Lanthanide (Eu3+/Tb3+)-Loaded γ-Cyclodextrin Nano-Aggregates for Smart Sensing of the Anticancer Drug Irinotecan
Abstract
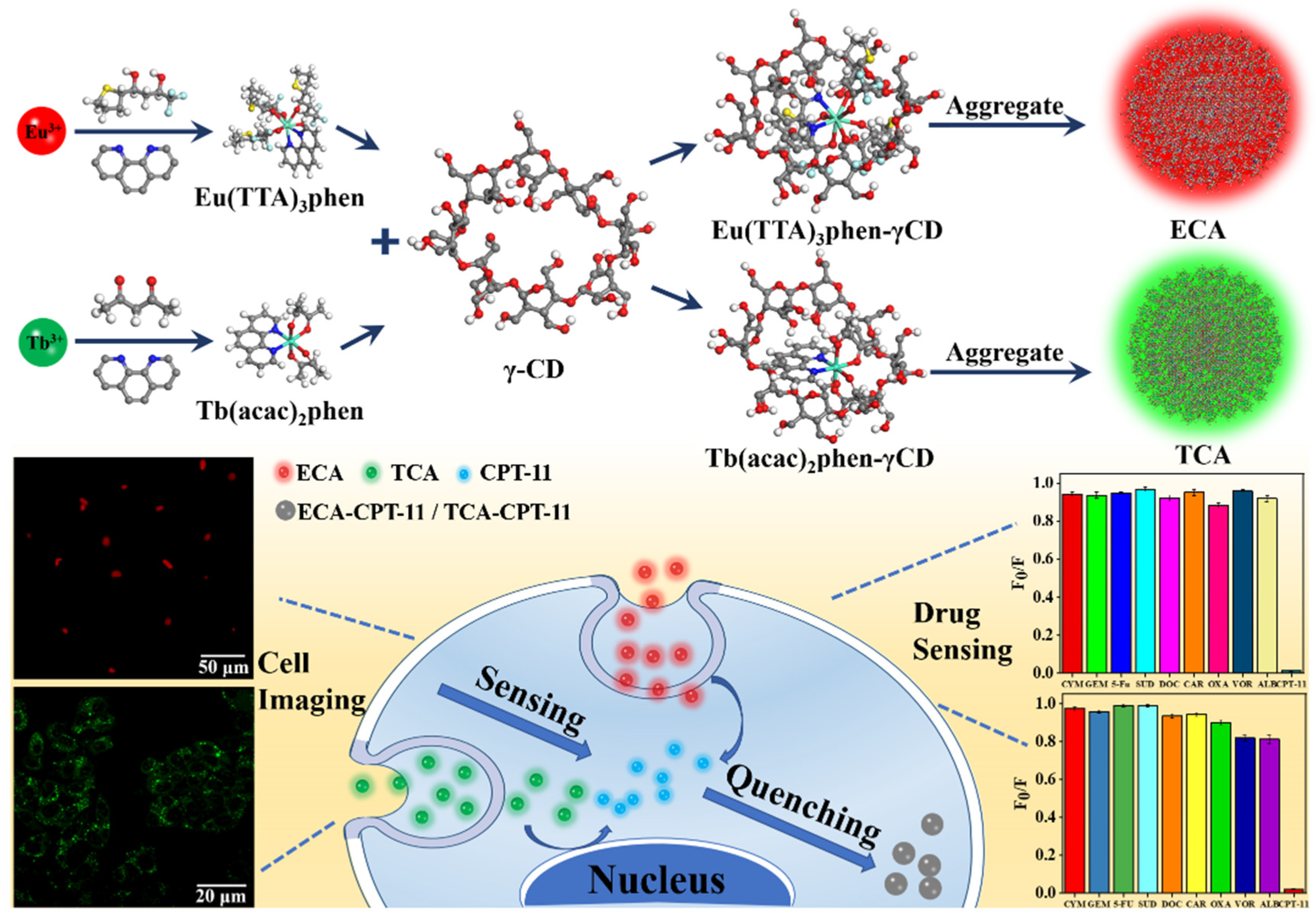
:1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of Lanthanide (Eu3+ and Tb3+)-Loaded γ-Cyclodextrin Nano-Aggregates
2.3. Fluorescence Properties of Lanthanide (Eu3+ and Tb3+)-Loaded γ-Cyclodextrin Nano-Aggregates
2.4. ECA and TCA Smart Sensing of Anticancer Drugs
2.4.1. Detection of Irinotecan (CPT-11)
2.4.2. Sensitivity of ECA and TCA to Other Anticancer Drugs
2.5. Cytocompatibility Measurement
2.6. Cell Imaging
2.7. Characterization of ECA and TCA
3. Results and Discussion
3.1. Lanthanide (Eu3+/Tb3+)-Loaded γ-Cyclodextrin Nano-Aggregates
3.2. Photophysical Properties of Lanthanide (Eu3+ and Tb3+)-Loaded γ-Cyclodextrin Nano-Aggregates
3.3. Smart Sensing Property of Lanthanide (Eu3+ and Tb3+)-Loaded γ-Cyclodextrin Nano-Aggregates for Anticancer Drug of Irinotecan (CPT-11)
3.4. Cytocompatibility of ECA and TCA
3.5. Detection of CPT-11 in Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogeser, M. From therapeutic drug monitoring to total drug monitoring and drug-omics. Clin. Chem. Lab. Med. 2020, 59, 287–290. [Google Scholar] [CrossRef]
- Luan, F.; He, X.; Zeng, N. Tetrandrine: A review of its anticancer potentials, clinical settings, pharmacokinetics and drug delivery systems. J. Pharm. Pharmacol. 2020, 72, 1491–1512. [Google Scholar] [CrossRef] [PubMed]
- Krens, S.D.; Lassche, G.; Jansman, F.G.A.; Desar, I.M.E.; Lankheet, N.A.G.; Burger, D.M.; Van Herpen, C.M.L.; Van Erp, N.P. Dose recommendations for anticancer drugs in patients with renal or hepatic impairment. Lancet Oncol. 2019, 20, e200–e207. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Li, L.; Zhang, R.; Xu, Z.P. Synergistic cancer photochemotherapy via layered double hydroxide-based trimodal nanomedicine at very low therapeutic doses. ACS Appl. Mater. Interfac. 2021, 13, 7115–7126. [Google Scholar] [CrossRef]
- Huang, W.; Chen, L.; Kang, L.; Jin, M.; Sun, P.; Xin, X.; Gao, Z.; Bae, Y.H. Nanomedicine-based combination anticancer therapy between nucleic acids and small-molecular drugs. Adv. Drug Deliv. Rev. 2017, 115, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Alvau, M.D.; Tartaggia, S.; Meneghello, A.; Casetta, B.; Calia, G.; Serra, P.A.; Polo, F.; Toffoli, G. Enzyme-based electrochemical biosensor for therapeutic drug monitoring of anticancer drug irinotecan. Anal. Chem. 2018, 90, 6012–6019. [Google Scholar] [CrossRef]
- Lavezzi, S.M.; Borella, E.; Carrara, L.; De Nicolao, G.; Magni, P.; Poggesi, I. Mathematical modeling of efficacy and safety for anticancer drugs clinical development. Expert Opin. Drug Discov. 2018, 13, 5–21. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Q.; Zhang, K.; Cheng, M.; Xin, X. Optimal switching control for drug therapy process in cancer chemotherapy. Eur. J. Control. 2018, 42, 49–58. [Google Scholar] [CrossRef]
- Komen, J.; Westerbeek, E.Y.; Kolkman, R.W.; Roesthuis, J.; Lievens, C.; Van den Berg, A.; Van der Meer, A.D. Controlled pharmacokinetic anti-cancer drug concentration profiles lead to growth inhibition of colorectal cancer cells in a microfluidic device. Lab Chip 2020, 20, 3167–3178. [Google Scholar] [CrossRef]
- Shindi, O.; Kanesan, J.; Kendall, G.; Ramanathan, A. The combined effect of optimal control and swarm intelligence on optimization of cancer chemotherapy. Comput. Methods Programs Biomed. 2020, 189, 105327. [Google Scholar] [CrossRef]
- Hifumi, T.; Miyoshi, N.; Kawaguchi, H.; Nomura, K.; Yasuda, N. Immunohistochemical detection of proteins associated with multidrug resistance to anti-cancer drugs in canine and feline primary pulmonary carcinoma. J. Vet. Med. Sci. 2010, 72, 665–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Crist, R.M.; McNeil, S.E.; Clogston, J.D. Ion quantification in liposomal drug products using high performance liquid chromatography. J. Pharm. Biomed. Anal. 2019, 165, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Shishehbore, M.R. A review on analytical methods with special reference to electroanalytical methods for the determination of some anticancer drugs in pharmaceutical and biological samples. Talanta 2021, 229, 122247. [Google Scholar] [CrossRef] [PubMed]
- Brothman, A.R.; Davis, T.P.; Duffy, J.J.; Lindell, T.J. Development of an Antibody to Actinomycin D and Its Application for the Detection of Serum Levels by Radioimmunoassay. Cancer Res. 1982, 42, 2960. [Google Scholar]
- Mullapudi, S.S.; Mitra, D.; Li, M.; Kang, E.-T.; Chiong, E.; Neoh, K.G. Potentiating anti-cancer chemotherapeutics and antimicrobials via sugar-mediated strategies. Mol. Syst. Des. Eng. 2020, 5, 772–791. [Google Scholar] [CrossRef]
- Meisenberg, C.; Ashour, M.E.; El-Shafie, L.; Liao, C.; Hodgson, A.; Pilborough, A.; Khurram, S.A.; Downs, J.A.; Ward, S.E.; El-Khamisy, S.F. Epigenetic changes in histone acetylation underpin resistance to the topoisomerase I inhibitor irinotecan. Nucleic Acids Res. 2017, 45, 1159–1176. [Google Scholar] [CrossRef] [Green Version]
- Bolat, G. Investigation of poly (CTAB-MWCNTs) composite based electrochemical DNA biosensor and interaction study with anticancer drug Irinotecan. Microchem. J. 2020, 159, 105426. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Iwasa, S.; Shoji, H.; Honma, Y.; Takashima, A.; Kato, K.; Hamaguchi, T.; Higuchi, K.; Boku, N. Association between UGT1A1 gene polymorphism and safety and efficacy of irinotecan monotherapy as the third-line treatment for advanced gastric cancer. Gastric Cancer 2019, 22, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Gold, H.T.; Hall, M.J.; Blinder, V.; Schackman, B.R. Cost effectiveness of pharmacogenetic testing for uridine diphosphate glucuronosyltransferase 1A1 before irinotecan administration for metastatic colorectal cancer. Cancer 2009, 115, 3858–3867. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.L.; Huang, C.W.; Lin, Y.W.; Wang, J.H.; Wu, C.C.; Sung, Y.C.; Chen, T.L.; Wang, H.M.; Tang, H.C.; Chen, J.B.; et al. Determination of the UGT1A1 polymorphism as guidance for irinotecan dose escalation in metastatic colorectal cancer treated with first-line bevacizumab and FOLFIRI (PURE FIST). Eur. J. Cancer 2020, 138, 19–29. [Google Scholar] [CrossRef]
- De Man, F.M.; Goey, A.K.L.; Van Schaik, R.H.N.; Mathijssen, R.H.J.; Bins, S. Individualization of irinotecan treatment: A review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin. Pharmacokinet. 2018, 57, 1229–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegrini, G.; Falcone, A.; Fioravanti, A.; Barletta, M.T.; Orlandi, P.; Loupakis, F.; Cerri, E.; Masi, G.; Di Paolo, A.; Kerbel, R.S.; et al. A pharmacokinetic and pharmacodynamic study on metronomic irinotecan in metastatic colorectal cancer patients. Br. J. Cancer 2008, 98, 1312–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, Q.; Liu, X.; Sun, Z.; Wang, H.; Jiang, J. A validated UPLC-MS/MS method to determine free and total irinotecan and its two metabolites in human plasma after intravenous administration of irinotecan hydrochloride liposome injection. J. Pharm. Biomed. Anal. 2019, 170, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Puscasu, A.; Zanchetta, M.; Posocco, B.; Bunka, D.; Tartaggia, S.; Toffoli, G. Development and validation of a selective SPR aptasensor for the detection of anticancer drug irinotecan in human plasma samples. Anal. Bioanal. Chem. 2021, 413, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Marangon, E.; Posocco, B.; Mazzega, E.; Toffoli, G. Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method for the simultaneous determination of irinotecan and its main metabolites in human plasma and its application in a clinical pharmacokinetic study. PLoS ONE 2015, 10, e0118194. [Google Scholar] [CrossRef]
- Bonazza, G.; Tartaggia, S.; Toffoli, G.; Polo, F.; Daniele, S. Voltammetric behaviour of the anticancer drug irinotecan and its metabolites in acetonitrile. Implications for electrochemical therapeutic drug monitoring. Electrochim. Acta 2018, 289, 483–493. [Google Scholar] [CrossRef]
- Aleem, A.R.; Ding, W.; Liu, J.; Li, T.; Guo, Y.; Wang, Q.; Wang, Y.; Wang, Y.; Rehman, F.U.L.; Kipper, M.J.; et al. Visible-light excitable Eu(3+)-induced hyaluronic acid-chitosan aggregates with heterocyclic ligands for sensitive and fast recognition of hazardous ions. Int. J. Biol. Macromol. 2021, 184, 188–199. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, X.; Xi, W.; Tang, M.; Liu, J.; Jiang, H.; Sun, L. Tailored upconversion nanomaterial: A hybrid nano fluorescent sensor for evaluating efficacy of lactate dehydrogenase inhibitors as anticancer drugs. Sens. Actuators B Chem. 2021, 345, 130417. [Google Scholar] [CrossRef]
- Aleem, A.R.; Liu, J.; Wang, J.; Wang, J.; Zhao, Y.; Wang, Y.; Wang, Y.; Wang, W.; Rehman, F.U.; Kipper, M.J.; et al. Selective sensing of Cu(2+) and Fe(3+) ions with vis-excitation using fluorescent Eu(3+)-Induced aggregates of polysaccharides (EIAP) in mammalian cells and aqueous systems. J. Hazard. Mater. 2020, 399, 122991. [Google Scholar] [CrossRef]
- Song, Z.; Wang, J.; Wang, J.; Liu, J.; Wang, X.; Wang, Y.; Aleem, A.R.; Kipper, M.J.; Belfiore, L.A.; Tang, J. Eu3+-induced polysaccharide nano-dumbbell aggregates (PNDA) as drug carriers to smartly report drug concentration through variable fluorescence. Sens. Actuators B Chem. 2021, 336, 129724. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Wang, J.; Wang, Y.; Cao, J.; Hou, L.; Ge, R.; Chi, J.; Huang, L.; Guo, J.; et al. Smart sensing of Cu2+ in living cells by water-soluble and nontoxic Tb3+/Eu3+-induced aggregates of polysaccharides through fluorescence imaging. J. Mater. Chem. C 2020, 8, 8171–8182. [Google Scholar] [CrossRef]
- Parveen, S.; Prasanna, P.K.; Chakraborty, S.; Giri, P.K. Stable deep blue emission with unity quantum yield in organic–inorganic halide perovskite 2D nanosheets doped with cerium and terbium at high concentrations. J. Mater. Chem. C 2021, 9, 2437–2454. [Google Scholar] [CrossRef]
- Zhao, Z.; Bian, M.; Lin, C.; Fu, X.; Yu, G.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. Efficient green OLEDs achieved by a terbium (III) complex with photoluminescent quantum yield close to 100%. Sci. China Chem. 2021, 64, 1504–1509. [Google Scholar] [CrossRef]
- Su, B.; Yang, W.; Wang, Y.; Huang, L.; Popat, K.C.; Kipper, M.J.; Belfiore, L.A.; Tang, J. Europium-functionalized luminescent titania nanotube arrays: Utilizing interactions with glucose, cholesterol and triglycerides for rapid detection application. Mater. Sci Eng. C Mater. Biol. Appl. 2020, 114, 111054. [Google Scholar] [CrossRef] [PubMed]
- Cernea, M.; Secu, M.; Radu, R.; Ganea, P.; Surdu, V.A.; Trusca, R.; Vasile, E.T.; Secu, E.C. Structural, electrical properties and photoluminescence analyses of the terbium doped barium titanate. J. Alloys Compd. 2021, 878, 160380. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Liu, J.; Wang, X.; Aleem, A.R.; Song, Z.; Kipper, M.J.; Tang, J. Smart sensing of bacterial contamination on fluorescent cotton fabrics (FCF) by nontoxic Eu3+-induced polyelectrolyte nano-aggregates (EIPAs). Dye. Pigment. 2020, 181, 108536. [Google Scholar] [CrossRef]
- Rong, M.; Ye, J.; Chen, B.; Wen, Y.; Deng, X.; Liu, Z.-Q. Ratiometric fluorescence detection of stringent ppGpp using Eu-MoS2 QDs test paper. Sens. Actuators B Chem. 2020, 309, 127807. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z.; Li, H. Water-soluble luminescent hybrid composites consisting of oligosilsesquioxanes and lanthanide complexes and their sensing ability for Cu2+. Chem.—A Eur. J. 2016, 22, 3037–3043. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Votava, M.; Ravoo, B.J. Principles and applications of cyclodextrin liquid crystals. Chem Soc. Rev. 2021, 50, 10009–10024. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xia, Y.; Su, L.; Wu, J. Modification of bacillus clarkii gamma-cyclodextrin glycosyltransferase and addition of complexing agents to increase gamma-cyclodextrin production. J. Agric. Food Chem 2020, 68, 12079–12085. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Nepogodiev, S.A.; Stoddart, J.F. Synthetic cyclic oligosaccharides. Chem. Rev. 1998, 98, 1919–1958. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Stoddart, J.F. Cyclodextrin metal-organic frameworks and their applications. Acc. Chem. Res. 2021, 54, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Schlatter, G.; Hebraud, A.; Vicini, S.; Castellano, M. Polymer-free cyclodextrin and natural polymer-cyclodextrin electrospun nanofibers: A comprehensive review on current applications and future perspectives. Carbohydr. Polym. 2021, 264, 118042. [Google Scholar] [CrossRef]
- Fan, W.; An, W.; Huo, M.; Xiao, D.; Lyu, T.; Cui, J. An integrated approach using ozone nanobubble and cyclodextrin inclusion complexation to enhance the removal of micropollutants. Water Res. 2021, 196, 117039. [Google Scholar] [CrossRef]
- Somsri, S.; Kuwamura, N.; Kojima, T.; Yoshinari, N.; Konno, T. Self-assembly of cyclic hexamers of gamma-cyclodextrin in a metallosupramolecular framework with d-penicillamine. Chem. Sci. 2020, 11, 9246–9253. [Google Scholar] [CrossRef]
- Majd, M.; Yazdanpanah, M.; Bayatloo, M.R.; Nojavan, S. Recent advances and applications of cyclodextrins in magnetic solid phase extraction. Talanta 2021, 229, 122296. [Google Scholar] [CrossRef]
- Dos Santos Lima, B.; Shanmugam, S.; De Souza Siqueira Quintans, J.; Quintans-Júnior, L.J.; De Souza Araújo, A.A. Inclusion complex with cyclodextrins enhances the bioavailability of flavonoid compounds: A systematic review. Phytochem. Rev. 2019, 18, 1337–1359. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Y.; Niu, Y.; Ke, Q.; Kou, X. Cyclodextrins as carriers for volatile aroma compounds: A review. Carbohydr. Polym. 2021, 269, 118292. [Google Scholar] [CrossRef]
- Noël, S.; Léger, B.; Ponchel, A.; Sadjadi, S.; Monflier, E. Cyclodextrins as multitask agents for metal nano-heterogeneous catalysis: A review. Environ. Chem. Lett. 2021, 19, 4327–4348. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Li, K.; Qin, Y.; Yu, H.; Li, P. Vascular targeted chitosan-derived nanoparticles as docetaxel carriers for gastric cancer therapy. Int. J. Biol. Macromol. 2019, 126, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kröger, M.; Liu, W.K. Shape effect in cellular uptake of PEGylated nanoparticles: Comparison between sphere, rod, cube and disk. Nanoscale 2015, 7, 16631–16646. [Google Scholar] [CrossRef] [PubMed]
- Pintor, A.V.B.; Queiroz, L.D.; Barcelos, R.; Primo, L.S.G.; Maia, L.C.; Alves, G.G. MTT versus other cell viability assays to evaluate the biocompatibility of root canal filling materials: A systematic review. Int. Endod. J. 2020, 53, 1348–1373. [Google Scholar] [CrossRef] [PubMed]








| Sample | CEu | CTb | CTTA | Cacac | CPhen | Cγ-CD |
|---|---|---|---|---|---|---|
| (mol/L) | (mol/L) | (mol/L) | (mol/L) | (mol/L) | (mol/L) | |
| A | 1.0 × 10−4 | 0 | 3.0 × 10−4 | 0 | 1.0 × 10−4 | 3.3 × 10−4 |
| B | 1.0 × 10−3 | 0 | 3.0 × 10−3 | 0 | 1.0 × 10−3 | 3.3 × 10−3 |
| C | 0 | 1.0 × 10−3 | 0 | 2.0 × 10−3 | 1.0 × 10−3 | 1.0 × 10−4 |
| D | 0 | 1.0 × 10−2 | 0 | 2.0 × 10−2 | 1.0 × 10−2 | 1.0 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Liu, J.; Tang, Q.; Li, C.; Zhang, Y.; Wang, Y.; Wang, Y.; Bi, Y.; Snow, C.D.; Kipper, M.J.; et al. Lanthanide (Eu3+/Tb3+)-Loaded γ-Cyclodextrin Nano-Aggregates for Smart Sensing of the Anticancer Drug Irinotecan. Int. J. Mol. Sci. 2022, 23, 6597. https://doi.org/10.3390/ijms23126597
Guo Y, Liu J, Tang Q, Li C, Zhang Y, Wang Y, Wang Y, Bi Y, Snow CD, Kipper MJ, et al. Lanthanide (Eu3+/Tb3+)-Loaded γ-Cyclodextrin Nano-Aggregates for Smart Sensing of the Anticancer Drug Irinotecan. International Journal of Molecular Sciences. 2022; 23(12):6597. https://doi.org/10.3390/ijms23126597
Chicago/Turabian StyleGuo, Yaowei, Jin Liu, Qinglin Tang, Cuicui Li, Yanying Zhang, Yao Wang, Yanxin Wang, Yupeng Bi, Christopher D. Snow, Matt J. Kipper, and et al. 2022. "Lanthanide (Eu3+/Tb3+)-Loaded γ-Cyclodextrin Nano-Aggregates for Smart Sensing of the Anticancer Drug Irinotecan" International Journal of Molecular Sciences 23, no. 12: 6597. https://doi.org/10.3390/ijms23126597
APA StyleGuo, Y., Liu, J., Tang, Q., Li, C., Zhang, Y., Wang, Y., Wang, Y., Bi, Y., Snow, C. D., Kipper, M. J., Belfiore, L. A., & Tang, J. (2022). Lanthanide (Eu3+/Tb3+)-Loaded γ-Cyclodextrin Nano-Aggregates for Smart Sensing of the Anticancer Drug Irinotecan. International Journal of Molecular Sciences, 23(12), 6597. https://doi.org/10.3390/ijms23126597








