High-Mobility Group Box 1 Inhibitor BoxA Alleviates Neuroinflammation-Induced Retinal Ganglion Cell Damage in Traumatic Optic Neuropathy
Abstract
:1. Introduction
2. Results
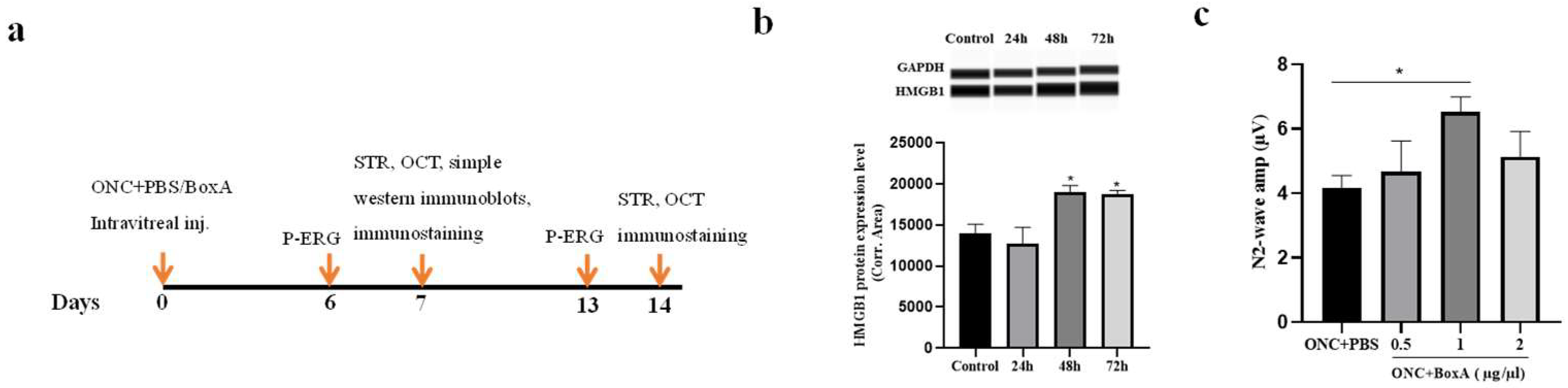
2.1. HMGB1 Is Released in Retina after ONC
2.2. Optimizing the Dosage of BoxA for Intravitreal Injection
2.3. Effects of BoxA in RGCs Function after ONC
2.4. Inhibition of HMGB1 Protects Retinal Structure and Reduces RGCs Apoptosis in Experimental ONC
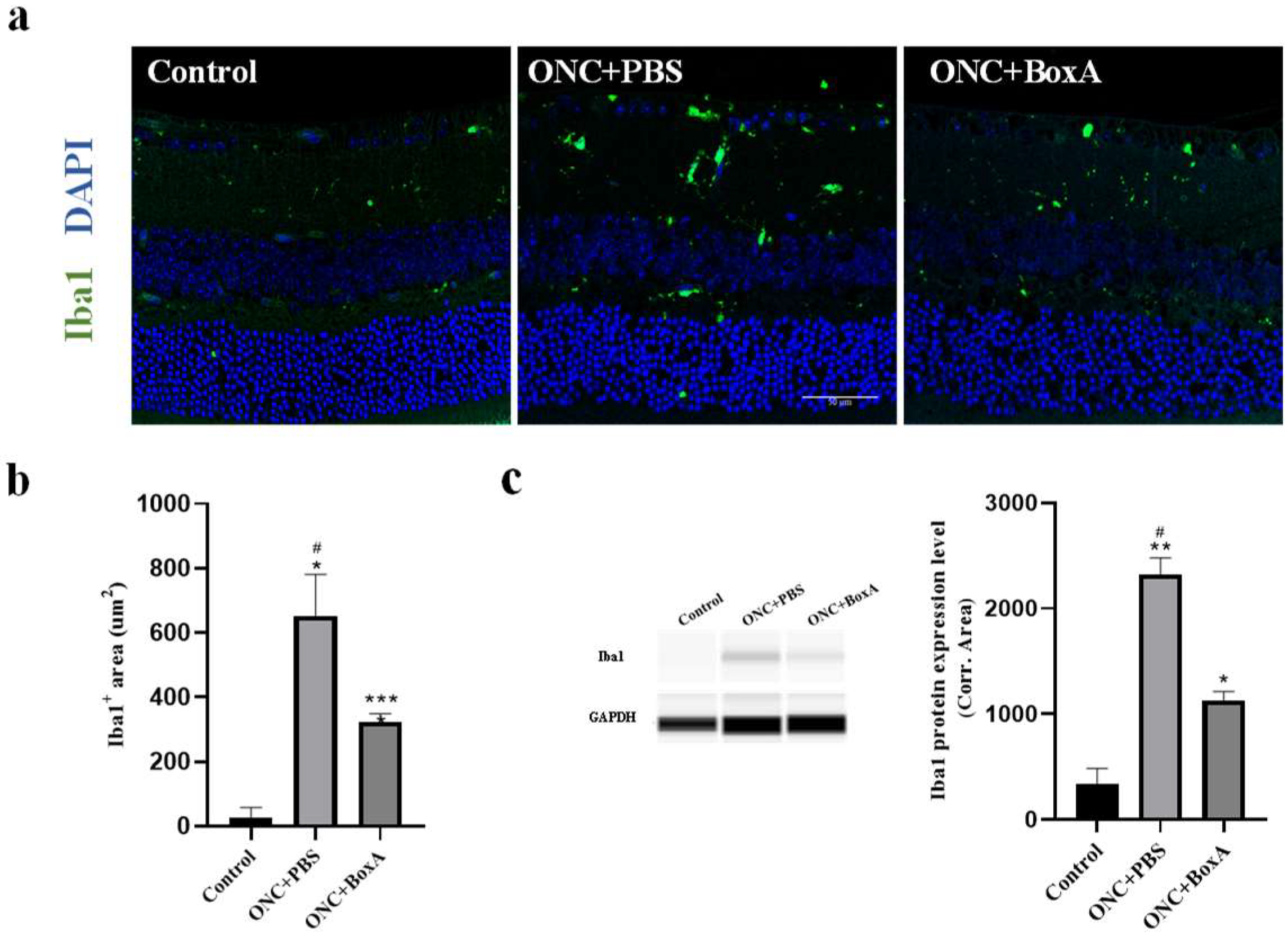
2.5. HMGB1 Inhibitor BoxA Inhibits the Activation of Retinal Microglia
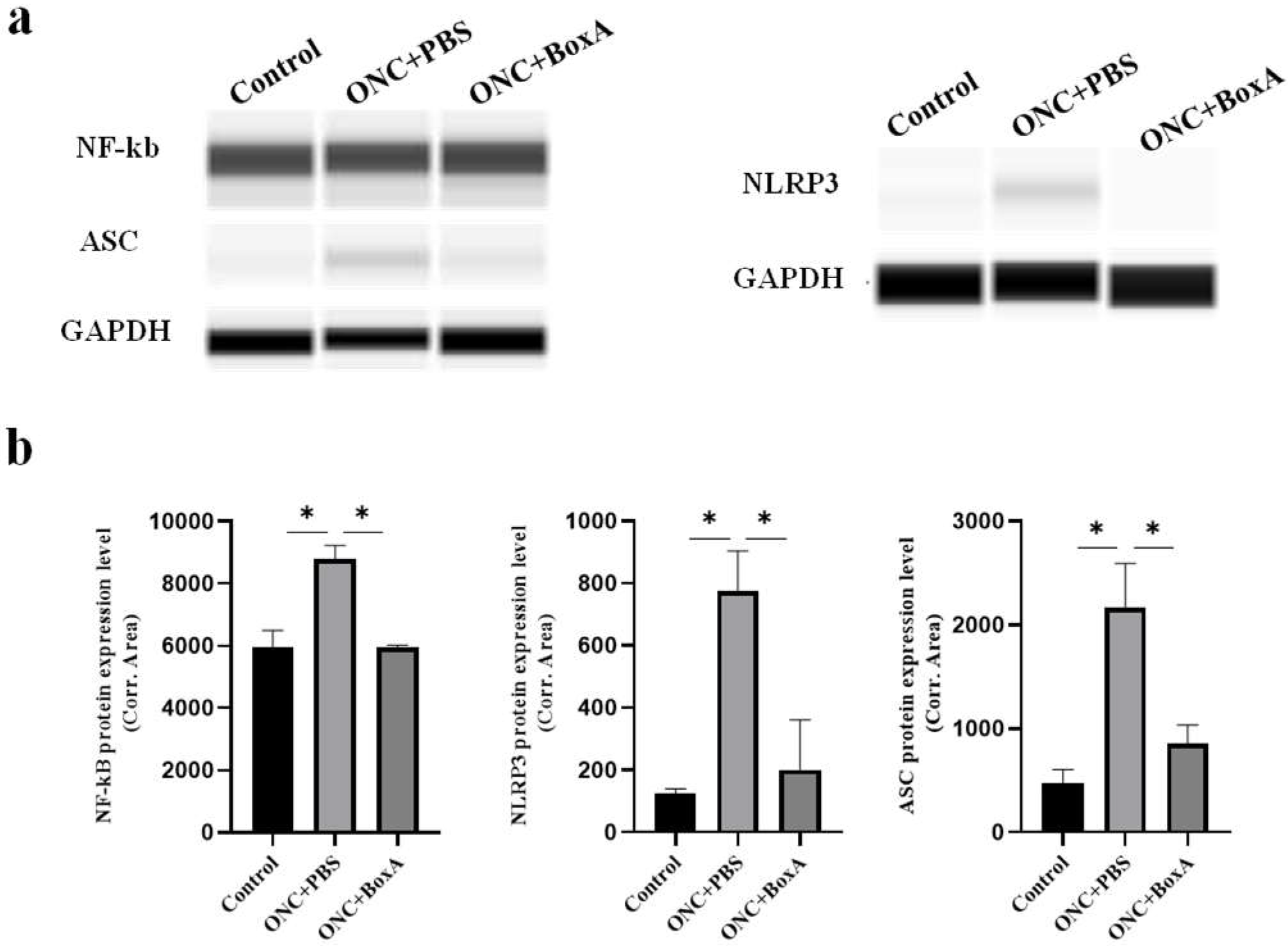
2.6. HMGB1 Mediates NLRP3 Inflammasome, NF-kB after ONC
3. Discussion
3.1. Protection of RGCs by BOXA in Traumatic Optic Neuropathy
3.2. Possible Mechanisms of BoxA Mediates the Neuroinflammation-Induced Damage
4. Materials and Methods
4.1. Animals
4.2. Study Design
4.3. Establishment of Optic Nerve Crush (ONC) Model
4.4. Pattern Electroretinogram (P-ERG)
4.5. Scotopic Threshold Responses (STR)
4.6. Optical Coherence Tomography (OCT)
4.7. Immunofluorescence
4.8. Flat-Mounted Retinas and RGC Quantification
4.9. Automated Western Immunoblotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McNair, N.D. Traumatic brain injury. Nurs. Clin. N. Am. 1999, 34, 637–659. [Google Scholar] [PubMed]
- Perry, M.; Dancey, A.; Mireskandari, K.; Oakley, P.; Davies, S.; Cameron, M. Emergency care in facial trauma—A maxillofacial and ophthalmic perspective. Injury 2005, 36, 875–896. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. Traumatic optic neuropathy. Ophthalmology 2000, 107, 814. [Google Scholar] [CrossRef]
- Thomas, C.N.; Thompson, A.M.; McCance, E.; Berry, M.; Logan, A.; Blanch, R.J.; Ahmed, Z. Caspase-2 Mediates Site-Specific Retinal Ganglion Cell Death After Blunt Ocular Injury. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4453–4462. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Zhang, H.; Zhai, Q.; Li, H.; Wang, C.; Wang, Y. Traumatic optic neuropathy: A review of current studies. Neurosurg. Rev. 2022, 45, 1895–1913. [Google Scholar] [CrossRef]
- Steinsapir, K.D.; Goldberg, R.A. Traumatic optic neuropathy. Surv. Ophthalmol. 1994, 38, 487–518. [Google Scholar] [CrossRef]
- Steinsapir, K.D.; Goldberg, R.A. Traumatic optic neuropathy: An evolving understanding. Am. J. Ophthalmol. 2011, 151, 928–933.e2. [Google Scholar] [CrossRef]
- Miller, N.R. Traumatic Optic Neuropathy. J. Neurol. Surg. Part B Skull Base 2021, 82, 107–115. [Google Scholar]
- Odhiambo, W.A.; Guthua, S.W.; Macigo, F.G.; Akama, M.K. Maxillofacial injuries caused by terrorist bomb attack in Nairobi, Kenya. Int. J. Oral Maxillofac. Surg. 2002, 31, 374–377. [Google Scholar] [CrossRef]
- Warner, N.; Eggenberger, E. Traumatic optic neuropathy: A review of the current literature. Curr. Opin. Ophthalmol. 2010, 21, 459–462. [Google Scholar] [CrossRef]
- Bastakis, G.G.; Ktena, N.; Karagogeos, D.; Savvaki, M. Models and treatments for traumatic optic neuropathy and demyelinating optic neuritis. Dev. Neurobiol. 2019, 79, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Levkovitch-Verbin, H. Animal models of optic nerve diseases. Eye 2004, 18, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Au, N.P.B.; Ma, C.H.E. Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy. Front. Immunol. 2022, 13, 860070. [Google Scholar] [CrossRef]
- Langmann, T. Microglia activation in retinal degeneration. J. Leukoc. Biol. 2007, 81, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Borggrewe, M.; Kooistra, S.M.; Noelle, R.J.; Eggen, B.J.L.; Laman, J.D. Exploring the VISTA of microglia: Immune checkpoints in CNS inflammation. J. Mol. Med. 2020, 98, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef]
- Xu, X.; Piao, H.N.; Aosai, F.; Zeng, X.Y.; Cheng, J.H.; Cui, Y.X.; Li, J.; Ma, J.; Piao, H.R.; Jin, X.; et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br. J. Pharmacol. 2020, 177, 5224–5245. [Google Scholar] [CrossRef]
- Steinle, J.J. Role of HMGB1 signaling in the inflammatory process in diabetic retinopathy. Cell. Signal. 2020, 73, 109687. [Google Scholar] [CrossRef]
- Tian, Y.; Cao, Y.; Chen, R.; Jing, Y.; Xia, L.; Zhang, S.; Xu, H.; Su, Z. HMGB1 A box protects neurons by potently inhibiting both microglia and T cell-mediated inflammation in a mouse Parkinson’s disease model. Clin. Sci. 2020, 134, 2075–2090. [Google Scholar] [CrossRef]
- Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.M.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2004, 101, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Kokkola, R.; Tabibzadeh, S.; Yang, R.; Ochani, M.; Qiang, X.; Harris, H.E.; Czura, C.J.; Wang, H.; Ulloa, L.; et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol. Med. 2003, 9, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Sitia, G.; Iannacone, M.; Müller, S.; Bianchi, M.E.; Guidotti, L.G. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J. Leukoc. Biol. 2007, 81, 100–107. [Google Scholar] [CrossRef] [PubMed]
- De Mori, R.; Straino, S.; Di Carlo, A.; Mangoni, A.; Pompilio, G.; Palumbo, R.; Bianchi, M.E.; Capogrossi, M.C.; Germani, A. Multiple effects of high mobility group box protein 1 in skeletal muscle regeneration. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2377–2383. [Google Scholar] [CrossRef] [Green Version]
- Dvoriantchikova, G.; Hernandez, E.; Grant, J.; Santos, A.R.; Yang, H.; Ivanov, D. The high-mobility group box-1 nuclear factor mediates retinal injury after ischemia reperfusion. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7187–7194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, B.; Eggenberger, E.; Kaufman, D. Current electrophysiology in ophthalmology: A review. Curr. Opin. Ophthalmol. 2012, 23, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Wang, X.; Chauhan, B.C.; Côté, P.D.; Tremblay, F. Contribution of retinal ganglion cells to the mouse electroretinogram. Doc. Ophthalmol. Adv. Ophthalmol. 2014, 128, 155–168. [Google Scholar] [CrossRef]
- Yukita, M.; Machida, S.; Nishiguchi, K.M.; Tsuda, S.; Yokoyama, Y.; Yasuda, M.; Maruyama, K.; Nakazawa, T. Molecular, anatomical and functional changes in the retinal ganglion cells after optic nerve crush in mice. Doc. Ophthalmol. Adv. Ophthalmol. 2015, 130, 149–156. [Google Scholar] [CrossRef]
- Chi, W.; Chen, H.; Li, F.; Zhu, Y.; Yin, W.; Zhuo, Y. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-κB pathway in acute glaucoma. J. Neuroinflamm. 2015, 12, 137. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, P.M.; Eby, M.T.; Jasmin, A.; Kumar, A.; Liu, L.; Hood, L. Activation of the NF-kappaB pathway by caspase 8 and its homologs. Oncogene 2000, 19, 4451–4460. [Google Scholar] [CrossRef] [Green Version]
- Bach, M.; Brigell, M.G.; Hawlina, M.; Holder, G.E.; Johnson, M.A.; McCulloch, D.L.; Meigen, T.; Viswanathan, S. ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc. Ophthalmol. Adv. Ophthalmol. 2013, 126, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; McDowell, C.M.; Zhang, Z.; Tebow, H.E.; Wordinger, R.J.; Clark, A.F. Monitoring retinal morphologic and functional changes in mice following optic nerve crush. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3766–3774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porciatti, V. Electrophysiological assessment of retinal ganglion cell function. Exp. Eye Res. 2015, 141, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puyang, Z.; Feng, L.; Chen, H.; Liang, P.; Troy, J.B.; Liu, X. Retinal Ganglion Cell Loss is Delayed Following Optic Nerve Crush in NLRP3 Knockout Mice. Sci. Rep. 2016, 6, 20998. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.R.; Benowitz, L.I.; Goldberg, J.L.; He, Z. Axon Regeneration in the Mammalian Optic Nerve. Annu. Rev. Vis. Sci. 2020, 6, 195–213. [Google Scholar] [CrossRef]
- Gotoh, Y.; Machida, S.; Tazawa, Y. Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch. Ophthalmol. 2004, 122, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yang, Y.L.; Zhang, S.S.; Liu, D.N.; Fang, L.H.; Du, G.H.; Wang, Y.H. RKC-B1 Blocks Activation of NF-κB and NLRP3 Signaling Pathways to Suppress Neuroinflammation in LPS-Stimulated Mice. Mar. Drugs 2021, 19, 429. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef] [PubMed]
- Yerramothu, P.; Vijay, A.K.; Willcox, M.D.P. Inflammasomes, the eye and anti-inflammasome therapy. Eye 2018, 32, 491–505. [Google Scholar] [CrossRef]
- Lin, S.; Mei, X. Role of NLRP3 Inflammasomes in Neuroinflammation Diseases. Eur. Neurol. 2020, 83, 576–580. [Google Scholar] [CrossRef]
- Chaon, B.C.; Lee, M.S. Is there treatment for traumatic optic neuropathy? Curr. Opin. Ophthalmol. 2015, 26, 445–449. [Google Scholar] [CrossRef]
- Jehle, T.; Dimitriu, C.; Auer, S.; Knoth, R.; Vidal-Sanz, M.; Gozes, I.; Lagrèze, W.A. The neuropeptide NAP provides neuroprotection against retinal ganglion cell damage after retinal ischemia and optic nerve crush. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Berkelaar, M.; Clarke, D.B.; Wang, Y.C.; Bray, G.; Aguayo, A. Axotomy results in delayed death and apoptosis in retinal ganglon cells in adult rats. J. Neurosci. 1994, 14, 4368–4374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porciatti, V. The mouse pattern electroretinogram. Doc. Ophthalmol. Adv. Ophthalmol. 2007, 115, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego-Ortega, A.; Vidal-Villegas, B.; Norte-Muñoz, M.; Salinas-Navarro, M.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M. 7,8-Dihydroxiflavone Maintains Retinal Functionality and Protects Various Types of RGCs in Adult Rats with Optic Nerve Transection. Int. J. Mol. Sci. 2021, 22, 11815. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X. Toll-like receptor-9 (TLR-9) deficiency alleviates optic nerve injury (ONI) by inhibiting inflammatory response in vivo and in vitro. Exp. Cell Res. 2020, 396, 112159. [Google Scholar] [CrossRef]
- Chien, J.Y.; Sheu, J.H.; Wen, Z.H.; Tsai, R.K.; Huang, S.P. Neuroprotective effect of 4-(Phenylsulfanyl)butan-2-one on optic nerve crush model in rats. Exp. Eye Res. 2016, 143, 148–157. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef]
- Nanki, K.; Fujii, M.; Shimokawa, M.; Matano, M.; Nishikori, S.; Date, S.; Takano, A.; Toshimitsu, K.; Ohta, Y.; Takahashi, S.; et al. Somatic inflammatory gene mutations in human ulcerative colitis epithelium. Nature 2020, 577, 254–259. [Google Scholar] [CrossRef]
- Nanki, K.; Toshimitsu, K.; Takano, A.; Fujii, M.; Shimokawa, M.; Ohta, Y.; Matano, M.; Seino, T.; Nishikori, S.; Ishikawa, K.; et al. Divergent Routes toward Wnt and R-spondin Niche Independency during Human Gastric Carcinogenesis. Cell 2018, 174, 856–869.e17. [Google Scholar] [CrossRef] [Green Version]
- Dahl, J.A.; Jung, I.; Aanes, H.; Greggains, G.D.; Manaf, A.; Lerdrup, M.; Li, G.; Kuan, S.; Li, B.; Lee, A.Y.; et al. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature 2016, 537, 548–552. [Google Scholar] [CrossRef]
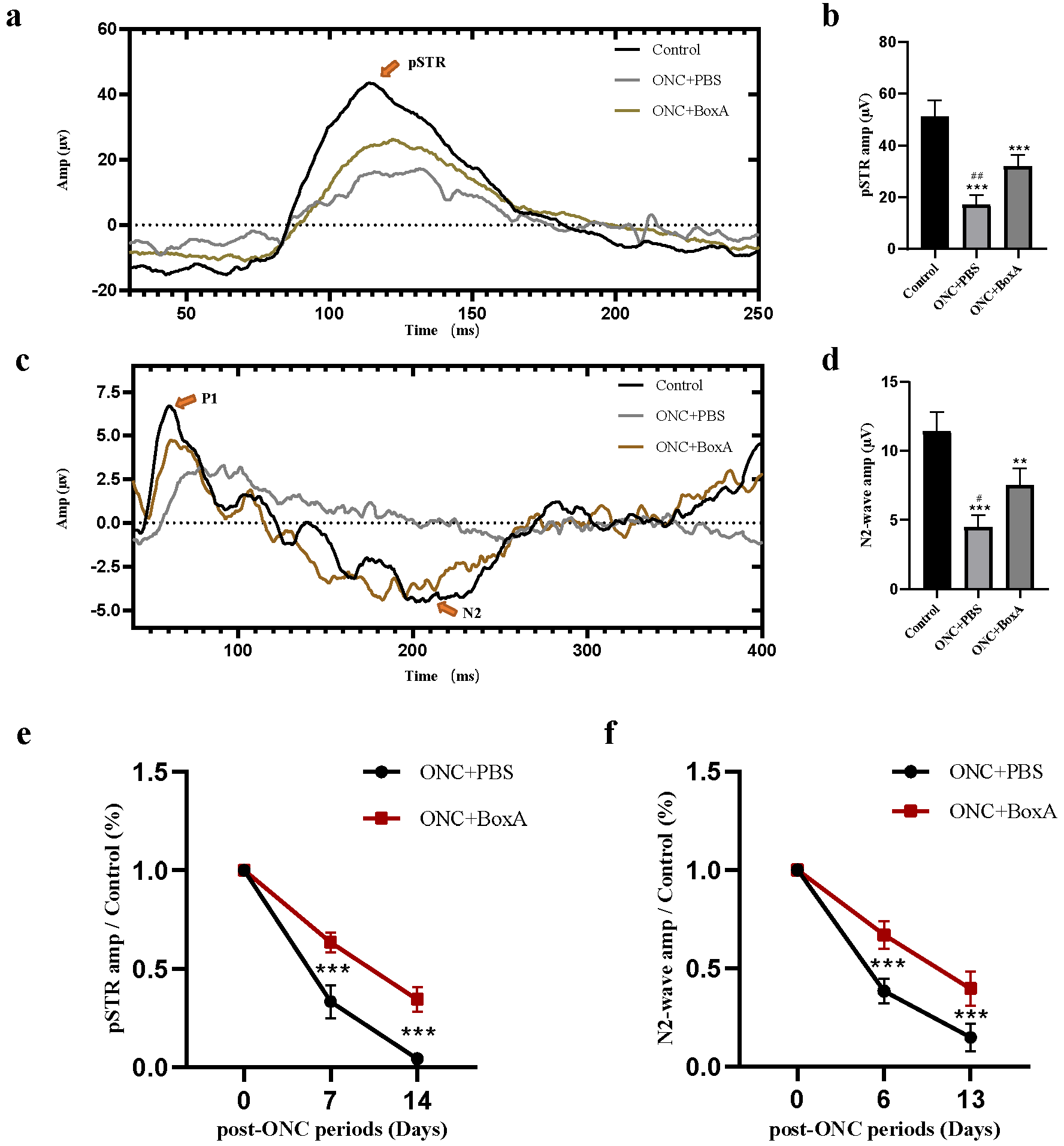
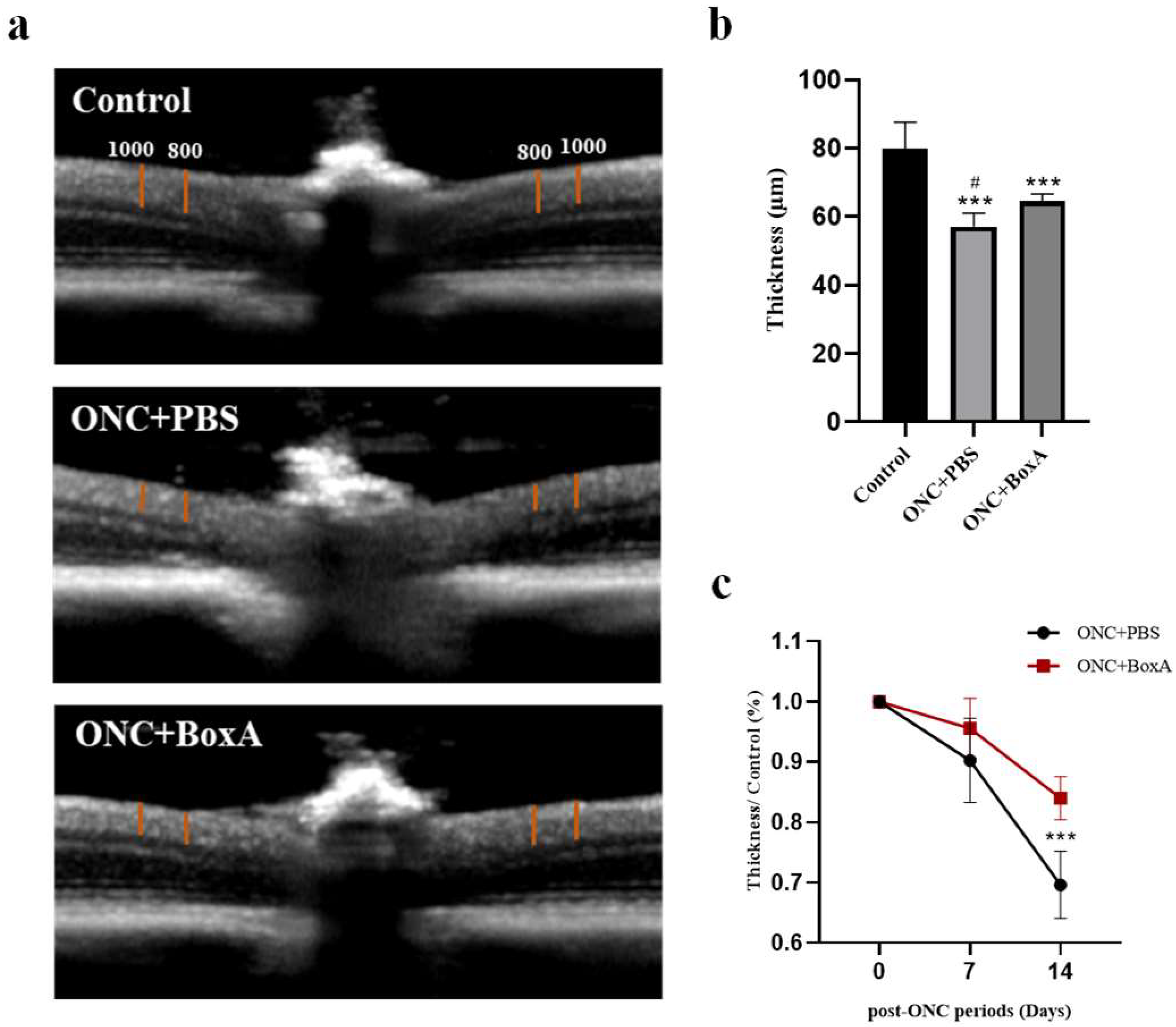

| Treatment | Mean Amplitude of ERG Waves ± SD (µv) | Thickness ± SD (µm) | |||
|---|---|---|---|---|---|
| N2 | pSTR | ||||
| Control | 11.44 ± 1.33 | 51.07 ± 6.20 | 79.87 ± 7.46 | ||
| ONC + PBS | 6 d | 4.47 ± 0.83 | 7 d | 17.23 ± 3.46 | 72.95 ± 3.95 |
| 13 d | 1.72 ± 0.73 | 14 d | 2.23 ± 0.61 | 57.14 ± 3.58 | |
| ONC + BoxA | 6 d | 7.54 ± 1.14 | 7 d | 31.88 ± 4.09 | 73.69 ± 4.91 |
| 13 d | 4.50 ± 1.14 | 14 d | 17.08 ± 2.93 | 64.66 ± 1.85 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Jin, J.; Su, W.; Shao, W.; Li, W.; Li, Z.; Yu, H.; Zheng, Y.; Zhong, L. High-Mobility Group Box 1 Inhibitor BoxA Alleviates Neuroinflammation-Induced Retinal Ganglion Cell Damage in Traumatic Optic Neuropathy. Int. J. Mol. Sci. 2022, 23, 6715. https://doi.org/10.3390/ijms23126715
Peng J, Jin J, Su W, Shao W, Li W, Li Z, Yu H, Zheng Y, Zhong L. High-Mobility Group Box 1 Inhibitor BoxA Alleviates Neuroinflammation-Induced Retinal Ganglion Cell Damage in Traumatic Optic Neuropathy. International Journal of Molecular Sciences. 2022; 23(12):6715. https://doi.org/10.3390/ijms23126715
Chicago/Turabian StylePeng, Jingyi, Jiayi Jin, Wenru Su, Wanwen Shao, Weihua Li, Zhiquan Li, Huan Yu, Yongxin Zheng, and Liuxueying Zhong. 2022. "High-Mobility Group Box 1 Inhibitor BoxA Alleviates Neuroinflammation-Induced Retinal Ganglion Cell Damage in Traumatic Optic Neuropathy" International Journal of Molecular Sciences 23, no. 12: 6715. https://doi.org/10.3390/ijms23126715






