Antitumor Effects of Orally Administered Rare Sugar D-Allose in Bladder Cancer
Abstract
:1. Introduction
2. Results
2.1. D-Allose Inhibits Bladder Cancer Cell Viability
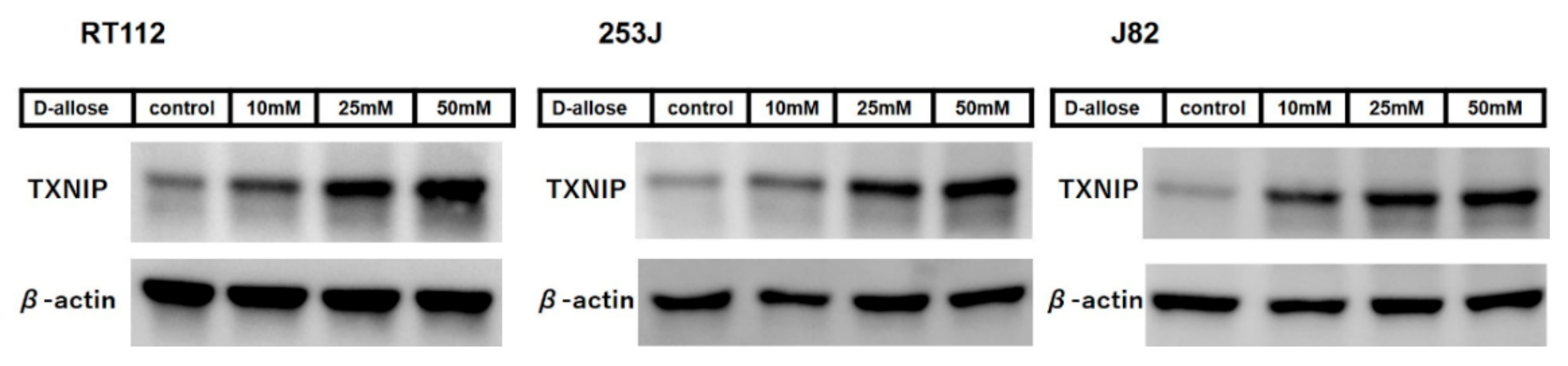
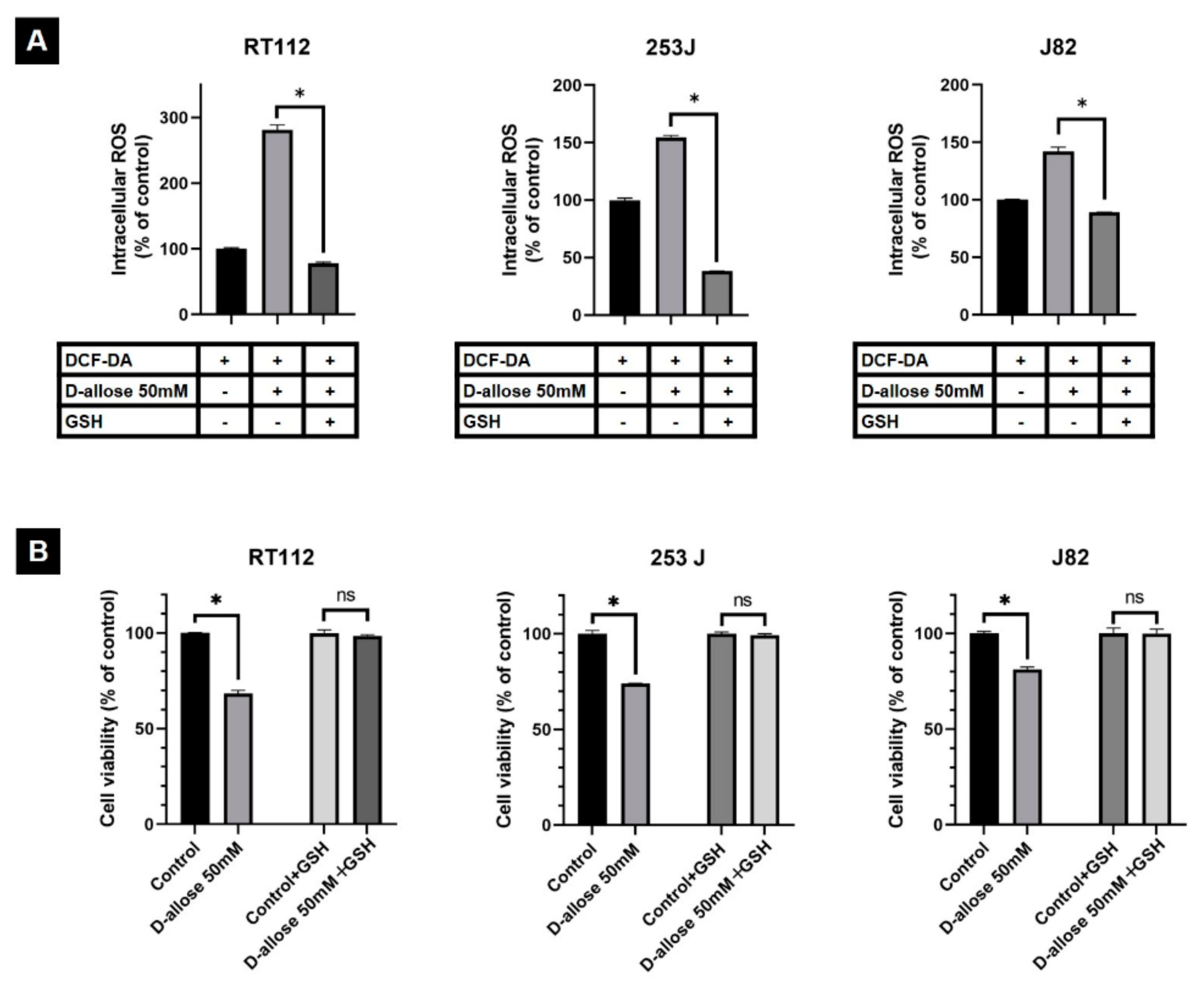
2.2. D-Allose Induces Intracellular ROS and Stimulates the TXNIP Expression in Bladder Cancer Cells
2.3. Suppression of D-Allose-Induced Antitumor Effects by Antioxidant Glutathione
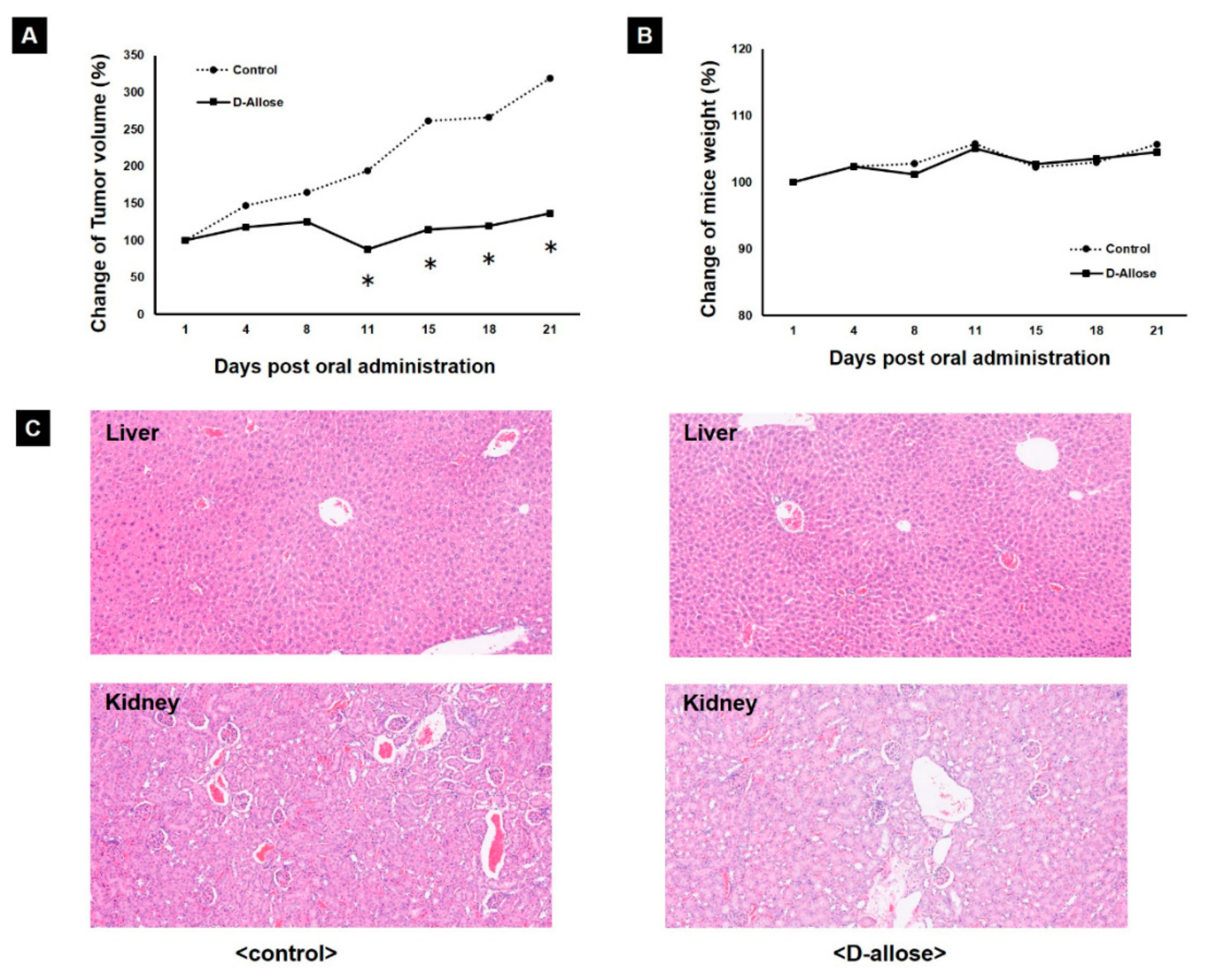
2.4. Tumor Growth Inhibition in a Preclinical Mouse Model by Oral Administration of D-Allose
3. Discussion
4. Materials and Methods
4.1. Reagents and Cell Lines
4.2. Cell Viability Assay
4.3. Intracellular ROS Evaluation
4.4. Co-Treatment with D-Allose and GSH
4.5. Western Blotting
4.6. In Vivo Xenograft Experiment
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Berdik, C. Unlocking bladder cancer. Nature 2017, 551, S34–S35. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute Surveillance. Epidemiology and End Results Program. SEER Cancer Statistics Factsheets: Bladder Cancer. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 1 May 2021).
- Izumori, K. Bioproduction strategies for rare hexose sugars. Naturwissenschaften 2002, 89, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Kanaji, N.; Kamitori, K.; Hossain, A.; Noguchi, C.; Katagi, A.; Kadowaki, N.; Tokuda, M. Additive antitumour effect of D-allose in combination with cisplatin in non-small cell lung cancer cells. Oncol. Rep. 2018, 39, 1292–1298. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, F.; Takata, M.; Kamitori, K.; Nonaka, M.; Dong, Y.; Sui, L.; Tokuda, M. Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27Kip1. Int. J. Oncol. 2008, 32, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Indo, K.; Hoshikawa, H.; Kamitori, K.; Yamaguchi, F.; Mori, T.; Tokuda, M.; Mori, N. Effects of D-allose in combination with docetaxel in human head and neck cancer cells. Int. J. Oncol. 2014, 45, 2044–2050. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, A.; Matsui, M.; Iwata, S.; Hirota, K.; Masutani, H.; Nakamura, H.; Takagi, Y.; Sono, H.; Gon, Y.; Yodoi, J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999, 274, 21645–21650. [Google Scholar] [CrossRef] [Green Version]
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Han, S.H.; Jeon, J.H.; Ju, H.R.; Jung, U.; Kim, K.Y.; Yoo, H.S.; Lee, Y.H.; Song, K.S.; Hwang, H.M.; Na, Y.S.; et al. VDUP1 upregulated by TGF-beta1 and 1,25-dihydorxyvitamin D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene 2003, 22, 4035–4046. [Google Scholar] [CrossRef] [Green Version]
- Escrich, E.; Moral, R.; García, G.; Costa, I.; Sánchez, J.A.; Solanas, M. Identification of novel differentially expressed genes by the effect of a high-fat n-6 diet in experimental breast cancer. Mol. Carcinog. 2004, 40, 73–78. [Google Scholar] [CrossRef]
- Ikarashi, M.; Takahashi, Y.; Ishii, Y.; Nagata, T.; Asai, S.; Ishikawa, K. Vitamin D3 up-regulated protein 1 (VDUP1) expression in gastrointestinal cancer and its relation to stage of disease. Anticancer Res. 2002, 22, 4045–4048. [Google Scholar]
- Takahashi, Y.; Nagata, T.; Ishii, Y.; Ikarashi, M.; Ishikawa, K.; Asai, S. Up-regulation of vitamin D3 up-regulated protein 1 gene in response to 5-fluorouracil in colon carcinoma SW620. Oncol. Rep. 2002, 9, 75–79. [Google Scholar] [CrossRef]
- Nishizawa, K.; Nishiyama, H.; Matsui, Y.; Kobayashi, T.; Saito, R.; Kotani, H.; Masutani, H.; Oishi, S.; Toda, Y.; Fujii, N.; et al. Thioredoxin-interacting protein suppresses bladder carcinogenesis. Carcinogenesis 2011, 32, 1459–1466. [Google Scholar] [CrossRef] [Green Version]
- Junn, E.; Han, S.H.; Im, J.Y.; Yang, Y.; Cho, E.W.; Um, H.D.; Kim, D.K.; Lee, K.W.; Han, P.L.; Rhee, S.G.; et al. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J. Immunol. 2000, 164, 6287–6295. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, A.; Masutani, H.; Nakamura, H.; Nishinaka, Y.; Yodoi, J. Redox regulation by thioredoxin and thioredoxin-binding proteins. IUBMB Life 2001, 52, 29–33. [Google Scholar] [CrossRef]
- Zhou, J.; Bi, C.; Cheong, L.L.; Mahara, S.; Liu, S.C.; Tay, K.G.; Koh, T.L.; Yu, Q.; Chng, W.J. The histone methyltransferase inhibitor, DZNep, up-regulates TXNIP, increases ROS production, and targets leukemia cells in AML. Blood 2011, 118, 2830–2839. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Rathore, K.; Wang, H.C. Differential induction of reactive oxygen species through ERK1/2 and Nox-1 by FK228 for selective apoptosis of oncogenic H-Ras-expressing human urinary bladder cancer J82 cells. J. Cancer Res. Clin. Oncol. 2011, 137, 471–480. [Google Scholar] [CrossRef]
- Takeuchi, H.; Taoka, R.; Mmeje, C.O.; Jinesh, G.G.; Safe, S.; Kamat, A.M. CDODA-Me decreases specificity protein transcription factors and induces apoptosis in bladder cancer cells through induction of reactive oxygen species. Urol. Oncol. 2016, 34, 337.e11–337.e18. [Google Scholar] [CrossRef]
- Mitani, T.; Hoshikawa, H.; Mori, T.; Hosokawa, T.; Tsukamoto, I.; Yamaguchi, F.; Kamitori, K.; Tokuda, M.; Mori, N. Growth inhibition of head and neck carcinomas by D-allose. Head Neck 2009, 31, 1049–1055. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- Zhou, J.; Chng, W.J. Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer. Mitochondrion 2013, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, Y.; Ueki, M.; Ueno, M.; Asaga, T.; Tokuda, M.; Shirakami, G. D-allose ameliorates cisplatin-induced nephrotoxicity in mice. Tohoku J. Exp. Med. 2012, 228, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohi, Y.; Taoka, R.; Zhang, X.; Matsuoka, Y.; Yoshihara, A.; Ibuki, E.; Haba, R.; Akimitsu, K.; Izumori, K.; Kakehi, Y.; et al. Antitumor Effects of Orally Administered Rare Sugar D-Allose in Bladder Cancer. Int. J. Mol. Sci. 2022, 23, 6771. https://doi.org/10.3390/ijms23126771
Tohi Y, Taoka R, Zhang X, Matsuoka Y, Yoshihara A, Ibuki E, Haba R, Akimitsu K, Izumori K, Kakehi Y, et al. Antitumor Effects of Orally Administered Rare Sugar D-Allose in Bladder Cancer. International Journal of Molecular Sciences. 2022; 23(12):6771. https://doi.org/10.3390/ijms23126771
Chicago/Turabian StyleTohi, Yoichiro, Rikiya Taoka, Xia Zhang, Yuki Matsuoka, Akihide Yoshihara, Emi Ibuki, Reiji Haba, Kazuya Akimitsu, Ken Izumori, Yoshiyuki Kakehi, and et al. 2022. "Antitumor Effects of Orally Administered Rare Sugar D-Allose in Bladder Cancer" International Journal of Molecular Sciences 23, no. 12: 6771. https://doi.org/10.3390/ijms23126771
APA StyleTohi, Y., Taoka, R., Zhang, X., Matsuoka, Y., Yoshihara, A., Ibuki, E., Haba, R., Akimitsu, K., Izumori, K., Kakehi, Y., & Sugimoto, M. (2022). Antitumor Effects of Orally Administered Rare Sugar D-Allose in Bladder Cancer. International Journal of Molecular Sciences, 23(12), 6771. https://doi.org/10.3390/ijms23126771





