Constructing a Carbon-Encapsulated Carbon Composite Material with Hierarchically Porous Architectures for Efficient Capacitive Storage in Organic Supercapacitors
Abstract
:1. Introduction
2. Results and Discussion
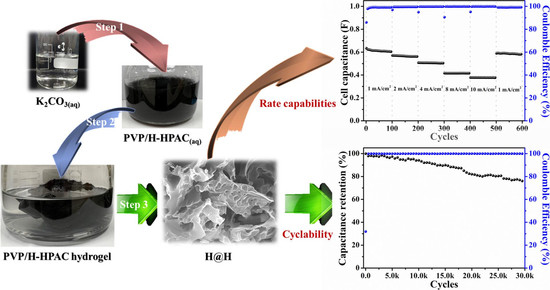
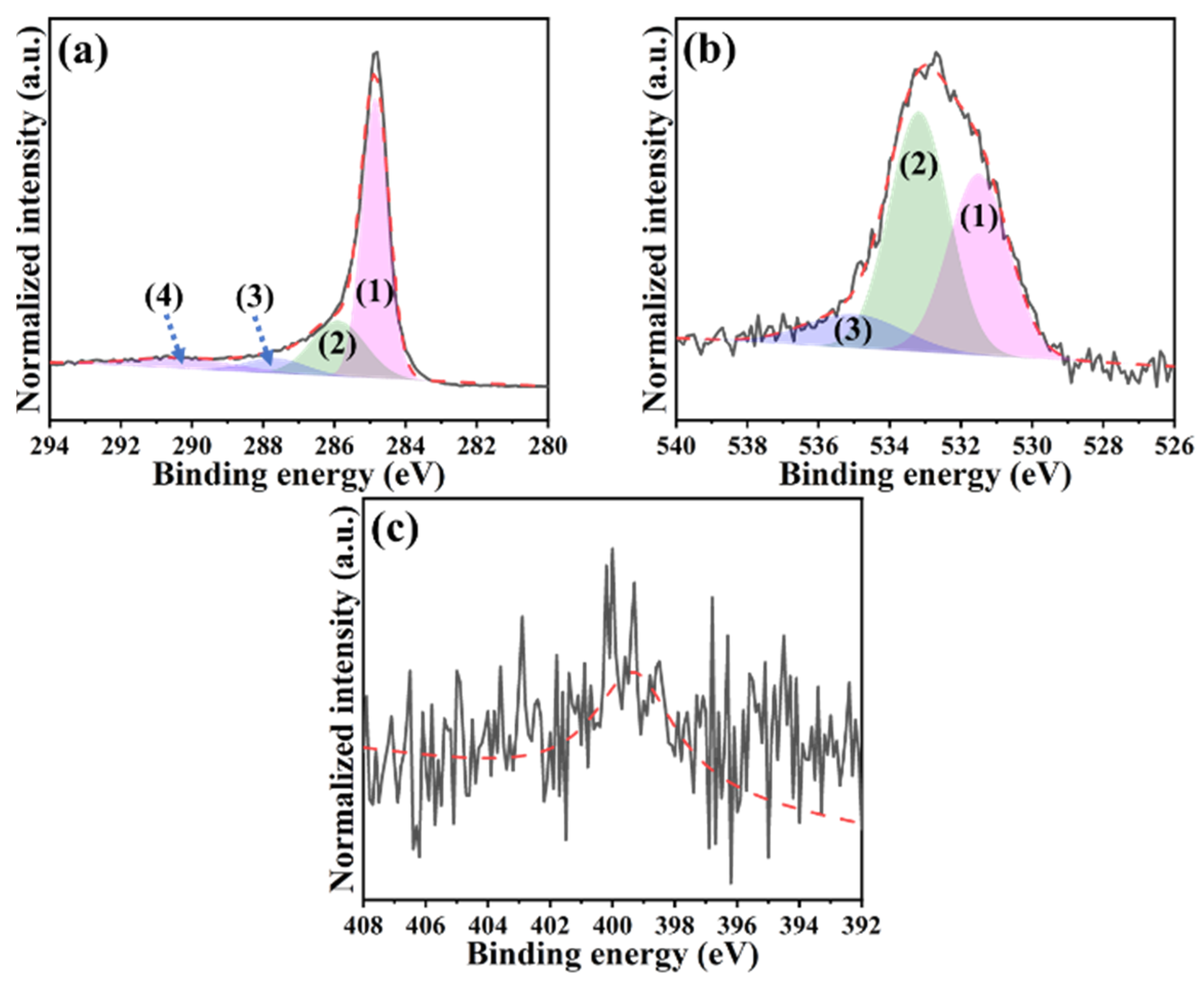
2.1. Characterizations of Hydrogel-Derived Hierarchical Porous Activated Carbon (H-HPAC)-Encapsulated H-HPAC (H@H) Composite Material
2.2. Electrochemical Performances of H@H in Symmetric Supercapacitor

3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Hydrogel-Derived Hierarchical Porous Activated Carbon (H-HPAC) Encapsulated H-HPAC (H@H) Composite Material
3.3. Characterizations
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Chen, G.Z. Supercapatteries as high-performance electrochemical energy storage devices. Electrochem. Energ. Rev. 2020, 3, 271–285. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R. Integration of supercapacitors and batteries towards high-performance hybrid energy storage devices. Int. J. Energy Res. 2021, 45, 1449–1479. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Santhosh, R.; Adduru, J.; Rao, T.N.; Karthik, M. Activated carbon fibres as high-performance supercapacitor electrodes with commercial level mass loading. Carbon 2018, 140, 465–476. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, J.; Li, Q.; Zhang, S.; Song, H.; Jia, D. Carbon materials for high mass-loading supercapacitors: Filling the gap between new materials and practical applications. J. Mater. Chem. A 2020, 8, 21930–21946. [Google Scholar] [CrossRef]
- He, W.; Chen, K.; Pathak, R.; Hummel, M.; Reza, K.M.; Ghimire, N.; Pokharel, J.; Lu, S.; Gu, Z.; Qiao, Q.; et al. High-mass-loading Sn-based anode boosted by pseudocapacitance for long-life sodium-ion batteries. Chem. Eng. J. 2021, 414, 128638. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chang-Jian, C.W.; Hsieh, T.H.; Huang, J.H.; Weng, H.C.; Hsiao, Y.S.; Syu, W.L.; Chen, C.P. High-performance Li-ion capacitor constructed from biomass-derived porous carbon and high-rate Li4Ti5O12. Appl. Surf. Sci. 2021, 543, 148717. [Google Scholar] [CrossRef]
- Hung, T.F.; Hsieh, T.H.; Tseng, F.S.; Wang, L.Y.; Yang, C.C.; Yang, C.C. High-mass loading hierarchically porous activated carbon electrode for pouch-type supercapacitors with propylene carbonate-based electrolyte. Nanomaterials 2021, 11, 785. [Google Scholar] [CrossRef]
- Guo, W.; Yu, C.; Li, S.; Qiu, J. Toward commercial-level mass-loading electrodes for supercapacitors: Opportunities, challenges and perspectives. Energy Environ. Sci. 2021, 14, 576–601. [Google Scholar] [CrossRef]
- Abdelaal, M.M.; Hung, T.C.; Mohamed, S.G.; Yang, C.C.; Hung, T.F. Two birds with one stone: Hydrogel-derived hierarchical porous activated carbon toward the capacitive performance for symmetric supercapacitors and lithium-ion capacitors. ACS Sustain. Chem. Eng. 2022, 10, 4717–4727. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Y.; Suresh, L.; Hao, A.; Bick, M.; Tan, S.C.; Chen, J. Carbon nanotube reinforced strong carbon matrix composites. ACS Nano 2020, 14, 9282–9319. [Google Scholar] [CrossRef]
- Han, Y.; Li, S.; Chen, F.; Zhao, T. Multi-scale alignment construction for strong and conductive carbon nanotube/carbon composites. Mater. Today Commun. 2016, 6, 56–68. [Google Scholar] [CrossRef]
- Lee, J.; Kim, T.; Jung, Y.; Jung, K.; Park, J.; Lee, D.M.; Jeong, H.S.; Hwang, J.Y.; Park, C.R.; Lee, K.H.; et al. High-strength carbon nanotube/carbon composite fibers via chemical vapor infiltration. Nanoscale 2016, 8, 18972–18979. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, F.; Song, Y.; Li, Y. Revitalizing carbon supercapacitor electrodes with hierarchical porous structures. J. Mater. Chem. A 2017, 5, 17705–17733. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.; Fu, Z.; Su, B.L. Hierarchically structured porous materials: Synthesis strategies and applications in energy storage. Natl. Sci. Rev. 2020, 7, 1667–1701. [Google Scholar] [CrossRef]
- Wu, J.; Xia, M.; Zhang, X.; Chen, Y.; Sun, F.; Wang, X.; Yang, H.; Chen, H. Hierarchical porous carbon derived from wood tar using crab as the template: Performance on supercapacitor. J. Power Source 2020, 455, 227982. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Alhebshi, N.A.; Alshareef, H.N. Synthesis strategies of porous carbon for supercapacitor applications. Small Methods 2020, 4, 1900853. [Google Scholar] [CrossRef]
- Díez, N.; Fuertes, A.B.; Sevilla, M. Molten salt strategies towards carbon materials for energy storage and conversion. Energy Storage Mater. 2021, 38, 50–69. [Google Scholar] [CrossRef]
- Díez, N.; Ferrero, G.A.; Fuertes, A.B.; Sevilla, M. Sustainable salt template-assisted chemical activation for the production of porous carbons with enhanced power handling ability in supercapacitors. Batter. Supercaps 2019, 2, 701–711. [Google Scholar] [CrossRef]
- Díez, N.; Ferrero, G.A.; Sevilla, M.; Fuertes, A.B. A sustainable approach to hierarchically porous carbons from tannic acid and their utilization in supercapacitive energy storage systems. J. Mater. Chem. A 2019, 7, 14280–14290. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, M.; Díez, N.; Fuertes, A.B. More sustainable chemical activation strategies for the production of porous carbons. ChemSusChem 2021, 14, 94–117. [Google Scholar] [CrossRef]
- Abdelaal, M.M.; Hung, T.C.; Mohamed, S.G.; Yang, C.C.; Huang, H.P.; Hung, T.F. A comparative study of the influence of nitrogen content and structural characteristics of NiS/nitrogen-doped carbon nanocomposites on capacitive performances in alkaline medium. Nanomaterials 2021, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- Plachy, T.; Kutalkova, E.; Skoda, D.; Holcapkova, P. Transformation of cellulose via two-step carbonization to conducting carbonaceous particles and their outstanding electrorheological performance. Int. J. Mol. Sci. 2022, 23, 5477. [Google Scholar] [CrossRef] [PubMed]
- Vatankhah, A.R.; Hosseini, M.A.; Malekiec, S. The characterization of gamma-irradiated carbon-nanostructured materials carried out using a multi-analytical approach including Raman spectroscopy. Appl. Surf. Sci. 2019, 488, 671–680. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chung, C.H.; Hsieh, T.H.; Lin, H.P. Green and highly-efficient microwave synthesis route for sulfur/carbon composite for Li-S battery. Int. J. Mol. Sci. 2022, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Zhu, Z.; Li, H.; Hu, Z.; Zhang, K.; Cheng, F.; Chen, J. Na3V2(PO4)3@C core-shell nanocomposites for rechargeable sodium-ion batteries. J. Mater. Chem. A 2014, 2, 8668–8675. [Google Scholar] [CrossRef]
- Zolkin, A.; Semerikova, A.; Chepkasov, S.; Khomyakov, M. Characteristics of the Raman spectra of diamond-like carbon films. Influence of methods of synthesis. Mater. Today Proc. 2017, 4, 11480–11485. [Google Scholar] [CrossRef]
- Hung, T.F.; Cheng, W.J.; Chang, W.S.; Yang, C.C.; Shen, C.C.; Kuo, Y.L. Ascorbic acid-assisted synthesis of mesoporous sodium vanadium phosphate nanoparticles with highly sp2-coordinated carbon coatings as efficient cathode materials for rechargeable sodium-ion batteries. Chem. Eur. J. 2016, 22, 10620–10626. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Ding, L.; Wu, X.; Guo, N.; Guo, J.; Hou, S.; Tong, F.; Jia, D.; Zhang, H. Coal-based 3D hierarchical porous carbon aerogels for high performance and super-long life supercapacitors. Sci. Rep. 2020, 10, 7022. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, S.C.; Lee, H.M.; Kim, B.J. Comparison of pore structures of cellulose-based activated carbon fibers and their applications for electrode materials. Int. J. Mol. Sci. 2022, 23, 3680. [Google Scholar] [CrossRef]
- Kubicka, M.; Bakierska, M.; Chudzik, K.; Świętosławski, M.; Molenda, M. Nitrogen-doped carbon aerogels derived from starch biomass with improved electrochemical properties for Li-ion batteries. Int. J. Mol. Sci. 2021, 22, 9918. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhao, L.; Yu, Z.; Zhang, M.; Li, S.; Zhang, R.; Ayub, M.; Ma, X.; Ning, G.; Xu, C. Multilayered graphene endowing superior dispersibility for excellent low temperature performance in lithium-ion capacitor as both anode and cathode. Chem. Eng. J. 2022, 429, 132358. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, L.; Liang, Y.; Chen, Q.; Li, Z.; Li, C.; Wang, Z.; Li, W. Coconut-based activated carbon fibers for efficient adsorption of various organic dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazar, P.; Mach, R.; Otyepka, M. Spectroscopic fingerprints of graphitic, pyrrolic, pyridinic, and chemisorbed nitrogen in N-doped graphene. J. Phys. Chem. C 2019, 123, 10695–10702. [Google Scholar] [CrossRef]
- Yang, I.; Kim, S.G.; Kwon, S.H.; Kim, M.S.; Jung, J.C. Relationships between pore size and charge transfer resistance of carbon aerogels for organic electric double-layer capacitor electrodes. Electrochim. Acta 2017, 223, 21–30. [Google Scholar] [CrossRef]
- Kim, Y.J.; Masutzawa, Y.; Ozaki, S.; Endo, M.; Dresselhaus, M.S. PVDC-based carbon material by chemical activation and its application to nonaqueous EDLC. J. Electrochem. Soc. 2004, 151, E199. [Google Scholar] [CrossRef]
- Lin, R.; Taberna, P.L.; Chmiola, J.; Guay, D.; Gogotsi, Y.; Simon, P. Microelectrode study of pore size, ion size, and solvent effects on the charge/discharge behavior of microporous carbons for electrical double-layer capacitors. J. Electrochem. Soc. 2009, 156, A7. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Cao, H.; Zhu, S.; Hou, L.; Yuan, C. Hierarchical micro-/mesoporous N- and O-enriched carbon derived from disposable cashmere: A competitive cost-effective material for high-performance electrochemical capacitors. Green Chem. 2015, 17, 2373–2382. [Google Scholar] [CrossRef]
- Bai, Q.; Li, H.; Zhang, L.; Li, C.; Shen, Y.; Uyama, H. Flexible solid-state supercapacitors derived from biomass konjac/polyacrylonitrile-based nitrogen-doped porous carbon. ACS Appl. Mater. Interfaces 2020, 12, 55913–55925. [Google Scholar] [CrossRef]
- Sun, F.; Wu, H.; Liu, X.; Liu, F.; Zhou, H.; Gao, J.; Lu, Y. Nitrogen-rich carbon spheres made by a continuous spraying process for high-performance supercapacitors. Nano Res. 2016, 9, 3209–3221. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, Y.; Wang, C.; Qiao, Z.; Chen, M. Fabrication of conductive carbonaceous spherical architecture from pitch by spray drying. Chem. Eng. Sci. 2015, 135, 109–116. [Google Scholar] [CrossRef]
- Yang, C.; Nguyen, Q.D.; Chen, T.; Helal, A.S.; Li, J.; Chang, J. Functional group-dependent supercapacitive and aging properties of activated carbon electrodes in organic electrolyte. ACS Sustain. Chem. Eng. 2018, 6, 1208–1214. [Google Scholar] [CrossRef]
- Chang, P.; Wang, C.; Kinumoto, T.; Tsumura, T.; Chen, M.; Toyoda, M. Frame-filling C/C composite for high-performance EDLCs with high withstanding voltage. Carbon 2018, 131, 184–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Jiao, M.; Zhang, J.; Chen, M.; Wang, C. Synthesis of size-controllable lignin-based nanospheres and its application in electrical double layer capacitors. ChemistrySelect 2020, 5, 8265–8273. [Google Scholar] [CrossRef]
- Ghosh, S.; Barg, S.; Jeong, S.M.; Ostrikov, K. Heteroatom-doped and oxygen-functionalized nanocarbons for high-performance supercapacitors. Adv. Energy Mater. 2020, 10, 2001239. [Google Scholar] [CrossRef]
- Samartzis, N.; Athanasiou, M.; Dracopoulos, V.; Yannopoulos, S.N.; Ioannides, T. Laser-assisted transformation of a phenol-based resin to high quality graphene-like powder for supercapacitor applications. Chem. Eng. J. 2022, 430, 133179. [Google Scholar] [CrossRef]





| Properties | SSA (m2/g) | Vt 1 (cm3/g) | Vultra 2 (cm3/g) | Vmicro 3 (cm3/g) | Vmeso 4 (cm3/g) | |
|---|---|---|---|---|---|---|
| Samples | ||||||
| H@H | 1316 | 0.62 | 0.21 | 0.51 | 0.11 | |
| H-HPAC 5 | 2012 | 1.16 | 0.11 | 0.69 | 0.47 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amirtha, R.M.; Hsu, H.-H.; Abdelaal, M.M.; Anbunathan, A.; Mohamed, S.G.; Yang, C.-C.; Hung, T.-F. Constructing a Carbon-Encapsulated Carbon Composite Material with Hierarchically Porous Architectures for Efficient Capacitive Storage in Organic Supercapacitors. Int. J. Mol. Sci. 2022, 23, 6774. https://doi.org/10.3390/ijms23126774
Amirtha RM, Hsu H-H, Abdelaal MM, Anbunathan A, Mohamed SG, Yang C-C, Hung T-F. Constructing a Carbon-Encapsulated Carbon Composite Material with Hierarchically Porous Architectures for Efficient Capacitive Storage in Organic Supercapacitors. International Journal of Molecular Sciences. 2022; 23(12):6774. https://doi.org/10.3390/ijms23126774
Chicago/Turabian StyleAmirtha, Rene Mary, Hao-Huan Hsu, Mohamed M. Abdelaal, Ammaiyappan Anbunathan, Saad G. Mohamed, Chun-Chen Yang, and Tai-Feng Hung. 2022. "Constructing a Carbon-Encapsulated Carbon Composite Material with Hierarchically Porous Architectures for Efficient Capacitive Storage in Organic Supercapacitors" International Journal of Molecular Sciences 23, no. 12: 6774. https://doi.org/10.3390/ijms23126774
APA StyleAmirtha, R. M., Hsu, H.-H., Abdelaal, M. M., Anbunathan, A., Mohamed, S. G., Yang, C.-C., & Hung, T.-F. (2022). Constructing a Carbon-Encapsulated Carbon Composite Material with Hierarchically Porous Architectures for Efficient Capacitive Storage in Organic Supercapacitors. International Journal of Molecular Sciences, 23(12), 6774. https://doi.org/10.3390/ijms23126774