Extracellular Vesicles in Facial Aesthetics: A Review
Abstract
:1. Introduction
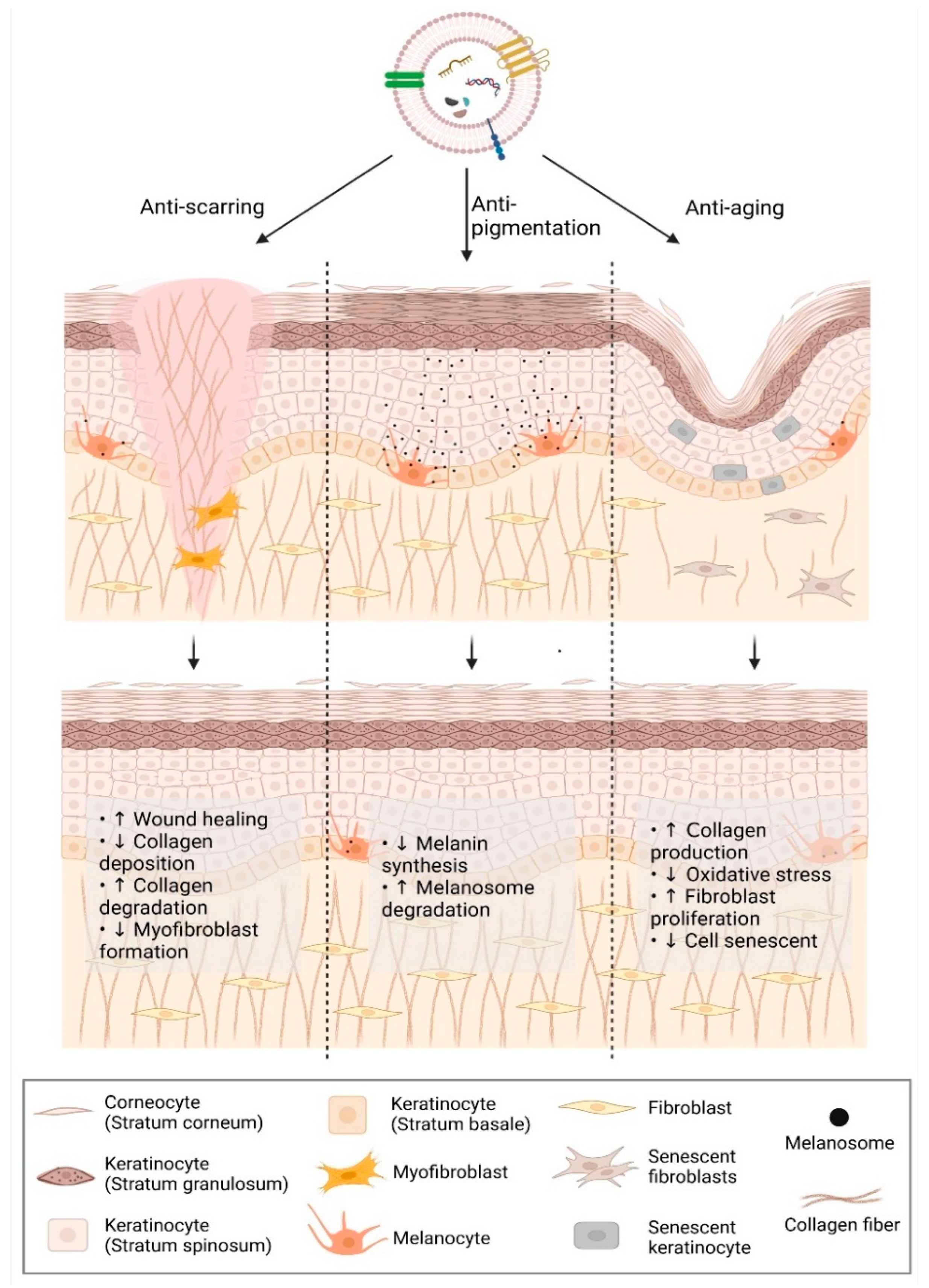
2. Applications of Extracellular Vesicles in Facial Aesthetics
2.1. Anti-Scarring
2.1.1. Scar Formation
2.1.2. Extracellular Vesicles in Reducing Scar Formation: Evidence and Clues
2.2. Anti-Aging
2.2.1. Skin Aging
2.2.2. EVs in Anti-Aging: Evidence and Clues
2.3. Anti-Pigmentation
2.3.1. Skin Pigmentation
2.3.2. Extracellular Vesicles in Regulation of Skin Pigmentation: Evidence and Clues
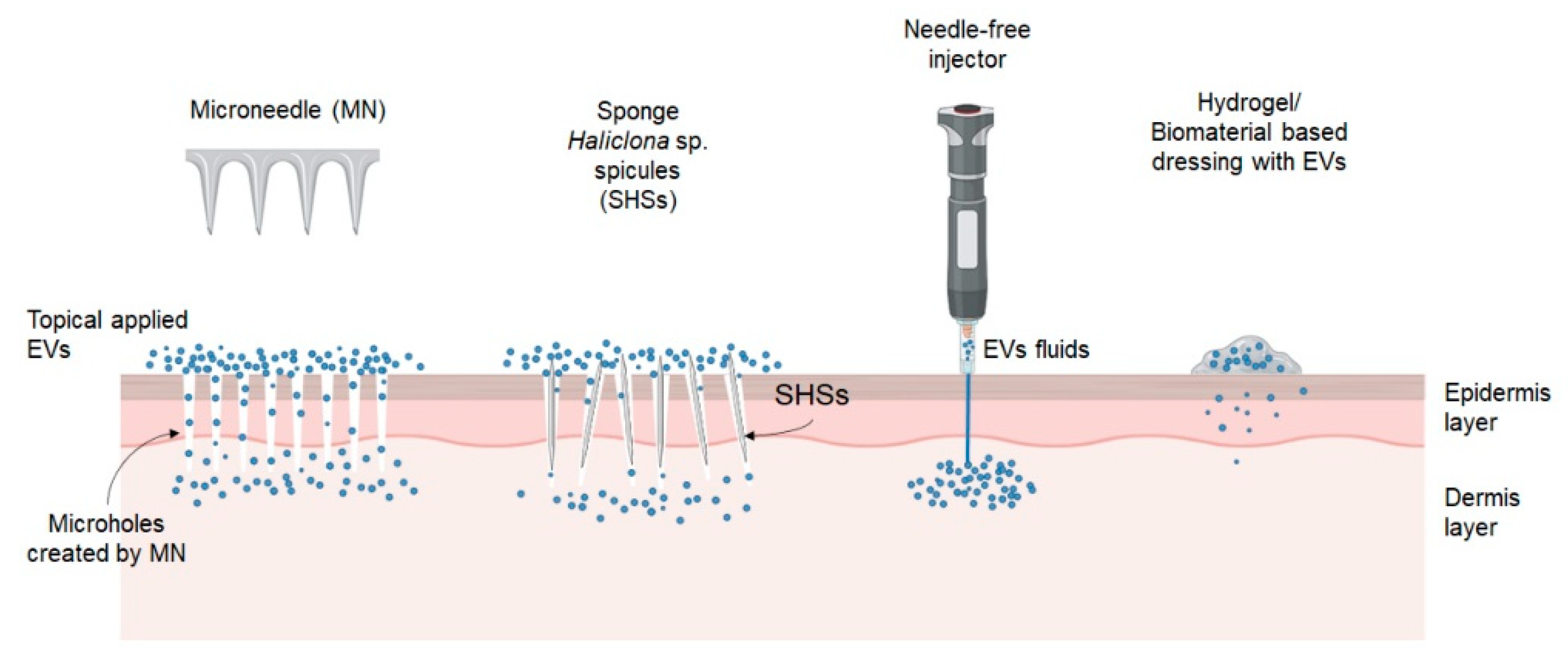
3. Advanced Delivery Strategy for EVs
3.1. Physical Penetration
3.2. Hydrogel/Biomaterials-Based Dressings
4. Plant EVs in Skin Improvement
5. Clinical Study of EVs
6. Limitation and Prospective
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, H.J.; Jung, M.S.; Hur, Y.K.; Jung, A.H. A study on clinical effectiveness of cosmetics containing human stem cell conditioned media. Biomed Dermatol. 2020, 4, 9. [Google Scholar] [CrossRef]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the development of new cosmetic formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Jatana, G.K.; Sonthalia, S. Cosmeceuticals; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, Z.A.A.; Mohd-Nasir, H.; Ahmad, A.; Mohd Setapar, S.H.; Peng, W.L.; Chuo, S.C.; Khatoon, A.; Umar, K.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Role of nanotechnology for design and development of cosmeceutical: Application in makeup and skin care. Front. Chem. 2019, 7, 739. [Google Scholar] [CrossRef]
- Sharma, A.; Madhunapantula, S.V.; Robertson, G.P. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expert Opin. Drug Metab. Toxicol. 2012, 8, 47–69. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem. Cell Res. Ther. 2019, 10, 359. [Google Scholar] [CrossRef]
- Ng, C.Y.; Chai, J.Y.; Foo, J.B.; Yahaya, N.H.M.; Yang, Y.; Ng, M.H.; Law, J.X. Potential of exosomes as cell-free therapy in articular cartilage regeneration: A review. Int. J. Nanomed. 2021, 16, 6749–6781. [Google Scholar] [CrossRef]
- Foo, J.B.; Looi, Q.H.; How, C.W.; Lee, S.H.; Al-Masawa, M.E.; Chong, P.P.; Law, J.X. Mesenchymal stem cell-derived exosomes and microRNAs in cartilage regeneration: Biogenesis, efficacy, miRNA enrichment and delivery. Pharmaceuticals 2021, 14, 1093. [Google Scholar] [CrossRef]
- Akers, J.C.; Gondo, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Kim, M.H.; Chung, C.; Oh, M.H.; Jun, J.H.; Ko, Y.; Lee, J.H. Extracellular vesicles from a three-dimensional culture of perivascular cells accelerate skin wound healing in a rat. Aesthetic Plast. Surg. 2021, 45, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.B.; Looi, Q.H.; Chong, P.P.; Hassan, N.H.; Yeo, G.E.C.; Ng, C.Y.; Koh, B.; How, C.W.; Lee, S.H.; Law, J.X. Comparing the therapeutic potential of stem cells and their secretory products in regenerative medicine. Stem Cells Int. 2021, 2021, 2616807. [Google Scholar] [CrossRef] [PubMed]
- Narauskaitė, D.; Vydmantaitė, G.; Rusteikaitė, J.; Sampath, R.; Rudaitytė, A.; Stašytė, G.; Aparicio Calvente, M.I.; Jekabsone, A. Extracellular Vesicles in Skin Wound Healing. Pharmaceuticals 2021, 14, 811. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xin, Y.; Zhang, Z.; Zou, X.; Xue, K.; Zhang, H.; Zhang, W.; Liu, K. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res. Ther. 2020, 11, 264. [Google Scholar] [CrossRef]
- Jang, B.; Chung, H.; Jung, H.; Song, H.K.; Park, E.; Choi, H.S.; Jung, K.; Choe, H.; Yang, S.; Oh, E.S. Extracellular vesicles from Korean Codium fragile and Sargassum fusiforme negatively regulate melanin synthesis. Mol. Cells 2021, 44, 736–745. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, G.; Huang, H.; Wang, K.; Wang, H.; Lang, M.; Gao, H.; Zhao, S. Sequential release of small extracellular vesicles from bilayered thiolated alginate/polyethylene glycol diacrylate hydrogels for scarless wound healing. ACS Nano 2021, 15, 6352–6368. [Google Scholar] [CrossRef]
- Subramaniam, T.; Fauzi, M.B.; Lokanathan, Y.; Law, J.X. The role of calcium in wound healing. Int. J. Mol. Sci. 2021, 22, 6486. [Google Scholar] [CrossRef]
- Lee, H.J.; Jang, Y.J. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int. J. Mol. Sci. 2018, 19, 711. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Wang, Z.; Sun, J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res. Ther. 2020, 11, 198. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical relevance of elastin in the structure and function of skin. Aesthetic Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef] [PubMed]
- Järveläinen, H.; Puolakkainen, P.; Pakkanen, S.; Brown, E.L.; Höök, M.; Iozzo, R.V.; Sage, E.H.; Wight, T.N. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair. Regen. 2006, 14, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, B.; Peng, L.; Gao, S.; Guo, J.; Zhu, X. Blockade of LINC01605-enriched exosome generation in M2 macrophages impairs M2 macrophage-induced proliferation, migration, and invasion of human dermal fibroblasts. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211016724. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.; Jayaraman, V.; Huelsmann, E.J.; Bonish, B.; Burgad, D.; Sivaramakrishnan, G.; Qin, S.; DiPietro, L.A.; Zloza, A.; Zhang, C.; et al. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS ONE 2014, 9, e91574. [Google Scholar] [CrossRef] [Green Version]
- Gharaee-Kermani, M.; Denholm, E.M.; Phan, S.H. Costimulation of fibroblast collagen and transforming growth factor β1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J. Biol. Chem. 1996, 271, 17779–17784. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.S.C.; Nelson, P.J.; Gro, H.; Weber, C. Implications for MCP-1 mediated wound injury repair and in vivo. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2085–2093. [Google Scholar] [CrossRef] [Green Version]
- Shan, S.; Zhang, Y.; Wu, M.; Yi, B.; Wang, J.; Li, Q. Naringenin attenuates fibroblast activation and inflammatory response in a mechanical stretch-induced hypertrophic scar mouse model. Mol. Med. Rep. 2017, 16, 4643–4649. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, Y.; Liu, T.; Wang, X.; Wang, H.; Song, H.; Wang, W. Exosomes derived from TSG-6 modified mesenchymal stromal cells attenuate scar formation during wound healing. Biochimie 2020, 177, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL- 17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2015, 142, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of dermis: Scarring and cells involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Ulrich, F.; Unglaub, F.; Piatkowski, A.; Pallua, N. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Auler, S.; Hugo, R.; Gonzalez, S. Beneficial regulation of matrix metalloproteinases for skin health. Enzym. Res. 2011, 2011, 427285. [Google Scholar] [CrossRef] [Green Version]
- Pilcher, B.K.; Dumin, J.A.; Sudbeck, B.D.; Krane, S.M.; Welgus, G.; Parks, W.C. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J. Cell Biol. 2017, 137, 1445–1457. [Google Scholar] [CrossRef]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Bullard, K.M.; Lund, L.; Mudgett, J.S.; Mellin, T.N.; Hunt, T.K.; Murphy, B.; Ronan, J.; Werb, Z.; Banda, M.J. Impaired wound contraction in stromelysin-1-deficient mice. Ann. Surg. 1999, 230, 260–265. [Google Scholar] [CrossRef]
- Yang, C.; Luo, L.; Bai, X.; Shen, K.; Liu, K.; Wang, J.; Hu, D. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch. Biochem. Biophys. 2020, 681, 108259. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.M.; Beanes, S.R.; Lee, H.; Zhang, X.; Soo, C.; Ting, K. Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plast. Reconstr. Surg. 2003, 111, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Z.; Hu, X.; Zhang, J.; Wang, Z.H.; Wu, S.; Yi, Y.Y. Extracellular Vesicles Derived from Human Adipose-Derived Stem Cell Prevent the Formation of Hypertrophic Scar in a Rabbit Model. Ann. Plast. Surg. 2020, 84, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Liu, Y.; Li, X.; Tang, L.; Duan, M.; Li, J.; Zhang, G. Exosomes Derived from Human Umbilical Cord Blood Mesenchymal Stem Cells Stimulate Regenerative Wound Healing via Transforming Growth Factor-β Receptor Inhibition. Stem Cell Res. Ther. 2021, 12, 434. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from Human Umbilical Cord Blood Accelerate Cutaneous Wound Healing through MiR-21-3p-Mediated Promotion of Angiogenesis and Fibroblast Function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Huang, Y.; Su, Y. Human umbilical cord mesenchymal stem cell-derived exosomes suppress dermal fibroblasts-myofibroblast transition via inhibiting the TGF-β1/Smad 2/3 signaling pathway. Exp. Mol. Pathol. 2020, 115, 104468. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, F.; Liu, Z.; Zuo, K.; Wang, B.; Zhang, Y.; Han, X.; Lian, A.; Wang, Y.; Liu, M.; et al. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 174. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Han, S.; Zhang, W.; Zhou, Q.; Guan, H.; Liu, J.; Shi, J.; Su, L.; Hu, D. Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. J. Mol. Histol. 2017, 48, 121–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Liu, Y.; Chen, Z.; Li, X.; Tang, L.; Li, J.; Duan, M.; Zhang, G. Human amniotic fluid stem cell-derived exosomes as a novel cell-free therapy for cutaneous regeneration. Front. Cell Dev. Biol. 2021, 9, 685873. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.K.; Kim, H.; Kim, T.M. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int. J. Mol. Sci. 2018, 19, 3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tutuianu, R.; Rosca, A.M.; Iacomi, D.M.; Simionescu, M.; Titorencu, I. Human mesenchymal stromal cell-derived exosomes promote in vitro wound healing by modulating the biological properties of skin keratinocytes and fibroblasts and stimulating angiogenesis. Int. J. Mol. Sci. 2021, 22, 6239. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.X.; Liao, X.; Li, S.H.; Jiang, X.; Li, Z.H.; Wu, Y.D.; Xiao, L.L.; Xie, G.H.; Song, J.X.; Liu, H.W. Antiaging properties of exosomes from adipose-derived mesenchymal stem cells in photoaged rat skin. Biomed. Res. Int. 2020, 2020, 6406395. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-J.; Kil, I.S.; Cho, E. Extracellular vesicles derived from senescent fibroblasts attenuate the dermal effect on keratinocyte differentiation. Int. J. Mol. Sci. 2020, 21, 1022. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Wang, X.; Si, Y.; Pang, J.; Liu, H.; Li, S.; Ding, Q.; Wang, Y. Exosome derived from ADSCs attenuates ultraviolet B-mediated photoaging in human dermal fibroblasts. Photochem. Photobiol. 2021, 97, 795–804. [Google Scholar] [CrossRef]
- Deng, M.; Yu, T.Z.; Li, D.; Wang, X.; Zhou, G.; Liu, W.; Cao, Y.; Xia, W.; Li, W.; Zhang, W.J. Human umbilical cord mesenchymal stem cell-derived and dermal fibroblast-derived extracellular vesicles protect dermal fibroblasts from ultraviolet radiation-induced photoaging in vitro. Photochem. Photobiol. Sci. 2020, 19, 406–414. [Google Scholar] [CrossRef]
- Oh, M.; Lee, J.; Kim, Y.J.; Rhee, W.J.; Park, J.H. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715. [Google Scholar] [CrossRef] [Green Version]
- Go, Y.Y.; Lee, C.M.; Ju, W.M.; Chae, S.W.; Song, J.J. Extracellular vesicles (secretomes) from human trophoblasts promote the regeneration of skin fibroblasts. Int. J. Mol. Sci. 2021, 22, 6959. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Han, X.; Sun, Y.; Sun, Z.; Li, L.; Zhou, X.; Jin, Q.; Fu, P.; Xu, W.; et al. HucMSC exosome-delivered 14-3-3ζ alleviates ultraviolet radiation-induced photodamage via SIRT1 pathway modulation. Aging 2021, 13, 11542–11563. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, L.; Gao, H.; Chang, L.; Yu, X.; Zhu, Z.; He, X.; Geng, J.; Dong, Y.; Li, H.; et al. Exosomal miRNA derived from keratinocytes regulates pigmentation in melanocytes. J. Dermatol. Sci. 2019, 93, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Y.; Guan, X.H.; Yu, Z.P.; Wu, J.; Huang, Q.M.; Deng, K.Y.; Xin, H.B. Human amniotic stem cells-derived exosmal miR-181a-5p and miR-199a inhibit melanogenesis and promote melanosome degradation in skin hyperpigmentation, respectively. Stem Cell Res. Ther. 2021, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Wäster, P.; Eriksson, I.; Vainikka, L.; Öllinger, K. Extracellular vesicles released by melanocytes after UVA irradiation promote intercellular signaling via miR21. Pigment. Cell Melanoma Res. 2020, 33, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ouyang, P.; He, G.; Wang, X.; Song, D.; Yang, Y.; He, X. Exosomes from MicroRNA-126 Overexpressing Mesenchymal Stem Cells Promote Angiogenesis by Targeting the PIK3R2-Mediated PI3K/Akt Signalling Pathway. J. Cell. Mol. Med. 2021, 25, 2148–2162. [Google Scholar] [CrossRef]
- Toriseva, M.; Laato, M.; Carpén, O.; Ruohonen, S.T.; Savontaus, E.; Inada, M.; Krane, S.M.; Kähäri, V.M. MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability. PLoS ONE 2012, 7, e42596. [Google Scholar]
- Wilgus, T.A. Inflammation as an orchestrator of cutaneous scar formation: A review of the literature. Plast. Aesthetic Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Jia, P.; Cheng, H.; Wang, C.; Chen, S.; Huang, H.; Han, Z.; Han, Z.C.; Marycz, K.; et al. Chitosan hydrogel-loaded MSC-derived extracellular vesicles promote skin rejuvenation by ameliorating the senescence of dermal fibroblasts. Stem Cell Res. Ther. 2021, 12, 196. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Cores, J.; Huang, K.; Su, T.; Dinh, P.U.; Cheng, K. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. Physiol. Behav. 2017, 13, 11273–11282. [Google Scholar] [CrossRef]
- Sugita, S.; Suzumura, T.; Nakamura, A.; Tsukiji, S.; Ujihara, Y.; Nakamura, M. Second harmonic generation light quantifies the ratio of type III to total (I + III) collagen in a bundle of collagen fiber. Sci. Rep. 2021, 11, 11874. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, P.; Wang, X. Skin Ageing and Cancer. In The Role of Matrix Metalloproteinase in Human Body Pathologies; InTech: London, UK, 2017; pp. 103–144. [Google Scholar]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Sa, Y.; Li, C.; Li, H.; Guo, H. TIMP-1 induces α-smooth muscle actin in fibroblasts to promote urethral scar formation. Cell Physiol. Biochem. 2015, 35, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, S.; Zhang, S.; Cai, G.; Jiang, H.; Su, H.; Li, X.; Hong, Q.; Zhang, X.; Chen, X. Tissue inhibitor of metalloproteinase-1 promotes NIH3T3 fibroblast proliferation by activating p-Akt and cell cycle progression. Mol. Cells 2011, 31, 225–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-β signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. Soc. Investig. Dermatol. 2002, 118, 211–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupa, D.M.W.; Kalfalah, F.; Safferling, K.; Boukamp, P.; Poschmann, G.; Volpi, E.; Götz-Rösch, C.; Bernerd, F.; Haag, L.; Huebenthal, U.; et al. Characterization of skin aging-associated secreted proteins (SAASP) produced by dermal fibroblasts isolated from intrinsically aged human skin. J. Investig. Dermatol. 2015, 135, 1954–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Mauro, T.M.; Dang, E.; Man, G.; Zhang, J.; Lee, D.; Wang, G.; Feingold, K.R.; Elias, P.M.; Man, M.Q. Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J. Investig. Dermatol. 2017, 137, 1277–1285. [Google Scholar] [CrossRef] [Green Version]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.H.; Lee, Y.B.; Kang, D.; Choi, E.; Nam, Y.; Lee, K.H.; You, H.J.; Kang, H.J.; An, S.H.; Jeon, H. Overcome the barriers of the skin: Exosome therapy. Biomater. Res. 2021, 25, 22. [Google Scholar] [CrossRef]
- Choi, J.S.; Cho, W.L.; Choi, Y.J.; Kim, J.D.; Park, H.A.; Kim, S.Y.; Park, J.H.; Jo, D.G.; Cho, Y.W. Functional recovery in photo-damaged human dermal fibroblasts by human adipose-derived stem cell extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1565885. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Inhibitors of melanogenesis: A patent review (2009–2014). Expert Opin. Ther. Pat. 2015, 25, 775–788. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-munoz, J.; Garcia-molina, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [Green Version]
- Videira IF dos, S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. De Dermatol. 2014, 88, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Li, Y.; Zhao, B.; Chen, T.; Dong, Y.; Fan, R.; Li, J.; Wang, H.; He, X. TRP-2 mediates coat color pigmentation in sheep skin. Mol. Med. Rep. 2018, 17, 5869–5877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.; Ko, H.J.; Kim, K.; Sohn, Y.; Min, S.Y.; Kim, J.A.; Na, D.; Yeon, J.H. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles 2020, 9, 1703480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murase, D.; Hachiya, A.; Takano, K.; Hicks, R.; Visscher, M.O.; Kitahara, T.; Hase, T.; Takema, Y.; Yoshimori, T. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J. Investig. Dermatol. 2013, 133, 2416–2424. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Lai, R.C.; Sim, W.K.; Choo, A.B.H.; Lane, E.B.; Lim, S.K. Topical application of mesenchymal stem cell exosomes alleviates the imiquimod induced psoriasis-like inflammation. Int. J. Mol. Sci. 2021, 22, 720. [Google Scholar] [CrossRef]
- Hao, Y.; Li, W.; Zhou, X.L.; Yang, F.; Qian, Z.Y. Microneedles-based transdermal drug delivery systems: A review. J. Biomed. Nanotechnol. 2017, 13, 1581–1597. [Google Scholar] [CrossRef]
- Cao, Z.; Jin, S.; Wang, P.; He, Q.; Yang, Y.; Gao, Z.; Wang, X. Microneedle based adipose derived stem cells-derived extracellular vesicles therapy ameliorates UV-induced photoaging in SKH-1 mice. J. Biomed. Mater. Res. 2021, 109, 1849–1857. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, L.; Li, F.R.; Li, X.; Wang, Z.; Zou, X.; Zhang, C.; Lv, K.; Zhou, B.; Mitragotri, S.; et al. Topical application of exosomes derived from human umbilical cord mesenchymal stem cells in combination with sponge spicules for treatment of photoaging. Int. J. Nanomed. 2020, 15, 2859–2872. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Ou, H.; Liu, C.; Zhang, Y.; Mitragotri, S.; Wang, D.; Chen, M. Skin delivery of hydrophilic biomacromolecules using marine sponge spicules. Mol. Pharm. 2017, 14, 3188–3200. [Google Scholar] [CrossRef]
- Ravi, A.; Sadhna, D.; Nagpaal, D.; Chawla, L. Needle free injection technology: A complete insight. Int. J. Pharm. Investig. 2015, 5, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Bahram, M.; Mohseni, N.; Moghtader, M. An introduction to hydrogels and some recent applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016; pp. 1–30. [Google Scholar]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds:an in vivo study. J. Biomed. Mater. Res.-Part A 2020, 108, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Nooshabadi, V.T.; Khanmohamadi, M.; Valipour, E.; Mahdipour, S.; Salati, A.; Malekshahi, Z.V.; Shafei, S.; Amini, E.; Farzamfar, S.; Ai, J. Impact of Exosome-Loaded Chitosan Hydrogel in Wound Repair and Layered Dermal Reconstitution in Mice Animal Model. J. Biomed. Mater. Res.-Part A 2020, 108, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gong, S.; Yao, W.; Yang, Z.; Wang, R.; Yu, Z.; Wei, M. Exosome loaded genipin crosslinked hydrogel facilitates full thickness cutaneous wound healing in rat animal model. Drug Deliv. 2021, 28, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Z.; Li, Y.; Wang, Y.; Li, Q.; Han, D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J. Mol. Histol. 2020, 51, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Gangadaran, P.; Rajendran, R.L.; Kim, H.M.; Oh, J.M.; Choi, K.Y.; Chung, H.Y.; Ahn, B.C. Extracellular vesicles derived from fibroblasts promote wound healing by optimizing fibroblast and endothelial cellular functions. Stem Cells 2021, 39, 266–279. [Google Scholar] [CrossRef]
- Duan, M.; Zhang, Y.; Zhang, H.; Meng, Y.; Qian, M.; Zhang, G. Epidermal stem cell-derived exosomes promote skin regeneration by downregulating transforming growth factor-β1 in wound healing. Stem Cell Res. Ther. 2020, 11, 452. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M.; Li, S. A sodium alginate-based sustained-release IPN hydrogel and its applications. RSC Adv. 2020, 10, 39722–39730. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Yue, K.; Santiago, G.T.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2016, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, K.; Láng, O.; Láng, J.; Perczel-Kovách, K.; Gyulai-Gaál, S.; Kádár, K.; Kőhidai, L.; Varga, G. A novel hydrogel scaffold for periodontal ligament stem cells. Interv. Med. Appl. Sci. 2018, 10, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant extracellular vesicles. Protoplasma 2019, 257, 3–12. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The antioxidant effect of small extracellular vesicles derived from Aloe vera peels for wound healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef]
- Urzì, O.; Raimondo, S.; Alessandro, R. Extracellular vesicles from plants: Current knowledge and open questions. Int. J. Mol. Sci. 2021, 22, 5366. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, Q.; Tang, J.; Xiong, L.; Li, L. Extracellular vesicles: Emerging therapeutics in cutaneous lesions. Int. J. Nanomed. 2021, 16, 6183–6202. [Google Scholar] [CrossRef]
- Cho, E.G.; Choi, S.Y.; Kim, H.; Choi, E.J.; Lee, E.J.; Park, P.J.; Ko, J.; Kim, K.P.; Baek, H.S. Panax ginseng-derived extracellular vesicles facilitate anti-senescence effects in human skin cells: An eco-friendly and sustainable way to use ginseng substances. Cells 2021, 10, 486. [Google Scholar] [CrossRef]
- Surjushe, A.; Vasani, R.; Saple, D. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef]
- Quispe, C.; Villalobos, M.; Bórquez, J.; Simirgiotis, M. Chemical composition and antioxidant activity of Aloe vera from the Pica Oasis (Tarapacá, Chile) by UHPLC-Q/Orbitrap/MS/MS. J. Chem. 2018, 2018, 6123850. [Google Scholar] [CrossRef] [Green Version]
- Ratan, A.Z.; Haidere, F.M.; Hong, H.Y.; Park, H.S.; Lee, O.J.; Lee, J.; Cho, Y.J. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef]
- Ru, W.; Wang, D.; Xu, Y.; He, X.; Sun, Y.E.; Qian, L.; Zhou, X.; Qin, Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.). Drug Discov. Ther. 2015, 9, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, B.S.; Lee, J.; Won, Y.; Duncan, D.I.; Jin, R.C.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Park, B.C.; et al. Skin brightening efficacy of exosomes derived from human adipose tissue-derived stem/stromal cells: A prospective, split-face, randomized placebo-controlled study. Cosmetics 2020, 7, 90. [Google Scholar] [CrossRef]
- Kwon, H.H.; Yang, S.H.; Lee, J.; Park, B.C.; Park, K.Y.; Jung, J.Y.; Youin, B.A.E.; Park, G.H. Combination treatment with human adipose tissue stem cell- derived exosomes and fractional CO2 laser for acne scars: A 12-week prospective, double-blind, randomized, split-face study. Acta Derm. Venereol. 2020, 100, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.; Kim, J.; Park, J. Strategies to enhance extracellular vesicle production. Tissue Eng. Regen. Med. 2021, 18, 513–524. [Google Scholar] [CrossRef]
- Gao, J.; Wang, S.; Wang, Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials 2017, 135, 62–73. [Google Scholar] [CrossRef]
- Wang, L.; Abhange, K.K.; Wen, Y.; Chen, Y.; Xue, F.; Wang, G.; Tong, J.; Zhu, C.; He, X.; Wan, Y. Preparation of engineered extracellular vesicles derived from human umbilical cord mesenchymal stem cells with ultrasonication for skin rejuvenation. ACS Omega 2019, 4, 22638–22645. [Google Scholar] [CrossRef] [Green Version]
- Pisano, S.; Pierini, I.; Gu, J.; Gazze, A.; Francis, L.W.; Gonzalez, D.; Conlan, R.S.; Corradetti, B. Immune (cell) derived exosome imetics (IDEM) as a treatment for ovarian cancer. Front. Cell Dev. Biol. 2020, 8, 932. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: General methodologies and latest trends. Biomed. Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Jeyaram, A.; Jay, S.M. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2019, 20, 1. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Richter, M.; Heinrich, E.; Koch, M.; Fuhrmann, G.; Friess, W. Enhancing the stabilization potential of lyophilization for extracellular vesicles. Adv. Healthc. Mater. 2022, 11, 2100538. [Google Scholar] [CrossRef]
- Ha, D.H.; Kim, S.D.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Lee, J.H.; Park, S.R.; Youn, J.; et al. Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul. Toxicol. Pharmacol. 2020, 115, 104686. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.D.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Nair, B.; Elmore, A.R. Final report on the safety assessment of Human Placental Protein, Hydrolyzed Human Placental Protein, Human Placental Enzymes, Human Placental Lipids, Human Umbilical Extract, Placental Protein, Hydrolyzed Placental Protein, Placental Enzymes, Placental Lipids, and umbilical extract. Int. J. Toxicol. 2002, 21, 81–91. [Google Scholar] [PubMed]

| Effect | Source of EVs | Model Used | Treatment Dose | Administration Method | Evidence and Clues | Reference |
|---|---|---|---|---|---|---|
| Scarless wound healing | ADSC-Exo | In vivo: Full-thickness dorsal wound in BALB/c mice model | 1000 µg/mL | IV | ↑ ratio of Col III:Col I, TGF-β3:TGF-β1, MMP-1 & MMP-3:TIMP-1 More flattened epidermal surface Well distributed collagen in the dermis with less cross-linking | [36] |
| In vitro: Fibroblasts | 0, 25, 50 & 100 µg/mL | - | ↓ myofibroblast differentiation ↓ α-SMA & Col1A1 ↑ TGF-β3, Col3A1, MMP-1 & MMP-3 | |||
| ADSC-Exo | In vivo: Excisional wound model in BALB/c mice | 700 μg/mL | SC | ↓ wound area ↓ Col I, Col III & α-SMA | [33] | |
| In vitro: HSF | 20 μg/mL | - | ↓ proliferation & migration ↓ Col I, Col III & α-SMA | |||
| ADSC-Exo | In vivo: Hypertrophic scar model in New Zealand rabbits | 0.1 mL | Local injection | ↓ α-SMA & Col I ↓ myofibroblast aggregation | [44] | |
| ADSC-Exo | In vivo: Full-thickness wound in BALB/C mice | 1000 µg/mL | SC | ↑ angiogenesis ↑ re-epithelialization rate ↓ scar area ↑ dermis thickness & collagen deposition | [45] | |
| In vitro: HDF | 0, 25, 50 & 100 μg/mL (Gene and protein expression analysis & Quantification of growth factor) 0, 50, and 100 μg/mL (proliferation & migration assay) | - | ↑ Col I, Col III, MMP-1, bFGF & TGF-β1 level ↓ α-SMA ↑ proliferation & migration | |||
| ADSC-MV | In vivo: Full-thickness wound in BALB/C mice | 50 μL | SC | ↑ re-epithelialization ↑ collagen deposition ↑ neovascularization ↓ wound edge (scar) | [46] | |
| In vitro: HUVEC, HaCaT, HDF | 0, 5 & 10 μg/mL (migration assay) 0 & 20 μg/mL (proliferation, angiogenesis assay, gene and protein expression analysis) | - | ↑ proliferation, migration & angiogenesis ↑ fibronectin in HUVEC, HaCaT & HDF ↑ Col I, Col III & elastin in HDF | |||
| UCB-MSC-Exo | In vivo: Full-thickness excisional wound in SD rats | 200 μg/mL | Tail vein injection | ↑ wound closure ↓ scar formation ↑ skin appendages regeneration ↑ angiogenesis Regulated collagen fibers distribution | [47] | |
| In vitro: HDF | 25 ng/mL | - | ↓ α-SMA & Col I ↑ proliferation & migration | |||
| UCB-Exo | In vivo: Full-thickness wound in C57BL/6 mice | 2000 μg/mL | Local injection | ↑ re-epithelialization ↑ angiogenesis ↓ scar width | [48] | |
| UC-MSC-Exo | In vitro: Fibroblasts | 50, 75 & 150 μg/mL | - | ↓ Col I, Col III & α-SMA | [49] | |
| UC-MSC-Exo | In vivo: Full-thickness excisional wound in BALB/C mice | 1000 μg/mL | SC | ↑ epidermal re-epithelialization & dermal angiogenesis ↓ α-SMA | [50] | |
| In vitro: HaCaT | 125, 250, 500, 1000 ng/mL | - | ↑ proliferation & migration ↓ apoptosis | |||
| hAEC-Exo | In vivo: Full-thickness excisional wound in SD rats | 25, 50 & 100 μg/mL | SC | Well-organized collagen fibers ↑ re-epithelialization | [51] | |
| In vitro: HDF | 25, 50 & 100 μg/mL | - | ↓ Col I & Col III ↑ MMP-1 & TIMP-1 ↑ proliferation & migration | |||
| hAFSC-Exo | In vivo: Full- thickness excisional wound in SD rats | 200 µg/mL | SC | Smoother wound edge ↑ hair follicle regeneration ↓ collagen fiber deposition ↑ nerve & vessel reconstruction ↑ cutaneous cell proliferation ↓ α-SMA, Col1A2, TGF-β1 & TIMP-1 ↑ Col3A1, TGF-β3, MMP-1 & MMP-3 | [52] | |
| In vitro: HDF | 10 & 25 ng/mL | - | ↓ α-SMA | |||
| TSG-6 modified MSC-Exo | In vivo: Full-thickness wound model in C57BL/6J mice | 1000 µg/mL | SC | ↓ MCP-1, TNF-α, IL-1β, IL-6, TGF-β1, Col I, Col III & α-SMA | [32] | |
| iPSC-MSC-Exo | In vitro: HaCaT, HDF | 10 & 20 µg/mL | - | ↑ proliferation & migration ↑ Col1A1, elastin & MMP-1 in HDF ↑ Col1A1 & elastin in HaCaT | [53] | |
| BM-MSC-Exo | In vitro: HaCaT, HDF, EAhy926 line, Human monocytic cell U937 | N/S | - | Not changes in TNF-α release by activated macrophages ↑ angiogenesis ↑ proliferation & migration of skin cells ↓ Col I, Col III, α-SMA, MMP-2 & MMP-14 as well as ↑ MMP-13 expression in myofibroblasts at the gene level ↑ Col I expression of myofibroblasts at the protein level ↑ decorin & fibronectin expression of fibroblasts at the protein level | [54] | |
| BM-MSC-Exo | In vivo: Full-thickness excisional wound in SD rat | 250 μg | IV | ↑ wound closure Restore skin function ↑ angiogenesis ↓ TGF-β1 | [20] | |
| In vitro: HaCaT, HDF | 25 μg/mL | - | ↑ proliferation | |||
| Anti-aging and promoting skin regeneration. | ADSC-Exo | In vivo: Photoaging SD rats | 100 μL | SC | ↓ epidermal thickness ↑ dermal thickness | [55] |
| In vitro: HDF | 12.5, 25, 50, 100 & 200 μg/mL | - | ↑ Col I ↓ Col III, MMP-1 & MMP-3 | |||
| ADSC-EV | In vivo: Photoaging BALB/c nude mice | 150 & 300 μg/mL | SC | ↓ skin wrinkle ↑ epidermal cell proliferation ↓ macrophage infiltration & ROS production | [15] | |
| In vitro: Photoaging HDF, 264.7 cells | 50, 100, 150 & 200 μg/mL (HDF activity) 100 & 200 μg/mL (Macrophage differentiation, gene expression analysis & protein expression analysis) | - | ↑ HDF activity & protected HDFs from UVB-induced senescence ↓ Col I ↑ MMP-3 ↓ M0 to M1 differentiation of macrophages ↑ SOD-1 & CAT Rescue HDFs from cell cycle arrest | |||
| ADSC-Exo | In vitro: UVB- irradiated HDF | N/S | - | ↑ cell migration & proliferation ↓ MMP-1, -2, -3 & -9 ↑ Col I, II, III, V & elastin ↑ TIMP-1 & TGF-β1 | [56] | |
| ADSC-Exo | In vitro: HDF | N/S | - | ↓ UVB-induced DNA damage, ROS production & MMP-1 ↑ procollagen type I | [57] | |
| UC-MSC-EV & Fb-EV | In vitro: HDF | 0, 0.5 & 5 μg/mL | - | ↓ ROS production ↑ proliferation ↑ GPX-1 & Col I ↓ MMP-1 Protect cells against UVB- induced cell death & cell cycle arrest Protect cells against UVB-induced photoaging through antioxidant activity | [58] | |
| iPSCs-Exo | In vitro: Photoaging and naturally senescent HDF | 20 × 108 particles/mL | - | ↓ cell damage ↓ SA-β-Gal & MMP-1/3 ↑ Col I | [59] | |
| TB-Exo | In vitro: Intrinsically/extrinsically senescent HNDF | 1 × 104 & 1 × 105 particles/mL | - | ↑ cell migration & proliferation ↑ Col I, Col III, elastin & fibronectin | [60] | |
| UC-MSC-Exo | Ex vivo: Photodamage skin model in SD rat | 200, 400 & 600 µg | - | ↓ skin photodamage | [61] | |
| In vitro: HaCaT | 600 µg | - | Protect cells from oxidative stress ↓ ROS production ↑ SIRT1 expression under oxidative stress condition Activate autophagy by delivery of 14-3-3ζ protein | |||
| Anti-pigmentation | Mouse keratinocyte-Exo | In vitro: Mouse melanocytes | N/S | - | ↓ TYR, TYRP1, TYRP2 & MITF ↓ melanin content | [62] |
| hAMSC- Exo | In vitro: B16F10 cells | N/S | - | ↓ TYR, TYRP1, TYRP2 & MITF ↓ melanin content ↑ LC3II ↓ p62 | [63] | |
| UVA-exposed melanocyte-EV | In vitro: Keratinocytes, melanoma cells | N/S | - | ↑ BCL-xL & BCL-2 in keratinocytes ↓ PDCD4 & PTEN in keratinocytes ↑ proliferation & migration of keratinocytes and melanocytes | [64] |
| Source of EVs + Treatment | Model Used | Treatment Dose | Administration | Result | Effect | Reference |
|---|---|---|---|---|---|---|
| ADSCs-EVs + MN | In vivo: UV-induced photoaging model in SKH-1 mice | 200 μL | Topical application | Least wrinkles Highest collagen density Organized collagen fibers ↓ cell infiltration ↓ epidermis thickness ↑ stratum corneum hydration ↓ TEWL ↑ recovery from the MN-induced injury | Anti-aging | [89] |
| UC-MSC-Exo + SHSs | In vivo: UV-induced photoaging model in Kunming mice | 1 mg/mL | Topical application | ↑ skin absorption of exosomes ↓ microwrinkle & epidermis thickness | Anti-aging | [90] |
| In vivo: Guinea pigs | 1 mg/mL | Topical application | Slightly irritating, but fast recovery | |||
| 3D HDF spheroids-Exo + needle-free injector | In vivo: UVB-induced photoaging model in nude mice | N/S | Needle-free injection | Least wrinkles Most dense collagen fibers Most compact stratum corneum ↓ epidermal thickness | Anti-aging | [69] |
| Source of EVs + Treatment | Model Used | Treatment Dose | Administration | Results | Effect | Reference |
|---|---|---|---|---|---|---|
| ADSC-Exo + Alg hydrogel | In vivo: Full-thickness excisional wound model in Wistar rats | 300 µL | Topical application | Cumulative release of Exo from hydrogel up to 172 h ↑ wound closure, collagen synthesis & angiogenesis ↓ wound size | Promote wound healing | [94] |
| SR-sEVs + BSSPD hydrogel | In vivo: Full-thickness skin defect model in rats | 1 × 1011 particles/mL | Topical application | Faster wound healing ↑ vascularization & angiogenesis Improve collagen fiber arrangement ↓ Col I/III collagen ratio Attenuated M2 polarization in later phase of wound healing | Promote scarless wound Healing | [17] |
| In vivo: Rabbit ears | 1 × 1011 particles/mL | Topical application | More ordered collagen arrangement ↓ scar elevation index | |||
| hEnSCs-Exo + CS-glycerol based hydrogel | In vivo: Full-thickness wound model in BALB/c mice | 100 µg/mL | Topical application | ↑ epidermal & skin appendages formation ↑ vascularization & angiogenesis ↑ wound closure ↓ wound size | Promote wound healing | [95] |
| hP-MSC-EV + CS hydrogel | In vivo: Natural aging FVB mice (48 weeks old) | 750 μg/mL | SC | ↑ skin appendages & epithelial thickness ↑ wound closure ↑ collagen bundles ↓ SA-β-gal ↑ Col I & Col III ↓ MMP-1, 2, 3 & 9 ↑ TIMP-1 & 2 | Skin rejuvenation | [68] |
| UC-MSC-Exo + CMCS/P407 hydrogel | In vivo: Full-thickness skin defect in SD rats | 20 μg/mL | Topical application | 85% cumulative release of Exo after 72 h ↑ wound closure rate, number of dermal appendages & collagen deposition, Well organized collagen fiber ↓ TNF-α & IL-1β | Promote wound healing | [96] |
| HUVEC-Exo + GelMA hydrogel | In vivo: Full-thickness wound model in SD rats | 1 × 108 particles/mL | Topical application | Controlled release of Exo until day 7 ↑ re-epithelialization, collagen alignment, deposition and maturity, & granulation tissue thickness ↑ Col I & Col III ↑ angiogenesis | Wound repair and regeneration | [97] |
| L929-EV + FG | In vivo: Full-thickness wound model in C57BL/6 mice | 5000 μg/mL | Topical application | ↑ wound closure rate ↑ collagen formation & maturation ↑ skin appendages & angiogenesis Minimum scarring | Promote scarless wound healing | [98] |
| EPSC-Exo + HydroMatrix | In vivo: Full-thickness skin defect in SD rats | 100 μg/mL | Local injection | ↑ nerve and vessel regeneration ↑ skin appendage regeneration ↓ myofiber formation ↑ Col III ↓ Col I & TGF-β1 | Promote scarless wound healing | [99] |
| PVC-EV + HydroMatrix | In vivo: Full-thickness skin defect in SD rats | 100 μg/mL | Local injection | ↑ wound contraction ↓ wound size ↑ α-SMA and TGF-β1 ↑ angiogenesis & VEGF | Promote wound healing | [12] |
| Source of EVs | Model Used | Treatment Dose | Administration | Result | Effect | Reference |
|---|---|---|---|---|---|---|
| EV from C. fragile and S. fusiforme | Clinical: 21 women (ages between 20 and 50 years) | 5 µg/mL | Topical application | ↑ skin brightness | Whitening | [16] |
| Ex vivo: MelanoDerm tissue | 5 µg/mL | - | ↓ melanin synthesis | |||
| In vitro: MNT-1 cells | 0,5, 25, 50 μg/mL (C. fragile) 0, 10, 50, 250 μg/mL (S. fusiforme) | - | ↓ α-MSH-mediated melanin synthesis ↓ MITF, TYR & TYRP1 | |||
| LEV and SEV from D. morbifera | Ex vivo: Neoderm-ME | 10 µg/mL | - | Lighter color ↓ melanin distribution in the epidermis | Anti-melanogenic | [85] |
| In vitro: B16BL6 melanoma cells | 1, 5 & 10 µg/mL (Melanin content measurement) 10, 50 & 100 µg/mL (TYR, TYRP-1, TYRP-2 & MITF protein or activity measurement) | - | ↓ melanin content ↓ TYR, TYRP-1, TYRP-2 & MITF | |||
| A-EV | In vitro: HaCaT & HDF | 1, 5, and 10 × 108 particles/mL (cell viability and SOD activity) 108 and 109 particles/mL (Scratch wound assay) | - | Non-cytotoxic ↑ migration ↑ Nrf2, HO-1, CAT & SOD | Antioxidant & skin regeneration | [107] |
| GrEV and GcEV from P. ginseng | In vitro: HEK, HDF & HEM | 1 & 10 µg/mL (Anti-senescence effect) 0.1, 1, 5, or 10 µg/mL (Melanin level) | - | Non-cytotoxic ↓ TYRP2, TYR & RAB27 ↑ HMGB1 ↓ SA-β-Gal ↓ TP53, CDKN1A, CDKN2A, MMP-1 & IL-8 ↓ melanin | Anti-senescence & anti-melanogenic | [110] |
| Source of EVs | Model Used | Treatment Dose | Administration | Result | Effect | Reference |
|---|---|---|---|---|---|---|
| ADSC-Exo | In vivo: 21 female with hyperpigmentation, aged 39–55 years | 2.0 × 1010 particles/mL | Topical application | ↓ melanin levels No cytotoxicity No adverse effect | Skin brightening effect | [115] |
| In vitro: B16F10 cells | 2.3 × 109–3.0 × 1011 particles/mL | - | ↓ melanin contents | |||
| ADSC-Exo | In vivo: 18 men and 7 women with atrophic acne scars, age 19–54 years, 12 with Fitzpatrick skin type III and 13 with type IV | 9.78 × 1010 particles/mL (for the day of FCL treatment) or 1.63 × 1010 particles/mL (for days subsequent to FCL treatment) | N/S | ↓ ECCA scores ↓ IGA scores ↓ atrophic scar volume, mean pore volume & skin surface roughness Milder adverse effects on Exo treated side which resolved within 5 days. | Scar reduction | [116] |
| Limitation | Recommendation |
|---|---|
| Scalable production of EVs | Application of strategies such as 3D culture using a bioreactor, physical stimulation (e.g., mechanical and electrical), chemical stimulation (e.g., drugs and small molecules), genetic manipulation to modulate the EV biogenesis and release pathway, and physiological modification (e.g., hypoxia and temperature) to up-scale the EV production |
| Storage and stability of EVs | Innovations in formulation or lyophilization |
| Dosage regime | Standardize the safe and effective dose |
| Safety | Perform more toxicity testing in animals (rodent and non-rodent models) |
| Cargo and mechanism of action | Identify the protein, nucleic acid, and lipid contents, followed by bioinformatics as well as in vitro and in vivo experiments to examine the mechanism of action |
| Lack of clinical data | Carried out more clinical studies |
| Uniformity in EV production | Development of standardized EV production, isolation, and storage protocols |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kee, L.T.; Ng, C.Y.; Al-Masawa, M.E.; Foo, J.B.; How, C.W.; Ng, M.H.; Law, J.X. Extracellular Vesicles in Facial Aesthetics: A Review. Int. J. Mol. Sci. 2022, 23, 6742. https://doi.org/10.3390/ijms23126742
Kee LT, Ng CY, Al-Masawa ME, Foo JB, How CW, Ng MH, Law JX. Extracellular Vesicles in Facial Aesthetics: A Review. International Journal of Molecular Sciences. 2022; 23(12):6742. https://doi.org/10.3390/ijms23126742
Chicago/Turabian StyleKee, Li Ting, Chiew Yong Ng, Maimonah Eissa Al-Masawa, Jhi Biau Foo, Chee Wun How, Min Hwei Ng, and Jia Xian Law. 2022. "Extracellular Vesicles in Facial Aesthetics: A Review" International Journal of Molecular Sciences 23, no. 12: 6742. https://doi.org/10.3390/ijms23126742
APA StyleKee, L. T., Ng, C. Y., Al-Masawa, M. E., Foo, J. B., How, C. W., Ng, M. H., & Law, J. X. (2022). Extracellular Vesicles in Facial Aesthetics: A Review. International Journal of Molecular Sciences, 23(12), 6742. https://doi.org/10.3390/ijms23126742









