Extracellular Histones Trigger Disseminated Intravascular Coagulation by Lytic Cell Death
Abstract
:1. Introduction
2. Results and Discussion
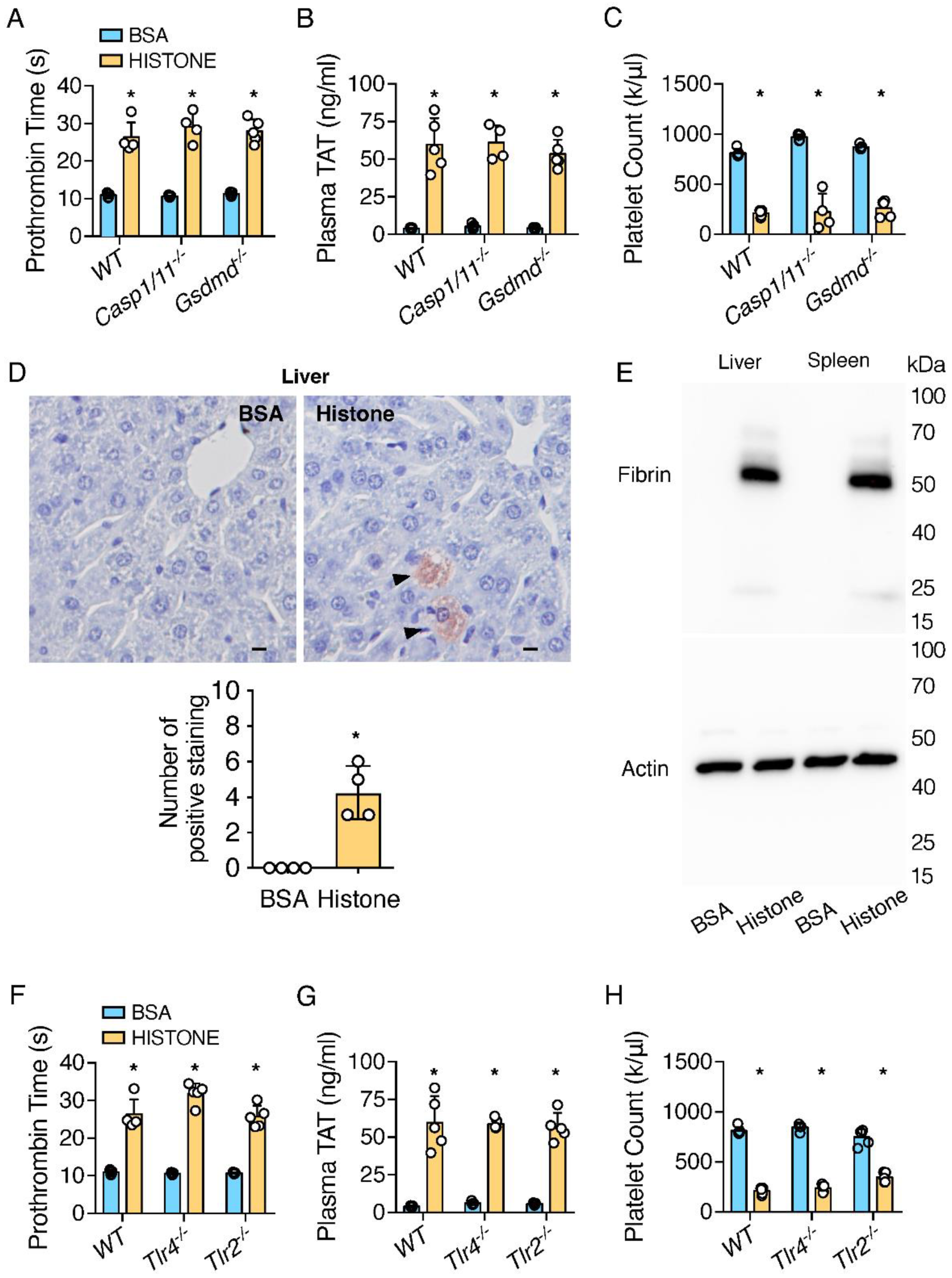
2.1. Extracellular Histones Trigger Coagulopathy Independent of TLR Receptors and Inflammatory Signaling Pathways
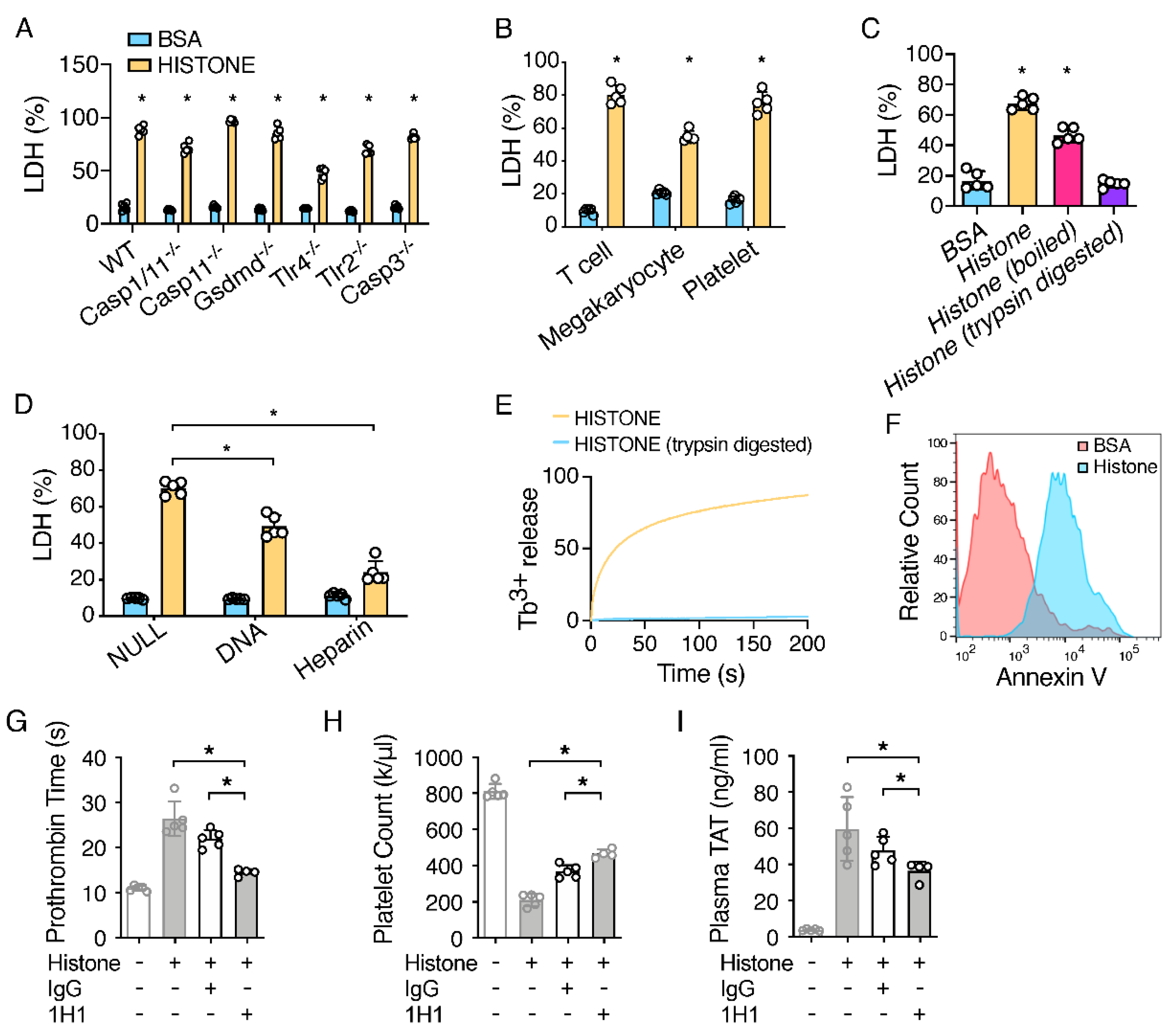
2.2. Extracellular Histones Lyse Macrophages and Expose PS
2.3. TF Neutralization Protects against Extracellular Histone-Induced Coagulopathy
3. Materials and Methods
3.1. Mice
3.2. In Vivo Challenges
3.3. Pharmacological TF Inhibition
3.4. Measurement of Coagulation
3.5. Tissue Preparation and Immunohistochemistry
3.6. BMDM Cultures
3.7. Detection of Fibrin in Tissues by Western Blot
3.8. Liposome Leakage Assay
3.9. Cytotoxicity Assays
3.10. Flow Cytometry
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrams, S.T.; Zhang, N.; Wang, G.; Toh, C.-H. Histone-Induced Lung Injury and Coagulation Activation Are Reduced by C-Reactive Protein in the Acute Phase Response. Blood 2013, 122, 457. [Google Scholar] [CrossRef]
- Kordbacheh, F.; O’Meara, C.H.; Coupland, L.A.; Lelliott, P.M.; Parish, C.R.J.B. Extracellular histones induce erythrocyte fragility and anemia. J. Am. Soc. Hematol. 2017, 130, 2884–2888. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Bhandari, A.A.; Wagner, D.D.J.B. Histones induce rapid and profound thrombocytopenia in mice. J. Am. Soc. Hematol. 2011, 118, 3708–3714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Noubouossie, D.F.; Whelihan, M.F.; Yu, Y.-B.; Sparkenbaugh, E.; Pawlinski, R.; Monroe, D.M.; Key, N.S. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood J. Am. Soc. Hematol. 2017, 129, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Monestier, M.; Esmon, N.L.; Esmon, C.T. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J. Immunol. 2011, 187, 2626–2631. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, T.A.; Wagner, D.D. Heparin Prevents Histone-Induced Thrombocytopenia and Mortality. Blood 2010, 116, 2106. [Google Scholar] [CrossRef]
- Michels, A.; Albánez, S.; Mewburn, J.; Nesbitt, K.; Gould, T.J.; Liaw, P.C.; James, P.D.; Swystun, L.L.; Lillicrap, D. Histones link inflammation and thrombosis through the induction of Weibel–Palade body exocytosis. J. Thromb. Haemost. 2016, 14, 2274–2286. [Google Scholar] [CrossRef]
- Ammollo, C.T.; Semeraro, F.; Xu, J.; Esmon, N.L.; Esmon, C.T. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J. Thromb. Haemost. 2011, 9, 1795–1803. [Google Scholar] [CrossRef]
- Semeraro, F.; Ammollo, C.T.; Morrissey, J.H.; Dale, G.L.; Friese, P.; Esmon, N.L.; Esmon, C.T. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet TLR2 and TLR4. Blood J. Am. Soc. Hematol. 2011, 118, 1952–1961. [Google Scholar] [CrossRef] [Green Version]
- Gould, T.J.; Lysov, Z.; Swystun, L.L.; Dwivedi, D.J.; Zarychanski, R.; Fox-Robichaud, A.E.; Liaw, P.C. Extracellular histones increase tissue factor activity and enhance thrombin generation by human blood monocytes. Shock. Inj. Inflamm. Sepsis Lab. Clin. Approaches 2016, 46, 655–662. [Google Scholar] [CrossRef]
- Kim, J.E.; Yoo, H.J.; Gu, J.Y.; Kim, H.K. Histones induce the procoagulant phenotype of endothelial cells through tissue factor up-regulation and thrombomodulin down-regulation. PLoS ONE 2016, 11, e0156763. [Google Scholar] [CrossRef] [Green Version]
- Nakahara, M.; Ito, T.; Kawahara, K.-i.; Yamamoto, M.; Nagasato, T.; Shrestha, B.; Yamada, S.; Miyauchi, T.; Higuchi, K.; Takenaka, T.; et al. Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS ONE 2013, 8, e75961. [Google Scholar] [CrossRef] [Green Version]
- Wada, H.; Wakita, Y.; Nakase, T.; Shimura, M.; Hiyoyama, K.; Nagaya, S.; Mori, Y.; Deguchi, K.; Shiku, H. Diagnosis of pre-disseminated intravascular coagulation stage with hemostatic molecular markers. The Mie DIC Study Group. Pol. J. Pharmacol. 1996, 48, 225–228. [Google Scholar]
- Koyama, K.; Madoiwa, S.; Nunomiya, S.; Koinuma, T.; Wada, M.; Sakata, A.; Ohmori, T.; Mimuro, J.; Sakata, Y. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: A prospective observational study. Crit. Care 2014, 18, R13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allam, R.; Darisipudi, M.N.; Tschopp, J.; Anders, H.J. Histones trigger sterile inflammation by activating the NLRP 3 inflammasome. Eur. J. Immunol. 2013, 43, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lu, W.; Zhang, Y.; Zhang, G.; Shi, X.; Hisada, Y.; Grover, S.P.; Zhang, X.; Li, L.; Xiang, B.; et al. Inflammasome Activation Triggers Blood Clotting and Host Death through Pyroptosis. Immunity 2019, 50, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Braster, Q.; Wichapong, K.; Lee, E.Y.; Teulon, J.M.; Berrebeh, N.; Winter, J.; Adrover, J.M.; Santos, G.S.; Froese, A.; et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 2019, 569, 236–240. [Google Scholar] [CrossRef]
- Marsman, G.; von Richthofen, H.; Bulder, I.; Lupu, F.; Hazelzet, J.; Luken, B.M.; Zeerleder, S. DNA and factor VII–activating protease protect against the cytotoxicity of histones. Blood Adv. 2017, 1, 2491–2502. [Google Scholar] [CrossRef]
- Kirchhofer, D.; Moran, P.; Bullens, S.; Peale, F.; Bunting, S. A monoclonal antibody that inhibits mouse tissue factor function. J. Thromb. Haemost. 2005, 3, 1098–1099. [Google Scholar] [CrossRef]
- Perdomo, J.; Leung, H.H.L.; Ahmadi, Z.; Yan, F.; Chong, J.J.H.; Passam, F.H.; Chong, B.H. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 2019, 10, 1322. [Google Scholar] [CrossRef] [Green Version]
- Abrams, S.T.; Su, D.; Sahraoui, Y.; Lin, Z.; Cheng, Z.; Nesbitt, K.; Alhamdi, Y.; Harrasser, M.; Du, M.; Foley, J.H.; et al. Assembly of alternative prothrombinase by extracellular histones initiates and disseminates intravascular coagulation. Blood 2021, 137, 103–114. [Google Scholar] [CrossRef]
- Davies, J.Q.; Gordon, S. Isolation and culture of murine macrophages. Methods Mol. Biol. 2005, 290, 91–103. [Google Scholar] [CrossRef]
- Xiang, B.; Zhang, G.; Guo, L.; Li, X.A.; Morris, A.J.; Daugherty, A.; Whiteheart, S.W.; Smyth, S.S.; Li, Z. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat. Commun. 2013, 4, 2657. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wu, C.; Li, L.; Pandeya, A.; Zhang, G.; Cui, J.; Kirchhofer, D.; Wood, J.P.; Smyth, S.S.; Wei, Y.; et al. Extracellular Histones Trigger Disseminated Intravascular Coagulation by Lytic Cell Death. Int. J. Mol. Sci. 2022, 23, 6800. https://doi.org/10.3390/ijms23126800
Zhang Y, Wu C, Li L, Pandeya A, Zhang G, Cui J, Kirchhofer D, Wood JP, Smyth SS, Wei Y, et al. Extracellular Histones Trigger Disseminated Intravascular Coagulation by Lytic Cell Death. International Journal of Molecular Sciences. 2022; 23(12):6800. https://doi.org/10.3390/ijms23126800
Chicago/Turabian StyleZhang, Yan, Congqing Wu, Lan Li, Ankit Pandeya, Guoying Zhang, Jian Cui, Daniel Kirchhofer, Jeremy P. Wood, Susan S. Smyth, Yinan Wei, and et al. 2022. "Extracellular Histones Trigger Disseminated Intravascular Coagulation by Lytic Cell Death" International Journal of Molecular Sciences 23, no. 12: 6800. https://doi.org/10.3390/ijms23126800
APA StyleZhang, Y., Wu, C., Li, L., Pandeya, A., Zhang, G., Cui, J., Kirchhofer, D., Wood, J. P., Smyth, S. S., Wei, Y., & Li, Z. (2022). Extracellular Histones Trigger Disseminated Intravascular Coagulation by Lytic Cell Death. International Journal of Molecular Sciences, 23(12), 6800. https://doi.org/10.3390/ijms23126800






