Urinary Tract Tumor Organoids Reveal Eminent Differences in Drug Sensitivities When Compared to 2-Dimensional Culture Systems
Abstract
:1. Introduction
2. Results
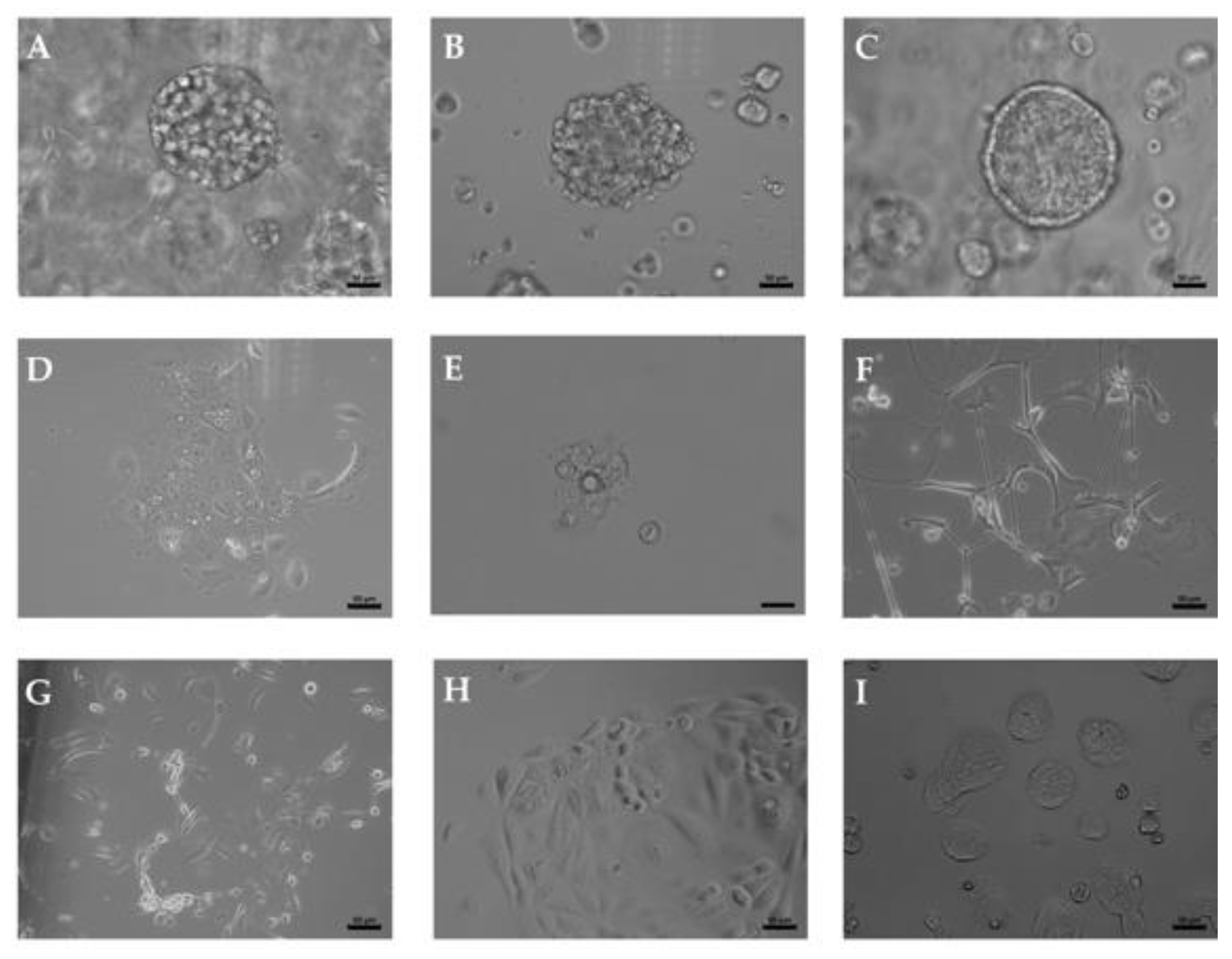
2.1. Upper Tract and Lower Tract Urinary Carcinoma Organoids
2.2. Comparing Different Viability Assays Using Urothelial Cells in Adherent Cultures
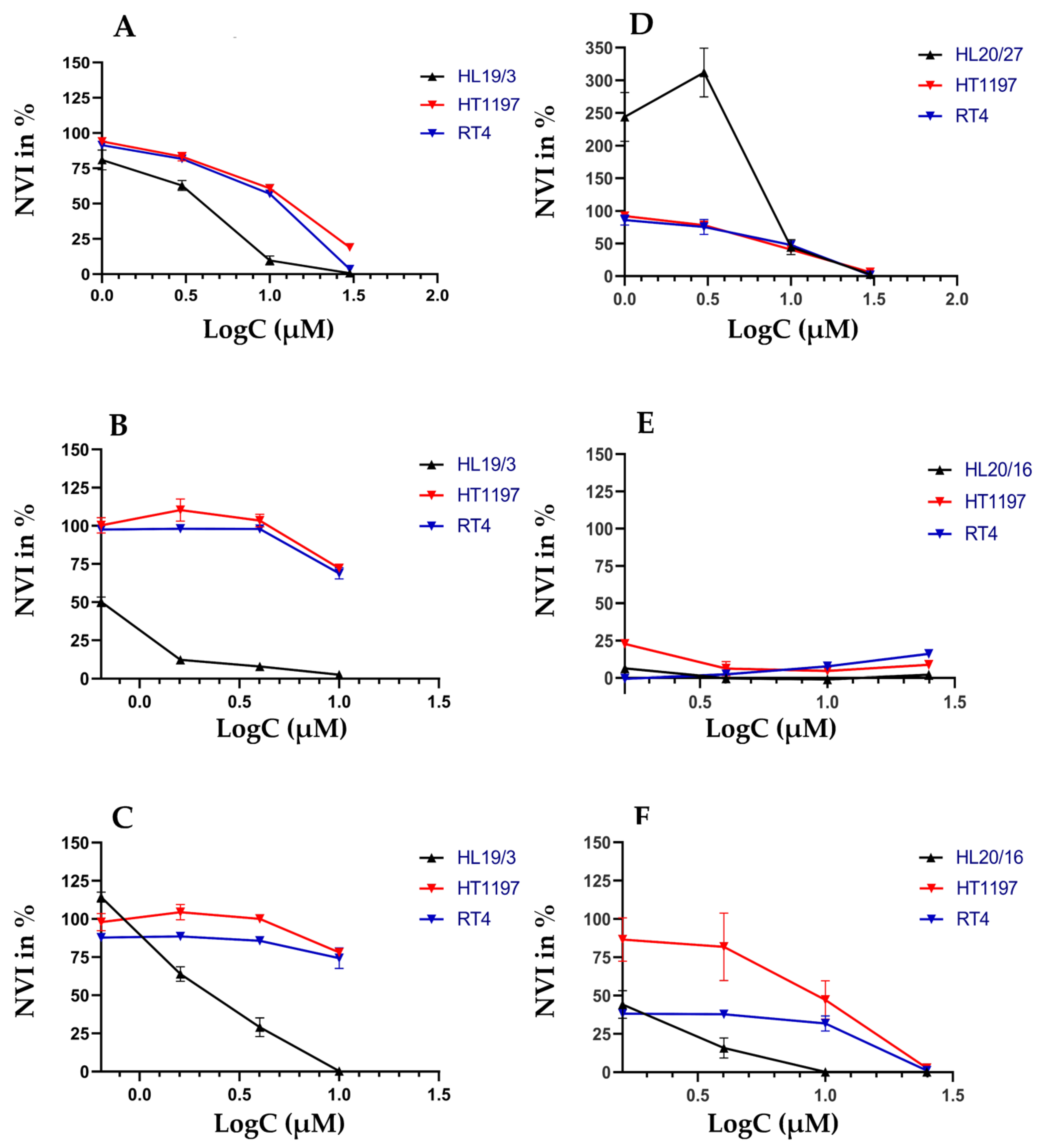
2.3. Individual Sensitivities to Cytotoxic Drugs in Two-Dimensional versus Three-Dimensional Cell Cultures
3. Discussion
4. Materials and Methods
4.1. Organoid Culture
4.2. Immunofluorescence Assay
4.3. Cytotoxicity Assay
4.3.1. Drug Testing in 2D Cell Culture
4.3.2. Drug Testing in Organoids
4.4. Data Processing and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Bladder cancer |
| BCO | Bladder cancer organoid (includes here organoids from RNU und TURBT surgeries) |
| CIS | Cisplatin |
| CSC | Cancer stem cell |
| CTG | CellTiterGlo (chemiluminescence assay) |
| IC50 | Inhibitory concentration to yield the half-maximal effect |
| MIBC | Muscle invasive bladder cancer |
| NUC | Normal urothelial cell |
| RNU | Radical nephroureterectomy |
| S63 | S63845 (a BH3 mimetic drug facilitating apoptosis) |
| TURBT | Transurethral resection of a bladder tumor |
| UC | Urothelial carcinoma |
| UTUC | Upper tract urothelial cancer (cell) |
| VTX | Venetoclax (a BCL-2 blocker facilitating apoptosis) |
| WST | Water soluble tetrazolium |
| 2D | Two-dimensional (standard cell culture) |
| 3D | Three-dimensional (s.c., organoid culture) |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Kirkali, Z.; Chan, T.; Manoharan, M.; Algaba, F.; Busch, C.; Cheng, L.; Kiemeney, L.; Kriegmair, M.; Montironi, R.; Murphy, W.M.; et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology 2005, 66, 4–34. [Google Scholar] [CrossRef]
- Cosentino, M.; Palou, J.; Gaya, J.M.; Breda, A.; Rodriguez-Faba, O.; Villavicencio-Mavrich, H. Upper urinary tract urothelial cell carcinoma: Location as a predictive factor for concomitant bladder carcinoma. World J. Urol. 2013, 31, 141–145. [Google Scholar] [CrossRef]
- Roupret, M.; Colin, P.; Yates, D.R. A new proposal to risk stratify urothelial carcinomas of the upper urinary tract (UTUCs) in a predefinitive treatment setting: Low-risk versus high-risk UTUCs. Eur. Urol. 2014, 66, 181–183. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Zigeuner, R.; Shariat, S.F.; van Rhijn, B.W.; Comperat, E.; Sylvester, R.J.; Kaasinen, E.; Bohle, A.; Palou Redorta, J.; et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur. Urol. 2013, 64, 639–653. [Google Scholar] [CrossRef]
- Matulewicz, R.S.; Steinberg, G.D. Non-muscle-invasive Bladder Cancer: Overview and Contemporary Treatment Landscape of Neoadjuvant Chemoablative Therapies. Rev. Urol. 2020, 22, 43–51. [Google Scholar]
- Resnick, M.J.; Bassett, J.C.; Clark, P.E. Management of superficial and muscle-invasive urothelial cancers of the bladder. Curr. Opin. Oncol. 2013, 25, 281–288. [Google Scholar] [CrossRef]
- Seisen, T.; Peyronnet, B.; Dominguez-Escrig, J.L.; Bruins, H.M.; Yuan, C.Y.; Babjuk, M.; Bohle, A.; Burger, M.; Comperat, E.M.; Cowan, N.C.; et al. Oncologic Outcomes of Kidney-sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur. Urol. 2016, 70, 1052–1068. [Google Scholar] [CrossRef]
- Apollo, A.; Ortenzi, V.; Scatena, C.; Zavaglia, K.; Aretini, P.; Lessi, F.; Franceschi, S.; Tomei, S.; Sepich, C.A.; Viacava, P.; et al. Molecular characterization of low grade and high grade bladder cancer. PLoS ONE 2019, 14, e0210635. [Google Scholar] [CrossRef]
- Vemana, G.; Kim, E.H.; Bhayani, S.B.; Vetter, J.M.; Strope, S.A. Survival Comparison Between Endoscopic and Surgical Management for Patients With Upper Tract Urothelial Cancer: A Matched Propensity Score Analysis Using Surveillance, Epidemiology and End Results-Medicare Data. Urology 2016, 95, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Ou, Y.C.; Hu, C.Y.; Cheng, H.L.; Yang, W.H. Long-term outcomes of total ureterectomy with ileal-ureteral substitution treatment for ureteral cancer: A single-center experience. BMC Urol. 2018, 18, 73. [Google Scholar] [CrossRef]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G.; et al. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.D.; Ng, C.K.; Shariat, S.F.; Margulis, V.; Montorsi, F.; Karakiewicz, P.; Upper-Tract Urothelial Carcinoma, C. Outcomes for patients with pT0 disease after radical nephroureterectomy for upper-tract urothelial carcinoma. BJU Int. 2009, 103, 3–4. [Google Scholar] [CrossRef]
- Ploussard, G.; Xylinas, E.; Lotan, Y.; Novara, G.; Margulis, V.; Roupret, M.; Matsumoto, K.; Karakiewicz, P.I.; Montorsi, F.; Remzi, M.; et al. Conditional survival after radical nephroureterectomy for upper tract carcinoma. Eur. Urol. 2015, 67, 803–812. [Google Scholar] [CrossRef]
- Moschini, M.; Xylinas, E.; Zamboni, S.; Mattei, A.; Niegisch, G.; Yu, E.Y.; Bamias, A.; Agarwal, N.; Sridhar, S.S.; Sternberg, C.N.; et al. Efficacy of Surgery in the Primary Tumor Site for Metastatic Urothelial Cancer: Analysis of an International, Multicenter, Multidisciplinary Database. Eur. Urol. Oncol. 2020, 3, 94–101. [Google Scholar] [CrossRef]
- Nazzani, S.; Preisser, F.; Mazzone, E.; Marchioni, M.; Bandini, M.; Tian, Z.; Mistretta, F.A.; Shariat, S.F.; Soulieres, D.; Montanari, E.; et al. Survival Effect of Chemotherapy in Metastatic Upper Urinary Tract Urothelial Carcinoma. Clin. Genitourin. Cancer 2019, 17, e97–e103. [Google Scholar] [CrossRef]
- Brausi, M.; Collette, L.; Kurth, K.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Newling, D.; Bouffioux, C.; Sylvester, R.J.; Group, E.G.-U.T.C.C. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: A combined analysis of seven EORTC studies. Eur. Urol. 2002, 41, 523–531. [Google Scholar] [CrossRef]
- Culp, S.H.; Dickstein, R.J.; Grossman, H.B.; Pretzsch, S.M.; Porten, S.; Daneshmand, S.; Cai, J.; Groshen, S.; Siefker-Radtke, A.; Millikan, R.E.; et al. Refining patient selection for neoadjuvant chemotherapy before radical cystectomy. J. Urol. 2014, 191, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Porten, S.; Siefker-Radtke, A.O.; Xiao, L.; Margulis, V.; Kamat, A.M.; Wood, C.G.; Jonasch, E.; Dinney, C.P.; Matin, S.F. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer 2014, 120, 1794–1799. [Google Scholar] [CrossRef]
- Hosogoe, S.; Hatakeyama, S.; Kusaka, A.; Hamano, I.; Iwamura, H.; Fujita, N.; Yamamoto, H.; Tobisawa, Y.; Yoneyama, T.; Yoneyama, T.; et al. Platinum-based Neoadjuvant Chemotherapy Improves Oncological Outcomes in Patients with Locally Advanced Upper Tract Urothelial Carcinoma. Eur. Urol. Focus 2018, 4, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Jokisch, J.F.; Karl, A.; Stief, C. Intravesical immunotherapy in nonmuscle invasive bladder cancer. Indian J. Urol. 2015, 31, 304–311. [Google Scholar] [CrossRef]
- Shintani, Y.; Sawada, Y.; Inagaki, T.; Kohjimoto, Y.; Uekado, Y.; Shinka, T. Intravesical instillation therapy with bacillus Calmette-Guerin for superficial bladder cancer: Study of the mechanism of bacillus Calmette-Guerin immunotherapy. Int. J. Urol. 2007, 14, 140–146. [Google Scholar] [CrossRef]
- Lee, R.K.; Abol-Enein, H.; Artibani, W.; Bochner, B.; Dalbagni, G.; Daneshmand, S.; Fradet, Y.; Hautmann, R.E.; Lee, C.T.; Lerner, S.P.; et al. Urinary diversion after radical cystectomy for bladder cancer: Options, patient selection, and outcomes. BJU Int. 2014, 113, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Ploussard, G.; Shariat, S.F.; Dragomir, A.; Kluth, L.A.; Xylinas, E.; Masson-Lecomte, A.; Rieken, M.; Rink, M.; Matsumoto, K.; Kikuchi, E.; et al. Conditional survival after radical cystectomy for bladder cancer: Evidence for a patient changing risk profile over time. Eur. Urol. 2014, 66, 361–370. [Google Scholar] [CrossRef]
- International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (Now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group; Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; Finnbladder; Norwegian Bladder Cancer Study Group; Club Urologico Espanol de Tratamiento Oncologico Group; Griffiths, G.; Hall, R.; et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J. Clin. Oncol. 2011, 29, 2171–2177. [Google Scholar] [CrossRef] [Green Version]
- Galsky, M.D.; Hoimes, C.J.; Necchi, A.; Shore, N.; Witjes, J.A.; Steinberg, G.; Bedke, J.; Nishiyama, H.; Fang, X.; Kataria, R.; et al. Perioperative pembrolizumab therapy in muscle-invasive bladder cancer: Phase III KEYNOTE-866 and KEYNOTE-905/EV-303. Future Oncol. 2021, 17, 3137–3150. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Flaig, T.W.; Mar, N.; Gourdin, T.S.; Srinivas, S.; Rosenberg, J.E.; Guseva, M.; Yu, Y.; Narayanan, S.; Hoimes, C.J. Study EV-103 Cohort H: Antitumor activity of neoadjuvant treatment with enfortumab vedotin monotherapy in patients (pts) with muscle invasive bladder cancer (MIBC) who are cisplatin-ineligible. J. Clin. Oncol. 2022, 40, 435. [Google Scholar] [CrossRef]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2021, 12, 648407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.D.; Jiang, L.H.; Zhong, S.L.; Li, J.; Sun, D.W.; Hou, J.C.; Wang, D.D.; Zhou, S.Y.; Tang, J.H. The role of long non-coding RNAs in drug resistance of cancer. Clin. Genet. 2021, 99, 84–92. [Google Scholar] [CrossRef]
- Long, X.; Xiong, W.; Zeng, X.; Qi, L.; Cai, Y.; Mo, M.; Jiang, H.; Zhu, B.; Chen, Z.; Li, Y. Cancer-associated fibroblasts promote cisplatin resistance in bladder cancer cells by increasing IGF-1/ERbeta/Bcl-2 signalling. Cell Death Dis. 2019, 10, 375. [Google Scholar] [CrossRef]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Yu, J.; Shi, J.; Wang, C.; Fu, W.; Chen, Z.; Yang, J. Cancer stem-like cells contribute to cisplatin resistance and progression in bladder cancer. Cancer Lett. 2012, 322, 70–77. [Google Scholar] [CrossRef]
- Massari, F.; Santoni, M.; Ciccarese, C.; Brunelli, M.; Conti, A.; Santini, D.; Montironi, R.; Cascinu, S.; Tortora, G. Emerging concepts on drug resistance in bladder cancer: Implications for future strategies. Crit. Rev. Oncol. Hematol. 2015, 96, 81–90. [Google Scholar] [CrossRef]
- Smith, A.G.; Macleod, K.F. Autophagy, cancer stem cells and drug resistance. J. Pathol. 2019, 247, 708–718. [Google Scholar] [CrossRef] [Green Version]
- van Kessel, K.E.; Zuiverloon, T.C.; Alberts, A.R.; Boormans, J.L.; Zwarthoff, E.C. Targeted therapies in bladder cancer: An overview of in vivo research. Nat. Rev. Urol. 2015, 12, 681–694. [Google Scholar] [CrossRef] [PubMed]
- McConkey, D.J.; Choi, W. Molecular Subtypes of Bladder Cancer. Curr. Oncol. Rep. 2018, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, K.M.; Theodorescu, D. Precision medicine for urothelial bladder cancer: Update on tumour genomics and immunotherapy. Nat. Rev. Urol. 2018, 15, 92–111. [Google Scholar] [CrossRef]
- Peng, M.; Xiao, D.; Bu, Y.; Long, J.; Yang, X.; Lv, S.; Yang, X. Novel Combination Therapies for the Treatment of Bladder Cancer. Front. Oncol. 2021, 10, 539527. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, A.P.; Metcalfe, A.D.; Romer, L.H.; Streuli, C.H. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J. Cell Biol. 2000, 149, 431–446. [Google Scholar] [CrossRef]
- Rainero, E.; Norman, J.C. Endosomal integrin signals for survival. Nat. Cell Biol. 2015, 17, 1373–1375. [Google Scholar] [CrossRef] [Green Version]
- John, B.A.; Said, N. Insights from animal models of bladder cancer: Recent advances, challenges, and opportunities. Oncotarget 2017, 8, 57766–57781. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.; Patel, A.; Heston, W.; Larchian, W. Mouse orthotopic models for bladder cancer research. BJU Int. 2009, 104, 1286–1291. [Google Scholar]
- Tian, H.; Lyu, Y.; Yang, Y.G.; Hu, Z. Humanized Rodent Models for Cancer Research. Front. Oncol. 2020, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Teicher, B.A. Tumor models for efficacy determination. Mol. Cancer Ther. 2006, 5, 2435–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerin, M.V.; Finisguerra, V.; Van den Eynde, B.J.; Bercovici, N.; Trautmann, A. Preclinical murine tumor models: A structural and functional perspective. eLife 2020, 9, e50740. [Google Scholar] [CrossRef]
- Banerjee, S.; Southgate, J. Bladder organoids: A step towards personalised cancer therapy? Transl. Androl. Urol. 2019, 8, S300–S302. [Google Scholar] [CrossRef]
- Abugomaa, A.; Elbadawy, M.; Yamanaka, M.; Goto, Y.; Hayashi, K.; Mori, T.; Uchide, T.; Azakami, D.; Fukushima, R.; Yoshida, T.; et al. Establishment of 2.5D organoid culture model using 3D bladder cancer organoid culture. Sci. Rep. 2020, 10, 9393. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Kretzschmar, K.; Clevers, H. Organoids: Modeling Development and the Stem Cell Niche in a Dish. Dev. Cell 2016, 38, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [Green Version]
- van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef] [Green Version]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 2018, 172, 373–386.e310. [Google Scholar] [CrossRef] [Green Version]
- Mullenders, J.; de Jongh, E.; Brousali, A.; Roosen, M.; Blom, J.P.A.; Begthel, H.; Korving, J.; Jonges, T.; Kranenburg, O.; Meijer, R.; et al. Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proc. Natl. Acad. Sci. USA 2019, 116, 4567–4574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Choi, S.; Kang, B.; Kong, J.; Kim, Y.; Yoon, W.H.; Lee, H.R.; Kim, S.; Kim, H.M.; Lee, H.; et al. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature 2020, 588, 664–669. [Google Scholar] [CrossRef]
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528.e517. [Google Scholar] [CrossRef] [Green Version]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.H.; Reynolds, C.P. Bcl-2 inhibitors: Targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 2009, 15, 1126–1132. [Google Scholar] [CrossRef] [Green Version]
- Scheffold, A.; Jebaraj, B.M.C.; Stilgenbauer, S. Venetoclax: Targeting BCL2 in Hematological Cancers. In Small Molecules in Hematology; Martens, U., Ed.; Springer: Cham, Switzerland, 2018; Volume 212, pp. 215–242. [Google Scholar]
- Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Maragno, A.L.; Le Toumelin-Braizat, G.; Chanrion, M.; Kelly, G.L.; Gong, J.N.; Moujalled, D.M.; et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016, 538, 477–482. [Google Scholar] [CrossRef]
- Kapalczynska, M.; Kolenda, T.; Przybyla, W.; Zajaczkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Blizniak, R.; Luczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Xylinas, E.; Hassler, M.R.; Zhuang, D.; Krzywinski, M.; Erdem, Z.; Robinson, B.D.; Elemento, O.; Clozel, T.; Shariat, S.F. An Epigenomic Approach to Improving Response to Neoadjuvant Cisplatin Chemotherapy in Bladder Cancer. Biomolecules 2016, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Vakhrusheva, O.; Markowitsch, S.D.; Slade, K.S.; Tsaur, I.; Cinatl, J.; Michaelis, M.; Efferth, T.; Haferkamp, A.; Juengel, E. Artesunate Impairs Growth in Cisplatin-Resistant Bladder Cancer Cells by Cell Cycle Arrest, Apoptosis and Autophagy Induction. Cells 2020, 9, 2643. [Google Scholar] [CrossRef]
- Masters, J.R.; Hepburn, P.J.; Walker, L.; Highman, W.J.; Trejdosiewicz, L.K.; Povey, S.; Parkar, M.; Hill, B.T.; Riddle, P.R.; Franks, L.M. Tissue culture model of transitional cell carcinoma: Characterization of twenty-two human urothelial cell lines. Cancer Res. 1986, 46, 3630–3636. [Google Scholar]
- Sabichi, A.; Keyhani, A.; Tanaka, N.; Delacerda, J.; Lee, I.L.; Zou, C.; Zhou, J.H.; Benedict, W.F.; Grossman, H.B. Characterization of a panel of cell lines derived from urothelial neoplasms: Genetic alterations, growth in vivo and the relationship of adenoviral mediated gene transfer to coxsackie adenovirus receptor expression. J. Urol. 2006, 175, 1133–1137. [Google Scholar] [CrossRef]
- Earl, J.; Rico, D.; Carrillo-de-Santa-Pau, E.; Rodriguez-Santiago, B.; Mendez-Pertuz, M.; Auer, H.; Gomez, G.; Grossman, H.B.; Pisano, D.G.; Schulz, W.A.; et al. The UBC-40 Urothelial Bladder Cancer Cell Line Index: A genomic resource for functional studies. BMC Genom. 2015, 16, 403, Erratum in BMC Genom. 2015, 16, 1019. [Google Scholar]
- Pohjala, L.; Tammela, P.; Samanta, S.K.; Yli-Kauhaluoma, J.; Vuorela, P. Assessing the data quality in predictive toxicology using a panel of cell lines and cytotoxicity assays. Anal. Biochem. 2007, 362, 221–228. [Google Scholar] [CrossRef]
- Schroterova, L.; Kralova, V.; Voracova, A.; Haskova, P.; Rudolf, E.; Cervinka, M. Antiproliferative effects of selenium compounds in colon cancer cells: Comparison of different cytotoxicity assays. Toxicol. Vitr. 2009, 23, 1406–1411. [Google Scholar] [CrossRef]
- Ulker, Z.; Alpsoy, L.; Mihmanli, A. Assessment of cytotoxic and apoptotic effects of benzaldehyde using different assays. Hum. Exp. Toxicol. 2013, 32, 858–864. [Google Scholar] [CrossRef]
- Braun, K.; Sturzel, C.M.; Biskupek, J.; Kaiser, U.; Kirchhoff, F.; Linden, M. Comparison of different cytotoxicity assays for in vitro evaluation of mesoporous silica nanoparticles. Toxicol. Vitr. 2018, 52, 214–221. [Google Scholar] [CrossRef]
- Cathomas, R.; Lorch, A.; Bruins, H.M.; Comperat, E.M.; Cowan, N.C.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Hernandez, V.; Espinos, E.L.; et al. The 2021 Updated European Association of Urology Guidelines on Metastatic Urothelial Carcinoma. Eur. Urol. 2022, 81, 95–103. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell Physiol. 2019, 234, 8381–8395. [Google Scholar] [CrossRef]
- Wang, K.J.; Wang, C.; Dai, L.H.; Yang, J.; Huang, H.; Ma, X.J.; Zhou, Z.; Yang, Z.Y.; Xu, W.D.; Hua, M.M.; et al. Targeting an Autocrine Regulatory Loop in Cancer Stem-like Cells Impairs the Progression and Chemotherapy Resistance of Bladder Cancer. Clin. Cancer Res. 2019, 25, 1070–1086. [Google Scholar] [CrossRef] [Green Version]
- Patani, N.; Martin, L.-A.; Dowsett, M. Biomarkers for the clinical management of breast cancer: International perspective. Int. J. Cancer 2012, 133, 1–13. [Google Scholar] [CrossRef]
- Sveen, A.; Kopetz, S.; Lothe, R.A. Biomarker-guided therapy for colorectal cancer: Strength in complexity. Nat. Rev. Clin. Oncol. 2020, 17, 11–32. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Yoshida, T.; Kates, M.; Fujita, K.; Bivalacqua, T.J.; McConkey, D.J. Predictive biomarkers for drug response in bladder cancer. Int. J. Urol. 2019, 26, 1044–1053. [Google Scholar] [CrossRef]
- Takahashi, T. Organoids for Drug Discovery and Personalized Medicine. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Abugomaa, A.; Elbadawy, M.; Yamawaki, H.; Usui, T.; Sasaki, K. Emerging Roles of Cancer Stem Cells in Bladder Cancer Progression, Tumorigenesis, and Resistance to Chemotherapy: A Potential Therapeutic Target for Bladder Cancer. Cells 2020, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Wensink, G.E.; Elias, S.G.; Mullenders, J.; Koopman, M.; Boj, S.F.; Kranenburg, O.W.; Roodhart, J.M.L. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis. Oncol. 2021, 5, 30. [Google Scholar] [CrossRef]
- Hofner, T.; Macher-Goeppinger, S.; Klein, C.; Schillert, A.; Eisen, C.; Wagner, S.; Rigo-Watermeier, T.; Baccelli, I.; Vogel, V.; Trumpp, A.; et al. Expression and prognostic significance of cancer stem cell markers CD24 and CD44 in urothelial bladder cancer xenografts and patients undergoing radical cystectomy. Urol. Oncol. 2014, 32, 678–686. [Google Scholar] [CrossRef]
- Geng, R.; Harland, N.; Montes-Mojarro, I.A.; Fend, F.; Aicher, W.K.; Stenzl, A.; Amen, B. CD24, a marker for an extended expansion potential of urothelial cancer cell organoids in vitro? Int. J. Mol. Sci. 2022, 23, 5453. [Google Scholar] [CrossRef]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Al-Ahmadie, H.; Choi, W.; Groeneveld, C.S.; et al. A consensus molecular classification of muscle-invasive bladder cancer. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Stepan, A.E.; Ciuca, M.; Simionescu, C.; Stepan, D.; Margaritescu, C. Immunoexpression of N-cadherin, Twist and Vimentin in Bladder Urothelial Carcinomas. Curr. Health Sci. J. 2015, 41, 219–226. [Google Scholar] [CrossRef]
- Vuoriluoto, K.; Haugen, H.; Kiviluoto, S.; Mpindi, J.P.; Nevo, J.; Gjerdrum, C.; Tiron, C.; Lorens, J.B.; Ivaska, J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 2011, 30, 1436–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jena, B.C.; Das, C.K.; Bharadwaj, D.; Mandal, M. Cancer associated fibroblast mediated chemoresistance: A paradigm shift in understanding the mechanism of tumor progression. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2020, 1874, 188416. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.; Wiemann, S. Cancer-Associated Fibroblasts: Implications for Cancer Therapy. Cancers 2021, 13, 3526. [Google Scholar] [CrossRef]
- Li, Y.; Lin, K.; Yang, Z.; Han, N.; Quan, X.; Guo, X.; Li, C. Bladder cancer stem cells: Clonal origin and therapeutic perspectives. Oncotarget 2017, 8, 66668–66679. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.M.; Chang, J.W. Bladder cancer initiating cells (BCICs) are among EMA-CD44v6+ subset: Novel methods for isolating undetermined cancer stem (initiating) cells. Cancer Investig. 2008, 26, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Espinosa, I.; Chao, M.; Wong, D.; Ailles, L.; Diehn, M.; Gill, H.; Presti, J., Jr.; Chang, H.Y.; van de Rijn, M.; et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14016–14021. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Sopko, N.A.; Kates, M.; Liu, X.; Joice, G.; McConkey, D.J.; Bivalacqua, T.J. Three-dimensional organoid culture reveals involvement of Wnt/beta-catenin pathway in proliferation of bladder cancer cells. Oncotarget 2018, 9, 11060–11070. [Google Scholar] [CrossRef] [Green Version]
- Porter, R.J.; Murray, G.I.; McLean, M.H. Current concepts in tumour-derived organoids. Br. J. Cancer 2020, 123, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Vachon, P.H. Integrin signaling, cell survival, and anoikis: Distinctions, differences, and differentiation. J. Signal. Transduct. 2011, 2011, 738137. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoudjit, F.; Vuori, K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene 2001, 20, 4995–5004. [Google Scholar] [CrossRef] [Green Version]
- Baumann, P.; Cremers, N.; Kroese, F.; Orend, G.; Chiquet-Ehrismann, R.; Uede, T.; Yagita, H.; Sleeman, J.P. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005, 65, 10783–10793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao-yang, Z.; Ke-sen, X.; Qing-si, H.; Wei-bo, N.; Jia-yong, W.; Yue-tang, M.; Jin-shen, W.; Guo-qiang, W.; Guang-yun, Y.; Jun, N. Signaling and regulatory mechanisms of integrin ανβ6 on the apoptosis of colon cancer cells. Cancer Lett. 2008, 266, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Alanko, J.; Mai, A.; Jacquemet, G.; Schauer, K.; Kaukonen, R.; Saari, M.; Goud, B.; Ivaska, J. Integrin endosomal signalling suppresses anoikis. Nat. Cell Biol. 2015, 17, 1412–1421. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Jin, W.; Xiao, W.; Deng, B.; Wu, D.; Zhi, J.; Wu, K.; Cao, X.; Chen, S.; Ding, Y.; et al. Integrin α5 promotes migration and cisplatin resistance in esophageal squamous cell carcinoma cells. Am. J. Cancer Res. 2019, 9, 2774–2788. [Google Scholar]
- Lu, Q.; Rounds, S. Focal adhesion kinase and endothelial cell apoptosis. Microvasc. Res. 2012, 83, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeSavage, B.L.; Suhar, R.A.; Broguiere, N.; Lutolf, M.P.; Heilshorn, S.C. Next-generation cancer organoids. Nat. Mater. 2022, 21, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Aicher, W.K.; Korn, M.; Reitnauer, L.; Maurer, F.B.; Hennenlotter, J.; Black, P.C.; Todenhofer, T.; Bedke, J.; Stenzl, A. Expression patterns of the immune checkpoint ligand CD276 in urothelial carcinoma. BMC Urol. 2021, 21, 60. [Google Scholar] [CrossRef] [PubMed]





| IC50 (µM) Cells, Time | Cisplatin WST/CTG | Venetoclax WST/CTG | S63845 WST/CTG |
|---|---|---|---|
| NUCs, day1 | 8.41/19.01 | 1.18/6.21 | ~4.15/3.29 |
| NUCs, day2 | ~9.71/3.67 | 0.21/0.74 | 2.37/2.12 |
| NUCs, day3 | ~9.83/3.52 | 6.1 × 10152/0.63 | 2.2 × 10151/2.41 |
| HT1197, day1 | 22.38/91.56 | 2.38/~252,423 | 16.26/~12.05 |
| HT1197, day2 | 10.50/17.76 | 4.6 × 10150/~12.16 | 5.48/~11.51 |
| HT1197, day3 | 7.18/12.05 | 0.38/~10.71 | 8.62/~11.49 |
| RT4, day1 | 18.85/20.45 | 41.88/~11.76 | 10.81/755.70 |
| RT4, day2 | 10.06/11.82 | 85.27/13.70 | 7.24/59.68 |
| RT4, day3 | 7.27/9.96 | 113.60/12.78 | 5 × 10143/195.00 |
| Cells, Culture | Cisplatin | Venetoclax | S63845 |
|---|---|---|---|
| Cells #56, 2D | 49.82 | 0.32 | 29.77 |
| BCO #56, 3D | 15.48 | 10.08 | 9.30 |
| Cells #140, 2D | 0.89 | 8.73 | 8.88 |
| BCO #140, 3D | 2.00 | 9.27 | 12.79 |
| Cells #147, 2D | 10.85 | 3.15 | 1.63 |
| BCO #147, 3D | ~30.35 | ~2.9 × 100.48 | ~ |
| Antibody | Supplier |
|---|---|
| Primary antibodies: | |
| Mouse-anti-CK antibody AE1/AE3 (MAB3412) | Millipore, Taufkirchen, Germany |
| Rabbit-anti-CK5 (905504) | BioLegend, San diego, CA, USA |
| Mouse anti-CK8 antibody (MA5-14088) | Invitrogen, Waltham, MA, USA |
| Mouse anti-CK20 (M7019) | Dako, Jena, Germany |
| Mouse Anti-Vimentin (550513), | Becton Dickinson, Heidelberg, Germany |
| Rabbit anti-FGFR3 | Invitrogen, Waltham, MA, USA |
| Secondary antibodies: | |
| Goat-anti-mouse IgG Cy3 | Jackson ImmunoResearch, Cambridgeshire, UK |
| Goat-anti-rabbit IgG Alexa FI.488 | Jackson ImmunoResearch, |
| DAPI | Jackson ImmunoResearch |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Amend, B.; Todenhöfer, T.; Lipke, N.; Aicher, W.K.; Fend, F.; Stenzl, A.; Harland, N. Urinary Tract Tumor Organoids Reveal Eminent Differences in Drug Sensitivities When Compared to 2-Dimensional Culture Systems. Int. J. Mol. Sci. 2022, 23, 6305. https://doi.org/10.3390/ijms23116305
Wei Y, Amend B, Todenhöfer T, Lipke N, Aicher WK, Fend F, Stenzl A, Harland N. Urinary Tract Tumor Organoids Reveal Eminent Differences in Drug Sensitivities When Compared to 2-Dimensional Culture Systems. International Journal of Molecular Sciences. 2022; 23(11):6305. https://doi.org/10.3390/ijms23116305
Chicago/Turabian StyleWei, Yi, Bastian Amend, Tilman Todenhöfer, Nizar Lipke, Wilhelm K. Aicher, Falko Fend, Arnulf Stenzl, and Niklas Harland. 2022. "Urinary Tract Tumor Organoids Reveal Eminent Differences in Drug Sensitivities When Compared to 2-Dimensional Culture Systems" International Journal of Molecular Sciences 23, no. 11: 6305. https://doi.org/10.3390/ijms23116305
APA StyleWei, Y., Amend, B., Todenhöfer, T., Lipke, N., Aicher, W. K., Fend, F., Stenzl, A., & Harland, N. (2022). Urinary Tract Tumor Organoids Reveal Eminent Differences in Drug Sensitivities When Compared to 2-Dimensional Culture Systems. International Journal of Molecular Sciences, 23(11), 6305. https://doi.org/10.3390/ijms23116305









