Molecular Characterization, Tissue Distribution Profile, and Nutritional Regulation of acsl Gene Family in Golden Pompano (Trachinotus ovatus)
Abstract
:1. Introduction
2. Results
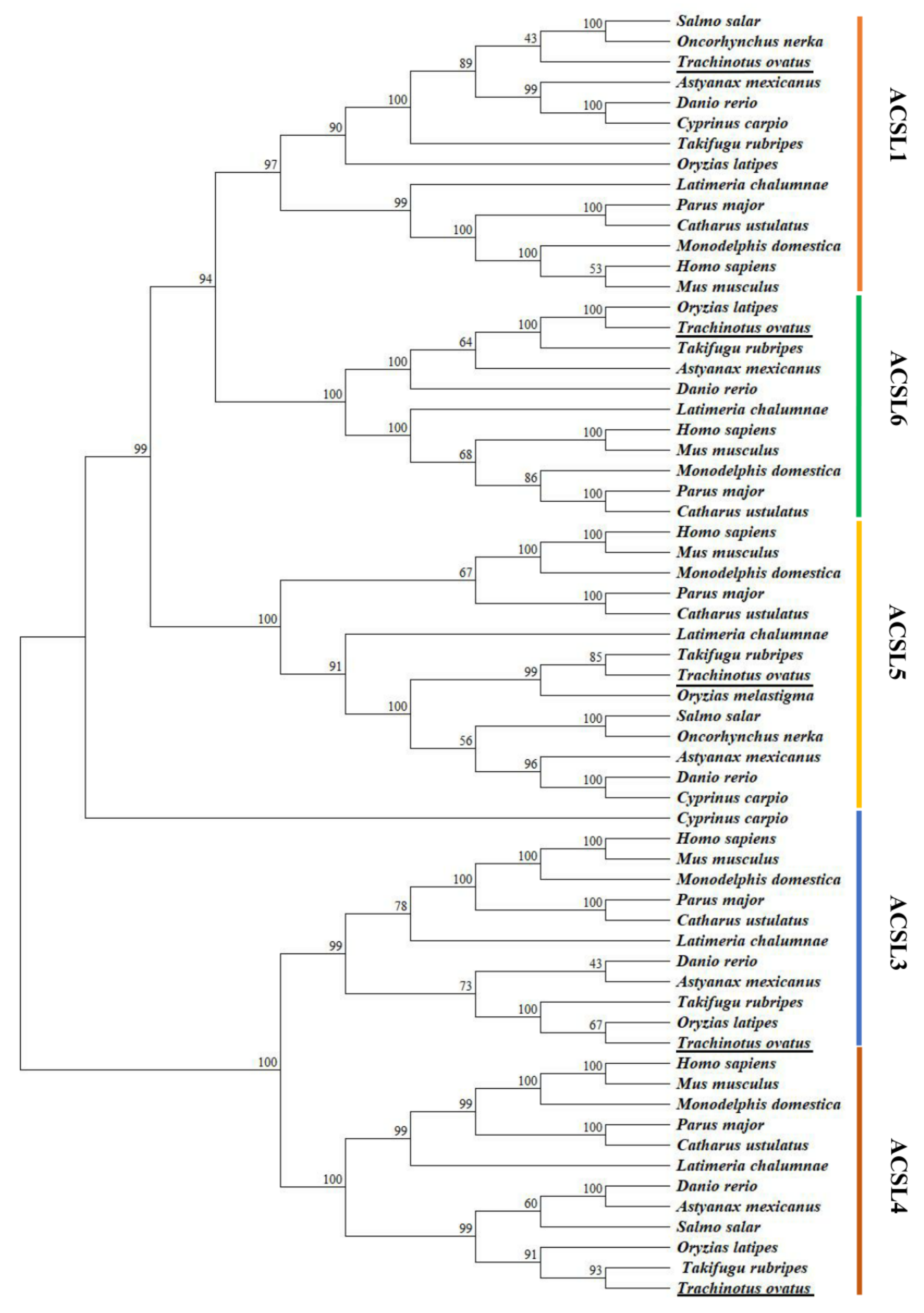
2.1. Molecular Characterization and Phylogenetic Analyses of acsl Genes
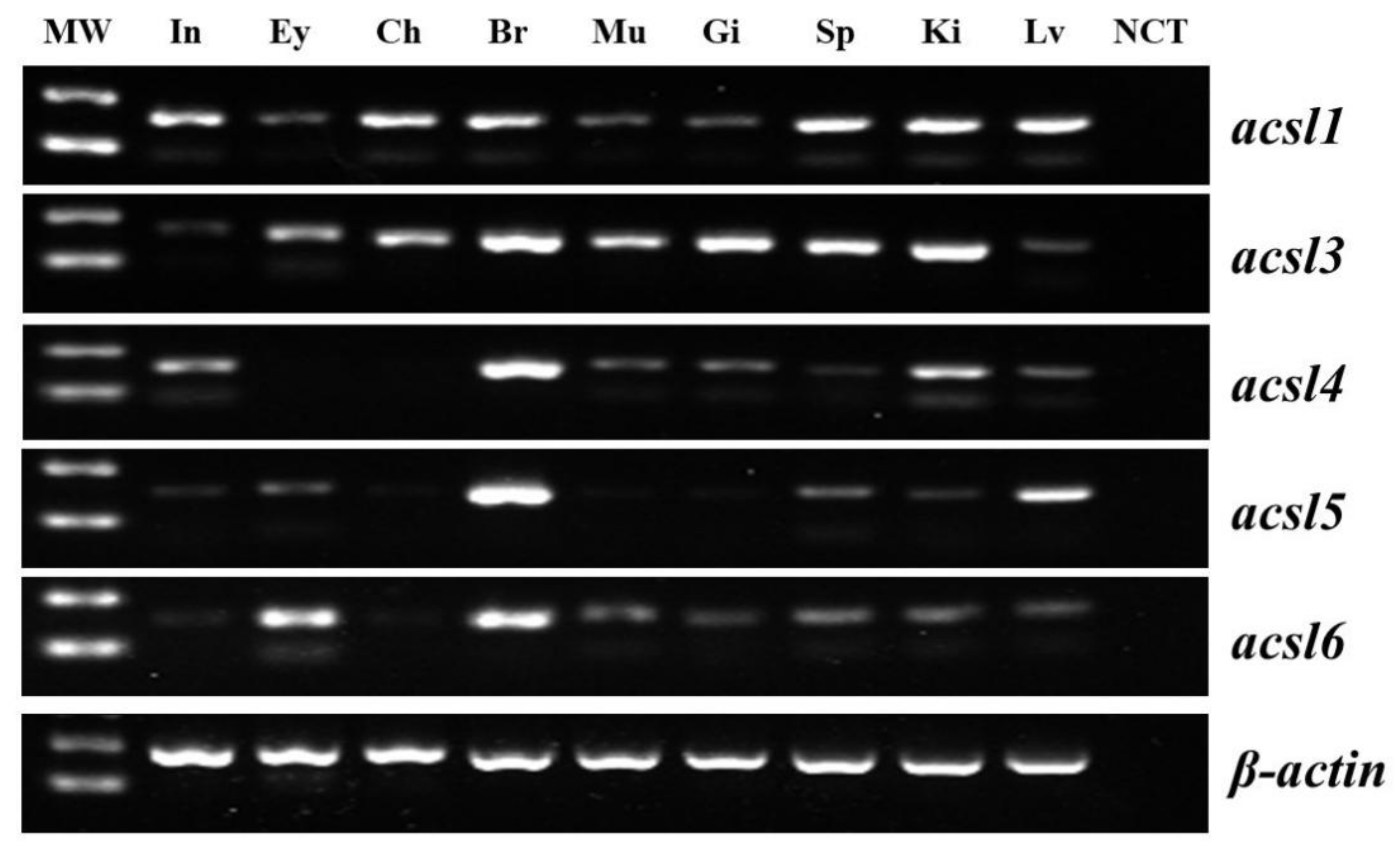
2.2. Tissue Distribution Pattern of acsl Genes
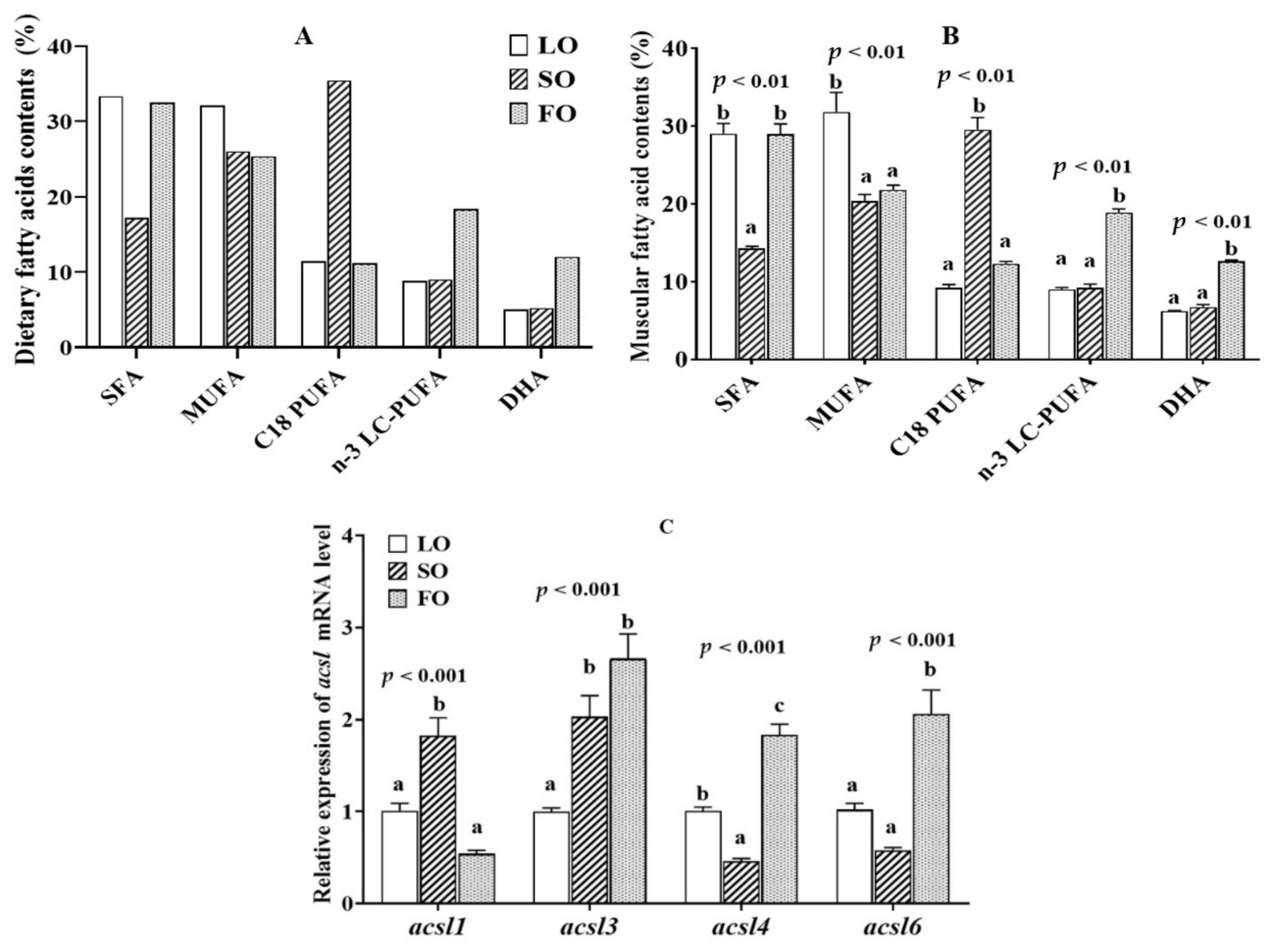
2.3. Muscular Fatty Acid Composition and acsl mRNA Level in the Fish Fed Diets with Different Lipid Sources
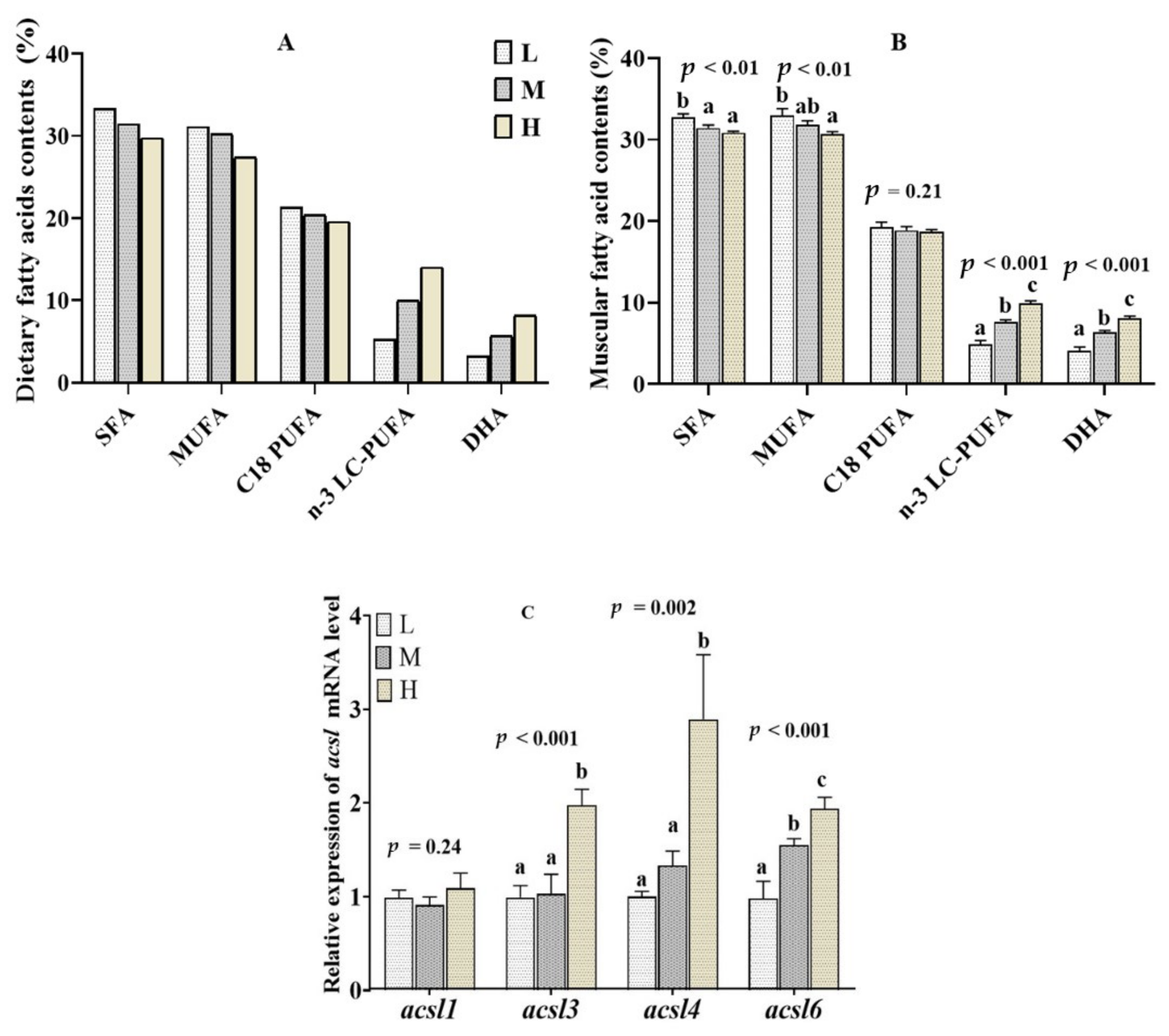
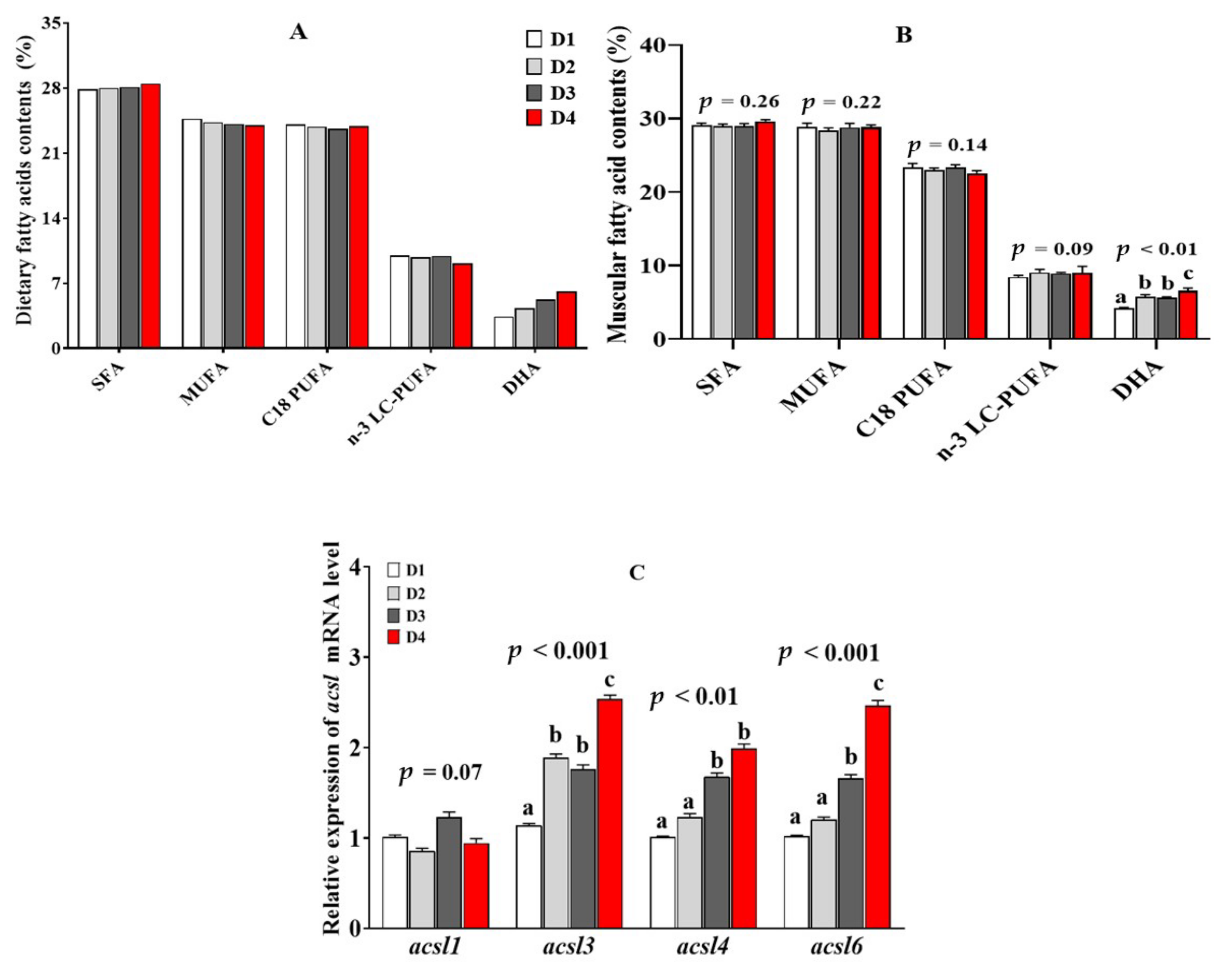
2.4. Muscular Fatty Acid Composition and acsl mRNA Level in the Fish Fed Diets with Different n-3 LC-PUFA Levels
2.5. Muscular Fatty Acid Composition and acsl mRNA Level in the Fish Fed Diets with Different DHA/EPA Ratios
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Animal Experiments
4.3. Trachinotus Ovatus acsl Genes Cloning
4.4. Phylogenetic Analysis
4.5. Tissue Distribution Pattern of Trachinotus ovatus acsl Genes
4.6. Quantitative Real-Time PCR Analysis
4.7. Fatty Acid Compositions Evaluation
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACSL | Long chain acyl-coA synthase |
| ARA | Arachidonic acid |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| LCFAs | Long chain Fatty acids |
| LC-PUFA | Long chain-polyunsaturated fatty acid |
| MUFA | Monounsaturated fatty acid |
| SFA | Saturated fatty acid |
References
- Ellis, J.M.; Frahm, J.L.; Li, L.O.; Coleman, R.A. Acyl-coenzyme A synthetases in metabolic control. Curr. Opin. Lipidol. 2010, 21, 212–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Marques, M.; Cunha, I.; Reis-Henriques, M.A.; Santos, M.M.; Castro, L.F.C. Diversity and history of the long-chain acyl-CoA synthetase (Acsl) gene family in vertebrates. BMC Evol. Biol. 2013, 13, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, J.; Bode, A.M.; Luo, X. ACSL family: The regulatory mechanisms and therapeutic implications in cancer. Eur. J. Pharmacol. 2021, 909, 174397. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, J.; Hooi, S.C.; Jiang, Y.M.; Lu, G.D. Fatty acid activation in carcinogenesis and cancer development: Essential roles of long-chain acyl-CoA synthetases. Oncol. Lett. 2018, 16, 1390–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Zha, J.; Yang, X.; Li, Q.; Zhang, Q.; Yin, A.; Beharry, Z.; Huang, H.; Huang, J.; Bartlett, M.; et al. Long-chain fatty acyl-CoA synthetase 1 promotes prostate cancer progression by elevation of lipogenesis and fatty acid beta-oxidation. Oncogene 2021, 40, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Van Horn, C.G.; Caviglia, J.M.; Li, L.O.; Wang, S.; Granger, D.A.; Coleman, R.A. Characterization of recombinant long-chain rat acyl-CoA synthetase isoforms 3 and 6: Identification of a novel variant of isoform 6. Biochemistry 2005, 44, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Kassan, A.; Herms, A.; Fernandez-Vidal, A.; Bosch, M.; Schieber, N.L.; Reddy, B.J.; Fajardo, A.; Gelabert-Baldrich, M.; Tebar, F.; Enrich, C.; et al. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J. Cell Biol. 2013, 203, 985–1001. [Google Scholar] [CrossRef] [Green Version]
- Klett, E.L.; Chen, S.; Yechoor, A.; Lih, F.B.; Coleman, R.A. Long-chain acyl-CoA synthetase isoforms differ in preferences for eicosanoid species and long-chain fatty acids. J. Lipid Res. 2017, 58, 884–894. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H.; Arasaki, K.; Ohsaki, Y.; Fujimoto, T.; Ohtomo, T.; Yamada, J.; Tagaya, M. Syntaxin 17 promotes lipid droplet formation by regulating the distribution of acyl-CoA synthetase 3. J. Lipid Res. 2018, 59, 805–819. [Google Scholar] [CrossRef]
- Lewin, T.M.; Van Horn, C.G.; Krisans, S.K.; Coleman, R.A. Rat liver acyl-CoA synthetase 4 is a peripheral-membrane protein located in two distinct subcellular organelles, peroxisomes, and mitochondrial-associated membrane. Arch. Biochem. Biophys. 2002, 404, 263–270. [Google Scholar] [CrossRef]
- Grevengoed, T.J.; Klett, E.L.; Coleman, R.A. Acyl-CoA metabolism and partitioning. Annu. Rev. Nutr. 2014, 34, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Kurotaki, A.; Kuwata, H.; Hara, S. Substrate Specificity of Human Long-Chain Acyl-CoA Synthetase ACSL6 Variants. Biol. Pharm. Bull. 2021, 44, 1571–1575. [Google Scholar] [CrossRef]
- Yan, S.; Yang, X.; Liu, H.; Fu, N.; Ouyang, Y.; Qing, K. Long-chain acyl-CoA synthetase in fatty acid metabolism involved in liver and other diseases: An update. World J. Gastroenterol. 2015, 21, 3492–3498. [Google Scholar] [CrossRef]
- Fernandez, R.F.; Kim, S.Q.; Zhao, Y.; Foguth, R.M.; Weera, M.M.; Counihan, J.L.; Nomura, D.K.; Chester, J.A.; Cannon, J.R.; Ellis, J.M. Acyl-CoA synthetase 6 enriches the neuroprotective omega-3 fatty acid DHA in the brain. Proc. Natl. Acad. Sci. USA 2018, 115, 12525–12530. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Chen, S.; Xu, J.; Yi, L.; Peng, Y.; Pan, Q.; Shen, X.; Dong, Z.; Zhang, X.; Wang, W. Molecular cloning and nutrient regulation analysis of long chain acyl-CoA synthetase 1 gene in grass carp, Ctenopharyngodon idella L. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 204, 61–68. [Google Scholar] [CrossRef]
- Xie, D.; He, Z.; Dong, Y.; Gong, Z.; Nie, G.; Li, Y. Molecular Cloning, Characterization, and expression regulation of acyl-CoA synthetase 6 gene and promoter in common carp Cyprinus carpio. Int. J. Mol. Sci. 2020, 21, 4736. [Google Scholar] [CrossRef]
- Chen, F.; Huang, X.; Zhu, H.; Li, Y.; Xu, C.; Xie, D. Molecular characterization and expression analysis of acyl-CoA synthetase 6 in golden pompano Trachinotus ovatus reveal its function in DHA enrichment. Aquaculture 2022, 551, 737966. [Google Scholar] [CrossRef]
- Li, M.; Zhang, M.; Ma, Y.; Ye, R.; Wang, M.; Chen, H.; Xie, D.; Dong, Y.; Ning, L.; You, C. Dietary supplementation with n-3 high unsaturated fatty acids decreases serum lipid levels and improves flesh quality in the marine teleost golden pompano Trachinotus ovatus. Aquaculture 2020, 516, 734632. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, C.; You, C.; Chen, B.; Wang, S.; Li, Y. Effects of different dietary ratios of docosahexaenoic to eicosapentaenoic acid (DHA/EPA) on the growth, non-specific immune indices, tissue fatty acid compositions and expression of genes related to LC-PUFA biosynthesis in juvenile golden pompano Trachinotus ovatus. Aquaculture 2019, 505, 488–495. [Google Scholar]
- Soupene, E.; Kuypers, F.A. Mammalian Long-Chain Acyl-CoA Synthetases. Exp. Biol. Med. 2008, 233, 507–521. [Google Scholar] [CrossRef] [Green Version]
- Li, L.O.; Ellis, J.M.; Paich, H.A.; Wang, S.; Gong, N.; Altshuller, G.; Thresher, R.J.; Koves, T.R.; Watkins, S.M.; Muoio, D.M.; et al. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J. Biol. Chem. 2009, 284, 27816–27826. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; He, L.; Yang, Y.; Chen, Y.; Song, Y.; Lu, X.; Liang, Y. The inhibition of Nrf2 accelerates renal lipid deposition through suppressing the ACSL1 expression in obesity-related nephropathy. Renal Failure 2019, 41, 821–831. [Google Scholar] [CrossRef] [Green Version]
- Killion, E.A.; Reeves, A.R.; El Azzouny, M.A.; Yan, Q.W.; Surujon, D.; Griffin, J.D.; Bowman, T.A.; Wang, C.; Matthan, N.R.; Klett, E.L.; et al. A role for long-chain acyl-CoA synthetase-4 (ACSL4) in diet-induced phospholipid remodeling and obesity-associated adipocyte dysfunction. Mol. Metab. 2018, 9, 43–56. [Google Scholar] [CrossRef]
- Jung, H.S.; Shimizu-Albergine, M.; Shen, X.; Kramer, F.; Shao, D.; Vivekanandan-Giri, A.; Pennathur, S.; Tian, R.; Kanter, J.E.; Bornfeldt, K.E. TNF-α induces acyl-CoA synthetase 3 to promote lipid droplet formation in human endothelial cells. J. Lipid Res. 2020, 61, 33–44. [Google Scholar] [CrossRef]
- Tian, W.; Wang, D.; Wang, Z.; Jiang, K.; Li, Z.; Tian, Y.; Kang, X.; Liu, X.; Li, H. Evolution, expression profile, and regulatory characteristics of ACSL gene family in chicken (Gallus gallus). Gene 2021, 764, 145094. [Google Scholar] [CrossRef]
- Singh, A.B.; Kan, C.F.K.; Kraemer, F.B.; Sobel, R.A.; Liu, J. Liver-specific knockdown of long-chain acyl-CoA synthetase 4 reveals its key role in VLDL-TG metabolism and phospholipid synthesis in mice fed a high-fat diet. Am. J. Physiol.-Endoc. Metab. 2019, 316, E880–E894. [Google Scholar] [CrossRef]
- Poppelreuther, M.; Rudolph, B.; Du, C.; Grossmann, R.; Becker, M.; Thiele, C.; Ehehalt, R.; Fullekrug, J. The N-terminal region of acyl-CoA synthetase 3 is essential for both the localization on lipid droplets and the function in fatty acid uptake. J. Lipid Res. 2012, 53, 888–900. [Google Scholar] [CrossRef] [Green Version]
- Oikawa, E.; Iijima, H.; Suzuki, T.; Sasano, H.; Sato, H.; Kamataki, A.; Nagura, H.; Kang, M.J.; Fujino, T.; Suzuki, H. A novel acyl-CoA synthetase, ACS5, expressed in intestinal epithelial cells and proliferating preadipocytes. J. Biochem. 1998, 124, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Meller, N.; Morgan, M.E.; Wong, W.P.; Altemus, J.B.; Sehayek, E. Targeting of Acyl-CoA synthetase 5 decreases jejunal fatty acid activation with no effect on dietary long-chain fatty acid absorption. Lipids Health Dis. 2013, 12, 88. [Google Scholar] [CrossRef] [Green Version]
- Li, L.O.; Mashek, D.G.; An, J.; Doughman, S.D.; Newgard, C.B.; Coleman, R.A. Overexpression of rat long chain acyl-coa synthetase 1 alters fatty acid metabolism in rat primary hepatocytes. J. Biol. Chem. 2006, 281, 37246–37255. [Google Scholar] [CrossRef] [Green Version]
- Ubellacker, J.M.; Tasdogan, A.; Ramesh, V.; Shen, B.; Mitchell, E.C.; Martin-Sandoval, M.S.; Gu, Z.; Mccormick, M.L.; Durham, A.B.; Spitz, D.R.; et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 2020, 585, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Kang, M.J.; Suzuki, H.; Iijima, H.; Yamamoto, T. Molecular characterization and expression of rat acyl-CoA synthetase 3. J. Biol. Chem. 1996, 271, 16748–16752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betancor, M.B.; Li, K.; Bucerzan, V.S.; Sprague, M.; Sayanova, O.; Usher, S.; Han, L.; Norambuena, F.; Torrissen, O.; Napier, J.A. Oil from transgenic Camelina sativa containing over 25% n-3 long-chain PUFA as the major lipid source in feed for Atlantic salmon (Salmo salar). Br. J. Nutr. 2018, 119, 1378–1392. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Lei, C.; Ji, H.; Jin, A. Role of cyclooxygenase-mediated metabolites in lipid metabolism and expression of some immune-related genes in juvenile grass carp (Ctenopharyngodon idellus) fed arachidonic acid. Fish Physiol. Biochem. 2017, 43, 703–717. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Itabe, H.; Kinoshita, T.; Homma, K.J.; Onoduka, J.; Mori, M.; Yamaguchi, S.; Makita, M.; Higashi, Y.; Yamashita, A.; et al. Involvement of ACSL in local synthesis of neutral lipids in cytoplasmic lipid droplets in human hepatocyte HuH7. J. Lipid Res. 2007, 48, 1280–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glencross, B.D.; De Santis, C.; Bicskei, B.; Taggart, J.B.; Bron, J.E.; Betancor, M.B.; Tocher, D.R. A comparative analysis of the response of the hepatic transcriptome to dietary docosahexaenoic acid in Atlantic salmon (Salmo salar) post-smolts. BMC Genom. 2015, 16, 684. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Lu, R.; Ji, H.; Sun, J.; Li, C.; Liu, P.; Lei, C.; Chen, L.; Du, Z. Comparative analysis of the hepatopancreas transcriptome of grass carp (Ctenopharyngodon idellus) fed with lard oil and fish oil diets. Gene 2015, 565, 192–200. [Google Scholar] [CrossRef]
- Shimbara-Matsubayashi, S.; Kuwata, H.; Tanaka, N.; Kato, M.; Hara, S. Analysis on the substrate specificity of recombinant human acyl-CoA synthetase ACSL4 variants. Biol. Pharm. Bull. 2019, 42, 850–855. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Zhang, H.; Hua, Z.; Zhu, Z.; Tao, J.; Xiao, H.; Zhang, L.; Bi, Y.; Wang, H. ACSL4 Directs Intramuscular Adipogenesis and Fatty Acid Composition in Pigs. Animals 2022, 12, 119. [Google Scholar] [CrossRef]
- Teodoro, B.G.; Sampaio, I.H.; Bomfim, L.H.; Queiroz, A.L.; Silveira, L.R.; Souza, A.O.; Fernandes, A.M.; Eberlin, M.N.; Huang, T.Y.; Zheng, D. Long-chain acyl-CoA synthetase 6 regulates lipid synthesis and mitochondrial oxidative capacity in human and rat skeletal muscle. J. Physiol. 2017, 595, 677–693. [Google Scholar] [CrossRef] [Green Version]
- Hale, B.J.; Fernandez, R.F.; Kim, S.Q.; Diaz, V.D.; Jackson, S.N.; Liu, L.; Brenna, J.T.; Hermann, B.P.; Geyer, C.B.; Ellis, J.M. Acyl-CoA synthetase 6 enriches seminiferous tubules with the ω-3 fatty acid docosahexaenoic acid and is required for male fertility in the mouse. J. Biol. Chem. 2019, 294, 14394–14405. [Google Scholar] [CrossRef]
- Shishikura, K.; Kuroha, S.; Matsueda, S.; Iseki, H.; Matsui, T.; Inoue, A.; Arita, M. Acyl-CoA synthetase 6 regulates long-chain polyunsaturated fatty acid composition of membrane phospholipids in spermatids and supports normal spermatogenic processes in mice. FASEB J. 2019, 33, 14194–14203. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Monroig, O.; Zhang, L.; Wang, S.; Zheng, X.; Dick, J.R.; You, C.; Tocher, D.R. Vertebrate fatty acyl desaturase with Δ4 activity. Proc. Natl. Acad. Sci. USA 2010, 107, 16840–16845. [Google Scholar] [CrossRef] [Green Version]
| Subject | Primer Name | Primer Sequence (5′-3′) | References/ Accession Number |
|---|---|---|---|
| RT-PCR | RT-acsl1-F | ATGCAGGCTCAGGAAGTCCTGAGAC | PRJNA574781 |
| RT-acsl1-R | TTAGATCTTAATTTTAGAATAAAGT | ||
| RT-acsl3-F | ATGAAGCTGAAGGAGGACCTGAA | PRJNA574781 | |
| RT-acsl3-R | TTATTTTCCACCGTACATTCTCTC | ||
| RT-acsl4-F | ATGGGTCTCCAGGCAGACTCAAC | PRJNA574781 | |
| RT-acsl4-R | TTATTTGCCCCCATACATCCTCT | ||
| RT-acsl5-F | ATGGAATTCCTTTTCCAGTTGCTC | PRJNA574781 | |
| RT-acsl5-R | TTATTGGATGTTAGCATATAGTT | ||
| RT-acsl6-F | ATGCTCGCATTCGTTTTGGTCTC | MN481524 | |
| RT-acsl6-R | TCACATGGAGATGCTGCTGTAGA | ||
| qPCR | acsl1-F | CTGAAGATCGTGGACAGGAAGAAGC | PRJNA574781 |
| acsl1-R | CAACCACACAGGAAGTCAGGGTCAG | ||
| acsl3-F | TGCCTATGCCAACAGTGACCAGTC | PRJNA574781 | |
| acsl3-R | ATCGCTCCAGTTTCGCTGAGATAGC | ||
| acsl4-F | AGGCAAGGACACGCTGGATAAG | PRJNA574781 | |
| acsl4-R | TCCAGTTCATTGTAGGACAGCCA | ||
| acsl6-F | GCCTCGTTGAGCGCGGCAAGGGCT | MN481524 | |
| acsl6-R | AAGCCTGAGAAATCAGCTACCACG | ||
| β-actin-F | TACGAGCTGCCTGACGGACA | Chen et al., 2022 | |
| β-actin-R | GGCTGTGATCTCCTTCTGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhu, H.; Huang, X.; Wang, A.; Xie, D. Molecular Characterization, Tissue Distribution Profile, and Nutritional Regulation of acsl Gene Family in Golden Pompano (Trachinotus ovatus). Int. J. Mol. Sci. 2022, 23, 6437. https://doi.org/10.3390/ijms23126437
Yang Z, Zhu H, Huang X, Wang A, Xie D. Molecular Characterization, Tissue Distribution Profile, and Nutritional Regulation of acsl Gene Family in Golden Pompano (Trachinotus ovatus). International Journal of Molecular Sciences. 2022; 23(12):6437. https://doi.org/10.3390/ijms23126437
Chicago/Turabian StyleYang, Zhigang, Hangbo Zhu, Xiaoping Huang, Aimin Wang, and Dizhi Xie. 2022. "Molecular Characterization, Tissue Distribution Profile, and Nutritional Regulation of acsl Gene Family in Golden Pompano (Trachinotus ovatus)" International Journal of Molecular Sciences 23, no. 12: 6437. https://doi.org/10.3390/ijms23126437






