The Unfolded Protein Response Sensor PERK Mediates Stiffness-Dependent Adaptation in Glioblastoma Cells
Abstract
:1. Introduction
2. Results
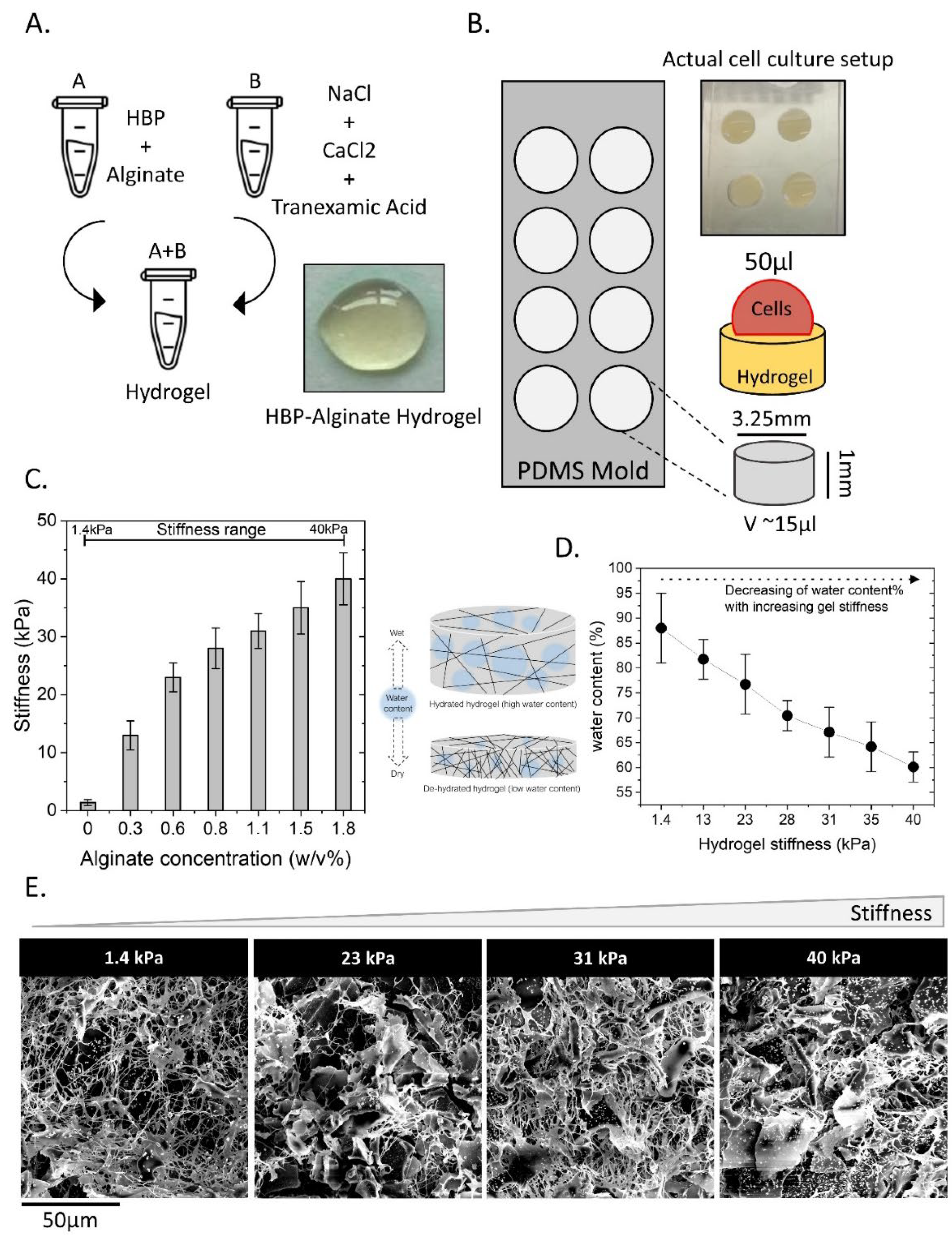
2.1. HBP/Alginate Hydrogel Characterization
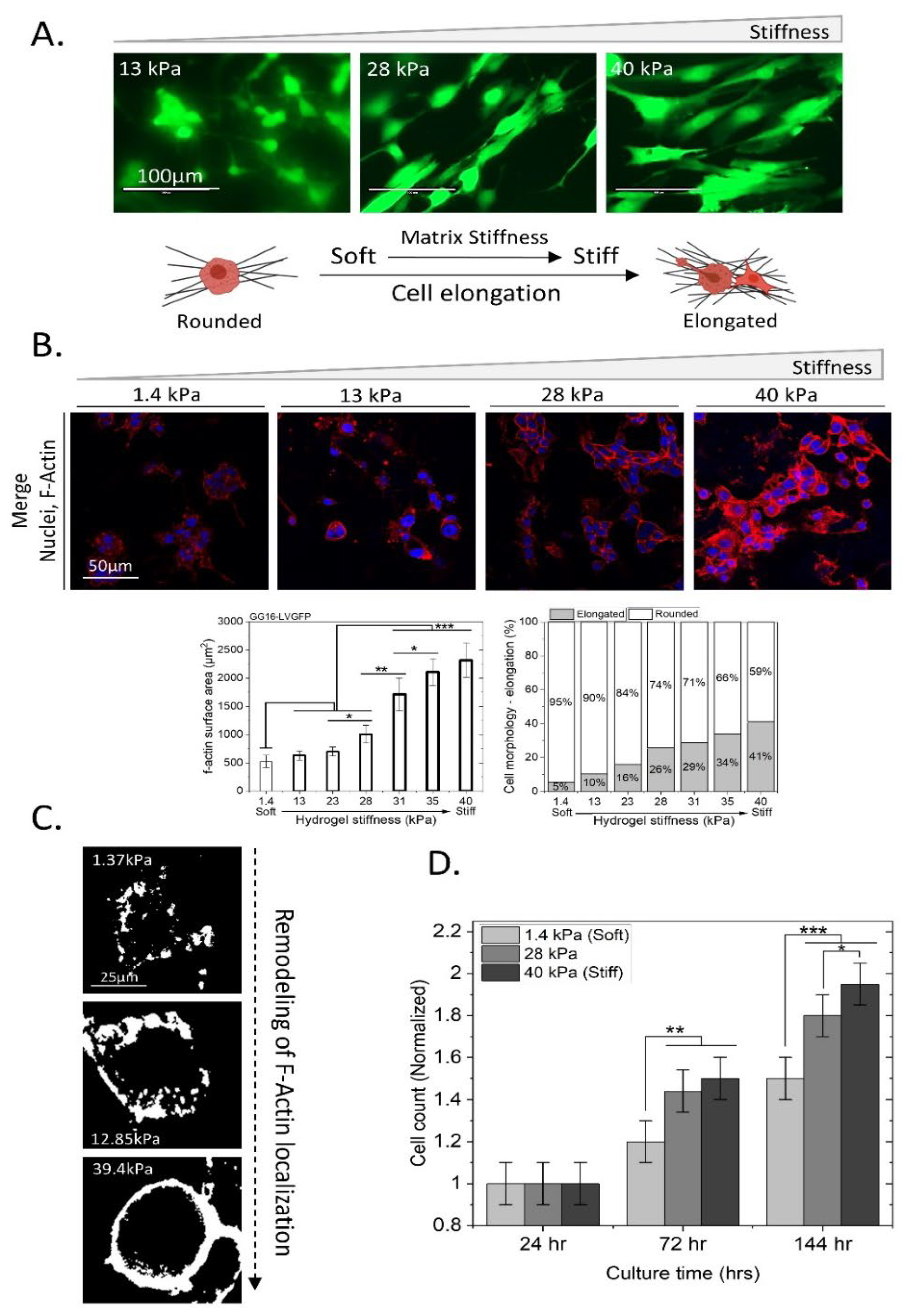
2.2. GSCs Adapt to Increasing Matrix Stiffness by Changing Cell Morphology, Increased F-Actin Expression, and Cell Proliferation
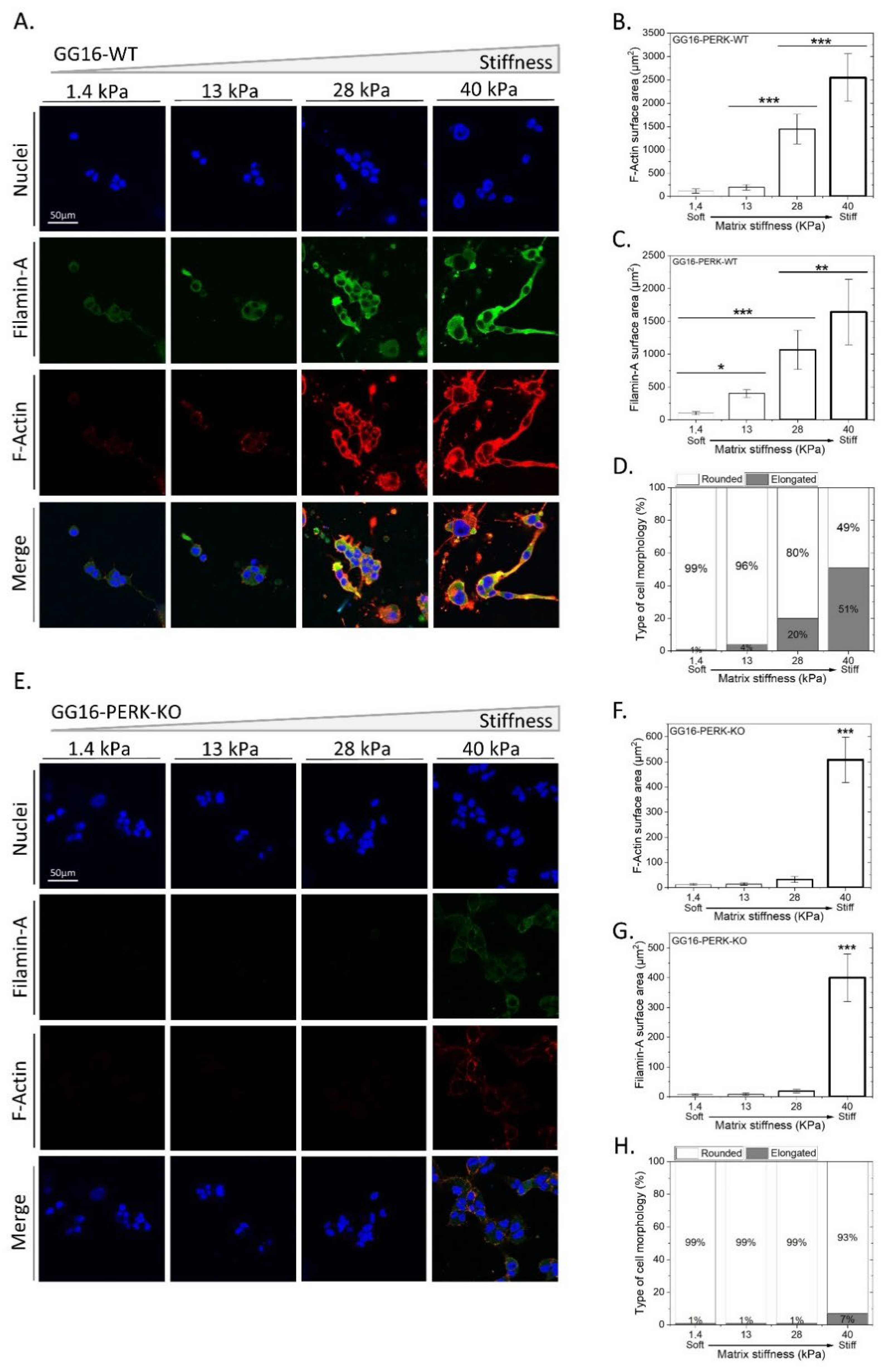
2.3. PERK Mediates Cellular Adaptation of GSCs to Increased Matrix Stiffness
2.4. Blocking F-Actin Polymerization in GG16 Cells Mimics the PERK-Deficient Phenotype by Failing to Adapt to Matrix Stiffness
2.5. Exploring the Involvement of UPR PERK Signaling in Cellar Adaptation of GSC to Increasing Matrix Stiffness
2.6. PERK Mediates Stiffness-Dependent GBM Cell Migration and Proliferation
3. Discussion
4. Materials and Methods
4.1. Preparation of Stiffness-Tunable Human Blood Plasma (HBP)/Alginate Hydrogel
4.2. Stiffness Measurements
4.3. Rheology
4.4. Scanning Electron Microscopy (SEM)
4.5. Water Content of the HBP/Alginate Hydrogel
4.6. Cell Culture
4.7. Migration and Proliferation Assays
4.8. Immunofluorescent Microscopy
4.9. Cellular Characterization and Microscopic Data Analysis
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro. Oncol. 2019, 21, V1–V100. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of Glioblastoma: State of the Art and Future Directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Arya, R.K.; Maheshwari, S.; Singh, A.; Meena, S.; Pandey, P.; Dormond, O.; Datta, D. Tumor Heterogeneity and Cancer Stem Cell Paradigm: Updates in Concept, Controversies and Clinical Relevance. Int. J. Cancer 2015, 136, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Dirkse, A.; Golebiewska, A.; Buder, T.; Nazarov, P.V.; Muller, A.; Poovathingal, S.; Brons, N.H.C.; Leite, S.; Sauvageot, N.; Sarkisjan, D.; et al. Stem Cell-Associated Heterogeneity in Glioblastoma Results from Intrinsic Tumor Plasticity Shaped by the Microenvironment. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Virolle, T. Cancer Stem Cells in Glioblastoma. Bull. Cancer 2017, 104, 1075–1079. [Google Scholar] [CrossRef]
- White, F.M.; Gatenby, R.A.; Fischbach, C. The Physics of Cancer. Cancer Res. 2019, 79, 2107–2110. [Google Scholar] [CrossRef]
- Wirtz, D.; Konstantopoulos, K.; Searson, P.C. The Physics of Cancer: The Role of Physical Interactions and Mechanical Forces in Metastasis. Nat. Rev. Cancer 2011, 11, 512–522. [Google Scholar] [CrossRef]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Physical Traits of Cancer. Science 2020, 370, eaaz0868. [Google Scholar] [CrossRef]
- Xia, S.; Lal, B.; Tung, B.; Wang, S.; Goodwin, C.R.; Laterra, J. Tumor Microenvironment Tenascin-C Promotes Glioblastoma Invasion and Negatively Regulates Tumor Proliferation. Neuro-Oncology 2016, 18, 507–517. [Google Scholar] [CrossRef]
- Virga, J.; Szivos, L.; Hortobágyi, T.; Chalsaraei, M.K.; Zahuczky, G.; Steiner, L.; Tóth, J.; Reményi-Puskár, J.; Bognár, L.; Klekner, A. Extracellular Matrix Differences in Glioblastoma Patients with Different Prognoses. Oncol. Lett. 2019, 17, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.M.; Przybyla, L.; Weaver, V.M. Tissue Mechanics Regulate Brain Development, Homeostasis and Disease. J. Cell Sci. 2017, 130, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Khoonkari, M.; Liang, D.; Kamperman, M.; Kruyt, F.A.E.; Rijn, P. Van Physics of Brain Cancer: Multiscale Alterations of Glioblastoma Cells under Extracellular Matrix Stiffening. Pharmaceutics 2022, 14, 1031. [Google Scholar] [CrossRef]
- Wolf, K.J.; Chen, J.; Coombes, J.D.; Aghi, M.K.; Kumar, S. Dissecting and Rebuilding the Glioblastoma Microenvironment with Engineered Materials. Nat. Rev. Mater. 2019, 4, 651–668. [Google Scholar] [CrossRef]
- Bunevicius, A.; Schregel, K.; Sinkus, R.; Golby, A.; Patz, S. REVIEW: MR Elastography of Brain Tumors. NeuroImage. Clin. 2020, 25, 102109. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; McGee, K.P.; Arani, A.; Lake, D.S.; Glaser, K.J.; Manduca, A.; Parney, I.F.; Ehman, R.L.; Huston, J. MR Elastography Analysis of Glioma Stiffness and IDH1-Mutation Status. Am. J. Neuroradiol. 2018, 39, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Belousov, A.; Titov, S.; Shved, N.; Garbuz, M.; Malykin, G.; Gulaia, V.; Kagansky, A.; Kumeiko, V. The Extracellular Matrix and Biocompatible Materials in Glioblastoma Treatment. Front. Bioeng. Biotechnol. 2019, 7, 341. [Google Scholar] [CrossRef]
- Miroshnikova, Y.A.; Mouw, J.K.; Barnes, J.M.; Pickup, M.W.; Lakins, J.N.; Kim, Y.; Lobo, K.; Persson, A.I.; Reis, G.F.; McKnigh, T.R.; et al. Tissue Mechanics Promote IDH1-Dependent HIF1α-Tenascin C Feedback to Regulate Glioblastoma Aggression. Nat. Cell Biol. 2016, 18, 1336–1345. [Google Scholar] [CrossRef]
- Cha, J.; Kim, P. Biomimetic Strategies for the Glioblastoma Microenvironment. Front. Mater. 2017, 4, 45. [Google Scholar] [CrossRef]
- Erickson, A.E.; Lan Levengood, S.K.; Sun, J.; Chang, F.C.; Zhang, M. Fabrication and Characterization of Chitosan–Hyaluronic Acid Scaffolds with Varying Stiffness for Glioblastoma Cell Culture. Adv. Healthc. Mater. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Beliveau, A.; Thomas, G.; Gong, J.; Wen, Q.; Jain, A. Aligned Nanotopography Promotes a Migratory State in Glioblastoma Multiforme Tumor Cells. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Sohrabi, A.; Seidlits, S.K. Integrating the Glioblastoma Microenvironment into Engineered Experimental Models. Futur. Sci. OA 2017, 3, FSO189. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Jeibmann, A.; Halama, K.; Witte, H.T.; Wälte, M.; Matzat, T.; Schillers, H.; Faber, C.; Senner, V.; Paulus, W.; et al. ECM Stiffness Regulates Glial Migration in Drosophila and Mammalian Glioma Models. Development 2014, 141, 3233–3242. [Google Scholar] [CrossRef]
- Razinia, Z.; Castagnino, P.; Xu, T.; Vázquez-Salgado, A.; Puré, E.; Assoian, R.K. Stiffness-Dependent Motility and Proliferation Uncoupled by Deletion of CD44. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Wang, C.; Sinha, S.; Jiang, X.; Murphy, L.; Fitch, S.; Wilson, C.; Grant, G.; Yang, F. Matrix Stiffness Modulates Patient-Derived Glioblastoma Cell Fates in Three-Dimensional Hydrogels. Tissue Eng. Part A 2020, 27, 390–401. [Google Scholar] [CrossRef]
- Wala, J.; Das, S. Mapping of Biomechanical Properties of Cell Lines on Altered Matrix Stiffness Using Atomic Force Microscopy. Biomech. Model. Mechanobiol. 2020, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.; Cha, J.; Park, J.; Choi, J.; Kang, S.G.; Kim, P. The Mode and Dynamics of Glioblastoma Cell Invasion into a Decellularized Tissue-Derived Extracellular Matrix-Based Three-Dimensional Tumor Model. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Kenche, H.; Zhao, H.; Li, J.; Stone, J. The Role of Extracellular Matrix Stiffness in Regulating Cytoskeletal Remodeling via Vinculin in Synthetic Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 2019, 508, 302–307. [Google Scholar] [CrossRef]
- Broders-Bondon, F.; Ho-Bouldoires, T.H.N.; Fernandez-Sanchez, M.E.; Farge, E. Mechanotransduction in Tumor Progression: The Dark Side of the Force. J. Cell Biol. 2018, 217, 1571–1587. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. Protein Misfolding in the Endoplasmic Reticulum as a Conduit to Human Disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Peñaranda Fajardo, N.M.; Meijer, C.; Kruyt, F.A.E. The Endoplasmic Reticulum Stress/Unfolded Protein Response in Gliomagenesis, Tumor Progression and as a Therapeutic Target in Glioblastoma. Biochem. Pharmacol. 2016, 118, 1–8. [Google Scholar] [CrossRef]
- Peñaranda-Fajardo, N.M.; Meijer, C.; Liang, Y.; Dijkstra, B.M.; Aguirre-Gamboa, R.; den Dunnen, W.F.A.; Kruyt, F.A.E. ER Stress and UPR Activation in Glioblastoma: Identification of a Noncanonical PERK Mechanism Regulating GBM Stem Cells through SOX2 Modulation. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Khoonkari, M.; Avril, T.; Chevet, E.; Kruyt, F.A.E. The Unfolded Protein Response as Regulator of Cancer Stemness and Differentiation: Mechanisms and Implications for Cancer Therapy. Biochem. Pharmacol. 2021, 192, 114737. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; McGrath, B.; Cavener, D.R. PERK (EIF2AK3) Regulates Proinsulin Trafficking and Quality Control in the Secretory Pathway. Diabetes 2010, 59, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, A.R.; Giordano, F.; Gerlo, S.; Segura, I.; Van Eygen, S.; Molenberghs, G.; Rocha, S.; Houcine, A.; Derua, R.; Verfaillie, T.; et al. The ER Stress Sensor PERK Coordinates ER-Plasma Membrane Contact Site Formation through Interaction with Filamin-A and F-Actin Remodeling. Mol. Cell 2017, 65, 885–899.e6. [Google Scholar] [CrossRef] [PubMed]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Mapping Physical Tumor Microenvironment and Drug Delivery. Clin. Cancer Res. 2019, 25, 2024–2026. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Y.; Zhao, H. The Effect of Matrix Stiffness on Biomechanical Properties of Chondrocytes. Acta Biochim. Biophys. Sin. (Shanghai) 2016, 48, 958–965. [Google Scholar] [CrossRef]
- Gardel, M.L.; Schneider, I.C.; Aratyn-Schaus, Y.; Waterman, C.M. Mechanical Integration of Actin and Adhesion Dynamics in Cell Migration. Annu. Rev. Cell Dev. Biol. 2010, 26, 315–333. [Google Scholar] [CrossRef]
- Stricker, J.; Falzone, T.; Gardel, M.L. Mechanics of the F-Actin Cytoskeleton. J. Biomech. 2010, 43, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, N.; Arani, A.; Perry, A.; Meyer, F.; Manduca, A.; Glaser, K.; Senjem, M.L.; Ehman, R.L.; Huston, J. MR Elastography Demonstrates Increased Brain Stiffness in Normal Pressure Hydrocephalus. Am. J. Neuroradiol. 2016, 37, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, K.; Chin, L.; Georges, P.C.; Byfield, F.J.; Bucki, R.; Kim, R.; Weaver, M.; Wells, R.G.; Marcinkiewicz, C.; Janmey, P.A. Compression Stiffening of Brain and Its Effect on Mechanosensing by Glioma Cells. N. J. Phys. 2014, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Malagón-Romero, D.; Hernández, N.; Cardozo, C.; Godoy-Silva, R.D. Rheological Characterization of a Gel Produced Using Human Blood Plasma and Alginate Mixtures. J. Mech. Behav. Biomed. Mater. 2014, 34, 171–180. [Google Scholar] [CrossRef]
- Sadeghi-Ataabadi, M.; Mostafavi-pour, Z.; Vojdani, Z.; Sani, M.; Latifi, M.; Talaei-Khozani, T. Fabrication and Characterization of Platelet-Rich Plasma Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2017, 71, 372–380. [Google Scholar] [CrossRef]
- Kim, Y.; Kumar, S. CD44-Mediated Adhesion to Hyaluronic Acid Contributes to Mechanosensing and Invasive Motility. Mol. Cancer Res. 2014, 12, 1416–1429. [Google Scholar] [CrossRef]
- Wu, H.; Wei, L.; Fan, F.; Ji, S.; Zhang, S.; Geng, J.; Hong, L.; Fan, X.; Chen, Q.; Tian, J.; et al. Integration of Hippo Signalling and the Unfolded Protein Response to Restrain Liver Overgrowth and Tumorigenesis. Nat. Commun. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Pegoraro, A.F.; Janmey, P.; Weitz, D.A. Mechanical Properties of the Cytoskeleton and Cells. Cold Spring Harb. Perspect. Biol. 2017, 9, a022038. [Google Scholar] [CrossRef]
- Schwarz, U.S.; Gardel, M.L. United We Stand—Integrating the Actin Cytoskeleton and Cell-Matrix Adhesions in Cellular Mechanotransduction. J. Cell Sci. 2012, 125, 3051–3060. [Google Scholar] [CrossRef]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal Crosstalk in Cell Migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Schmoller, K.M.; Lieleg, O.; Bausch, A.R. Structural and Viscoelastic Properties of Actin/Filamin Networks: Cross-Linked versus Bundled Networks. Biophys. J. 2009, 97, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.; An, K.M.; Esue, O.; Wirtz, D. The Bimodal Role of Filamin in Controlling the Architecture and Mechanics of F-Actin Networks. J. Biol. Chem. 2004, 279, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Sixt, M. Mechanisms of 3D Cell Migration. Nat. Rev. Mol. Cell Biol. 2019, 20, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Urra, H.; Henriquez, D.R.; Cánovas, J.; Villarroel-campos, D.; Carreras-sureda, A.; Pulgar, E.; Molina, E.; Hazari, Y.M.; Limia, C.M.; Alvarez-rojas, S.; et al. IRE1α governs cytoskeleton remodelling and Cell Migration Through a Direct Interaction with filamin A. Nat. Cell Biol. 2018, 20, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Karabiyik Acar, O.; Kayitmazer, A.B.; Torun Kose, G. Hyaluronic Acid/Chitosan Coacervate-Based Scaffolds. Biomacromolecules 2018, 19, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.V.; Conroy, S.; Tomar, T.; Eggens-Meijer, E.; Bhat, K.; Copray, S.; Walenkamp, A.M.E.; Boddeke, E.; Balasubramanyian, V.; Wagemakers, M.; et al. TGF-β Is an Inducer of ZEB1-Dependent Mesenchymal Transdifferentiation in Glioblastoma That Is Associated with Tumor Invasion. Cell Death Dis. 2014, 5, e1443. [Google Scholar] [CrossRef]
- Wierenga, A.T.J.; Vellenga, E.; Schuringa, J.J. Convergence of Hypoxia and TGFβ Pathways on Cell Cycle Regulation in Human Hematopoietic Stem/Progenitor Cells. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoonkari, M.; Liang, D.; Lima, M.T.; van der Land, T.; Liang, Y.; Sun, J.; Dolga, A.; Kamperman, M.; van Rijn, P.; Kruyt, F.A.E. The Unfolded Protein Response Sensor PERK Mediates Stiffness-Dependent Adaptation in Glioblastoma Cells. Int. J. Mol. Sci. 2022, 23, 6520. https://doi.org/10.3390/ijms23126520
Khoonkari M, Liang D, Lima MT, van der Land T, Liang Y, Sun J, Dolga A, Kamperman M, van Rijn P, Kruyt FAE. The Unfolded Protein Response Sensor PERK Mediates Stiffness-Dependent Adaptation in Glioblastoma Cells. International Journal of Molecular Sciences. 2022; 23(12):6520. https://doi.org/10.3390/ijms23126520
Chicago/Turabian StyleKhoonkari, Mohammad, Dong Liang, Marina Trombetta Lima, Tjitze van der Land, Yuanke Liang, Jianwu Sun, Amalia Dolga, Marleen Kamperman, Patrick van Rijn, and Frank A. E. Kruyt. 2022. "The Unfolded Protein Response Sensor PERK Mediates Stiffness-Dependent Adaptation in Glioblastoma Cells" International Journal of Molecular Sciences 23, no. 12: 6520. https://doi.org/10.3390/ijms23126520
APA StyleKhoonkari, M., Liang, D., Lima, M. T., van der Land, T., Liang, Y., Sun, J., Dolga, A., Kamperman, M., van Rijn, P., & Kruyt, F. A. E. (2022). The Unfolded Protein Response Sensor PERK Mediates Stiffness-Dependent Adaptation in Glioblastoma Cells. International Journal of Molecular Sciences, 23(12), 6520. https://doi.org/10.3390/ijms23126520







