Human Amniotic Fluid Stem Cells Ameliorate Thioglycollate-Induced Peritonitis by Increasing Tregs in Mice
Abstract
1. Introduction
2. Results
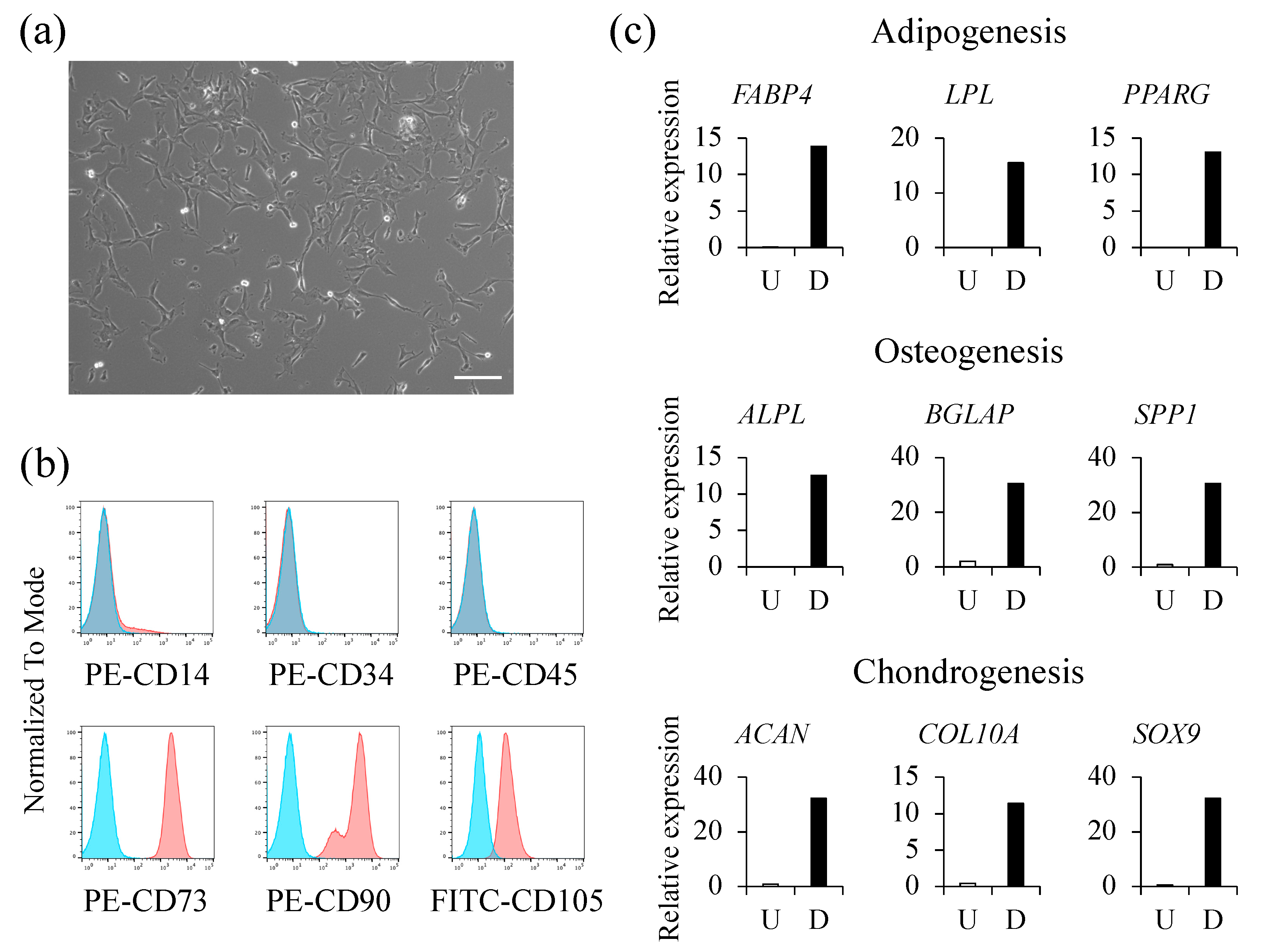
2.1. Isolation and Characterization of hAFSCs
2.2. Treatment with hAFSCs Modulates Peritoneal Inflammation
2.3. Engrafted hAFSCs form Aggregates in the Peritoneal Cavity
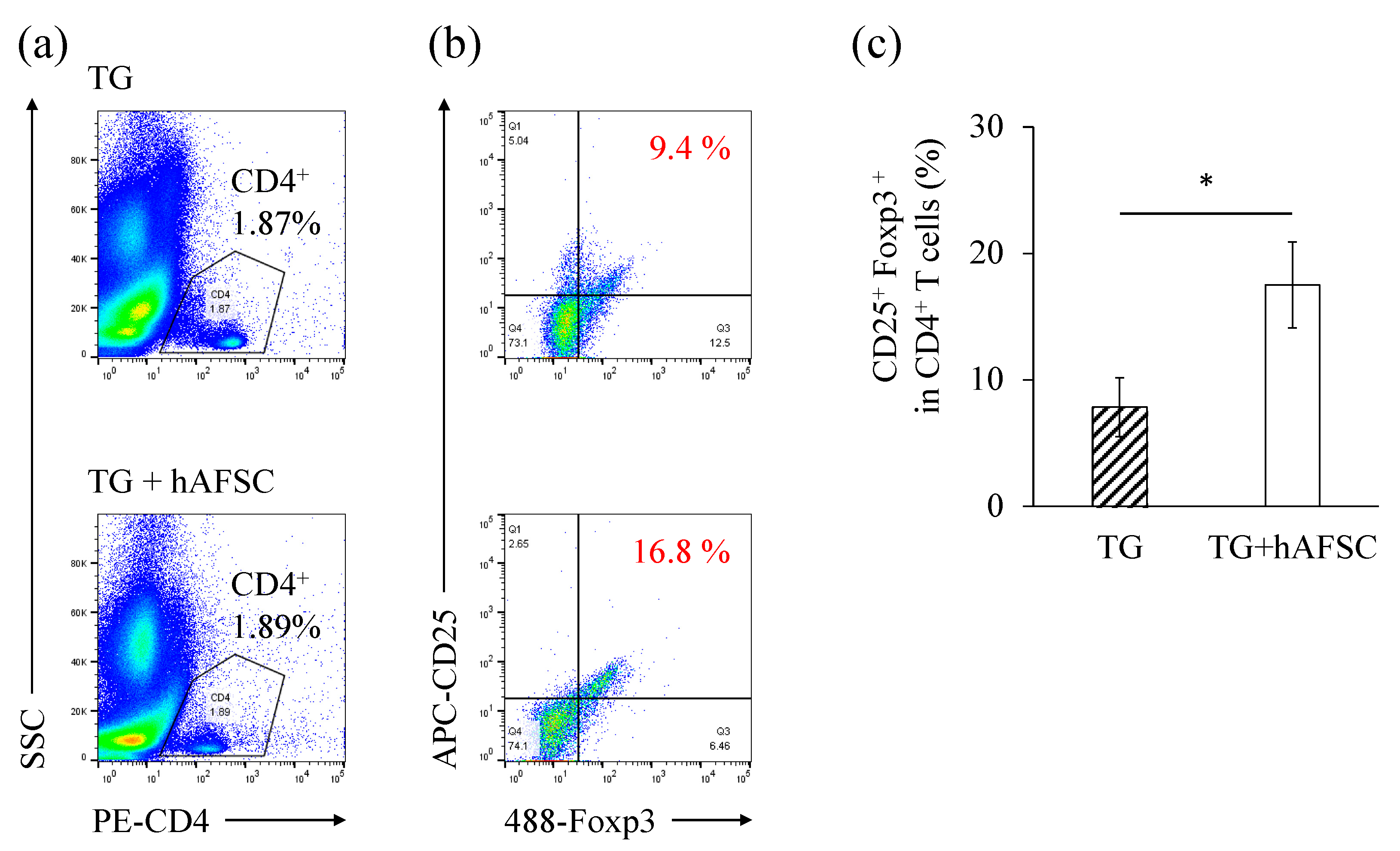
2.4. Administration of hAFSCs Increases the Number of Regulatory T Cells in the Abdominal Cavity
3. Discussion
4. Materials and Methods
4.1. Isolation, Culture, and Characterization of hAFSCs
4.2. Animals and TG Medium Injection into the Peritoneal
4.3. Collection of Lavage Cell
4.4. Tracking hAFSCs after Intraperitoneal Administration and Immunofluorescence Staining of Cell Aggregates
4.5. Flow Cytometry
4.6. Real-Time qPCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haynesworth, S.E.; Goshima, J.; Goldberg, V.M.; Caplan, A.I. Characterization of cells with osteogenic potential from human marrow. Bone 1992, 13, 81–88. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Romanov, Y.A.; Svintsitskaya, V.A.; Smirnov, V.N. Searching for alternative sources of postnatal human mesenchymal stem cells: Candidate MSC-like cells from umbilical cord. Stem Cells 2003, 21, 105–110. [Google Scholar] [CrossRef] [PubMed]
- In’t Anker, P.S.; Scherjon, S.A.; van der Keur, C.K.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- De Coppi, P.; Bartsch, G., Jr.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Morata-Tarifa, C.; Macías-Sánchez, M.D.M.; Gutiérrez-Pizarraya, A.; Sanchez-Pernaute, R. Mesenchymal stromal cells for the prophylaxis and treatment of graft-versus-host disease—A meta-analysis. Stem Cell Res. Ther. 2020, 11, 64. [Google Scholar] [CrossRef]
- Najar, M.; Raicevic, G.; Fayyad-Kazan, H.; Bron, D.; Toungouz, M.; Lagneaux, L. Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy 2016, 18, 160–171. [Google Scholar] [CrossRef]
- Wang, L.T.; Ting, C.H.; Yen, M.L.; Liu, K.J.; Sytwu, H.K.; Wu, K.K.; Yen, B.L. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: Review of current clinical trials. J. Biomed. Sci. 2016, 23, 76. [Google Scholar] [CrossRef]
- Zheng, X.; Hermann, D.M.; Bähr, M.; Doeppner, T.R. The role of small extracellular vesicles in cerebral and myocardial ischemia—Molecular signals, treatment targets, and future clinical translation. Stem Cells 2021, 39, 403–413. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, S. Differentiation of cardiomyocytes from amniotic fluid-derived mesenchymal stem cells by combined induction with transforming growth factor β1 and 5-azacytidine. Mol. Med. Rep. 2017, 16, 5887–5893. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.; Imberti, B.; Pozzobon, M.; Piccoli, M.; De Coppi, P.; Atala, A.; Gagliardini, E.; Xinaris, C.; Benedetti, V.; Fabricio, A.S.; et al. Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev. 2012, 21, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Ochiai, D.; Masuda, H.; Sato, Y.; Otani, T.; Fukutake, M.; Ikenoue, S.; Miyakoshi, K.; Okano, H.; Tanaka, M. In utero amniotic fluid stem cell therapy protects against myelomeningocele via spinal cord coverage and hepatocyte growth factor secretion. Stem Cells Transl. Med. 2019, 8, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Ochiai, D.; Sato, Y.; Kanzaki, S.; Ikenoue, S.; Kasuga, Y.; Tanaka, M. Prophylactic therapy with human amniotic fluid stem cells improves long-term cognitive impairment in rat neonatal sepsis survivors. Int. J. Mol. Sci. 2020, 21, 9590. [Google Scholar] [CrossRef] [PubMed]
- Fukutake, M.; Ochiai, D.; Masuda, H.; Abe, Y.; Sato, Y.; Otani, T.; Sakai, S.; Aramaki-Hattori, N.; Shimoda, M.; Matsumoto, T.; et al. Human amniotic fluid stem cells have a unique potential to accelerate cutaneous wound healing with reduced fibrotic scarring like a fetus. Hum. Cell 2019, 32, 51–63. [Google Scholar] [CrossRef]
- Ochiai, D.; Abe, Y.; Fukutake, M.; Sato, Y.; Ikenoue, S.; Kasuga, Y.; Masuda, H.; Tanaka, M. Cell sheets using human amniotic fluid stem cells reduce tissue fibrosis in murine full-thickness skin wounds. Tissue Cell 2021, 68, 101472. [Google Scholar] [CrossRef]
- Otani, T.; Ochiai, D.; Masuda, H.; Abe, Y.; Fukutake, M.; Matsumoto, T.; Miyakoshi, K.; Tanaka, M. The neurorestorative effect of human amniotic fluid stem cells on the chronic phase of neonatal hypoxic-ischemic encephalopathy in mice. Pediatr. Res. 2019, 85, 97–104. [Google Scholar] [CrossRef]
- Sato, Y.; Ochiai, D.; Abe, Y.; Masuda, H.; Fukutake, M.; Ikenoue, S.; Kasuga, Y.; Shimoda, M.; Kanai, Y.; Tanaka, M. Prophylactic therapy with human amniotic fluid stem cells improved survival in a rat model of lipopolysaccharide-induced neonatal sepsis through immunomodulation via aggregates with peritoneal macrophages. Stem Cell Res. Ther. 2020, 11, 300. [Google Scholar] [CrossRef]
- Iyer, S.S.; Rojas, M. Anti-inflammatory effects of mesenchymal stem cells: Novel concept for future therapies. Expert Opin. Biol. Ther. 2008, 8, 569–581. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Basic Immunology E-Book: Functions and Disorders of the Immune System; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ghionzoli, M.; Cananzi, M.; Zani, A.; Rossi, C.A.; Leon, F.F.; Pierro, A.; Eaton, S.; De Coppi, P. Amniotic fluid stem cell migration after intraperitoneal injection in pup rats: Implication for therapy. Pediatr. Surg. Int. 2010, 26, 79–84. [Google Scholar] [CrossRef]
- van Till, J.W.; Van Veen, S.Q.; van Ruler, O.; Lamme, B.; Gouma, D.J.; Boermeester, M.A. The innate immune response to secondary peritonitis. Shock 2007, 28, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, A.; Cottone, L.; Monno, A.; Manfredi, A.A.; Rovere-Querini, P. The peritoneum: Healing, immunity, and diseases. J. Pathol. 2017, 243, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Melnicoff, M.J.; Horan, P.K.; Morahan, P.S. Kinetics of changes in peritoneal cell populations following acute inflammation. Cell. Immunol. 1989, 118, 178–191. [Google Scholar] [CrossRef]
- Remick, D.G.; Newcomb, D.E.; Bolgos, G.L.; Call, D.R. Comparison of the mortality and inflammatory response of two models of sepsis: Lipopolysaccharide vs. cecal ligation and puncture. Shock 2000, 13, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.P.; Heard, S.O. Laboratory models of sepsis and septic shock. J. Surg. Res. 1990, 49, 186–196. [Google Scholar] [CrossRef]
- Frimodt-Møller, N.; Knudsen, J.D.; Espersen, F. Chapter 14. The mouse peritonitis/sepsis model. In Handbook of Animal Models of Infection; Zak, O., Sande, M.A., Eds.; Academic Press: London, UK, 1999; pp. 127–136. [Google Scholar]
- Stommel, M.W.; Strik, C.; van Goor, H. Response to pathological processes in the peritoneal cavity—Sepsis, tumours, adhesions, and ascites. Semin. Pediatr. Surg. 2014, 23, 331–335. [Google Scholar] [CrossRef]
- Bazhanov, N.; Ylostalo, J.H.; Bartosh, T.J.; Tiblow, A.; Mohammadipoor, A.; Foskett, A.; Prockop, D.J. Intraperitoneally infused human mesenchymal stem cells form aggregates with mouse immune cells and attach to peritoneal organs. Stem Cell Res. Ther. 2016, 7, 27. [Google Scholar] [CrossRef]
- Wang, N.; Li, Q.; Zhang, L.; Lin, H.; Hu, J.; Li, D.; Shi, S.; Cui, S.; Zhou, J.; Ji, J.; et al. Mesenchymal stem cells attenuate peritoneal injury through secretion of TSG-6. PLoS ONE 2012, 7, e43768. [Google Scholar] [CrossRef]
- Sala, E.; Genua, M.; Petti, L.; Anselmo, A.; Arena, V.; Cibella, J.; Zanotti, L.; D’Alessio, S.; Scaldaferri, F.; Luca, G.; et al. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology 2015, 149, 163–176.e20. [Google Scholar] [CrossRef]
- Gosemann, J.H.; Kuebler, J.F.; Pozzobon, M.; Neunaber, C.; Hensel, J.H.; Ghionzoli, M.; de Coppi, P.; Ure, B.M.; Holze, G. Activation of regulatory T cells during inflammatory response is not an exclusive property of stem cells. PLoS ONE 2012, 7, e35512. [Google Scholar] [CrossRef]
- Santana, A.C.; Dellê, H.; Cavaglieri, R.C.; Lopes, M.A.; Francisco, R.P.; Zugaib, M.; Bydlowski, S.P.; Noronha, I.L. Protective effects of human amniotic fluid stem cells in a model of aorta allograft vasculopathy in rats. Transplant. Proc. 2012, 44, 2490–2494. [Google Scholar] [CrossRef] [PubMed]
- Perin, L.; Sedrakyan, S.; Giuliani, S.; Da Sacco, S.; Carraro, G.; Shiri, L.; Lemley, K.V.; Rosol, M.; Wu, S.; Atala, A.; et al. Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis. PLoS ONE 2010, 5, e9357. [Google Scholar] [CrossRef] [PubMed]
- Trohatou, O.; Anagnou, N.P.; Roubelakis, M.G. Human amniotic fluid stem cells as an attractive tool for clinical applications. Curr. Stem Cell Res. Ther. 2013, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Gallily, R.; Warwick, A.; Bang, F.B. Effect of cortisone on genetic resistance to mouse hepatitis virus in vivo and in vitro. Proc. Natl. Acad. Sci. USA 1964, 51, 1158–1164. [Google Scholar] [CrossRef]
- Kwak, T.K.; Jang, H.S.; Lee, M.G.; Jung, Y.S.; Kim, D.O.; Kim, Y.B.; Kim, J.I.; Kang, H. Effect of orally administered Atractylodes macrocephala Koidz Water extract on macrophage and T cell inflammatory response in mice. Evid. Based Complement. Alternat. Med. 2018, 2018, 4041873. [Google Scholar] [CrossRef]
- Wan, H.; Coppens, J.M.; van Helden-Meeuwsen, C.G.; Leenen, P.J.; van Rooijen, N.; Khan, N.A.; Kiekens, R.C.; Benner, R.; Versnel, M.A. Chorionic gonadotropin alleviates thioglycollate-induced peritonitis by affecting macrophage function. J. Leukoc. Biol. 2009, 86, 361–370. [Google Scholar] [CrossRef][Green Version]
- Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006, 6, 295–307. [Google Scholar] [CrossRef]
- Romano, M.; Tung, S.L.; Smyth, L.A.; Lombardi, G. Treg therapy in transplantation: A general overview. Transpl. Int. 2017, 30, 745–753. [Google Scholar] [CrossRef]
- Edinger, M.; Hoffmann, P. Regulatory T cells in stem cell transplantation: Strategies and first clinical experiences. Curr. Opin. Immunol. 2011, 23, 679–684. [Google Scholar] [CrossRef]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+ CD25(High) forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef]
- Ghannam, S.; Pène, J.; Moquet-Torcy, G.; Jorgensen, C.; Yssel, H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010, 185, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, J.; Zhang, P.; Cheuk, Y.C.; Jiang, Y.; Wang, J.; Xu, S.; Rong, R. Mesenchymal stem cell protects injured renal tubular epithelial cells by regulating mTOR-mediated Th17/Treg axis. Front. Immunol. 2021, 12, 684197. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Bi, Y.; Lin, X.; Liang, H.; Yang, D.; Zhang, X.; Ke, J.; Xiao, J.; Chen, Z.; Chen, W.; Zhang, X.; et al. Human adipose tissue-derived mesenchymal stem cells in Parkinson’s disease: Inhibition of T Helper 17 cell differentiation and regulation of immune balance Towards a regulatory T cell phenotype. Clin. Interv. Aging 2020, 15, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Mareschi, K.; Castiglia, S.; Sanavio, F.; Rustichelli, D.; Muraro, M.; Defedele, D.; Bergallo, M.; Fagioli, F. Immunoregulatory effects on T lymphocytes by human mesenchymal stromal cells isolated from bone marrow, amniotic fluid, and placenta. Exp. Hematol. 2016, 44, 138–150.e1. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, Y.; Ochiai, D.; Taguchi, M.; Kanzaki, S.; Ikenoue, S.; Kasuga, Y.; Tanaka, M. Human Amniotic Fluid Stem Cells Ameliorate Thioglycollate-Induced Peritonitis by Increasing Tregs in Mice. Int. J. Mol. Sci. 2022, 23, 6433. https://doi.org/10.3390/ijms23126433
Abe Y, Ochiai D, Taguchi M, Kanzaki S, Ikenoue S, Kasuga Y, Tanaka M. Human Amniotic Fluid Stem Cells Ameliorate Thioglycollate-Induced Peritonitis by Increasing Tregs in Mice. International Journal of Molecular Sciences. 2022; 23(12):6433. https://doi.org/10.3390/ijms23126433
Chicago/Turabian StyleAbe, Yushi, Daigo Ochiai, Masako Taguchi, Seiji Kanzaki, Satoru Ikenoue, Yoshifumi Kasuga, and Mamoru Tanaka. 2022. "Human Amniotic Fluid Stem Cells Ameliorate Thioglycollate-Induced Peritonitis by Increasing Tregs in Mice" International Journal of Molecular Sciences 23, no. 12: 6433. https://doi.org/10.3390/ijms23126433
APA StyleAbe, Y., Ochiai, D., Taguchi, M., Kanzaki, S., Ikenoue, S., Kasuga, Y., & Tanaka, M. (2022). Human Amniotic Fluid Stem Cells Ameliorate Thioglycollate-Induced Peritonitis by Increasing Tregs in Mice. International Journal of Molecular Sciences, 23(12), 6433. https://doi.org/10.3390/ijms23126433





