependence. Mol. Pharmacol. 2018, 93, 49–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legakis, L.P.; Negus, S.S. Repeated Morphine Produces Sensitization to Reward and Tolerance to Antiallodynia in Male and Female Rats with Chemotherapy-Induced Neuropathy. J. Pharmacol. Exp. Ther. 2018, 365, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Zhou, D.; Pan, Z.; Yang, G.; Zhang, H.; Ji, L.; Mao, Z. CAIXplatins: Highly Potent Platinum(IV) Prodrugs {Selective} against Carbonic Anhydrase IX for the Treatment of Hypoxic Tumors. Angew. Chem. Int. Ed. 2020, 59, 18556–18562. [Google Scholar] [CrossRef]
- Balayssac, D.; Ferrier, J.; Descoeur, J.; Ling, B.; Pezet, D.; Eschalier, A.; Authier, N. Chemotherapy-induced peripheral neuropathies: From clinical relevance to preclinical evidence. Expert Opin. Drug Saf. 2011, 10, 407–417. [Google Scholar] [CrossRef]
- Micheli, L.; Rajagopalan, R.; Lucarini, E.; Toti, A.; Parisio, C.; Carrino, D.; Pacini, A.; Ghelardini, C.; Rajagopalan, P.; Di Cesare Mannelli, L. Pain Relieving and Neuroprotective Effects of Non-opioid Compound, DDD-028, in the Rat {Model} of Paclitaxel-Induced Neuropathy. Neurotherapeutics 2021, 18, 2008–2020. [Google Scholar] [CrossRef]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Pacini, A.; Bonaccini, L.; Zanardelli, M.; Mello, T.; Ghelardini, C. Morphologic Features and Glial Activation in Rat Oxaliplatin-Dependent Neuropathic Pain. J. Pain 2013, 14, 1585–1600. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, V.; Tanga, F.; DeLeo, J.A. Inhibition of Microglial Activation Attenuates the Development but Not {Existing} Hypersensitivity in a Rat Model of Neuropathy. J. Pharmacol. Exp. Ther. 2003, 306, 624–630. [Google Scholar] [CrossRef] [Green Version]
- Di Cesare Mannelli, L.; Pacini, A.; Micheli, L.; Tani, A.; Zanardelli, M.; Ghelardini, C. Glial role in oxaliplatin-induced neuropathic pain. Exp. Neurol. 2014, 261, 22–33. [Google Scholar] [CrossRef]
- Ghandour, M.S.; Langley, O.K.; Zhu, X.L.; Waheed, A.; Sly, W.S. Carbonic anhydrase IV on brain capillary endothelial cells: A marker associated with the blood-brain barrier. Proc. Natl. Acad. Sci. USA 1992, 89, 6823–6827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branca, J.J.V.; Maresca, M.; Morucci, G.; Becatti, M.; Paternostro, F.; Gulisano, M.; Ghelardini, C.; Salvemini, D.; Di Cesare Mannelli, L.; Pacini, A. Oxaliplatin-induced blood brain barrier loosening: A new point of view on chemotherapy-induced neurotoxicity. Oncotarget 2018, 9, 23426–23438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khuankaew, C.; Sawaddiruk, P.; Surinkaew, P.; Chattipakorn, N.; Chattipakorn, S.C. Possible roles of mitochondrial dysfunction in neuropathy. Int. J. Neurosci. 2021, 131, 1019–1041. [Google Scholar] [CrossRef] [PubMed]
- McCormick, B.; Lowes, D.A.; Colvin, L.; Torsney, C.; Galley, H.F. MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model. Br. J. Anaesth. 2016, 117, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Carozzi, V.A.; Canta, A.; Chiorazzi, A. Chemotherapy-induced peripheral neuropathy: {What} do we know about mechanisms? Neurosci. Lett. 2015, 596, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Polomano, R.C.; Mannes, A.J.; Clark, U.S.; Bennett, G.J. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 2001, 94, 293–304. [Google Scholar] [CrossRef]
- Micheli, L.; Di Cesare Mannelli, L.; Rizzi, A.; Guerrini, R.; Trapella, C.; Calò, G.; Ghelardini, C. Intrathecal administration of nociceptin/orphanin FQ receptor agonists in rats: A strategy to relieve chemotherapy-induced neuropathic hypersensitivity. Eur. J. Pharmacol. 2015, 766, 155–162. [Google Scholar] [CrossRef]
- Baptista-de-Souza, D.; Di Cesare Mannelli, L.; Zanardelli, M.; Micheli, L.; Nunes-de-Souza, R.L.; Canto-de-Souza, A.; Ghelardini, C. Serotonergic modulation in neuropathy induced by oxaliplatin: Effect on the 5HT2C receptor. Eur. J. Pharmacol. 2014, 735, 141–149. [Google Scholar] [CrossRef]
- Lucarini, E.; Pagnotta, E.; Micheli, L.; Parisio, C.; Testai, L.; Martelli, A.; Calderone, V.; Matteo, R.; Lazzeri, L.; Di Cesare Mannelli, L.; et al. Eruca sativa Meal against Diabetic Neuropathic Pain: An H(2)S-Mediated Effect of Glucoerucin. Molecules 2019, 24, 3006. [Google Scholar] [CrossRef] [Green Version]
- Testai, L.; Piragine, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Di Cesare Mannelli, L.; Brogi, S.; et al. The Citrus Flavonoid Naringenin Protects the Myocardium from Ageing-Dependent Dysfunction: Potential Role of SIRT1. Oxid. Med. Cell. Longev. 2020, 2020, 4650207. [Google Scholar] [CrossRef] [Green Version]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP: Implementing guidelines on reporting research using animals (ARRIVE etc.). Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheli, L.; Testai, L.; Angeli, A.; Carrino, D.; Pacini, A.; Margiotta, F.; Flori, L.; Supuran, C.T.; Calderone, V.; Ghelardini, C.; et al. Inhibitors of Mitochondrial Human Carbonic Anhydrases VA and VB as a Therapeutic Strategy against Paclitaxel-Induced Neuropathic Pain in Mice. Int. J. Mol. Sci. 2022, 23, 6229. https://doi.org/10.3390/ijms23116229
Micheli L, Testai L, Angeli A, Carrino D, Pacini A, Margiotta F, Flori L, Supuran CT, Calderone V, Ghelardini C, et al. Inhibitors of Mitochondrial Human Carbonic Anhydrases VA and VB as a Therapeutic Strategy against Paclitaxel-Induced Neuropathic Pain in Mice. International Journal of Molecular Sciences. 2022; 23(11):6229. https://doi.org/10.3390/ijms23116229
Chicago/Turabian StyleMicheli, Laura, Lara Testai, Andrea Angeli, Donatello Carrino, Alessandra Pacini, Francesco Margiotta, Lorenzo Flori, Claudiu T. Supuran, Vincenzo Calderone, Carla Ghelardini, and et al. 2022. "Inhibitors of Mitochondrial Human Carbonic Anhydrases VA and VB as a Therapeutic Strategy against Paclitaxel-Induced Neuropathic Pain in Mice" International Journal of Molecular Sciences 23, no. 11: 6229. https://doi.org/10.3390/ijms23116229
APA StyleMicheli, L., Testai, L., Angeli, A., Carrino, D., Pacini, A., Margiotta, F., Flori, L., Supuran, C. T., Calderone, V., Ghelardini, C., & Di Cesare Mannelli, L. (2022). Inhibitors of Mitochondrial Human Carbonic Anhydrases VA and VB as a Therapeutic Strategy against Paclitaxel-Induced Neuropathic Pain in Mice. International Journal of Molecular Sciences, 23(11), 6229. https://doi.org/10.3390/ijms23116229










