How to Restore Oxidative Balance That Was Disrupted by SARS-CoV-2 Infection
Abstract
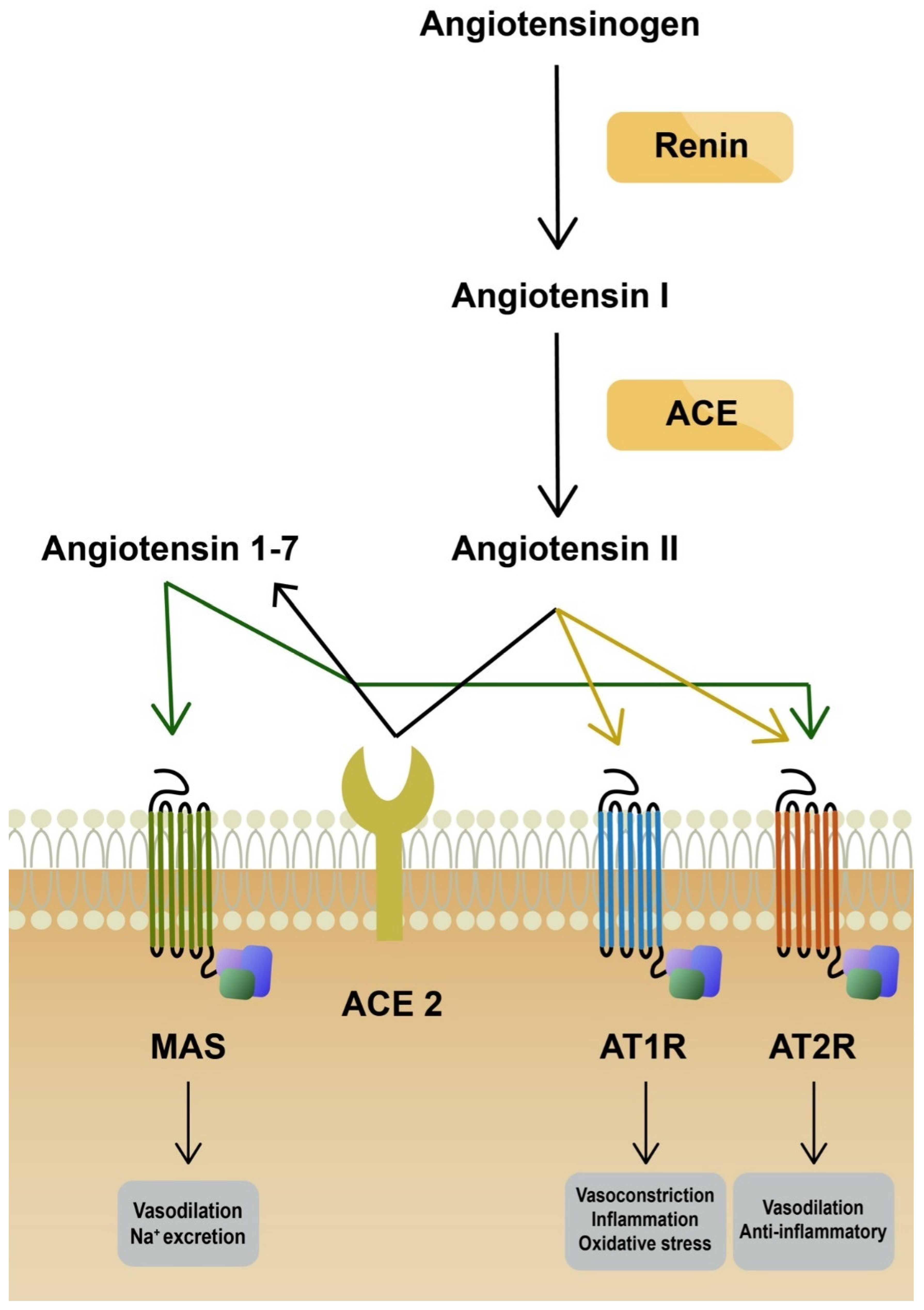
:1. Introduction
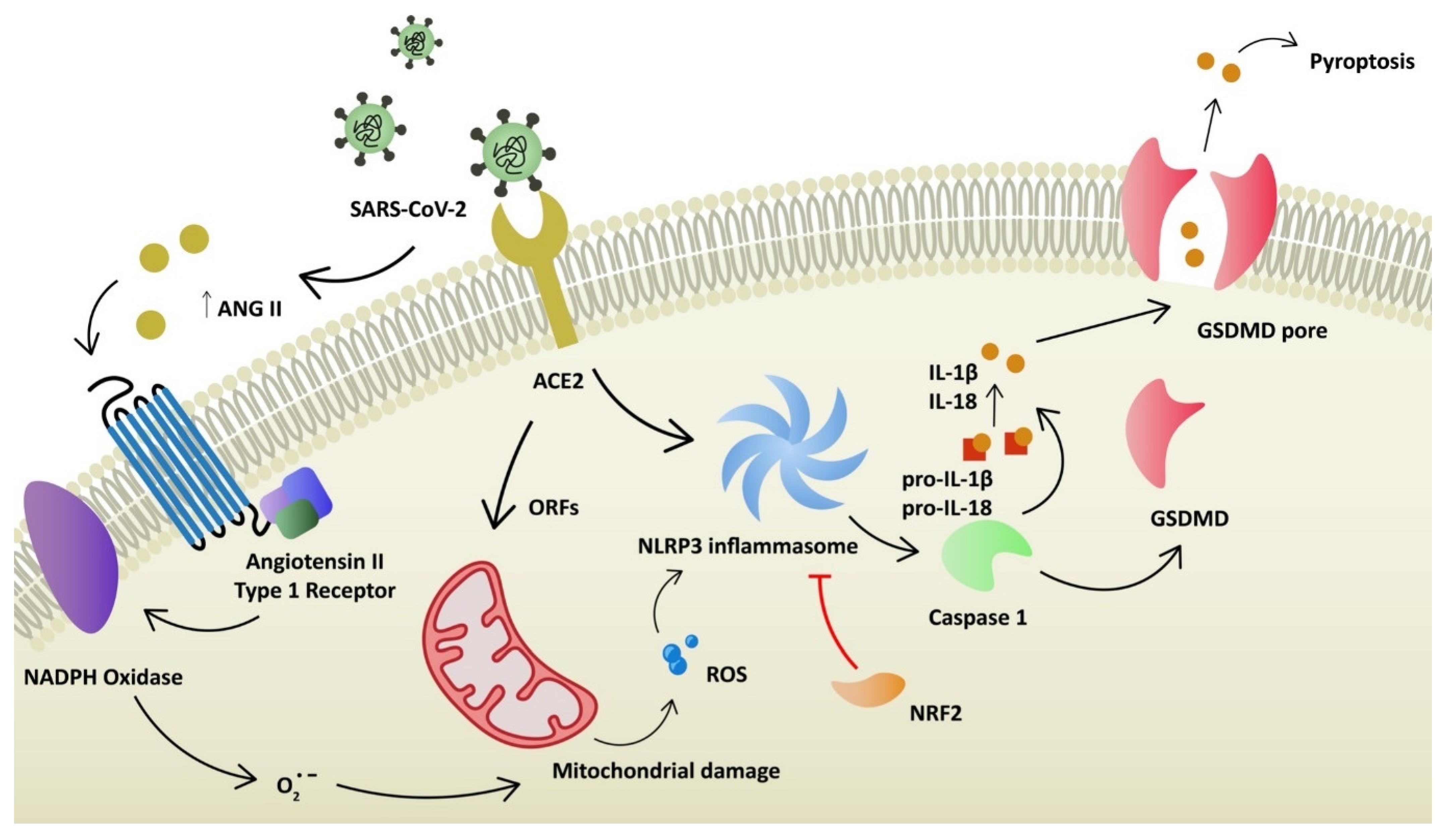
2. COVID-19 Pathophysiology and Oxidative Stress
3. Glutathione
4. AT1R Antagonists
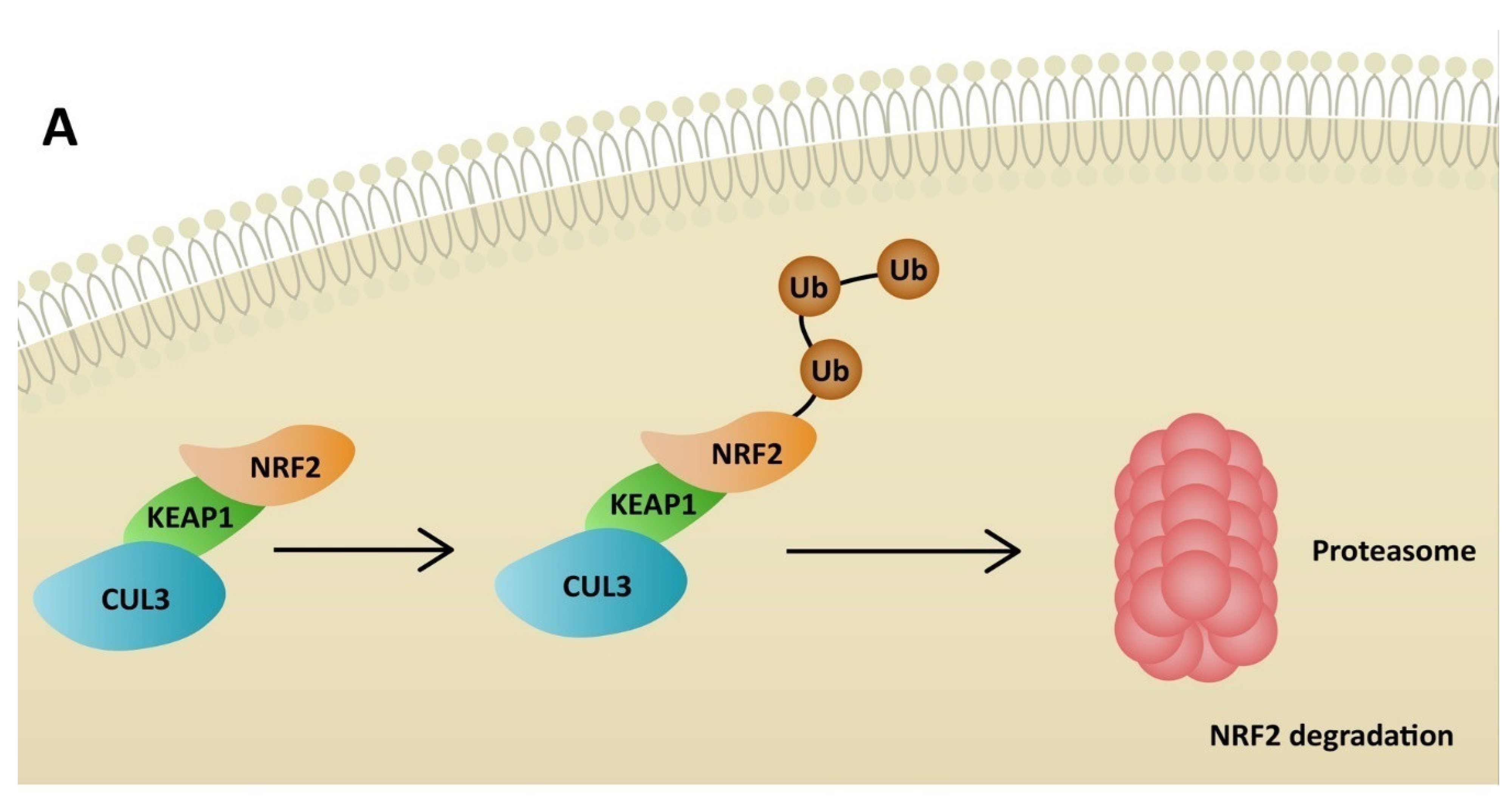
5. NRF2 Activators
6. NLRP3 Antagonists
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020, 19, 155–170. [Google Scholar] [CrossRef]
- Mohamadian, M.; Chiti, H.; Shoghli, A.; Biglari, S.; Parsamanesh, N.; Esmaeilzadeh, A. COVID-19: Virology, Biology and Novel Laboratory Diagnosis. J. Gene Med. 2021, 23, e3303. [Google Scholar] [CrossRef] [PubMed]
- Gustine, J.N.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2020, 191, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Marzi, A.; Munster, V. Functional Assessment of Cell Entry and Receptor Usage for SARS-CoV-2 and Other Lineage B Betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The Protein Expression Profile of ACE2 in Human Tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035. [Google Scholar] [CrossRef]
- Nitin, P.; Nandhakumar, R.; Vidhya, B.; Rajesh, S.; Sakunthala, A. COVID-19: Invasion, Pathogenesis and Possible Cure—A Review. J. Virol. Methods 2022, 300, 114434. [Google Scholar]
- Wang, S.; Qiu, Z.; Hou, Y.; Deng, X.; Xu, W.; Zheng, T.; Wu, P.; Xie, S.; Bian, W.; Zhang, C.; et al. AXL Is a Candidate Receptor for SARS-CoV-2 That Promotes Infection of Pulmonary and Bronchial Epithelial Cells. Cell Res. 2021, 31, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Sharma, K.; Singh, H.; Silakari, O. The Interplay between Inflammatory Pathways and COVID-19: A Critical Review on Pathogenesis and Therapeutic Options. Microb. Pathog. 2020, 150, 104673. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and Biochemical Indexes from 2019-NCoV Infected Patients Linked to Viral Loads and Lung Injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Fujioka, S.; Takahashi, R.; Oe, T. Angiotensin II-Induced Oxidative Stress in Human Endothelial Cells: Modification of Cellular Molecules through Lipid Peroxidation. Chem. Res. Toxicol. 2019, 32, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Mamun, A.; Dominic, A.; Le, N.-T. SARS-CoV-2 Mediated Endothelial Dysfunction: The Potential Role of Chronic Oxidative Stress. Front. Physiol. 2021, 11, 605908. [Google Scholar] [CrossRef]
- Papola, F.; Biancofiore, V.; Angeletti, C.; Grimaldi, A.; Carucci, A.C.; Cofini, V.; Necozione, S.; Rosciano, A.; Marinangeli, F.; Cervelli, C. Anti-AT1R Autoantibodies and Prediction of the Severity of Covid-19. Hum. Immunol. 2022, 83, 130–133. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef] [PubMed]
- Ajaz, S.; McPhail, M.J.; Singh, K.K.; Mujib, S.; Trovato, F.M.; Napoli, S.; Agarwal, K. Mitochondrial Metabolic Manipulation by SARS-CoV-2 in Peripheral Blood Mononuclear Cells of Patients with COVID-19. Am. J. Physiol. Cell Physiol. 2021, 320, C57–C65. [Google Scholar] [CrossRef]
- Singh, K.K.; Chaubey, G.; Chen, J.Y.; Suravajhala, P. Decoding SARS-CoV-2 Hijacking of Host Mitochondria in Pathogenesis of COVID-19. Am. J. Physiol. Cell Physiol. 2020, 319, 258–267. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D Is an Executor of Pyroptosis and Required for Interleukin-1β Secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Pan, P.; Shen, M.; Yu, Z.; Ge, W.; Chen, K.; Tian, M.; Xiao, F.; Wang, Z.; Wang, J.; Jia, Y.; et al. SARS-CoV-2 N Protein Promotes NLRP3 Inflammasome Activation to Induce Hyperinflammation. Nat. Commun. 2021, 12, 4664. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hauenstein, A.V. The NLRP3 Inflammasome: Mechanism of Action, Role in Disease and Therapies. Mol. Aspects Med. 2020, 76, 100889. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.-J.; Lee, Y.-B.; Kim, S.-H.; Jin, H.K.; Bae, J.; Hong, C.-W. Nucleocapsid and Spike Proteins of SARS-CoV-2 Drive Neutrophil Extracellular Trap Formation. Immune Netw. 2021, 21, e16. [Google Scholar] [CrossRef]
- Arcanjo, A.; Logullo, J.; Menezes, C.C.B.; de Souza Carvalho Giangiarulo, T.C.; dos Reis, M.C.; de Castro, G.M.M.; da Silva Fontes, Y.; Todeschini, A.R.; Freire-de-Lima, L.; Decoté-Ricardo, D.; et al. The Emerging Role of Neutrophil Extracellular Traps in Severe Acute Respiratory Syndrome Coronavirus 2 (COVID-19). Sci. Rep. 2020, 10, 19630. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2017, 18, 134–147. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Mehri, F.; Rahbar, A.H.; Ghane, E.T.; Souri, B.; Esfahani, M. Changes in Oxidative Markers in COVID-19 Patients. Arch. Med. Res. 2021, 52, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Mdkhana, B.; Hussain Alsayed, H.A.; Alsafar, H.; Alrais, Z.F.; Hamid, Q.; Halwani, R. Upregulation of Oxidative Stress Gene Markers during SARS-COV-2 Viral Infection. Free Radic. Biol. Med. 2021, 172, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Y.; Kani, Y.A.; Iliya, S.; Muhammad, J.B.; Binji, A.; El-Fulaty Ahmad, A.; Kabir, M.B.; Umar Bindawa, K.; Ahmed, A. Deficiency of Antioxidants and Increased Oxidative Stress in COVID-19 Patients: A Cross-Sectional Comparative Study in Jigawa, Northwestern Nigeria. SAGE Open Med. 2021, 9, 205031212199124. [Google Scholar] [CrossRef]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy-Bien, J.-P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Goff, C.L.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Vovk, M.A.; Batalova, A.A.; Jenkins, R.O.; Goncharov, N.V. The Universal Soldier: Enzymatic and Non-Enzymatic Antioxidant Functions of Serum Albumin. Antioxidants 2020, 9, 966. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannellotto, M.; Duarte, M.; Keller, G.; Larrea, R.; Cunto, E.; Chediack, V.; Mansur, M.; Brito, D.M.; García, E.; Di Salvo, H.E.; et al. Hyperbaric Oxygen as an Adjuvant Treatment for Patients with COVID-19 Severe Hypoxaemia: A Randomised Controlled Trial. Emerg. Med. J. 2021, 39, 88–93. [Google Scholar] [CrossRef] [PubMed]
- De Wolde, S.D.; Hulskes, R.H.; Weenink, R.P.; Hollmann, M.W.; Van Hulst, R.A. The Effects of Hyperbaric Oxygenation on Oxidative Stress, Inflammation and Angiogenesis. Biomolecules 2021, 11, 1210. [Google Scholar] [CrossRef]
- Luo, P.; Ding, Y.; He, Y.; Chen, D.; He, Q.; Huang, Z.; Huang, S.; Lei, W. Hydrogen-Oxygen Therapy Alleviates Clinical Symptoms in Twelve Patients Hospitalized with COVID-19: A Retrospective Study of Medical Records. Medicine 2022, 101, 27759. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The Central Role of Glutathione in the Pathophysiology of Human Diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Naghashpour, M.; Ghiassian, H.; Mobarak, S.; Adelipour, M.; Piri, M.; Seyedtabib, M.; Golabi, S. Profiling Serum Levels of Glutathione Reductase and Interleukin-10 in Positive and Negative-PCR COVID-19 Outpatients: A Comparative Study from Southwestern Iran. J. Med. Virol. 2022, 94, 1457–1464. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, H.; Yang, Y.; Yang, Y.; Jiao, Y.; Tay, F.R.; Chen, J. Biological Activities and Potential Oral Applications of N-Acetylcysteine: Progress and Prospects. Oxidative Med. Cell. Longev. 2018, 2018, 2835787. [Google Scholar] [CrossRef]
- Rushworth, G.F.; Megson, I.L. Existing and Potential Therapeutic Uses for N-Acetylcysteine: The Need for Conversion to Intracellular Glutathione for Antioxidant Benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.L.; Soriano, J.B.; González, Y.; Lumbreras, S.; Ancochea, J.; Echeverry, C.; Rodríguez, J.M. Use of N-Acetylcysteine at High Doses as an Oral Treatment for Patients Hospitalized with COVID-19. Sci. Prog. 2022, 105, 00368504221074574. [Google Scholar] [CrossRef] [PubMed]
- De Alencar, J.C.G.; Moreira, C.D.L.; Müller, A.D.; Chaves, C.E.; Fukuhara, M.A.; da Silva, E.A.; Miyamoto, M.D.F.S.; Pinto, V.B.; Bueno, C.G.; Lazar Neto, F.; et al. Double-Blind, Randomized, Placebo-Controlled Trial with N-Acetylcysteine for Treatment of Severe Acute Respiratory Syndrome Caused by Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 72, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.; Lashgari, M.; Sedighi, L.; Rahimi-bashar, F.; Poorolajal, J.; Mehrpooya, M. A Pilot Study on Intravenous N-Acetylcysteine Treatment in Patients with Mild-To-Moderate COVID19-Associated Acute Respiratory Distress Syndrome. Pharmacol. Rep. 2021, 73, 1650–1659. [Google Scholar] [CrossRef]
- Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef] [Green Version]
- Zhong, M.; Sun, A.; Xiao, T.; Yao, G.; Sang, L.; Zheng, X.; Zhang, J.; Jin, X.; Xu, L.; Yang, W.; et al. A Randomized, Single-Blind, Group Sequential, Active-Controlled Study to Evaluate the Clinical Efficacy and Safety of α-Lipoic Acid for Critically Ill Patients with Coronavirus Disease 2019 (COVID-19). Front. Med. 2022, 8, 566609. [Google Scholar] [CrossRef]
- Uberti, F.; Ruga, S.; Farghali, M.; Galla, R.; Molinari, C. A Combination of α-Lipoic Acid (ALA) and Palmitoylethanolamide (PEA) Blocks Endotoxin-Induced Oxidative Stress and Cytokine Storm: A Possible Intervention for COVID-19. J. Diet. Suppl. 2021, 1, 1–23. [Google Scholar] [CrossRef]
- Horowitz, R.I.; Freeman, P.R.; Bruzzese, J. Efficacy of Glutathione Therapy in Relieving Dyspnea Associated with COVID-19 Pneumonia: A Report of 2 Cases. Respir. Med. Case Rep. 2020, 30, 101063. [Google Scholar] [CrossRef]
- Mil, K.M.; Gryciuk, M.E.; Pawlukianiec, C.; Żendzian-Piotrowska, M.; Ładny, J.R.; Zalewska, A.; Maciejczyk, M. Pleiotropic Properties of Valsartan: Do They Result from the Antiglycooxidant Activity? Literature Review and in Vitro Study. Oxidative Med. Cell. Longev. 2021, 2021, 5575545. [Google Scholar] [CrossRef] [PubMed]
- Wienen, W.; Entzeroth, M.; Meel, J.C.A.; Stangier, J.; Busch, U.; Ebner, T.; Schmid, J.; Lehmann, H.; Matzek, K.; Kempthorne-Rawson, J.; et al. A Review on Telmisartan: A Novel, Long-Acting Angiotensin II-Receptor Antagonist. Cardiovasc. Drug Rev. 2006, 18, 127–154. [Google Scholar] [CrossRef]
- Antar, S.A.; Abdo, W.; Taha, R.S.; Farage, A.E.; El-Moselhy, L.E.; Amer, M.E.; Abdel Monsef, A.S.; Abdel Hamid, A.M.; Kamel, E.M.; Ahmeda, A.F.; et al. Telmisartan Attenuates Diabetic Nephropathy by Mitigating Oxidative Stress and Inflammation, and Upregulating Nrf2/HO-1 Signaling in Diabetic Rats. Life Sci. 2022, 291, 120260. [Google Scholar] [CrossRef]
- Eslami, H.; Sharifi, A.M.; Rahimi, H.; Rahati, M. Protective Effect of Telmisartan against Oxidative Damage Induced by High Glucose in Neuronal PC12 Cell. Neurosci. Lett. 2014, 558, 31–36. [Google Scholar] [CrossRef]
- Reus, P.; Schneider, A.-K.; Ulshöfer, T.; Henke, M.; Bojkova, D.; Cinatl, J.; Ciesek, S.; Geisslinger, G.; Laux, V.; Grättinger, M.; et al. Characterization of ACE Inhibitors and AT1R Antagonists with Regard to Their Effect on ACE2 Expression and Infection with SARS-CoV-2 Using a Caco-2 Cell Model. Life 2021, 11, 810. [Google Scholar] [CrossRef]
- Duarte, M.; Pelorosso, F.; Nicolosi, L.N.; Victoria Salgado, M.; Vetulli, H.; Aquieri, A.; Azzato, F.; Castro, M.; Coyle, J.; Davolos, I.; et al. Telmisartan for Treatment of Covid-19 Patients: An Open Multicenter Randomized Clinical Trial. EClinicalMedicine 2021, 37, 100962. [Google Scholar] [CrossRef]
- Unger, T. Inhibiting Angiotensin Receptors in the Brain: Possible Therapeutic Implications. Curr. Med. Res. Opin. 2003, 19, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Hongwei, W.; Ueno, H.; Mizuta, M.; Nakazato, M. Candesartan Attenuates Fatty Acid-Induced Oxidative Stress and NAD(P)H Oxidase Activity in Pancreatic Beta-Cells. Diabetes Res. Clin. Pract. 2010, 90, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Basmadjian, O.M.; Occhieppo, V.B.; Marchese, N.A.; Silvero, C.M.J.; Becerra, M.C.; Baiardi, G.; Bregonzio, C. Amphetamine Induces Oxidative Stress, Glial Activation and Transient Angiogenesis in Prefrontal Cortex via AT1-R. Front. Pharmacol. 2021, 12, 647747. [Google Scholar] [CrossRef] [PubMed]
- Dohi, Y.; Ohashi, M.; Sugiyama, M.; Takase, H.; Sato, K.; Ueda, R. Candesartan Reduces Oxidative Stress and Inflammation in Patients with Essential Hypertension. Hypertens. Res. 2003, 26, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Elkahloun, A.G.; Saavedra, J.M. Candesartan Could Ameliorate the COVID-19 Cytokine Storm. Biomed. Pharmacother. 2020, 131, 110653. [Google Scholar] [CrossRef] [PubMed]
- Lukito, A.A.; Widysanto, A.; Lemuel, T.A.Y.; Prasetya, I.B.; Massie, B.; Yuniarti, M.; Lumbuun, N.; Pranata, R.; Meidy, C.; Wahjoepramono, E.J.; et al. Candesartan as a Tentative Treatment for COVID-19: A Prospective Non-Randomized Open-Label Study. Int. J. Infect. Dis. 2021, 108, 159–166. [Google Scholar] [CrossRef]
- Chu, K.Y.; Leung, P.S. Angiotensin II Type 1 Receptor Antagonism Mediates Uncoupling Protein 2-Driven Oxidative Stress and Ameliorates Pancreatic Islet β-Cell Function in Young Type 2 Diabetic Mice. Antioxid. Redox Signal. 2007, 9, 869–878. [Google Scholar] [CrossRef]
- Salmani, H.; Hosseini, M.; Baghcheghi, Y.; Moradi-Marjaneh, R.; Mokhtari-Zaer, A. Losartan Modulates Brain Inflammation and Improves Mood Disorders and Memory Impairment Induced by Innate Immune Activation: The Role of PPAR-γ Activation. Cytokine 2020, 125, 154860. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Yang, H.; Xue, Q.-L.; Chuang, Y.-F.; Roy, C.N.; Abadir, P.; Walston, J.D. Losartan Improves Measures of Activity, Inflammation, and Oxidative Stress in Older Mice. Exp. Gerontol. 2014, 58, 174–178. [Google Scholar] [CrossRef] [Green Version]
- Puskarich, M.A.; Ingraham, N.E.; Merck, L.H.; Driver, B.E.; Wacker, D.A.; Black, L.P.; Jones, A.E.; Fletcher, C.V.; South, A.M.; Murray, T.A.; et al. Efficacy of Losartan in Hospitalized Patients with COVID-19–Induced Lung Injury. JAMA Netw. Open 2022, 5, 222735. [Google Scholar] [CrossRef]
- Quijano, A.; Diaz-Ruiz, C.; Lopez-Lopez, A.; Villar-Cheda, B.; Muñoz, A.; Rodriguez-Perez, A.I.; Labandeira-Garcia, J.L. Angiotensin Type-1 Receptor Inhibition Reduces NLRP3 Inflammasome Upregulation Induced by Aging and Neurodegeneration in the Substantia Nigra of Male Rodents and Primary Mesencephalic Cultures. Antioxidants 2022, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennig, P.; Garstkiewicz, M.; Grossi, S.; Di Filippo, M.; French, L.E.; Beer, H.-D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018, 19, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef] [Green Version]
- Gümüş, H.; Erat, T.; Öztürk, İ.; Demir, A.; Koyuncu, I. Oxidative Stress and Decreased Nrf2 Level in Pediatric Patients with COVID-19. J. Med. Virol. 2022, 94, 2259–2264. [Google Scholar] [CrossRef]
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV2-Mediated Suppression of NRF2-Signaling Reveals Potent Antiviral and Anti-Inflammatory Activity of 4-Octyl-Itaconate and Dimethyl Fumarate. Nat. Commun. 2020, 11, 4938. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The Therapeutic Effect of Resveratrol: Focusing on the Nrf2 Signaling Pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef] [PubMed]
- De Ligt, M.; Hesselink, M.K.C.; Jorgensen, J.; Hoebers, N.; Blaak, E.E.; Goossens, G.H. Resveratrol Supplementation Reduces ACE2 Expression in Human Adipose Tissue. Adipocyte 2021, 10, 408–411. [Google Scholar] [CrossRef]
- Pasquereau, S.; Nehme, Z.; Haidar Ahmad, S.; Daouad, F.; Van Assche, J.; Wallet, C.; Schwartz, C.; Rohr, O.; Morot-Bizot, S.; Herbein, G. Resveratrol Inhibits HCoV-229E and SARS-CoV-2 Coronavirus Replication in Vitro. Viruses 2021, 13, 354. [Google Scholar] [CrossRef]
- Mazarakis, N.; Higgins, R.A.; Anderson, J.; Toh, Z.Q.; Luwor, R.B.; Snibson, K.J.; Karagiannis, T.C.; Do, L.A.H.; Licciardi, P.V. The Effects of the Dietary Compound L-Sulforaphane against Respiratory Pathogens. Int. J. Antimicrob. Agents 2021, 58, 106460. [Google Scholar] [CrossRef] [PubMed]
- Gasparello, J.; D’Aversa, E.; Papi, C.; Gambari, L.; Grigolo, B.; Borgatti, M.; Finotti, A.; Gambari, R. Sulforaphane Inhibits the Expression of Interleukin-6 and Interleukin-8 Induced in Bronchial Epithelial IB3-1 Cells by Exposure to the SARS-CoV-2 Spike Protein. Phytomedicine 2021, 87, 153583. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Bullen, C.K.; Villabona-Rueda, A.F.; Thompson, E.A.; Turner, M.L.; Merino, V.F.; Yan, Y.; Kim, J.; Davis, S.L.; Komm, O.; et al. Sulforaphane Exhibits Antiviral Activity against Pandemic SARS-CoV-2 and Seasonal HCoV-OC43 Coronaviruses in Vitro and in Mice. Commun. Biol. 2022, 5, 242. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, R.; Qiao, W.; Zhang, W.; Chen, J.; Mao, L.; Goltzman, D.; Miao, D. 1,25-Dihydroxyvitamin D Exerts an Antiaging Role by Activation of Nrf2-Antioxidant Signaling and Inactivation of P16/P53-Senescence Signaling. Aging Cell 2019, 18, 12951. [Google Scholar] [CrossRef]
- Cui, C.; Wang, C.; Jin, F.; Yang, M.; Kong, L.; Han, W.; Jiang, P. Calcitriol Confers Neuroprotective Effects in Traumatic Brain Injury by Activating Nrf2 Signaling through an Autophagy-Mediated Mechanism. Mol. Med. 2021, 27, 118. [Google Scholar] [CrossRef]
- Elamir, Y.M.; Amir, H.; Lim, S.; Rana, Y.P.; Lopez, C.G.; Feliciano, N.V.; Omar, A.; Grist, W.P.; Via, M.A. A Randomized Pilot Study Using Calcitriol in Hospitalized COVID-19 Patients. Bone 2022, 154, 116175. [Google Scholar] [CrossRef]
- Oristrell, J.; Oliva, J.C.; Subirana, I.; Casado, E.; Domínguez, D.; Toloba, A.; Aguilera, P.; Esplugues, J.; Fafián, P.; Grau, M. Association of Calcitriol Supplementation with Reduced COVID-19 Mortality in Patients with Chronic Kidney Disease: A Population-Based Study. Biomedicines 2021, 9, 509. [Google Scholar] [CrossRef]
- Gora, I.M.; Ciechanowska, A.; Ladyzynski, P. NLRP3 Inflammasome at the Interface of Inflammation, Endothelial Dysfunction, and Type 2 Diabetes. Cells 2021, 10, 314. [Google Scholar] [CrossRef]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B. Metformin and Resveratrol Inhibit Drp1-Mediated Mitochondrial Fission and Prevent ER Stress-Associated NLRP3 Inflammasome Activation in the Adipose Tissue of Diabetic Mice. Mol. Cell. Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Duan, F.; Li, W.; Wang, Y.; Zeng, C.; Hu, J.; Li, H.; Zhang, X.; Chen, Y.; Tan, H. Metformin Inhibited Nod-like Receptor Protein 3 Inflammasomes Activation and Suppressed Diabetes-Accelerated Atherosclerosis in ApoE−/− Mice. Biomed. Pharmacother. 2019, 119, 109410. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.; Liu, Y.; Rundberg Nilsson, A.; Gatchalian, R.; Crother, T.R.; Tourtellotte, W.G.; Zhang, Y.; Aleman-Muench, G.R.; Lewis, G.; Chen, W.; et al. Metformin Inhibition of Mitochondrial ATP and DNA Synthesis Abrogates NLRP3 Inflammasome Activation and Pulmonary Inflammation. Immunity 2021, 54, 1463–1477. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, A.; Randall, M.D. Does Metformin Affect Outcomes in COVID-19 Patients with New or Pre-Existing Diabetes Mellitus? A Systematic Review and Meta-Analysis. Br. J. Clin. Pharmacol. 2022, 88, 2642–2656. [Google Scholar] [CrossRef]
- Yu, Y.-W.; Que, J.-Q.; Liu, S.; Huang, K.-Y.; Qian, L.; Weng, Y.-B.; Rong, F.-N.; Wang, L.; Zhou, Y.-Y.; Xue, Y.-J.; et al. Sodium-Glucose Co-Transporter-2 Inhibitor of Dapagliflozin Attenuates Myocardial Ischemia/Reperfusion Injury by Limiting NLRP3 Inflammasome Activation and Modulating Autophagy. Front. Cardiovasc. Med. 2022, 8, 768214. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Esterline, R.; Furtado, R.H.M.; Oscarsson, J.; Gasparyan, S.B.; Koch, G.G.; Martinez, F.; Mukhtar, O.; Verma, S.; Chopra, V.; et al. Dapagliflozin in Patients with Cardiometabolic Risk Factors Hospitalised with COVID-19 (DARE-19): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Diabetes Endocrinol. 2021, 9, 586–594. [Google Scholar] [CrossRef]
- Darakhshan, S.; Pour, A.B. Tranilast: A Review of Its Therapeutic Applications. Pharmacol. Res. 2015, 91, 15–28. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, H.; Chen, Y.; Wang, X.; Yang, Y.; Tao, J.; Deng, X.; Liang, G.; Zhang, H.; Jiang, W.; et al. Tranilast Directly TargetsNLRP3 to Treat Inflammasome-Driven Diseases. EMBO Mol. Med. 2018, 10, e8689. [Google Scholar] [CrossRef] [PubMed]
- Saeedi-Boroujeni, A.; Nashibi, R.; Ghadiri, A.A.; Nakajima, M.; Salmanzadeh, S.; Mahmoudian-Sani, M.-R.; Hanafi, M.G.; Sharhani, A.; Khodadadi, A. Tranilast as an Adjunctive Therapy in Hospitalized Patients with Severe COVID-19: A Randomized Controlled Trial. Arch. Med. Res. 2022, 53, 368–377. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Lee, B.-J.; Tran, T.T.D. Current Developments in the Oral Drug Delivery of Fucoidan. Int. J. Pharm. 2021, 598, 120371. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Pan, X.; Wang, J.; Li, X.; Yang, S.; Yin, R.; Ma, A.; Zhu, X. Fucoidan Inhibits NLRP3 Inflammasome Activation by Enhancing P62/SQSTM1-Dependent Selective Autophagy to Alleviate Atherosclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 3186306. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Resendiz, K.J.G.; Toledo-Ibarra, G.A.; Ruiz-Manzano, R.; Giron Perez, D.A.; Covantes-Rosales, C.E.; Benitez-Trinidad, A.B.; Ramirez-Ibarra, K.M.; Hermosillo Escobedo, A.T.; González-Navarro, I.; Ventura-Ramón, G.H.; et al. Ex Vivo Treatment with Fucoidan of Mononuclear Cells from SARS-CoV-2 Infected Patients. Int. J. Environ. Health Res. 2021, 1–19. [Google Scholar] [CrossRef]
- Yim, S.-K.; Kim, K.; Kim, I.-H.; Chun, S.-H.; Oh, T.-H.; Kim, J.-U.; Kim, J.-W.; Jung, W.-H.; Moon, H.-S.; Ku, B.-S.; et al. Inhibition of SARS-CoV-2 Virus Entry by the Crude Polysaccharides of Seaweeds and Abalone Viscera in Vitro. Mar. Drugs 2021, 19, 219. [Google Scholar] [CrossRef]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Casirivimab and Imdevimab in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2022, 399, 665–676. [Google Scholar] [CrossRef]
- Mohiuddin Chowdhury, A.T.M.; Kamal, A.; Abbas, K.U.; Talukder, S.; Karim, M.R.; Ali, M.A.; Nuruzzaman, M.; Li, Y.; He, S. Efficacy and Outcome of Remdesivir and Tocilizumab Combination against Dexamethasone for the Treatment of Severe COVID-19: A Randomized Controlled Trial. Front. Pharmacol. 2022, 13, 690726. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiełbowski, K.; Herian, M.; Pawlik, A. How to Restore Oxidative Balance That Was Disrupted by SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 6377. https://doi.org/10.3390/ijms23126377
Kiełbowski K, Herian M, Pawlik A. How to Restore Oxidative Balance That Was Disrupted by SARS-CoV-2 Infection. International Journal of Molecular Sciences. 2022; 23(12):6377. https://doi.org/10.3390/ijms23126377
Chicago/Turabian StyleKiełbowski, Kajetan, Mariola Herian, and Andrzej Pawlik. 2022. "How to Restore Oxidative Balance That Was Disrupted by SARS-CoV-2 Infection" International Journal of Molecular Sciences 23, no. 12: 6377. https://doi.org/10.3390/ijms23126377
APA StyleKiełbowski, K., Herian, M., & Pawlik, A. (2022). How to Restore Oxidative Balance That Was Disrupted by SARS-CoV-2 Infection. International Journal of Molecular Sciences, 23(12), 6377. https://doi.org/10.3390/ijms23126377





