Killing Two Birds with One Stone: Potential Therapies Targeting Psoriasis and Atherosclerosis at the Same Time
Abstract
:1. Introduction
2. Mechanistic Associations between Psoriasis and Cardiometabolic Diseases
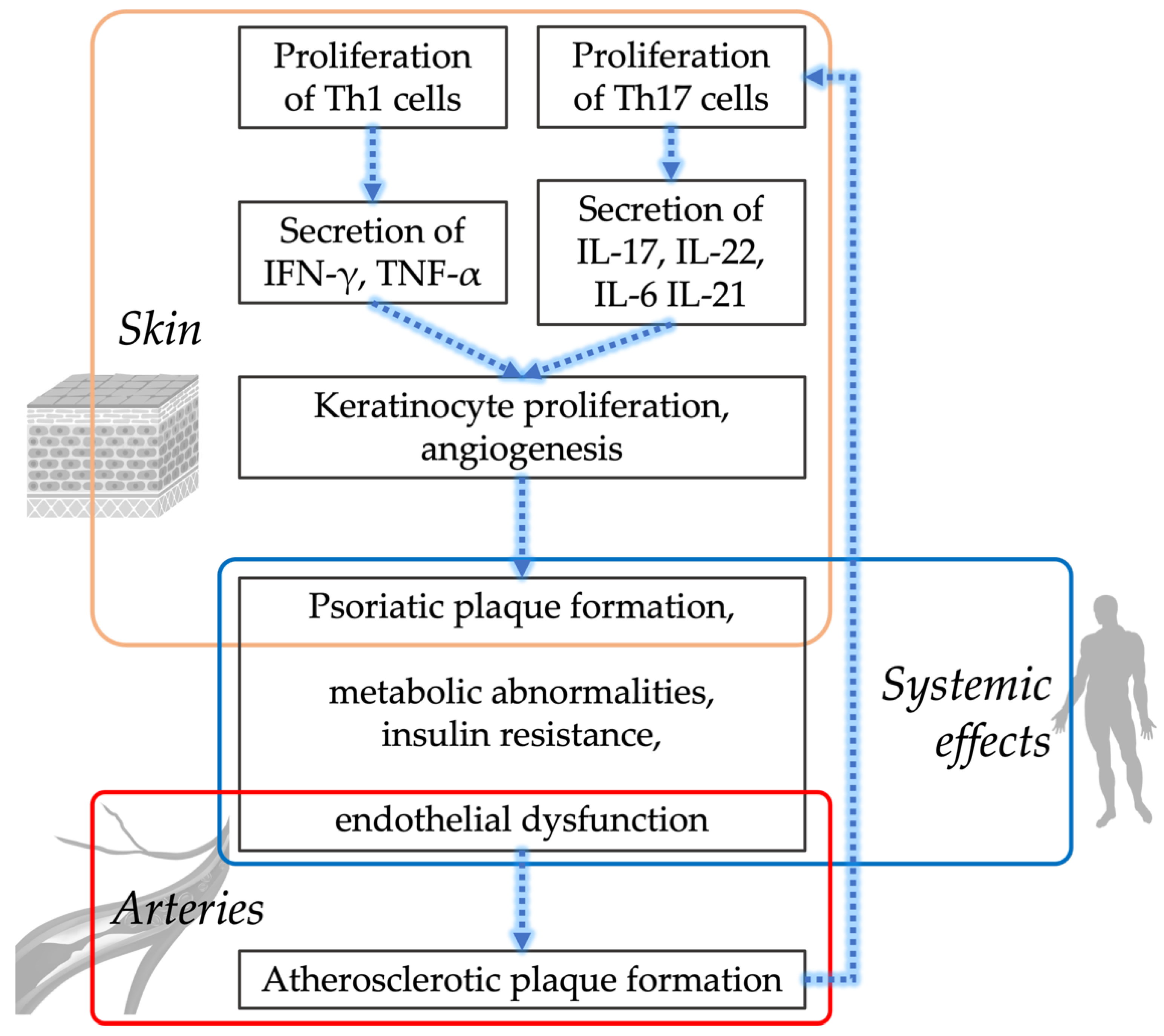
3. Cardiometabolic Phenotype of Patients with Psoriasis
- Patients with psoriasis are frequently overweight [36];
- They frequently have arterial hypertension, which is usually difficult to control (arterial hypertension in patients with psoriasis often requires a dual or triple therapy regimen with a probability of 16.5 times higher of a triple antihypertensive regimen compared to controls) [37];
- They have dyslipidemia with an atherogenic lipid profile (hypertriglyceridemia, low high-density lipoprotein (HDL), harmful qualitative changes in low-density lipoprotein particles (LDL), and postprandial hyperlipidemia); this atherogenic lipid profile predisposes to earlier formation of atherosclerotic plaques [38];
- Due to systemic inflammation, they are also commonly affected by insulin resistance or even type 2 diabetes (prevalence of 11.6% according to a recent systematic review by Holm et al.) [39];
- Patients with psoriasis are believed to be physically less active; however, according to a recent meta-analysis, there were no significant differences in physical activity level between those with or without psoriasis, but the subgroup analysis revealed that patients with psoriasis were significantly less likely to exercise vigorously compared to controls [40]. Vigorous physical activity is independently associated with a reduced incidence of psoriasis [41]. Furthermore, patients with a larger skin area affected by psoriasis and self-awareness performed lower intensity physical activity [40]. There are also several factors that influence physical activity in a person, including obesity, gender, age, financial situation, and in the case of psoriasis, probably also the psychological impact of psoriasis and the distribution of the psoriatic lesion (visible versus nonvisible areas or functional);
- Smoking is also common among patients with psoriasis, which in addition predisposes them to accelerated cardiovascular disease. Plus, the other way around, smoking represents an independent risk factor for the development of psoriasis [42];
- Patients with psoriasis often have unbalanced diets that could influence the incidence and severity of psoriasis and its comorbidities. Their diet often has a higher intake of saturated fatty acids, noncomplex sugars, red meat, and alcohol, which further exacerbate psoriasis and its comorbidities by activating the inflammasome cascade, tumor necrosis factor-α (TNF-α)–interleukin (IL) 23–IL-17 axis, generation of reactive oxygen species, intestinal dysbiosis, and suppression of regulatory T cells [43];
- Depression is another frequent comorbidity in patients with psoriasis. Psoriasis alone can affect mental health; however, systemic inflammation through TNF may have an adverse effect on neurocognitive functions. Extensive data also suggest that depression increases the risk of cardiovascular morbidity and mortality [44]. The risk is already increased in mild depression such as vital exhaustion. The link between depression and atherosclerosis might be inflammation and endothelial dysfunction that are already present in patients with psoriasis [45].
4. Common Ground for Targeting Psoriasis and Atherosclerosis at the Same Time
5. Anti-Psoriatic Treatment and Its Influence on Atherosclerosis
5.1. Traditional Systemic Agents
5.1.1. Methotrexate
5.1.2. Retinoids (Acitretin)
5.1.3. Cyclosporine
5.1.4. Fumaric Acid Esters
5.2. Biologic Therapy
5.2.1. Tumor Necrosis Factor-α Inhibitors (TNF-α Inhibitors) (Adalimumab, Etanercept, Infliximab, and Certolizumab)
5.2.2. Anti-Interleukin-12/-23p40 Agents (Anti-IL-12/23p40) (Ustekinumab)
5.2.3. Anti-IL-17 Agents (Ixekizumab, Secukinumab, and Brodalumab)
5.2.4. Anti-IL-23p19 Agents (Guselkumab, Risankizumab, and Tildrakizumab)
5.2.5. JAK Inhibitors (Tofacitinib)
5.2.6. Apremilast
5.3. Rarely Used Systemic Agents
Colchicine
5.4. Summary of Antipsoriatic Treatment and Its Association with Atherosclerosis
6. Medications Used in the Prevention of Atherosclerotic Cardiovascular Disease That Have an Impact on Psoriasis
6.1. Statins
6.2. Antihypertensive Agents
6.3. Aspirin and Nonsteroidal Anti-Inflammatory Drugs
6.4. Antihyperglycemic Agents
6.4.1. Metformin
6.4.2. GLP-1 Receptor Agonists
6.4.3. Thiazolidinediones
6.4.4. SGLT-2 Inhibitors
6.5. Inhibitors of IL-1β (Anakinra, Canakinumab, and Rilonacept)
6.6. Summary of Preventive Anti-Atherosclerotic Agents and Their Associations with Psoriasis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flammer, A.J.; Ruschitzka, F. Psoriasis and Atherosclerosis: Two Plaques, One Syndrome? Eur. Heart J. 2012, 33, 1989–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Kerkhof, P.C.M. Psoriasis More than Skin Deep. J. Dermatol. Treat. 2008, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Wierzbowska-Drabik, K.; Lesiak, A.; Skibińska, M.; Niedźwiedź, M.; Kasprzak, J.D.; Narbutt, J. Psoriasis and Atherosclerosis—Skin, Joints, and Cardiovascular Story of Two Plaques in Relation to the Treatment with Biologics. Int. J. Mol. Sci. 2021, 22, 10402. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Lan, C.-C.E. Psoriasis and Cardiovascular Comorbidities: Focusing on Severe Vascular Events, Cardiovascular Risk Factors and Implications for Treatment. Int. J. Mol. Sci. 2017, 18, 2211. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S.B.; Lebwohl, M.G. Psoriasis: Which Therapy for Which Patient. J. Am. Acad. Dermatol. 2019, 80, 27–40. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; van Voorhees, A.S.; Gelfand, J.M. Psoriasis and Comorbid Diseases. J. Am. Acad. Dermatol. 2017, 76, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Masson, W.; Lobo, M.; Molinero, G. Psoriasis and Cardiovascular Risk: A Comprehensive Review. Adv. Ther. 2020, 37, 2017–2033. [Google Scholar] [CrossRef] [Green Version]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Boechat, J.L. Psoriatic March, Skin Inflammation and Cardiovascular Events—Two Plaques for One Syndrome. Int. J. Cardiovasc. Sci. 2020, 33. [Google Scholar] [CrossRef]
- Holvoet, P. Stress in Obesity and Associated Metabolic and Cardiovascular Disorders. Scientifica (Cairo) 2012, 2012, 205027. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.W.; Voyles, S.V.; Armstrong, E.J.; Fuller, E.N.; Rutledge, J.C. A Tale of Two Plaques: Convergent Mechanisms of T-Cell-Mediated Inflammation in Psoriasis and Atherosclerosis. Exp. Dermatol. 2011, 20, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Kagen, M.H.; McCormick, T.S.; Cooper, K.D. Regulatory T Cells in Psoriasis. In Cytokines as Potential Therapeutic Targets for Inflammatory Skin Diseases; Springer: Berlin/Heidelberg, Germany; pp. 193–209.
- Mor, A.; Luboshits, G.; Planer, D.; Keren, G.; George, J. Altered Status of CD4+CD25+ Regulatory T Cells in Patients with Acute Coronary Syndromes. Eur. Heart J. 2006, 27, 2530–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N. Engl. J. Med. 2009, 361, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Cytokine Network and T Cell Immunity in Atherosclerosis. Semin. Immunopathol. 2009, 31, 23–33. [Google Scholar] [CrossRef]
- Späh, F. Inflammation in Atherosclerosis and Psoriasis: Common Pathogenic Mechanisms and the Potential for an Integrated Treatment Approach. Br. J. Dermatol. 2008, 159, 10–17. [Google Scholar] [CrossRef]
- Karbach, S.; Croxford, A.L.; Oelze, M.; Schüler, R.; Minwegen, D.; Wegner, J.; Koukes, L.; Yogev, N.; Nikolaev, A.; Reißig, S.; et al. Interleukin 17 Drives Vascular Inflammation, Endothelial Dysfunction, and Arterial Hypertension in Psoriasis-Like Skin Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2658–2668. [Google Scholar] [CrossRef] [Green Version]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease: Pathophysiological, Genetic, and Therapeutic Insights: A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Kakarala, C.L.; Hassan, M.; Belavadi, R.; Gudigopuram, S.V.R.; Raguthu, C.C.; Gajjela, H.; Kela, I.; Sange, I. Beyond the Skin Plaques: Psoriasis and Its Cardiovascular Comorbidities. Cureus 2021, 13, e19679. [Google Scholar] [CrossRef]
- Hao, Y.; Zhu, Y.; Zou, S.; Zhou, P.; Hu, Y.; Zhao, Q.; Gu, L.; Zhang, H.; Wang, Z.; Li, J. Metabolic Syndrome and Psoriasis: Mechanisms and Future Directions. Front. Immunol. 2021, 12, 711060. [Google Scholar] [CrossRef]
- Buerger, C.; Richter, B.; Woth, K.; Salgo, R.; Malisiewicz, B.; Diehl, S.; Hardt, K.; Boehncke, S.; Boehncke, W.-H. Interleukin-1β Interferes with Epidermal Homeostasis through Induction of Insulin Resistance: Implications for Psoriasis Pathogenesis. J. Investig. Dermatol. 2012, 132, 2206–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlüter, K.; Diehl, S.; Lang, V.; Kaufmann, R.; Boehncke, W.; Bürger, C. Insulin Resistance May Contribute to Upregulation of Adhesion Molecules on Endothelial Cells in Psoriatic Plaques. Acta Derm. Venereol. 2016, 96, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannu, S.; Rosmarin, D. Psoriasis in Patients with Metabolic Syndrome or Type 2 Diabetes Mellitus: Treatment Challenges. Am. J. Clin. Dermatol. 2021, 22, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Sereflican, B.; Goksugur, N.; Bugdayci, G.; Polat, M.; Haydar Parlak, A. Serum Visfatin, Adiponectin, and Tumor Necrosis Factor Alpha (TNF-α) Levels in Patients with Psoriasis and Their Correlation with Disease Severity. Acta Dermatovenerol. Croat. 2016, 24, 13–19. [Google Scholar]
- Bai, F.; Zheng, W.; Dong, Y.; Wang, J.; Garstka, M.A.; Li, R.; An, J.; Ma, H. Serum Levels of Adipokines and Cytokines in Psoriasis Patients: A Systematic Review and Meta-Analysis. Oncotarget 2018, 9, 1266–1278. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of Adiponectin Receptors That Mediate Antidiabetic Metabolic Effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef]
- Langley, R.G.; Ellis, C.N. Evaluating Psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J. Am. Acad. Dermatol. 2004, 51, 563–569. [Google Scholar] [CrossRef]
- Gerdes, S.; Pinter, A.; Biermann, M.; Papavassilis, C.; Reinhardt, M. Adiponectin Levels in a Large Pooled Plaque Psoriasis Study Population. J. Dermatol. Treat. 2020, 31, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Fleissner, F.; Thum, T. Critical Role of the Nitric Oxide/Reactive Oxygen Species Balance in Endothelial Progenitor Dysfunction. Antioxid. Redox Signal. 2011, 15, 933–948. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F. Role of Oxidative Modifications in Atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Nguyen, H.; Chiasson, V.L.; Chatterjee, P.; Kopriva, S.E.; Young, K.J.; Mitchell, B.M. Interleukin-17 Causes Rho-Kinase-Mediated Endothelial Dysfunction and Hypertension. Cardiovasc. Res. 2013, 97, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal Relationships Between Insulin Resistance and Endothelial Dysfunction. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef] [PubMed]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial Function in Cardiovascular Medicine: A Consensus Paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valaiyaduppu Subas, S.; Mishra, V.; Busa, V.; Antony, I.; Marudhai, S.; Patel, M.; Cancarevic, I. Cardiovascular Involvement in Psoriasis, Diagnosing Subclinical Atherosclerosis, Effects of Biological and Non-Biological Therapy: A Literature Review. Cureus 2020, 12, e11173. [Google Scholar] [CrossRef]
- Blegvad, C.; Nybo Andersen, A.-M.; Groot, J.; Zachariae, C.; Barker, J.; Skov, L. Clinical Characteristics Including Cardiovascular and Metabolic Risk Factors in Adolescents with Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1516–1523. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Lin, S.W.; Chambers, C.J.; Sockolov, M.E.; Chin, D.L. Psoriasis and Hypertension Severity: Results from a Case-Control Study. PLoS ONE 2011, 6, e18227. [Google Scholar] [CrossRef] [Green Version]
- Akkara Veetil, B.M.; Matteson, E.L.; Maradit-Kremers, H.; McEvoy, M.T.; Crowson, C.S. Trends in Lipid Profiles in Patients with Psoriasis: A Population-Based Analysis. BMC Dermatol. 2012, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Holm, J.G.; Thomsen, S.F. Type 2 Diabetes and Psoriasis: Links and Risks. Psoriasis Targets Ther. 2019, 9, 159163. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Sun, X.Y.; Miao, X.; Xu, R.; Ma, T.; Zhang, Y.N.; Li, H.J.; Li, B.; Li, X. Association between Physical Activity and Risk of Prevalent Psoriasis. Medicine 2018, 97, e11394. [Google Scholar] [CrossRef]
- Frankel, H.C.; Han, J.; Li, T.; Qureshi, A.A. The Association Between Physical Activity and the Risk of Incident Psoriasis. Arch. Dermatol. 2012, 148, 943. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.W.; Harskamp, C.T.; Dhillon, J.S.; Armstrong, E.J. Psoriasis and Smoking: A Systematic Review and Meta-analysis. Br. J. Dermatol. 2014, 170, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Psoriasis. Int. J. Mol. Sci. 2020, 21, 5405. [Google Scholar] [CrossRef] [PubMed]
- So, H.; Tam, L.-S. Cardiovascular Disease and Depression in Psoriatic Arthritis: Multidimensional Comorbidities Requiring Multidisciplinary Management. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101689. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Kollia, N.; Tousoulis, D. The Link between Depression and Atherosclerosis through the Pathways of Inflammation and Endothelium Dysfunction. Maturitas 2018, 109, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of Future Cardiovascular Outcomes by Flow-Mediated Vasodilatation of Brachial Artery: A Meta-Analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef]
- Blacher, J.; Asmar, R.; Djane, S.; London, G.M.; Safar, M.E. Aortic Pulse Wave Velocity as a Marker of Cardiovascular Risk in Hypertensive Patients. Hypertension 1999, 33, 1111–1117. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, H.; Wang, X.; Kvist-Hansen, A.; Krakauer, M.; Gørtz, P.M.; McCauley, B.D.; Skov, L.; Becker, C.; Hansen, P.R. Biomarkers of Subclinical Atherosclerosis in Patients with Psoriasis. Sci. Rep. 2021, 11, 21438. [Google Scholar] [CrossRef]
- Goldberg, R.B. Cytokine and Cytokine-Like Inflammation Markers, Endothelial Dysfunction, and Imbalanced Coagulation in Development of Diabetes and Its Complications. J. Clin. Endocrinol. Metab. 2009, 94, 3171–3182. [Google Scholar] [CrossRef] [Green Version]
- Krahel, J.A.; Baran, A.; Kamiński, T.W.; Maciaszek, M.; Flisiak, I. Methotrexate Decreases the Level of PCSK9—A Novel Indicator of the Risk of Proatherogenic Lipid Profile in Psoriasis. The Preliminary Data. J. Clin. Med. 2020, 9, 910. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef]
- Rallidis, L.S. Tracing New Atherogenic Properties of PCSK9: Another Insight into the Pathogenesis of Atherosclerosis ‘the Minotaur’s Labyrinth’. Hell. J. Cardiol. 2019, 60, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, P.S.; Rizk, D.; Lowe, N.J. Retinoid Therapy for Psoriasis. Dermatol. Clin. 2004, 22, 467–476. [Google Scholar] [CrossRef]
- Nozaki, Y.; Yamagata, T.; Sugiyama, M.; Ikoma, S.; Kinoshita, K.; Funauchi, M. Anti-Inflammatory Effect of All-Trans-Retinoic Acid in Inflammatory Arthritis. Clin. Immunol. 2006, 119, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.S.; Ertugrul, D.T.; Kalkan, G.; Bilgili, S.G.; Celik, H.T.; Takci, Z.; Balahoroglu, R.; Calka, O. The Effect of Acitretin Treatment on Insulin Resistance, Retinol-Binding Protein-4, Leptin, and Adiponectin in Psoriasis Vulgaris: A Noncontrolled Study. Dermatology 2013, 227, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, W.T.; Blanchard, P.-G.; Turcotte, V.; Laplante, M.; Sariahmetoglu, M.; Brindley, D.N.; Deshaies, Y. Depot-Specific Effects of the PPARγ Agonist Rosiglitazone on Adipose Tissue Glucose Uptake and Metabolism. J. Lipid Res. 2009, 50, 1185–1194. [Google Scholar] [CrossRef] [Green Version]
- Kockx, M.; Jessup, W.; Kritharides, L. Cyclosporin A and Atherosclerosis—Cellular Pathways in Atherogenesis. Pharmacol. Ther. 2010, 128, 106–118. [Google Scholar] [CrossRef]
- Rosmarin, D.M.; Lebwohl, M.; Elewski, B.E.; Gottlieb, A.B. Cyclosporine and Psoriasis: 2008 National Psoriasis Foundation Consensus Conference. J. Am. Acad. Dermatol. 2010, 62, 838–853. [Google Scholar] [CrossRef]
- Ahlehoff, O.; Skov, L.; Gislason, G.; Gniadecki, R.; Iversen, L.; Bryld, L.E.; Lasthein, S.; Lindhardsen, J.; Kristensen, S.L.; Torp-Pedersen, C.; et al. Cardiovascular Outcomes and Systemic Anti-Inflammatory Drugs in Patients with Severe Psoriasis: 5-Year Follow-up of a Danish Nationwide Cohort. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1128–1134. [Google Scholar] [CrossRef]
- Özdemir, M.; Yuksel, M.; Gokbel, H.; Okudan, N.; Mevlitoglu, İ. Serum Leptin, Adiponectin, Resistin and Ghrelin Levels in Psoriatic Patients Treated with Cyclosporin. J. Dermatol. 2012, 39, 443–448. [Google Scholar] [CrossRef]
- Balak, D. Fumaric Acid Esters in the Management of Psoriasis. Psoriasis: Targets Ther. 2015, 9, 51490. [Google Scholar] [CrossRef] [Green Version]
- Ahlehoff, O.; Skov, L.; Gislason, G.; Lindhardsen, J.; Kristensen, S.L.; Iversen, L.; Lasthein, S.; Gniadecki, R.; Dam, T.N.; Torp-Pedersen, C.; et al. Cardiovascular Disease Event Rates in Patients with Severe Psoriasis Treated with Systemic Anti-Inflammatory Drugs: A Danish Real-World Cohort Study. J. Intern. Med. 2013, 273, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Schottelius, A.J.G.; Moldawer, L.L.; Dinarello, C.A.; Asadullah, K.; Sterry, W.; Edwards, C.K. Biology of Tumor Necrosis Factor-Alpha- Implications for Psoriasis. Exp. Dermatol. 2004, 13, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lin, N.; Li, L.; Li, Y. The Effect of TNF Inhibitors on Cardiovascular Events in Psoriasis and Psoriatic Arthritis: An Updated Meta-Analysis. Clin. Rev. Allergy Immunol. 2016, 51, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Plomgaard, P.; Bouzakri, K.; Krogh-Madsen, R.; Mittendorfer, B.; Zierath, J.R.; Pedersen, B.K. Tumor Necrosis Factor-α Induces Skeletal Muscle Insulin Resistance in Healthy Human Subjects via Inhibition of Akt Substrate 160 Phosphorylation. Diabetes 2005, 54, 2939–2945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Juanatey, C.; Vazquez-Rodriguez, T.R.; Miranda-Filloy, J.A.; Gomez-Acebo, I.; Testa, A.; Garcia-Porrua, C.; Sanchez-Andrade, A.; Llorca, J.; González-Gay, M.A. Anti-TNF-Alpha-Adalimumab Therapy Is Associated with Persistent Improvement of Endothelial Function without Progression of Carotid Intima-Media Wall Thickness in Patients with Rheumatoid Arthritis Refractory to Conventional Therapy. Mediat. Inflamm. 2012, 2012, 674265. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, J.M.; Shin, D.B.; Alavi, A.; Torigian, D.A.; Werner, T.; Papadopoulos, M.; Takeshita, J.; Noe, M.H.; Dey, A.K.; Playford, M.P.; et al. A Phase IV, Randomized, Double-Blind, Placebo-Controlled Crossover Study of the Effects of Ustekinumab on Vascular Inflammation in Psoriasis (the VIP-U Trial). J. Investig. Dermatol. 2020, 140, 85–93.e2. [Google Scholar] [CrossRef] [Green Version]
- Weber, B.; Merola, J.F.; Husni, M.E.; di Carli, M.; Berger, J.S.; Garshick, M.S. Psoriasis and Cardiovascular Disease: Novel Mechanisms and Evolving Therapeutics. Curr. Atheroscler. Rep. 2021, 23, 67. [Google Scholar] [CrossRef]
- Gagliani, N.; Vesely, M.C.A.; Iseppon, A.; Brockmann, L.; Xu, H.; Palm, N.W.; de Zoete, M.R.; Licona-Limón, P.; Paiva, R.S.; Ching, T.; et al. Th17 Cells Transdifferentiate into Regulatory T Cells during Resolution of Inflammation. Nature 2015, 523, 221–225. [Google Scholar] [CrossRef]
- Kvist-Hansen, A.; Hansen, P.R.; Skov, L. Systemic Treatment of Psoriasis with JAK Inhibitors: A Review. Dermatol. Ther. 2020, 10, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Fatkhullina, A.R.; Peshkova, I.O.; Dzutsev, A.; Aghayev, T.; McCulloch, J.A.; Thovarai, V.; Badger, J.H.; Vats, R.; Sundd, P.; Tang, H.-Y.; et al. An Interleukin-23-Interleukin-22 Axis Regulates Intestinal Microbial Homeostasis to Protect from Diet-Induced Atherosclerosis. Immunity 2018, 49, 943–957.e9. [Google Scholar] [CrossRef] [Green Version]
- Kume, K.; Amano, K.; Yamada, S.; Kanazawa, T.; Ohta, H.; Hatta, K.; Amano, K.; Kuwaba, N. Tofacitinib Improves Atherosclerosis despite Up-Regulating Serum Cholesterol in Patients with Active Rheumatoid Arthritis: A Cohort Study. Rheumatol. Int. 2017, 37, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Schafer, P. Apremilast Mechanism of Action and Application to Psoriasis and Psoriatic Arthritis. Biochem. Pharmacol. 2012, 83, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Barbarroja, N.; de la Rosa, I.A.; de la Rosa-Garrido, M.D.; Perez-Sanchez, C.; Abalos-Aguilera, M.C.; Ruiz-Ponce, M.; Jiménez-Gómez, Y.; Escudero-Contreras, A.; Collantes-Estévez, E.; Lopez-Pedrera, C. Interplay Among Inflammation, Adipokines and Endothelial Dysfunction in Patients with Psoriatic Arthritis. Relationship with Cardiovascular and Metabolic Comorbidities. Modulation By Apremilast [Abstract]. Arthritis Rheumatol. 2018, 70, 1662. [Google Scholar]
- Robinson, K.P.; Chan, J.J. Colchicine in Dermatology: A Review. Australas. J. Dermatol. 2018, 59, 278–285. [Google Scholar] [CrossRef]
- Sardana, K.; Sinha, S.; Sachdeva, S. Colchicine in Dermatology: Rediscovering an Old Drug with Novel Uses. Indian Dermatol. Online J. 2020, 11, 693–700. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Zhang, F.; He, Q.; Qin, C.H.; Little, P.J.; Weng, J.; Xu, S. Therapeutic Potential of Colchicine in Cardiovascular Medicine: A Pharmacological Review. Acta Pharmacol. Sin. 2022, 1–18. [Google Scholar] [CrossRef]
- Elnabawi, Y.A.; Dey, A.K.; Goyal, A.; Groenendyk, J.W.; Chung, J.H.; Belur, A.D.; Rodante, J.; Harrington, C.L.; Teague, H.L.; Baumer, Y.; et al. Coronary Artery Plaque Characteristics and Treatment with Biologic Therapy in Severe Psoriasis: Results from a Prospective Observational Study. Cardiovasc. Res. 2019, 115, 721–728. [Google Scholar] [CrossRef]
- Gisondi, P.; Cazzaniga, S.; Chimenti, S.; Giannetti, A.; MacCarone, M.; Picardo, M.; Girolomoni, G.; Naldi, L.; Griseta, V.; Miracapillo, A.; et al. Metabolic Abnormalities Associated with Initiation of Systemic Treatment for Psoriasis: Evidence from the Italian Psocare Registry. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e30–e41. [Google Scholar] [CrossRef]
- Masson, W.; Lobo, M.; Molinero, G.; Rossi, E. Should All Patients with Psoriasis Receive Statins? Analysis According to Different Strategies. An. Bras. Dermatol. 2019, 94, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.J.; Gotto, A.M.; Basson, C.T. The Evolving Role of Statins in the Management of Atherosclerosis. J. Am. Coll Cardiol 2000, 35, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Socha, M.; Pietrzak, A.; Grywalska, E.; Pietrzak, D.; Matosiuk, D.; Kiciński, P.; Rolinski, J. The Effect of Statins on Psoriasis Severity: A Meta-Analysis of Randomized Clinical Trials. Arch. Med. Sci. 2020, 16, 90343. [Google Scholar] [CrossRef] [PubMed]
- Sarian, O.I.; Bolotna, L.A. Treatment of Endothelial Dysfunction with Rosuvastatin in Patients with Psoriasis. Arch. Balk. Med. Union 2019, 54, 25–31. [Google Scholar] [CrossRef]
- Wu, S.; Han, J.; Li, W.-Q.; Qureshi, A.A. Hypertension, Antihypertensive Medication Use, and Risk of Psoriasis. JAMA Dermatol. 2014, 150, 957. [Google Scholar] [CrossRef] [PubMed]
- Baccino, D.; Merlo, G.; Cozzani, E.; Rosa, G.M.; Tini, G.; Burlando, M.; Parodi, A. Cutaneous Effects of Antihypertensive Drugs. G. Ital. Di Dermatol. E Venereol. 2020, 155, S0392–S0488. [Google Scholar] [CrossRef]
- Effects of an Angiotensin-Converting–Enzyme Inhibitor, Ramipril, on Cardiovascular Events in High-Risk Patients. N. Engl. J. Med. 2000, 342, 145–153. [CrossRef]
- Abdelaziz, H.K.; Saad, M.; Pothineni, N.V.K.; Megaly, M.; Potluri, R.; Saleh, M.; Kon, D.L.C.; Roberts, D.H.; Bhatt, D.L.; Aronow, H.D.; et al. Aspirin for Primary Prevention of Cardiovascular Events. J. Am. Coll. Cardiol. 2019, 73, 2915–2929. [Google Scholar] [CrossRef]
- Wu, S.; Han, J.; Qureshi, A. Use of Aspirin, Non-Steroidal Anti-Inflammatory Drugs, and Acetaminophen (Paracetamol), and Risk of Psoriasis and Psoriatic Arthritis: A Cohort Study. Acta Derm. Venereol. 2015, 95, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-E.; Choi, M.S. A Molecular Perspective on the Potential Benefits of Metformin for the Treatment of Inflammatory Skin Disorders. Int. J. Mol. Sci. 2020, 21, 8960. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity. JAMA 2021, 325, 1414. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, Mortality, and Kidney Outcomes with GLP-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Stanescu, A.M.A.; Simionescu, A.A.; Florea, M.; Diaconu, C.C. Is Metformin a Possible Beneficial Treatment for Psoriasis? A Scoping Review. J. Pers. Med. 2021, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhansali, A. Randomized Placebo Control Study of Insulin Sensitizers (Metformin and Pioglitazone) in Psoriasis Patients with Metabolic Syndrome (Topical Treatment Cohort). BMC Dermatol. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes—State-of-the-Art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Buysschaert, M.; Tennstedt, D.; Preumont, V. Improvement of Psoriasis during Exenatide Treatment in a Patient with Diabetes. Diabetes Metab. 2012, 38, 86–88. [Google Scholar] [CrossRef]
- Buysschaert, M.; Baeck, M.; Preumont, V.; Marot, L.; Hendrickx, E.; van Belle, A.; Dumoutier, L. Improvement of Psoriasis during Glucagon-like Peptide-1 Analogue Therapy in Type 2 Diabetes Is Associated with Decreasing Dermal Γδ T-Cell Number: A Prospective Case-Series Study. Br. J. Dermatol. 2014, 171, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Faurschou, A.; Knop, F.K.; Thyssen, J.P.; Zachariae, C.; Skov, L.; Vilsbøll, T. Improvement in Psoriasis after Treatment with the Glucagon-like Peptide-1 Receptor Agonist Liraglutide. Acta Diabetol. 2014, 51, 147–150. [Google Scholar] [CrossRef]
- Patle, C.B.; de Lemos, J.A.; Wyne, K.L.; McGuire, D.K. Thiazolidinediones and Risk for Atherosclerosis: Pleiotropic Effects of PPARγ Agonism. Diabetes Vasc. Dis. Res. 2006, 3, 65–71. [Google Scholar] [CrossRef]
- Mansouri, B.; Menter, A. Reporting of MABp1 for the Treatment of Psoriasis. JAMA Dermatol. 2015, 151, 1143. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kishi, S.; Ninomiya, K.; Tomii, D.; Koseki, K.; Sato, Y.; Okuno, T.; Sato, K.; Koike, H.; Yahagi, K.; et al. Impact of Abdominal Fat Distribution, Visceral Fat, and Subcutaneous Fat on Coronary Plaque Scores Assessed by 320-Row Computed Tomography Coronary Angiography. Atherosclerosis 2019, 287, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Balci, A.; Balci, D.D.; Yonden, Z.; Korkmaz, I.; Yenin, J.Z.; Celik, E.; Okumus, N.; Egilmez, E. Increased Amount of Visceral Fat in Patients with Psoriasis Contributes to Metabolic Syndrome. Dermatology 2010, 220, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, X.; Ilyas, I.; Zheng, X.; Luo, S.; Little, P.J.; Kamato, D.; Sahebkar, A.; Wu, W.; Weng, J.; et al. Impact of Sodium Glucose Cotransporter 2 (SGLT2) Inhibitors on Atherosclerosis: From Pharmacology to Pre-Clinical and Clinical Therapeutics. Theranostics 2021, 11, 4502–4515. [Google Scholar] [CrossRef]
- Bujak, M.; Frangogiannis, N.G. The Role of IL-1 in the Pathogenesis of Heart Disease. Arch. Immunol. Ther. Exp. 2009, 57, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Samuel, M.; Tardif, J.-C. Lessons Learned from Large Cardiovascular Outcome Trials Targeting Inflammation in Cardiovascular Disease (CANTOS, CIRT, COLCOT and LoDoCo2). Future Cardiol. 2021, 17, 411–414. [Google Scholar] [CrossRef]
- Prodanowich, S.; Ma, F.; Taylor, J.; Pezon, C.; Fasihi, T.; Kirsner, R. Methotrexate Reduces Incidence of Vascular Diseases in Veterans with Psoriasis or Rheumatoid Arthritis. J. Am. Acad. Dermatol. 2005, 52, 262–267. [Google Scholar] [CrossRef]
- Dehpouri, T.; Rahmatpour Rokni, G.; Ahangar Narenjbon, N.; Goldust, M.; Yamauchi, P.S.; Wollina, U.; Lotti, T.; Kircik, L.; di Lernia, V.G.; Sonthalia, S.; et al. Evaluation of the Glycemic Effect of Methotrexate in Psoriatic Arthritis Patients with Metabolic Syndrome: A Pilot Study. Dermatol. Rep. 2019, 11, 7965. [Google Scholar] [CrossRef]
- van den Berg, W.B.; McInnes, I.B. Th17 Cells and IL-17 A—Focus on Immunopathogenesis and Immunotherapeutics. Semin. Arthritis Rheum. 2013, 43, 158–170. [Google Scholar] [CrossRef]
- Liu, N.; Su, D.; Liu, K.; Liu, B.; Wang, S.; Zhang, X. The Effects of IL-17/IL-17R Inhibitors on Atherosclerosis in Psoriasis and Psoriatic Arthritis. Medicine 2021, 100, e24549. [Google Scholar] [CrossRef]
- Deng, M.; Su, D.; Xu, S.; Little, P.J.; Feng, X.; Tang, L.; Shen, A. Metformin and Vascular Diseases: A Focused Review on Smooth Muscle Cell Function. Front. Pharmacol. 2020, 11, 00635. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Paolisso, P.; Sacra, C.; Mauro, C.; Minicucci, F.; Portoghese, M.; Rizzo, M.R.; Barbieri, M.; Sasso, F.C.; D’Onofrio, N.; et al. Effects of Metformin Therapy on Coronary Endothelial Dysfunction in Patients With Prediabetes With Stable Angina and Nonobstructive Coronary Artery Stenosis: The CODYCE Multicenter Prospective Study. Diabetes Care 2019, 42, 1946–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radbakhsh, S.; Atkin, S.L.; Simental-Mendia, L.E.; Sahebkar, A. The Role of Incretins and Incretin-Based Drugs in Autoimmune Diseases. Int. Immunopharmacol. 2021, 98, 107845. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes. JAMA Cardiol. 2021, 6, 148. [Google Scholar] [CrossRef]

| Agent(s) | Anti-Psoriatic Effects | Clinical Outcomes in Psoriasis | Anti-Atherosclerotic Effects | Clinical Outcomes in Atherosclerosis | ||
|---|---|---|---|---|---|---|
| Psoriasis treatment | Traditional systemic agents | Methotrexate | Anti-inflammatory action (↓ leukocytes, ↓ IL-1 and IL-6, ↑ IL-4 and IL-10, ↓ Th1 cells) [50] | Decrease in PASI [50,51,109,110] | Anti-inflammatory action (↓ IL-6, CRP and IL-1β) [51,108] ↓ PCSK9 level [50] | Reduction of cardiovascular risk if elevated inflammatory burden [50,51,109,110] |
| Retinoids | Anti-inflammatory action (↓ IL-6, IL-12, TNF-α) [53,54,55,56] Modulation of keratinocyte differentiation [53] | Decrease in PASI [53,58] | Reduction in leptin levels [55] Improvement of insulin resistance [55]; Redistribution of fat from visceral to subcutaneous area [55] | Unknown effect on MACE | ||
| Cyclosporine | Inhibition of T-cell function [58]; Down-regulation of ICAM-1 on keratinocytes and endothelial cells [58] Reduction of expression of Th17 pathway genes [58] ↓ TNF-α, IL-17, IL-22, IL-23p19 [58] ↓VEGF [58] | Decrease in PASI; useful in the management of psoriatic crises or as a bridge therapy [58,59] | Increase adiponectin and resistin [60] | Unknown effect on MACE | ||
| Fumaric acid esters | Immunomodulatory, anti-inflammatory and antioxidant effects that are not yet completely understood [4,61] | Decrease in PASI [4] | ↓ CRP and ↑ adiponectin levels [4] | Unknown effect on MACE | ||
| Biologic agents | TNF-α inhibitors | Anti-inflammatory action (↓ IL-1, IL-6, IL-8, ICAM-1, P-selectin and E-selectin [5,63] | Decrease in PASI [5,63] | Improvement in insulin sensitivity [65] Adalimumab improves endothelial function and carotid arterial stiffness [64,66] | Reduction of cardiovascular events and mortality [64] Reduction of coronary artery plaque burden after one year of treatment [80] | |
| Anti-IL-12/23p40 agents | Inhibition of Th1 and Th17 response [68] ↓ TNF-α, IL-1β, IL-17A, IL-6, VCAM-1 [67] | Decrease in PASI [68] | Transient decrease in aortic vascular inflammation [67] ↓ TNF-α, IL-1β, IL-17A, IL-6, VCAM-1 [67] | Reduction of coronary artery plaque burden after one year of treatment [80] | ||
| Anti-IL-17 agents | Inhibition of IL-17A [68] Reduction of recruitment of Th17 cells, neutrophils, and dendritic cells to the skin [111] | Decrease in PASI; superior to anti-TNF-α and anti-IL-12/23p40 [68] | ↑ IL-1 and IL-6 secretion [111] up-regulation of inducible NOS [111] inhibition of MMPs in endothelial cells [111,112] Induction of pro-atherogenic gut microbiota that could have a pro-atherogenic effect [71] | Reduction of coronary artery plaque burden after one year of treatment [80] No significant increase in MACE | ||
| Anti-IL-23p19 agents | Inhibition of Th17 cell development and propagation [68] ↓ IL-17, IL-21 and IL-22 [68] | Decrease in PASI [68] | Unknown effect on MACE | |||
| Others | JAK inhibitors | Inhibition of signal pathway and gene transcription involved in inflammatory cytokine production [68,70] | Decrease in PASI [68,70] | Reduction of carotid intima-media thickness [72] Limitation of vascular damage [72] ↑ fasting total cholesterol levels [72] | Improved cardiovascular risk in one study [72] | |
| Apremilast | ↑ cAMP resulting in immunomodulation [73] ↓TNF-α, IL-10 and IL-23 [73] ↓ expression of inducible NOS [68,73] | Decrease in PASI [68,70] | Decrease in insulin resistance, levels of apolipoprotein B, body mass index [74] Improvement of endothelial function [74] | No increased risk for MACE [5] | ||
| Rarely used systemic agent | Colchicine | Inhibition of inflammasome [75] ↓ IL-1, IL-6, TNF-α [75,76] | Improvement of pustular psoriasis and palmoplantar pustulosis [75] | Inhibition of endothelial cell dysfunction and inflammation [79] Inhibition of smooth muscle cell proliferation and migration [79] Inhibition macrophage chemotaxis, migration, and adhesion [79] Inhibition of platelet activation [8,77,78,79] | Reduction in MACE [8,77,78] Recommendation in secondary prevention in patients with high cardiovascular risk [8] | |
| Cardiometabolic treatment | Anti-atherosclerotic treatment | Statins | Reduction of inflammation and angiogenesis [84,85] ↓ IL-6 and hsCRP [84,85] | Decrease in PASI [84] | ↓ LDL [83] Improvement of endothelial dysfunction [83] Inhibition of cellular migration [83] Inhibition of plaque thrombogenicity [8,83] | Reduction in MACE [8] |
| Antihypertensive agents | Beta-blockers can block beta-adrenergic receptors in skin resulting in ↓cAMP which triggers pro-inflammatory state with stimulation of keratinocyte proliferation; With respect to ACE inhibitors, probably complex pathways are involved which are yet unknown. | Might trigger psoriasis (especially beta-blockers, less likely ACE inhibitors) Unknown effect on PASI | Suppression of vasoconstriction, smooth muscle proliferation, connective tissue synthesis, and chemotaxis of monocytes by angiotensin II [8,88] | Reduction in MACE [8] | ||
| Aspirin | Insufficient data | Possible induction or exacerbation of psoriasis—insufficient data | Antithrombotic effect [8] | Used in the secondary prevention of atherosclerosis; Lowers the risk of nonfatal myocardial infarction and ischemic stroke [89] No reduction in all-cause or cardiovascular mortality [8,89] | ||
| Antihyperglicemic agents | Metformin | Anti-inflammatory action [95,113] ↓ IL-1β [93,94,97,98] | Possible decrease in PASI [95] | Improvement of insulin resistance [113] ↓ LDL and total cholesterol [113] Improvement of endothelial dysfunction [113] Improvement of function of vascular smooth muscle cells [113] Inhibition of cardiac remodeling [113] | Reduction in MACE in patients with pre-diabetes [114] | |
| GLP-1 receptor agonists | Anti-inflammatory action by ↓ IL-17 [98,99,100] | Decrease in PASI [98,99,100] | ↓ blood pressure [93] ↓ body weight [93] Improvement of dyslipidemia [93] ↑ NO [93] Inhibits of adhesion and procoagulant factors [96,97] | Reduction of MACE in patients with type 2 diabetes [103] | ||
| Thiazolidinediones | Anti-inflammatory effects: Reduction of lymphocyte migration [115] Reduction of macrophage activation [115] Decrease in IL-17 expression [115] Modulation of monocyte cytokine secretion [7,95,115] | Increase in PASI [7,95] | Improvement of insulin sensitivity [101] Redistribution of fat from visceral to subcutaneous depot [101] ↓ blood pressure [101] Improvement of endothelial function [101] | Reduction of MACE in patients with type 2 diabetes [101,107] | ||
| SGLT-2 inhibitors | Unknown—no data | Insufficient data | Inhibition of vascular inflammation [116] Reduction of oxidative stress [116] Improvement of endothelial dysfunction [116] Prevention of platelet activation [116] Reduction of foam cell formation [116] Decrease in inflammasome activation [105,116] | Reduction of MACE in patients with type 2 diabetes [105] | ||
| Other | IL-1β inhibitors | Inhibition of systemic inflammation [102] | Mild decrease in PASI [102] | Inhibition of the formation of atheromatous lesions [112] Decrease in vascular inflammation [112] Plaque stabilization [112] Prevention of harmful cardiac remodeling by inhibition of MMPs [107] | Reduction of recurrent cardiovascular events in patients with previous acute myocardial infarction [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merzel Šabović, E.K.; Starbek Zorko, M.; Janić, M. Killing Two Birds with One Stone: Potential Therapies Targeting Psoriasis and Atherosclerosis at the Same Time. Int. J. Mol. Sci. 2022, 23, 6648. https://doi.org/10.3390/ijms23126648
Merzel Šabović EK, Starbek Zorko M, Janić M. Killing Two Birds with One Stone: Potential Therapies Targeting Psoriasis and Atherosclerosis at the Same Time. International Journal of Molecular Sciences. 2022; 23(12):6648. https://doi.org/10.3390/ijms23126648
Chicago/Turabian StyleMerzel Šabović, Eva Klara, Mateja Starbek Zorko, and Miodrag Janić. 2022. "Killing Two Birds with One Stone: Potential Therapies Targeting Psoriasis and Atherosclerosis at the Same Time" International Journal of Molecular Sciences 23, no. 12: 6648. https://doi.org/10.3390/ijms23126648
APA StyleMerzel Šabović, E. K., Starbek Zorko, M., & Janić, M. (2022). Killing Two Birds with One Stone: Potential Therapies Targeting Psoriasis and Atherosclerosis at the Same Time. International Journal of Molecular Sciences, 23(12), 6648. https://doi.org/10.3390/ijms23126648







