Characteristics of Graphene Oxide for Gene Transfection and Controlled Release in Breast Cancer Cells
Abstract
:1. Introduction
2. Characteristics of GO as a Nanocarrier
2.1. Size and Two-Dimensional Structure
2.2. Functionalization
2.2.1. Covalent Modification
2.2.2. Non-Covalent Modification
2.2.3. Targeting Strategy
2.2.4. Gene Loading Efficiency of GO-Based Carriers
3. Toxicity
3.1. In Vitro Toxicity
3.1.1. Dose-, Time-, and Cell-Line-Dependent Cytotoxicity
3.1.2. Charge- and Functionalization-Dependent Cytotoxicity
3.1.3. Size-Dependent Cytotoxicity
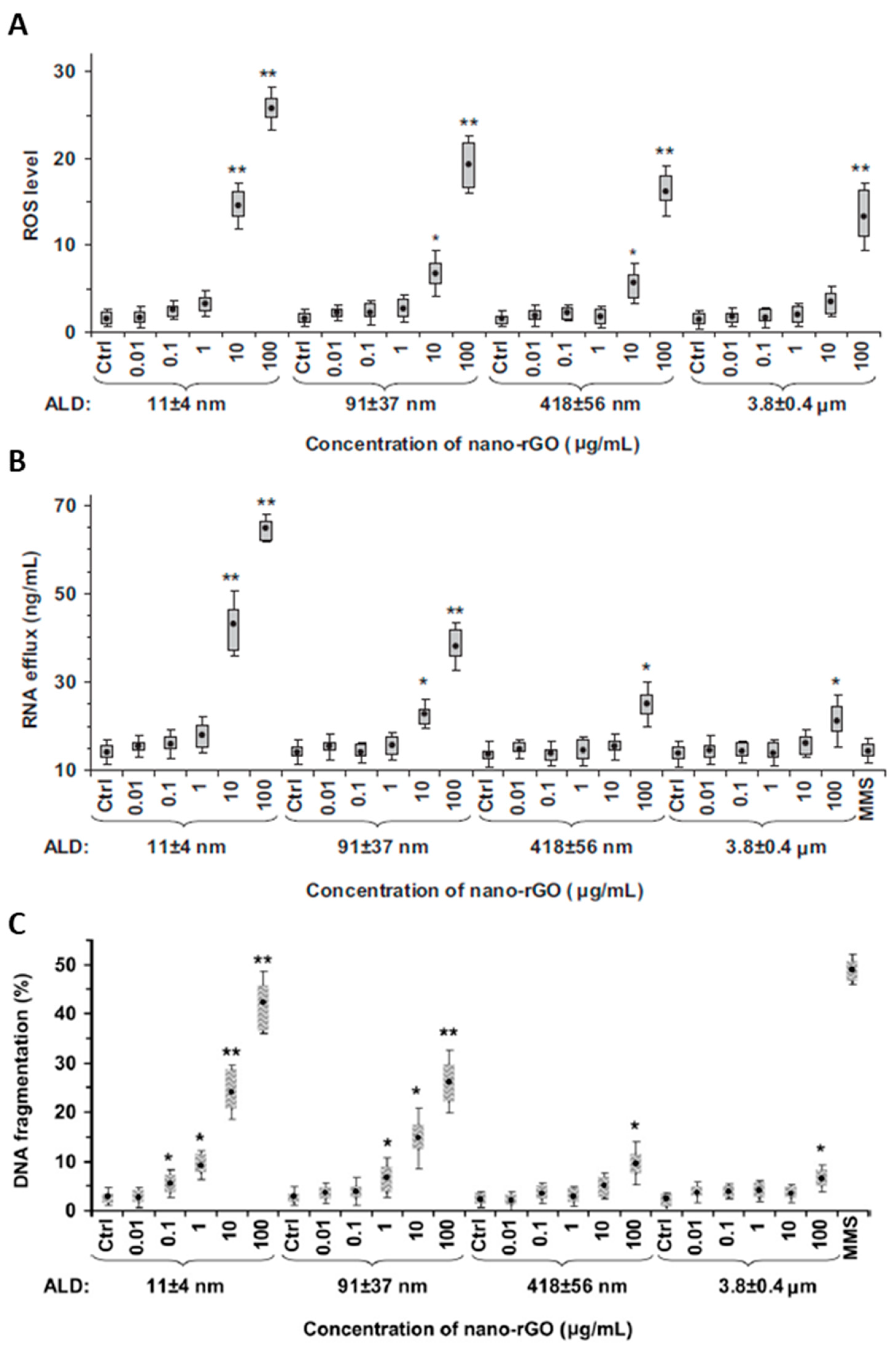
3.1.4. Oxidative Stress
3.1.5. GO Clearance from Cells and Biodegradation
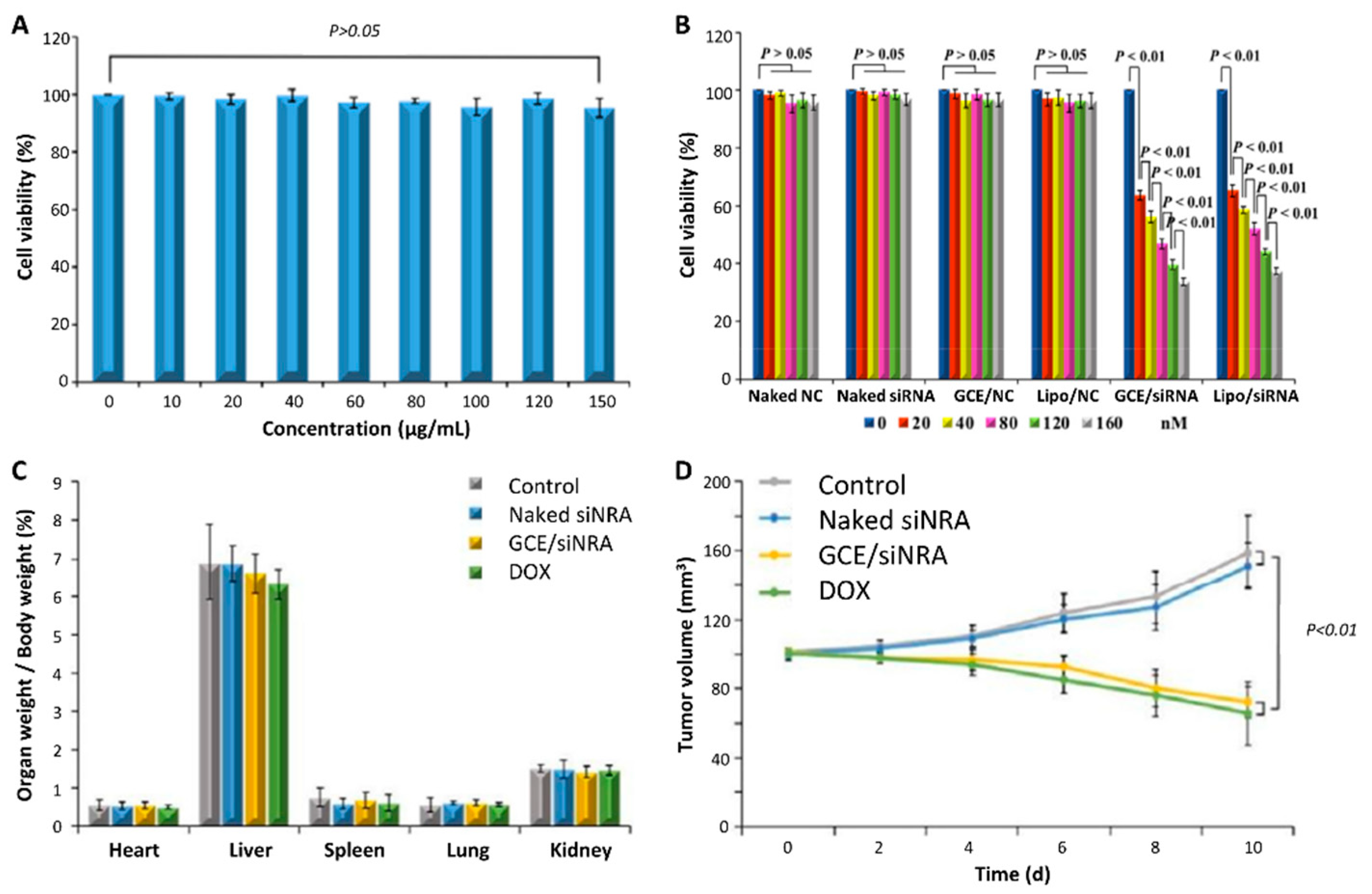
3.2. In Vivo Toxicity
3.2.1. Inflammatory Response
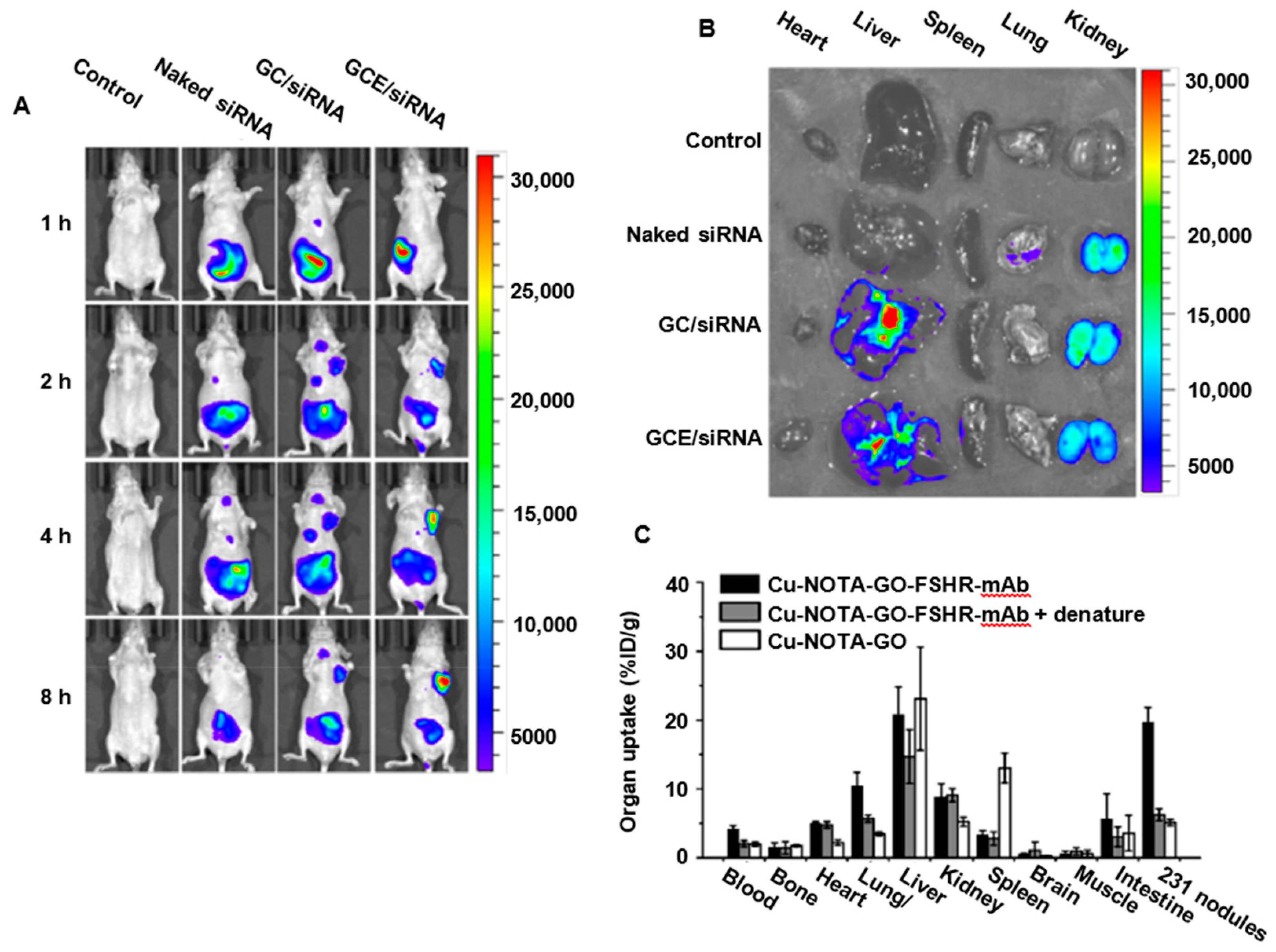
3.2.2. GO Biodistribution and Clearance from the Body
4. Controlled Release Strategies
4.1. pH-Sensitive Platforms for Control Release
4.2. Reducible Intracellular Environment
4.3. Enzyme-Induced Tumor Initiation
4.4. Near IR Stimuli and Treatment
4.5. Heat Treatment
4.6. Competitive Molecules
5. Challenges and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Singh, S.K.; Singh, S.; Lillard, J.W., Jr.; Singh, R. Drug delivery approaches for breast cancer. Int. J. Nanomed. 2017, 12, 6205–6218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Si, M.; Xue, H.Y.; Wong, H.L. Nanomedicine applications in the treatment of breast cancer: Current state of the art. Int. J. Nanomed. 2017, 12, 5879–5892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, H.-Y.; Bendayan, R.; Wong, H.-L. Chapter 5—MDR reversal for effective chemotherapy in breast cancer. In Drug Efflux Pumps in Cancer Resistance Pathways: From Molecular Recognition and Characterization to Possible Inhibition Strategies in Chemotherapy; Sosnik, A., Bendayan, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 7, pp. 121–147. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Xu, F.; Peng, H.; Luo, Y.; Tian, X.; Battaglia, G.; Zhang, H.; Gong, Q.; Gu, Z.; Luo, K. Stimuli-responsive polymeric prodrug-based nanomedicine delivering nifuroxazide and doxorubicin against primary breast cancer and pulmonary metastasis. J. Control. Release 2020, 318, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Madni, A.; Correia, A.; Rehman, M.; Balasubramanian, V.; Khan, M.M.; Santos, H.A. Lipid-polymer hybrid nanoparticles for controlled delivery of hydrophilic and lipophilic doxorubicin for breast cancer therapy. Int. J. Nanomed. 2019, 14, 4961–4974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, H.; Liu, S.; Wei, T.; Cheng, Q.; Siegwart, D.J. Theranostic dendrimer-based lipid nanoparticles containing PEGylated BODIPY dyes for tumor imaging and systemic mRNA delivery in vivo. J. Control. Release 2020, 325, 198–205. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, J.; Yao, M.; Palmerston Mendes, L.; Sarisozen, C.; Mao, S.; Torchilin, V.P. Charge reversible hyaluronic acid-modified dendrimer-based nanoparticles for siMDR-1 and doxorubicin co-delivery. Eur. J. Pharm. Biopharm. 2020, 154, 43–49. [Google Scholar] [CrossRef]
- Kong, T.; Hao, L.; Wei, Y.; Cai, X.; Zhu, B. Doxorubicin conjugated carbon dots as a drug delivery system for human breast cancer therapy. Cell Prolif. 2018, 51, e12488. [Google Scholar] [CrossRef] [Green Version]
- Pardo, J.; Peng, Z.; Leblanc, R.M. Cancer Targeting and Drug Delivery Using Carbon-Based Quantum Dots and Nanotubes. Molecules 2018, 23, 378. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Hwang, J.; Choi, Y.; Kwon, Y.; Jang, J.; Yoon, S.; Choi, J. Effective Delivery of Anti-Cancer Drug Molecules with Shape Transforming Liquid Metal Particles. Cancers 2019, 11, 1666. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Wang, H.; Zhu, D.; Wan, Y.; Yin, L. Combined effects of avasimibe immunotherapy, doxorubicin chemotherapy, and metal-organic frameworks nanoparticles on breast cancer. J. Cell Physiol. 2020, 235, 4814–4823. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzada, T.; Reid, G.; McKenzie, D.R. Fundamentals of siRNA and miRNA therapeutics and a review of targeted nanoparticle delivery systems in breast cancer. Biophys. Rev. 2018, 10, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Cheang, T.Y.; Lei, Y.Y.; Zhang, Z.Q.; Zhou, H.Y.; Ye, R.Y.; Lin, Y.; Wang, S. Graphene oxide-hydroxyapatite nanocomposites effectively deliver HSV-TK suicide gene to inhibit human breast cancer growth. J. Biomater. Appl. 2018, 33, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; Hennink, W.E.; Storm, G. Chapter 5: Drug Targeting Systems: Fundamentals and Applications to Parenteral Drug Delivery. In Drug Delivery and Targeting: For Pharmacists and Pharmaceutical Scientists, 1st ed.; Hillery, A.M., Lloyd, A.W., Swarbrick, J., Eds.; Taylor & Francis Publishing: New York, NY, USA, 2001; pp. 105–130. [Google Scholar] [CrossRef]
- Wu, C.; Tian, Y.; Zhang, Y.; Xu, J.; Wang, Y.; Guan, X.; Li, T.; Yang, H.; Li, S.; Qin, X.; et al. Acid-Triggered Charge-Convertible Graphene-Based All-in-One Nanocomplex for Enhanced Genetic Phototherapy of Triple-Negative Breast Cancer. Adv. Healthc. Mater. 2020, 9, e1901187. [Google Scholar] [CrossRef]
- Yadav, N.; Kumar, N.; Prasad, P.; Shirbhate, S.; Sehrawat, S.; Lochab, B. Stable Dispersions of Covalently Tethered Polymer Improved Graphene Oxide Nanoconjugates as an Effective Vector for siRNA Delivery. ACS Appl. Mater. Interfaces 2018, 10, 14577–14593. [Google Scholar] [CrossRef]
- Ma, K.; Li, W.; Zhu, G.; Chi, H.; Yin, Y.; Li, Y.; Zong, Y.; Guo, Z.; Wang, L.; Xu, W.; et al. PEGylated DOX-coated nano graphene oxide as pH-responsive multifunctional nanocarrier for targeted drug delivery. J. Drug Target. 2021, 29, 884–891. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Manchanda, S.; Chandra, A.; Ali, J.; Deb, P.K. Chapter 5—Overview of different carrier systems for advanced drug delivery. In Drug Delivery Systems; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 179–233. [Google Scholar] [CrossRef]
- Mendes, R.G.; Bachmatiuk, A.; Büchner, B.; Cuniberti, G.; Rümmeli, M.H. Carbon nanostructures as multi-functional drug delivery platforms. J. Mater. Chem. B 2013, 1, 401–428. [Google Scholar] [CrossRef]
- McCallion, C.; Burthem, J.; Rees-Unwin, K.; Golovanov, A.; Pluen, A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur. J. Pharm. Biopharm. 2016, 104, 235–250. [Google Scholar] [CrossRef]
- Shariare, M.H.; Masum, A.A.; Alshehri, S.; Alanazi, F.K.; Uddin, J.; Kazi, M. Preparation and Optimization of PEGylated Nano Graphene Oxide-Based Delivery System for Drugs with Different Molecular Structures Using Design of Experiment (DoE). Molecules 2021, 26, 1457. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schniepp, H.C.; Adamson, D.H. Characterization of graphene oxide: Variations in reported approaches. Carbon 2019, 154, 510–521. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Brodie, B.C. XIII. On the atomic weight of graphite. Phil. Trans. R. Soc. 1859, 149, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Shirvalilou, S.; Khoei, S.; Khoee, S.; Raoufi, N.J.; Karimi, M.R.; Shakeri-Zadeh, A. Development of a magnetic nano-graphene oxide carrier for improved glioma-targeted drug delivery and imaging: In vitro and in vivo evaluations. Chem. Biol. Interact. 2018, 295, 97–108. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Wang, M.H. Preparation, characterization and anti-cancer activity of graphene oxide--silver nanocomposite. J. Photochem. Photobiol. B 2020, 210, 111984. [Google Scholar] [CrossRef]
- Rawal, A.; Che Man, S.H.; Agarwal, V.; Yao, Y.; Thickett, S.C.; Zetterlund, P.B. Structural Complexity of Graphene Oxide: The Kirigami Model. ACS Appl. Mater. Interfaces 2021, 13, 18255–18263. [Google Scholar] [CrossRef]
- Tufano, I.; Vecchione, R.; Netti, P.A. Methods to Scale Down Graphene Oxide Size and Size Implication in Anti-cancer Applications. Front. Bioeng. Biotechnol. 2020, 8, 613280. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Narayan, R.J. Crossing the blood–brain barrier with graphene nanostructures. Mater. Today 2021, 51, 393–401. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Koiwai, N.; Ohtsuka, Y.; Miyamoto, M.; Sasakawa, S. Phagocytosis of latex particles by leucocytes. I. Dependence of phagocytosis on the size and surface potential of particles. Biomaterials 1986, 7, 61–66. [Google Scholar] [CrossRef]
- Champion, J.A.; Walker, A.; Mitragotri, S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 2008, 25, 1815–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, H.; Wei, W.; Yue, Z.; Lv, P.; Wang, L.; Ma, G.; Su, Z. Particle size affects the cellular response in macrophages. Eur. J. Pharm. Sci. 2010, 41, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Zhi, F.; Dong, H.; Jia, X.; Guo, W.; Lu, H.; Yang, Y.; Ju, H.; Zhang, X.; Hu, Y. Functionalized graphene oxide mediated adriamycin delivery and miR-21 gene silencing to overcome tumor multidrug resistance in vitro. PLoS ONE 2013, 8, e60034. [Google Scholar] [CrossRef] [Green Version]
- Hirano, A.; Kawanami, T.; Llena, J.F. Electron microscopy of the blood-brain barrier in disease. Microsc. Res. Tech. 1994, 27, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [Green Version]
- Engin, A.B.; Nikitovic, D.; Neagu, M.; Henrich-Noack, P.; Docea, A.O.; Shtilman, M.I.; Golokhvast, K.; Tsatsakis, A.M. Mechanistic understanding of nanoparticles’ interactions with extracellular matrix: The cell and immune system. Part. Fibre Toxicol. 2017, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Prokop, A.; Davidson, J.M. Nanovehicular intracellular delivery systems. J. Pharm. Sci. 2008, 97, 3518–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Singh, M.K.; Nayak, M.K.; Kumari, S.; Grácio, J.J.A.; Dash, D. Size distribution analysis and physical/fluorescence characterization of graphene oxide sheets by flow cytometry. Carbon 2011, 49, 684–692. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Zheng, X.; Liu, C.; Cui, X.; Zheng, W. Size distribution-controlled preparation of graphene oxide nanosheets with different C/O ratios. Mater. Chem. Phys. 2013, 139, 8–11. [Google Scholar] [CrossRef]
- Shao, G.; Lu, Y.; Wu, F.; Yang, C.; Zeng, F.; Wu, Q. Graphene oxide: The mechanisms of oxidation and exfoliation. J. Mater. Sci. 2012, 47, 4400–4409. [Google Scholar] [CrossRef]
- Shearer, C.J.; Slattery, A.D.; Stapleton, A.J.; Shapter, J.G.; Gibson, C.T. Accurate thickness measurement of graphene. Nanotechnology 2016, 27, 125704. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhou, T.; Deng, S.; Zong, G.; Yao, X.; Fu, Q. Size-specified graphene oxide sheets: Ultrasonication assisted preparation and characterization. J. Mater. Sci. 2014, 49, 1785–1793. [Google Scholar] [CrossRef]
- Sierra, U.; Álvarez, P.; Santamaría, R.; Granda, M.; Blanco, C.; Menéndez, R. A multi-step exfoliation approach to maintain the lateral size of graphene oxide sheets. Carbon 2014, 80, 830–832. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Q.; Cui, C.; Li, J.; Wang, Y. Anti-HER2 functionalized graphene oxide as survivin-siRNA delivery carrier inhibits breast carcinoma growth in vitro and in vivo. Drug Des. Devel. Ther. 2018, 12, 2841–2855. [Google Scholar] [CrossRef] [Green Version]
- Di Santo, R.; Digiacomo, L.; Palchetti, S.; Palmieri, V.; Perini, G.; Pozzi, D.; Papi, M.; Caracciolo, G. Microfluidic manufacturing of surface-functionalized graphene oxide nanoflakes for gene delivery. Nanoscale 2019, 11, 2733–2741. [Google Scholar] [CrossRef]
- Mirzaie, V.; Ansari, M.; Nematollahi-Mahani, S.N.; Moballegh Nasery, M.; Karimi, B.; Eslaminejad, T.; Pourshojaei, Y. Nano-Graphene Oxide-supported APTES-Spermine, as Gene Delivery System, for Transfection of pEGFP-p53 into Breast Cancer Cell Lines. Drug Des. Devel. Ther. 2020, 14, 3087–3097. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, G.; Gong, Y.; Zhang, Y.; Liang, X.; Yang, L. Functionalized Folate-Modified Graphene Oxide/PEI siRNA Nanocomplexes for Targeted Ovarian Cancer Gene Therapy. Nanoscale Res. Lett. 2020, 15, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Ge, X.; Cui, C.; Zhang, Y.; Wang, Y.; Wang, X.; Sun, Q. Preparation and Characterization of Functionalized Graphene Oxide Carrier for siRNA Delivery. Int. J. Mol. Sci. 2018, 19, 3202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Zong, C.; Shen, H.; Liu, M.; Chen, B.; Ren, B.; Zhang, Z. Mechanism of Cellular Uptake of Graphene Oxide Studied by Surface-Enhanced Raman Spectroscopy. Small 2012, 8, 2577–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nejabat, M.; Charbgoo, F.; Ramezani, M. Graphene as multifunctional delivery platform in cancer therapy. J. Biomed. Mater. Res. A 2017, 105, 2355–2367. [Google Scholar] [CrossRef]
- Coleman, B.R.; Knight, T.; Gies, V.; Jakubek, Z.J.; Zou, S. Manipulation and Quantification of Graphene Oxide Flake Size: Photoluminescence and Cytotoxicity. ACS Appl. Mater. Interfaces 2017, 9, 28911–28921. [Google Scholar] [CrossRef] [Green Version]
- Bidram, E.; Sulistio, A.; Amini, A.; Fu, Q.; Qiao, G.G.; Stewart, A.; Dunstan, D.E. Fractionation of graphene oxide single nano-sheets in water-glycerol solutions using gradient centrifugation. Carbon 2016, 103, 363–371. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-Graphene Oxide for Cellular Imaging and Drug Delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Tang, Y.; Liu, T.; Chen, Q.; Zhou, X.; Wang, N.; Ma, M.; Cheng, Y.; Chen, H. Engineering graphene oxide with ultrasmall SPIONs and smart drug release for cancer theranostics. Chem. Commun. 2019, 55, 1963–1966. [Google Scholar] [CrossRef]
- Assali, A.; Akhavan, O.; Adeli, M.; Razzazan, S.; Dinarvand, R.; Zanganeh, S.; Soleimani, M.; Dinarvand, M.; Atyabi, F. Multifunctional core-shell nanoplatforms (gold@graphene oxide) with mediated NIR thermal therapy to promote miRNA delivery. Nanomedicine 2018, 14, 1891–1903. [Google Scholar] [CrossRef]
- Basu, A.; Upadhyay, P.; Ghosh, A.; Chattopadhyay, D.; Adhikary, A. Folic-Acid-Adorned PEGylated Graphene Oxide Interferes with the Cell Migration of Triple Negative Breast Cancer Cell Line, MDAMB-231 by Targeting miR-21/PTEN Axis through NFκB. ACS Biomater. Sci. Eng. 2019, 5, 373–389. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Wang, Y.; Yang, X.; Yang, H.; Cui, C. Anti-EpCAM functionalized graphene oxide vector for tumor targeted siRNA delivery and cancer therapy. Asian J. Pharm. Sci. 2021, 16, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Izadi, S.; Moslehi, A.; Kheiry, H.; Karoon Kiani, F.; Ahmadi, A.; Masjedi, A.; Ghani, S.; Rafiee, B.; Karpisheh, V.; Hajizadeh, F.; et al. Codelivery of HIF-1α siRNA and Dinaciclib by Carboxylated Graphene Oxide-Trimethyl Chitosan-Hyaluronate Nanoparticles Significantly Suppresses Cancer Cell Progression. Pharm. Res. 2020, 37, 196. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Gadeval, A.; Raval, N.; Kalia, K.; Tekade, R.K. Laser activatable nanographene colloids for chemo-photothermal combined gene therapy of triple-negative breast cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 5, 112605. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Zhang, W.; Liu, H.; Jiang, J.J.; Wang, W.J.; Jia, Z.Y. Cell-Penetrating Peptide-Modified Graphene Oxide Nanoparticles Loaded with Rictor siRNA for the Treatment of Triple-Negative Breast Cancer. Drug Des. Devel. Ther. 2021, 15, 4961–4972. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, D.; Zeng, K.; Li, D.; Qin, L.; Cai, Y.; Jin, J. Simultaneous Delivery of antimiR-21 and Doxorubicin by Graphene Oxide for Reducing Toxicity in Cancer Therapy. ACS Omega 2020, 5, 14437–14443. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Meng, D.; Zheng, X.; Zhang, Y.; Chen, H. Graphene-based materials: A new tool to fight against breast cancer. Int. J. Pharm. 2021, 603, 120644. [Google Scholar] [CrossRef]
- Imani, R.; Shao, W.; Taherkhani, S.; Emami, S.H.; Prakash, S.; Faghihi, S. Dual-functionalized graphene oxide for enhanced siRNA delivery to breast cancer cells. Colloids Surf. B Biointerfaces 2016, 147, 315–325. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kim, H.Y.; Li, F.; Park, J.Y.; Kim, D.; Park, J.H.; Han, H.S.; Byun, J.W.; Lee, Y.S.; Jeong, J.M.; et al. In vivo visualization of endogenous miR-21 using hyaluronic acid-coated graphene oxide for targeted cancer therapy. Biomaterials 2017, 121, 144–154. [Google Scholar] [CrossRef]
- Bahreyni, A.; Yazdian-Robati, R.; Hashemitabar, S.; Ramezani, M.; Ramezani, P.; Abnous, K.; Taghdisi, S.M. A new chemotherapy agent-free theranostic system composed of graphene oxide nano-complex and aptamers for treatment of cancer cells. Int. J. Pharm. 2017, 526, 391–399. [Google Scholar] [CrossRef]
- Li, Q.; Xiu, Y.; Zhang, X.; Liu, R.; Du, Q.; Shun, X.; Chen, S.; Li, W. Preparation of 99mTc-C60(OH)x and its biodistribution studies. Nucl. Med. Biol. 2002, 29, 707–710. [Google Scholar] [CrossRef]
- Ji, Z.Q.; Sun, H.; Wang, H.; Xie, Q.; Liu, Y.; Wang, Z. Biodistribution and tumor uptake of C60(OH)xin mice. J. Nanopart. Res. 2006, 8, 53–63. [Google Scholar] [CrossRef]
- Yang, S.-T.; Guo, W.; Lin, Y.; Deng, X.-Y.; Wang, H.-F.; Sun, H.-F.; Liu, Y.-F.; Wang, X.; Wang, W.; Chen, M.; et al. Biodistribution of Pristine Single-Walled Carbon Nanotubes In Vivo. J. Phys.Chem. C 2007, 111, 17761–17764. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. Sci. Rep. 2020, 10, 2717. [Google Scholar] [CrossRef] [PubMed]
- Trusek, A.; Kijak, E.; Granicka, L. Graphene oxide as a potential drug carrier—Chemical carrier activation, drug attachment and its enzymatic controlled release. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111240. [Google Scholar] [CrossRef]
- Karki, N.; Tiwari, H.; Tewari, C.; Rana, A.; Pandey, N.; Basak, S.; Sahoo, N.G. Functionalized graphene oxide as a vehicle for targeted drug delivery and bioimaging applications. J. Mater. Chem. B 2020, 8, 8116–8148. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Hou, W.; Zhou, J.; Song, L. Effects of particle size and pH value on the hydrophilicity of graphene oxide. Appl. Surf. Sci. 2013, 273, 118–121. [Google Scholar] [CrossRef]
- Shih, C.J.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-dependent behavior of graphene oxide aqueous solutions: A comparative experimental and molecular dynamics simulation study. Langmuir 2012, 28, 235–241. [Google Scholar] [CrossRef]
- Karki, N.; Tiwari, H.; Pal, M.; Chaurasia, A.; Bal, R.; Joshi, P.; Sahoo, N.G. Functionalized graphene oxides for drug loading, release and delivery of poorly water soluble anticancer drug: A comparative study. Colloids Surf. B Biointerfaces 2018, 169, 265–272. [Google Scholar] [CrossRef]
- Farjadian, F.; Abbaspour, S.; Sadatlu, M.A.A.; Mirkiani, S.; Ghasemi, A.; Hoseini-Ghahfarokhi, M.; Mozaffari, N.; Karimi, M.; Hamblin, M.R. Recent Developments in Graphene and Graphene Oxide: Properties, Synthesis, and Modifications: A Review. ChemistrySelect 2020, 5, 10200–10219. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Yang, H.; Luo, J. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, L.; Wei, P.; Zhou, R.; Hua, C.; Xiao, M.; Tu, Y.; Gu, Z.; Wei, T. PEG-GO@XN nanocomposite suppresses breast cancer metastasis via inhibition of mitochondrial oxidative phosphorylation and blockade of epithelial-to-mesenchymal transition. Eur. J. Pharmacol. 2021, 895, 173866. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Guo, Y.; Wang, C.; Xu, J.; Wu, J.; Kirk, T.B.; Ma, D.; Xue, W. A polyamidoamne dendrimer functionalized graphene oxide for DOX and MMP-9 shRNA plasmid co-delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 572–585. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, Z.; Li, H.; Hao, Y.; Liu, C.; Zhu, L.; Liu, J.; Lu, B.; Li, R. Multifunctional Nanographene Oxide for Targeted Gene-Mediated Thermochemotherapy of Drug-resistant Tumour. Sci. Rep. 2017, 7, 43506. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-P.; Hung, C.-M.; Hsu, Y.-C.; Zhong, C.-Y.; Wang, W.-R.; Chang, C.-C.; Lee, M.-J. Suppression of Breast Cancer Cell Migration by Small Interfering RNA Delivered by Polyethylenimine-Functionalized Graphene Oxide. Nanoscale Res. Lett. 2016, 11, 247. [Google Scholar] [CrossRef] [Green Version]
- Alibolandi, M.; Mohammadi, M.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Fabrication of aptamer decorated dextran coated nano-graphene oxide for targeted drug delivery. Carbohydr. Polym. 2017, 155, 218–229. [Google Scholar] [CrossRef]
- Charmi, J.; Nosrati, H.; Mostafavi Amjad, J.; Mohammadkhani, R.; Danafar, H. Polyethylene glycol (PEG) decorated graphene oxide nanosheets for controlled release curcumin delivery. Heliyon 2019, 5, e01466. [Google Scholar] [CrossRef] [Green Version]
- Maleki, P.; Sadeghi, Z.; Shahryar Rahpeyma, S.; Taheri, M.; Raheb, J. MTT assay dataset of polyethylenimine coated graphenoxide nanosheets on breast cancer cell lines (MCF7, MDA-MB-231, MDA-MB-468). Hum. Antibodies 2020, 28, 197–202. [Google Scholar] [CrossRef]
- Mirzaie, Z.; Reisi-Vanani, A.; Barati, M. Polyvinyl alcohol-sodium alginate blend, composited with 3D-graphene oxide as a controlled release system for curcumin. J. Drug Deliv. Sci. Technol. 2019, 50, 380–387. [Google Scholar] [CrossRef]
- Lu, Y.J.; Yang, H.W.; Hung, S.C.; Huang, C.Y.; Li, S.M.; Ma, C.C.; Chen, P.Y.; Tsai, H.C.; Wei, K.C.; Chen, J.P. Improving thermal stability and efficacy of BCNU in treating glioma cells using PAA-functionalized graphene oxide. Int. J. Nanomed. 2012, 7, 1737–1747. [Google Scholar] [CrossRef] [Green Version]
- Saravanabhavan, S.S.; Rethinasabapathy, M.; Zsolt, S.; Kalambettu, A.B.; Elumalai, S.; Janakiraman, M.; Huh, Y.S.; Natesan, B. Graphene oxide functionalized with chitosan based nanoparticles as a carrier of siRNA in regulating Bcl-2 expression on Saos-2 & MG-63 cancer cells and its inflammatory response on bone marrow derived cells from mice. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Bart, J.; Tiggelaar, R.; Yang, M.; Schlautmann, S.; Zuilhof, H.; Gardeniers, H. Room-temperature intermediate layer bonding for microfluidic devices. Lab Chip 2009, 9, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control. Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Imani, R.; Prakash, S.; Vali, H.; Faghihi, S. Polyethylene glycol and octa-arginine dual-functionalized nanographene oxide: An optimization for efficient nucleic acid delivery. Biomater. Sci. 2018, 6, 1636–1650. [Google Scholar] [CrossRef]
- Wang, B.; Su, X.; Liang, J.; Yang, L.; Hu, Q.; Shan, X.; Wan, J.; Hu, Z. Synthesis of polymer-functionalized nanoscale graphene oxide with different surface charge and its cellular uptake, biosafety and immune responses in Raw264.7 macrophages. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 514–522. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Palmieri, V.; Di Pietro, L.; Perini, G.; Barba, M.; Parolini, O.; De Spirito, M.; Lattanzi, W.; Papi, M. Graphene Oxide Nano-Concentrators Selectively Modulate RNA Trapping According to Metal Cations in Solution. Front. Bioeng. Biotechnol. 2020, 8, 421. [Google Scholar] [CrossRef]
- Fong, J.F.Y.; Ng, Y.H.; Ng, S.M. Chapter 7—Carbon dots as a new class of light emitters for biomedical diagnostics and therapeutic applications. In Fullerens, Graphenes and Nanotubes; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 227–295. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.T. Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications. Molecules 2021, 26, 2797. [Google Scholar] [CrossRef]
- Yang, L.; Kim, T.H.; Cho, H.Y.; Luo, J.; Lee, J.M.; Chueng, S.D.; Hou, Y.; Yin, P.T.; Han, J.; Kim, J.H.; et al. Hybrid Graphene-Gold Nanoparticle-based Nucleic Acid Conjugates for Cancer-Specific Multimodal Imaging and Combined Therapeutics. Adv. Funct. Mater. 2021, 31, 2006918. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Parvez, K.; Nafiujjaman, M.; Revuri, V.; Khan, H.A.; Feng, X.; Lee, Y.-K. Bioapplication of graphene oxide derivatives: Drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015, 5, 42141–42161. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Hung, M.-C. The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016, 35, 575–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, J.; Paiva, A.; Cabral Campello, M.P.; Paulo, A.; Mergny, J.-L.; Salgado, G.F.; Queiroz, J.A.; Cruz, C. Aptamer-based Targeted Delivery of a G-quadruplex Ligand in Cervical Cancer Cells. Sci. Rep. 2019, 9, 7945. [Google Scholar] [CrossRef] [Green Version]
- Antwi-Boasiako, A.A.; Dunn, D.; Dasary, S.S.R.; Jones, Y.K.; Barnes, S.L.; Singh, A.K. Bioconjugated graphene oxide-based Raman probe for selective identification of SKBR3 breast cancer cells. J. Raman Spectrosc. 2017, 48, 1056–1064. [Google Scholar] [CrossRef]
- Saeed, A.A.; Sánchez, J.L.A.; O’Sullivan, C.K.; Abbas, M.N. DNA biosensors based on gold nanoparticles-modified graphene oxide for the detection of breast cancer biomarkers for early diagnosis. Bioelectrochemistry 2017, 118, 91–99. [Google Scholar] [CrossRef]
- Ali, M.A.; Mondal, K.; Jiao, Y.; Oren, S.; Xu, Z.; Sharma, A.; Dong, L. Microfluidic Immuno-Biochip for Detection of Breast Cancer Biomarkers Using Hierarchical Composite of Porous Graphene and Titanium Dioxide Nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 20570–20582. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Akhavan, A. Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials 2012, 33, 8017–8025. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Zhao, Y.; Bai, Y.; Chen, P.; Xia, T.; Wang, D. Response of MicroRNAs to In Vitro Treatment with Graphene Oxide. ACS Nano 2014, 8, 2100–2110. [Google Scholar] [CrossRef]
- Ribeiro, B.F.M.; Souza, M.M.; Fernandes, D.S.; do Carmo, D.R.; Machado-Santelli, G.M. Graphene oxide-based nanomaterial interaction with human breast cancer cells. J. Biomed. Mater. Res. A 2020, 108, 863–870. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, X.; Jiang, J.; Wang, Y.; Jiang, H.; Zhang, J.; Nie, X.; Liu, B. Systematic Assessment of the Toxicity and Potential Mechanism of Graphene Derivatives In Vitro and In Vivo. Toxicol. Sci. 2019, 167, 269–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, H.; Wei, W.; Yue, Z.; Wang, B.; Luo, N.; Gao, Y.; Ma, D.; Ma, G.; Su, Z. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials 2012, 33, 4013–4021. [Google Scholar] [CrossRef]
- Krętowski, R.; Jabłońska-Trypuć, A.; Cechowska-Pasko, M. The Preliminary Study on the Proapoptotic Effect of Reduced Graphene Oxide in Breast Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 12593. [Google Scholar] [CrossRef] [PubMed]
- Farell, M.; Self, A.; Guza, C.; Song, H.; Apollon, L.; Gomez, E.W.; Kumar, M. Lipid-Functionalized Graphene Loaded with hMnSOD for Selective Inhibition of Cancer Cells. ACS Appl. Mater. Interfaces 2020, 12, 12407–12416. [Google Scholar] [CrossRef] [PubMed]
- Han, X.M.; Zheng, K.W.; Wang, R.L.; Yue, S.F.; Chen, J.; Zhao, Z.W.; Song, F.; Su, Y.; Ma, Q. Functionalization and optimization-strategy of graphene oxide-based nanomaterials for gene and drug delivery. Am. J. Transl. Res. 2020, 12, 1515–1534. [Google Scholar] [PubMed]
- Lalwani, G.; D’Agati, M.; Khan, A.M.; Sitharaman, B. Toxicology of graphene-based nanomaterials. Adv. Drug Deliv. Rev. 2016, 105, 109–144. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.M.; Lalwani, G.; Zhang, K.; Yang, J.Y.; Neville, K.; Sitharaman, B. Cell specific cytotoxicity and uptake of graphene nanoribbons. Biomaterials 2013, 34, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, J.; Lee, M.; Choi, H.C.; Kim, W.J. Stimuli-Regulated Enzymatically Degradable Smart Graphene-Oxide-Polymer Nanocarrier Facilitating Photothermal Gene Delivery. Adv. Healthc. Mater. 2016, 5, 1918–1930. [Google Scholar] [CrossRef]
- Gies, V.; Lopinski, G.; Augustine, J.; Cheung, T.; Kodra, O.; Zou, S. The impact of processing on the cytotoxicity of graphene oxide. Nanoscale Adv. 2019, 1, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Gies, V.; Zou, S. Systematic toxicity investigation of graphene oxide: Evaluation of assay selection, cell type, exposure period and flake size. Toxicol. Res. 2018, 7, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, N.; Eom, H.J.; Choi, J. A systems toxicology approach to the surface functionality control of graphene-cell interactions. Biomaterials 2014, 35, 110–1127. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O. Graphene scaffolds in progressive nanotechnology/stem cell-based tissue engineering of the nervous system. J. Mater. Chem. B 2016, 4, 3169–3190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Yang, S.T.; Liu, J.H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef]
- Lammel, T.; Boisseaux, P.; Fernández-Cruz, M.L.; Navas, J.M. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part. Fibre Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Liu, M.; Zhang, L.; Huang, J.; Yao, J.; Zhang, Z. Polyethylenimine-functionalized graphene oxide as an efficient gene delivery vector. J. Mater. Chem. 2011, 21, 7736–7741. [Google Scholar] [CrossRef]
- Li, R.; Guiney, L.M.; Chang, C.H.; Mansukhani, N.D.; Ji, Z.; Wang, X.; Liao, Y.P.; Jiang, W.; Sun, B.; Hersam, M.C.; et al. Surface Oxidation of Graphene Oxide Determines Membrane Damage, Lipid Peroxidation, and Cytotoxicity in Macrophages in a Pulmonary Toxicity Model. ACS Nano 2018, 12, 1390–1402. [Google Scholar] [CrossRef]
- Ghamkhari, A.; Abbaspour-Ravasjani, S.; Talebi, M.; Hamishehkar, H.; Hamblin, M.R. Development of a graphene oxide-poly lactide nanocomposite as a Smart Drug Delivery System. Int. J. Biol. Macromol. 2021, 169, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Jaiswal, A. DNA binding and NIR triggered DNA release from quaternary ammonium modified poly(allylamine hydrochloride) functionalized and folic acid conjugated reduced graphene oxide nanocomposites. Int. J. Biol. Macromol. 2020, 153, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Peruzynska, M.; Cendrowski, K.; Barylak, M.; Tkacz, M.; Piotrowska, K.; Kurzawski, M.; Mijowska, E.; Drozdzik, M. Comparative in vitro study of single and four layer graphene oxide nanoflakes—Cytotoxicity and cellular uptake. Toxicol. In Vitro 2017, 41, 205–213. [Google Scholar] [CrossRef]
- Zhao, C.; Song, X.; Liu, Y.; Fu, Y.; Ye, L.; Wang, N.; Wang, F.; Li, L.; Mohammadniaei, M.; Zhang, M.; et al. Synthesis of graphene quantum dots and their applications in drug delivery. J. Nanobiotechnol. 2020, 18, 142. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Rao, C.N.R.; Koyakutty, M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 2011, 3, 2461–2464. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Zhou, X.; Han, T.; Xin, X.; Wu, J.; Zhang, J.; Guo, S. Enhancing Cell Nucleus Accumulation and DNA Cleavage Activity of Anti-Cancer Drug via Graphene Quantum Dots. Sci. Rep. 2013, 3, 2852. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, O.; Ghaderi, E.; Emamy, H.; Akhavan, F. Genotoxicity of graphene nanoribbons in human mesenchymal stem cells. Carbon 2013, 54, 419–431. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Ristic, B.; Harhaji-Trajkovic, L.; Bosnjak, M.; Dakic, I.; Mijatovic, S.; Trajkovic, V. Modulation of Cancer Cell Autophagic Responses by Graphene-Based Nanomaterials: Molecular Mechanisms and Therapeutic Implications. Cancers 2021, 13, 4145. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Fu, Y.; Wei, T.; Le Guyader, L.; Gao, G.; Liu, R.S.; Chang, Y.Z.; Chen, C. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials 2012, 33, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, C.; Li, Z.; Lu, Z.; Li, Y.; Yin, J.-J.; Zhou, Y.-T.; Gao, X.; Fang, Y.; Nie, G.; et al. Unraveling Stress Induced Toxicity Properties of Graphene Oxide and the Underlying Mechanism. Adv. Mater. 2012, 24, 5391–5397. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Kim, J.H. Green synthesis of graphene and its cytotoxic effects in human breast cancer cells. Int. J. Nanomed. 2013, 8, 1015–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yan, J. Superficial synthesis of photoactive copper sulfide quantum dots loaded nano-graphene oxide sheets combined with near infrared (NIR) laser for enhanced photothermal therapy on breast cancer in nursing care management. J. Photochem. Photobiol. B 2019, 192, 68–73. [Google Scholar] [CrossRef]
- Allen, B.L.; Kichambare, P.D.; Gou, P.; Vlasova, I.I.; Kapralov, A.A.; Konduru, N.; Kagan, V.E.; Star, A. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008, 8, 3899–3903. [Google Scholar] [CrossRef]
- Allen, B.L.; Kotchey, G.P.; Chen, Y.; Yanamala, N.V.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 17194–17205. [Google Scholar] [CrossRef]
- Andón, F.T.; Kapralov, A.A.; Yanamala, N.; Feng, W.; Baygan, A.; Chambers, B.J.; Hultenby, K.; Ye, F.; Toprak, M.S.; Brandner, B.D.; et al. Biodegradation of single-walled carbon nanotubes by eosinophil peroxidase. Small 2013, 9, 2721–2729. [Google Scholar] [CrossRef]
- Kagan, V.E.; Konduru, N.V.; Feng, W.; Allen, B.L.; Conroy, J.; Volkov, Y.; Vlasova, I.I.; Belikova, N.A.; Yanamala, N.; Kapralov, A.; et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 2010, 5, 354–359. [Google Scholar] [CrossRef]
- Kurapati, R.; Mukherjee, S.P.; Martín, C.; Bepete, G.; Vázquez, E.; Pénicaud, A.; Fadeel, B.; Bianco, A. Degradation of Single-Layer and Few-Layer Graphene by Neutrophil Myeloperoxidase. Angew. Chem. Int. Ed. Engl. 2018, 57, 11722–11727. [Google Scholar] [CrossRef]
- Luan, X.; Martín, C.; Zhang, P.; Li, Q.; Vacchi, I.A.; Delogu, L.G.; Mai, Y.; Bianco, A. Degradation of Structurally Defined Graphene Nanoribbons by Myeloperoxidase and the Photo-Fenton Reaction. Angew. Chem. Int. Ed. Engl. 2020, 59, 18515–18521. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Gliga, A.R.; Lazzaretto, B.; Brandner, B.; Fielden, M.; Vogt, C.; Newman, L.; Rodrigues, A.F.; Shao, W.; Fournier, P.M.; et al. Graphene oxide is degraded by neutrophils and the degradation products are non-genotoxic. Nanoscale 2018, 10, 1180–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurapati, R.; Martìn, C.; Palermo, V.; Nishina, Y.; Bianco, A. Biodegradation of graphene materials catalyzed by human eosinophil peroxidase. Faraday Discuss. 2021, 227, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety Assessment of Graphene-Based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-S.; Zepp, R.G. Reactivity of graphene oxide with reactive oxygen species (hydroxyl radical, singlet oxygen, and superoxide anion). Environ. Sci. Nano 2019, 6, 3734–3744. [Google Scholar] [CrossRef] [Green Version]
- Campbell, E.; Hasan, M.T.; Pho, C.; Callaghan, K.; Akkaraju, G.R.; Naumov, A.V. Graphene Oxide as a Multifunctional Platform for Intracellular Delivery, Imaging, and Cancer Sensing. Sci. Rep. 2019, 9, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lázaro, I.; Vranic, S.; Marson, D.; Rodrigues, A.F.; Buggio, M.; Esteban-Arranz, A.; Mazza, M.; Posocco, P.; Kostarelos, K. Graphene oxide as a 2D platform for complexation and intracellular delivery of siRNA. Nanoscale 2019, 11, 13863–13877. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Li, Y.; Wang, H.; Stewart, S.; Van der Jeught, K.; Agarwal, P.; Zhang, Y.; Liu, S.; Zhao, G.; et al. Precise targeting of POLR2A as a therapeutic strategy for human triple negative breast cancer. Nat. Nanotechnol. 2019, 14, 388–397. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. Graphene oxide touches blood: In vivo interactions of bio-coronated 2D materials. Nanoscale Horiz. 2019, 4, 273–290. [Google Scholar] [CrossRef]
- Amrollahi-Sharifabadi, M.; Koohi, M.K.; Zayerzadeh, E.; Hablolvarid, M.H.; Hassan, J.; Seifalian, A.M. In vivo toxicological evaluation of graphene oxide nanoplatelets for clinical application. Int. J. Nanomed. 2018, 13, 4757–4769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, L.; Jasim, D.A.; Prestat, E.; Lozano, N.; de Lazaro, I.; Nam, Y.; Assas, B.M.; Pennock, J.; Haigh, S.J.; Bussy, C.; et al. Splenic Capture and In Vivo Intracellular Biodegradation of Biological-Grade Graphene Oxide Sheets. ACS Nano 2020, 14, 10168–10186. [Google Scholar] [CrossRef] [PubMed]
- Alsaedi, I.I.J.; Taqi, Z.J.; Abdul Hussien, A.M.; Sulaiman, G.M.; Jabir, M.S. Graphene nanoparticles induces apoptosis in MCF-7 cells through mitochondrial damage and NF-KB pathway. Mater. Res. Express 2019, 6, 095413. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Khorsandi, K.; Hosseinzadeh, G. Graphene oxide-methylene blue nanocomposite in photodynamic therapy of human breast cancer. J. Biomol. Struct. Dyn. 2018, 36, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, J.; Zhang, G.; Zhang, Y.; Li, S.; Nie, D. Photothermal/pH Dual-Responsive Drug Delivery System of Amino-Terminated HBP-Modified rGO and the Chemo-Photothermal Therapy on Tumor Cells. Nanoscale Res. Lett. 2018, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Lazarovits, J.; Chen, Y.Y.; Sykes, E.A.; Chan, W.C.W. Nanoparticle–blood interactions: The implications on solid tumour targeting. Chem. Commun. 2015, 51, 2756–2767. [Google Scholar] [CrossRef]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernández, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. Biomed. Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Cui, G.; Wu, J.; Lin, J.; Liu, W.; Chen, P.; Yu, M.; Zhou, D.; Yao, G. Graphene-based nanomaterials for breast cancer treatment: Promising therapeutic strategies. J. Nanobiotechnol. 2021, 19, 211. [Google Scholar] [CrossRef]
- Liang, S.; Xu, S.; Zhang, D.; He, J.; Chu, M. Reproductive toxicity of nanoscale graphene oxide in male mice. Nanotoxicology 2015, 9, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Hougaard, K.S.; Kishimoto, A.; Honda, K. Reproductive and developmental toxicity of carbon-based nanomaterials: A literature review. Nanotoxicology 2016, 10, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, X.; Zhang, J.; Yin, Y.; Zuo, T.; Wang, Y.; Yang, X.; Shen, Q. Inhibiting pulmonary metastasis of breast cancer based on dual-targeting graphene oxide with high stability and drug loading capacity. Nanomedicine 2018, 14, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Feng, L.; Dougherty, C.A.; Luker, K.E.; Chen, D.; Cauble, M.A.; Banaszak Holl, M.M.; Luker, G.D.; Ross, B.D.; Liu, Z.; et al. In vivo targeting of metastatic breast cancer via tumor vasculature-specific nano-graphene oxide. Biomaterials 2016, 104, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Fu, Y.; Rong, L.; Yang, X.; Li, Y.; Wang, L.; Wu, W. Evaluating the cytotoxicity of graphene oxide using embryonic stem cells-derived cells. J. Biomed. Mater. Res. A 2020, 108, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.S.; Gharbi, S.; Jafarinejad-Farsangi, S.; Ansari-Asl, Z.; Dezfuli, A.S. Secondary toxic effect of graphene oxide and graphene quantum dots alters the expression of miR-21 and miR-29a in human cell lines. Toxicol. In Vitro 2020, 65, 104796. [Google Scholar] [CrossRef]
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.; Valle, R.P.; Zuo, Y.Y.; Xia, T.; Liu, S. Crucial Role of Lateral Size for Graphene Oxide in Activating Macrophages and Stimulating Pro-inflammatory Responses in Cells and Animals. ACS Nano 2015, 9, 10498–10515. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Chen, Z.; Liang, Y.; Zhang, Y.; Bai, Y.; Zhang, J.; Li, Y. Enhanced chemotherapy efficacy by co-delivery of shABCG2 and doxorubicin with a pH-responsive charge-reversible layered graphene oxide nanocomplex. J. Mater. Chem. B 2015, 3, 6462–6472. [Google Scholar] [CrossRef]
- Wang, H.; Sun, D.; Zhao, N.; Yang, X.; Shi, Y.; Li, J.; Su, Z.; Wei, G. Thermo-sensitive graphene oxide–polymer nanoparticle hybrids: Synthesis, characterization, biocompatibility and drug delivery. J. Mater. Chem. B 2014, 2, 1362–1370. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, G.; Li, H. Thermal-responsive nanocomposite hydrogel based on graphene oxide-polyvinyl alcohol/poly (N-isopropylacrylamide). IOP Conf. Ser. Mater. Sci. Eng. 2017, 274, 012115. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Lang, L.; Yang, T.; Zhao, X.; Cai, C.; Liu, Z.; Ding, P. Nuclear localization signal peptide enhances transfection efficiency and decreases cytotoxicity of poly(agmatine/N,N’-cystamine-bis-acrylamide)/pDNA complexes. J. Cell. Biochem. 2019, 120, 16967–16977. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, M.; Wang, L.; Iqbal, Z.; Zhu, K.; Yang, Y.; Li, Y. Size-transformable nanohybrids with pH/redox/enzymatic sensitivity for anticancer therapy. J. Mater. Chem. B 2021, 9, 4319–4328. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Zhang, Y. Controlled precipitation of solubilized carbon nanotubes by delamination of DNA. J. Phys. Chem. B 2006, 110, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.J.; Chen, Y.C.; Hsieh, P.Y.; Liu, S.R.; Wu, S.P.; Hsieh, Y.Z.; Hsu, H.Y. Graphene Oxide Based Nanocarrier Combined with a pH-Sensitive Tracer: A Vehicle for Concurrent pH Sensing and pH-Responsive Oligonucleotide Delivery. ACS Appl. Mater. Interfaces 2015, 7, 11467–11475. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.C.; Souza, P.R.; Vilsinski, B.H.; Winkler, M.E.G.; Bruschi, M.L.; Radovanovic, E.; Muniz, E.C.; Caetano, W.; Valente, A.J.M.; Martins, A.F. Thermo- and pH-Responsive Gelatin/Polyphenolic Tannin/Graphene Oxide Hydrogels for Efficient Methylene Blue Delivery. Molecules 2021, 26, 4529. [Google Scholar] [CrossRef]
- Bina, A.; Raissi, H.; Hashemzadeh, H.; Farzad, F. Conjugation of a smart polymer to doxorubicin through a pH-responsive bond for targeted drug delivery and improving drug loading on graphene oxide. RSC Adv. 2021, 11, 18809–18817. [Google Scholar] [CrossRef]
- Karimi, S.; Namazi, H. Fe3O4@PEG-coated dendrimer modified graphene oxide nanocomposite as a pH-sensitive drug carrier for targeted delivery of doxorubicin. J. Alloys Compd. 2021, 879, 160426. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Q.; Mei, L. pH-Sensitive nanoscale materials as robust drug delivery systems for cancer therapy. Chin. Chem. Lett. 2020, 31, 1345–1356. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Gao, J.; Cao, Z.; Jiang, Q.; Liu, J. pH and redox dual-responsive multifunctional gene delivery with enhanced capability of transporting DNA into the nucleus. Colloids Surf. B Biointerfaces 2017, 153, 111–122. [Google Scholar] [CrossRef]
- An, J.; Gou, Y.; Yang, C.; Hu, F.; Wang, C. Synthesis of a biocompatible gelatin functionalized graphene nanosheets and its application for drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2827–2837. [Google Scholar] [CrossRef]
- Gadeval, A.; Maheshwari, R.; Raval, N.; Kalyane, D.; Kalia, K.; Tekade, R.K. Green graphene nanoplates for combined photo-chemo-thermal therapy of triple-negative breast cancer. Nanomedicine 2020, 15, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Gai, L.X.; Wang, W.Q.; Wu, X.; Su, X.J.; Yang, F.C. NIR absorbing reduced graphene oxide for photothermal radiotherapy for treatment of esophageal cancer. J. Photochem. Photobiol. B 2019, 194, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Liu, J.; Li, J.; Zhang, Z.; Weng, Y.; Yuan, B.; Yang, K.; Ma, Y. Tunable dual-stimuli response of a microgel composite consisting of reduced graphene oxide nanoparticles and poly(N-isopropylacrylamide) hydrogel microspheres. J. Mater. Chem. B 2014, 2, 3791–3798. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.T.; Chen, C.H.; Chen, J.P. Intratumoral Delivery of Doxorubicin on Folate-Conjugated Graphene Oxide by In-Situ Forming Thermo-Sensitive Hydrogel for Breast Cancer Therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liu, L.; Jiang, C. Charge-reversal nanoparticles: Novel targeted drug delivery carriers. Acta Pharm. Sin. B 2016, 6, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, X.; Liu, Z.; Ma, Y.; Huang, Y.; Chen, Y. High-Efficiency Loading and Controlled Release of Doxorubicin Hydrochloride on Graphene Oxide. J. Phys. Chem. C 2008, 112, 17554–17558. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef]
- Abbasian, M.; Roudi, M.M.; Mahmoodzadeh, F.; Eskandani, M.; Jaymand, M. Chitosan-grafted-poly(methacrylic acid)/graphene oxide nanocomposite as a pH-responsive de novo cancer chemotherapy nanosystem. Int. J. Biol. Macromol. 2018, 118, 1871–1879. [Google Scholar] [CrossRef]
- Diaz-Diestra, D.; Thapa, B.; Badillo-Diaz, D.; Beltran-Huarac, J.; Morell, G.; Weiner, B.R. Graphene Oxide/ZnS:Mn Nanocomposite Functionalized with Folic Acid as a Nontoxic and Effective Theranostic Platform for Breast Cancer Treatment. Nanomaterials 2018, 8, 484. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Amani, A.M.; Babapoor, A.; Arjmand, O. Applications of graphene oxide in case of nanomedicines and nanocarriers for biomolecules: Review study. Drug Metab. Rev. 2019, 51, 12–41. [Google Scholar] [CrossRef]
- Asantewaa, G.; Harris, I.S. Glutathione and its precursors in cancer. Curr. Opin. Biotechnol. 2021, 68, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Zhao, J.-J.; Zhang, R.; Li, H.; Chen, B.; Zhang, L.-L.; Yang, H. Multifunctionalization of graphene and graphene oxide for controlled release and targeted delivery of anticancer drugs. Am. J. Transl. Res. 2017, 9, 5197–5219. [Google Scholar] [PubMed]
- Qi, L.-Y.; Wang, Y.; Hu, L.-F.; Zhao, P.-S.; Yu, H.-Y.; Xing, L.; Gao, X.-D.; Cao, Q.-R.; Jiang, H.-L. Enhanced nuclear gene delivery via integrating and streamlining intracellular pathway. J. Control. Release 2022, 341, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Song, A.; Li, Z.; Luan, Y. Redox-Sensitive Prodrug Molecules Meet Graphene Oxide: An Efficient Graphene Oxide-Based Nanovehicle toward Cancer Therapy. ACS Biomater. Sci. Eng. 2019, 5, 1384–1391. [Google Scholar] [CrossRef]
- Davoodi, P.; Srinivasan, M.P.; Wang, C.H. Synthesis of intracellular reduction-sensitive amphiphilic polyethyleneimine and poly(ε-caprolactone) graft copolymer for on-demand release of doxorubicin and p53 plasmid DNA. Acta Biomater. 2016, 39, 79–93. [Google Scholar] [CrossRef]
- Trusek, A.; Kijak, E. Drug Carriers Based on Graphene Oxide and Hydrogel: Opportunities and Challenges in Infection Control Tested by Amoxicillin Release. Materials 2021, 14, 3182. [Google Scholar] [CrossRef]
- Lee, S.J.; Yhee, J.Y.; Kim, S.H.; Kwon, I.C.; Kim, K. Biocompatible gelatin nanoparticles for tumor-targeted delivery of polymerized siRNA in tumor-bearing mice. J. Control. Release 2013, 172, 358–366. [Google Scholar] [CrossRef]
- Madkhali, O.; Mekhail, G.; Wettig, S.D. Modified gelatin nanoparticles for gene delivery. Int. J. Pharm. 2019, 554, 224–234. [Google Scholar] [CrossRef]
- Monroe, J.D.; Belekov, E.; Er, A.O.; Smith, M.E. Anticancer Photodynamic Therapy Properties of Sulfur-Doped Graphene Quantum Dot and Methylene Blue Preparations in MCF-7 Breast Cancer Cell Culture. Photochem. Photobiol. 2019, 95, 1473–1481. [Google Scholar] [CrossRef]
- Shaheen, F.; Hammad Aziz, M.; Fakhar-E-Alam, M.; Atif, M.; Fatima, M.; Ahmad, R.; Hanif, A.; Anwar, S.; Zafar, F.; Abbas, G.; et al. An In Vitro Study of the Photodynamic Effectiveness of GO-Ag Nanocomposites against Human Breast Cancer Cells. Nanomaterials 2017, 7, 401. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Chen, Y.C.; Huang, P.I. Preparation of Multifunctional Dopamine-Coated Zerovalent Iron/Reduced Graphene Oxide for Targeted Phototheragnosis in Breast Cancer. Nanomaterials 2020, 10, 1957. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.J.; Lan, Y.H.; Chuang, C.C.; Lu, W.T.; Chan, L.Y.; Hsu, P.W.; Chen, J.P. Injectable Thermo-Sensitive Chitosan Hydrogel Containing CPT-11-Loaded EGFR-Targeted Graphene Oxide and SLP2 shRNA for Localized Drug/Gene Delivery in Glioblastoma Therapy. Int. J. Mol. Sci. 2020, 21, 7111. [Google Scholar] [CrossRef] [PubMed]

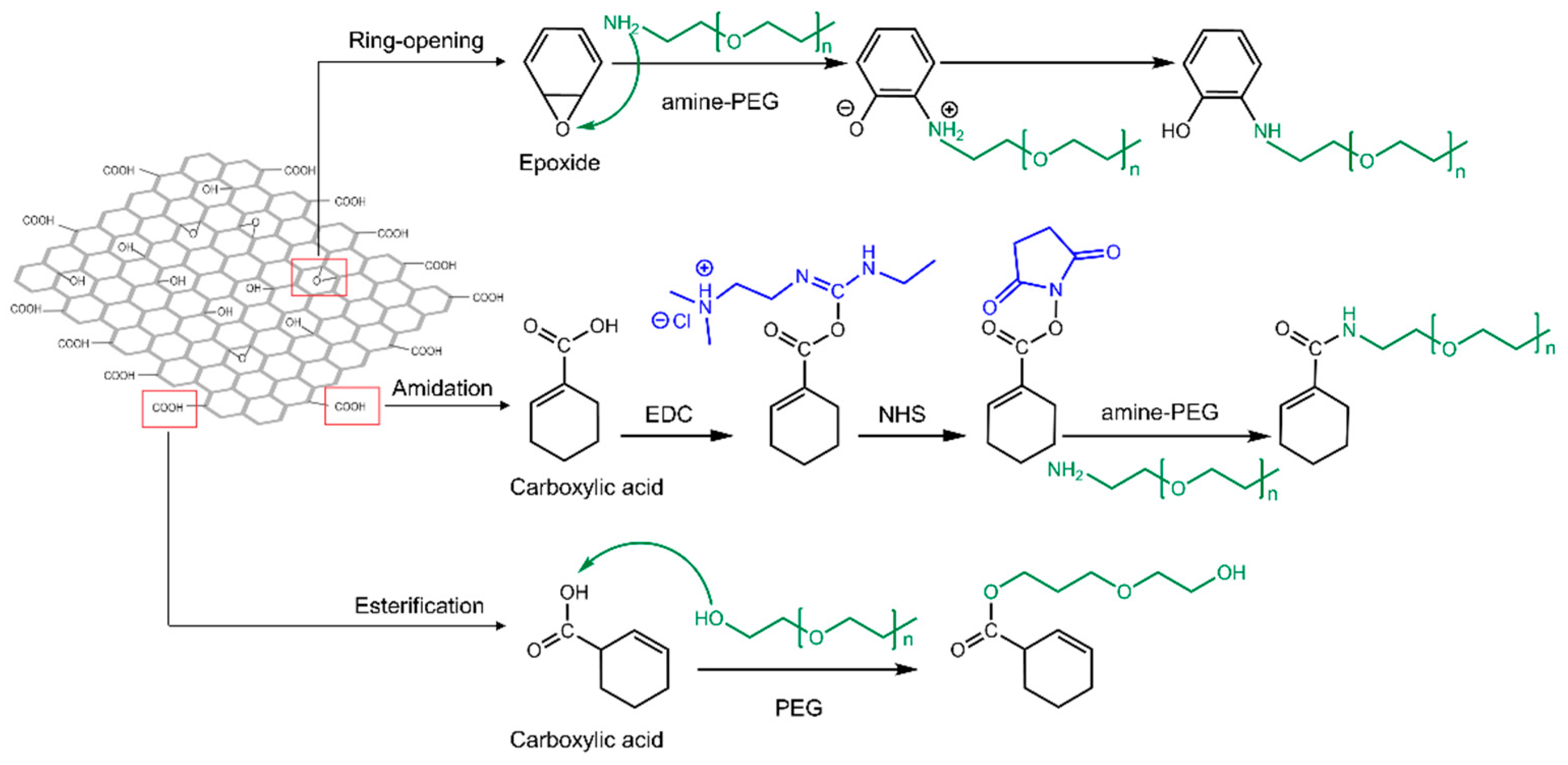



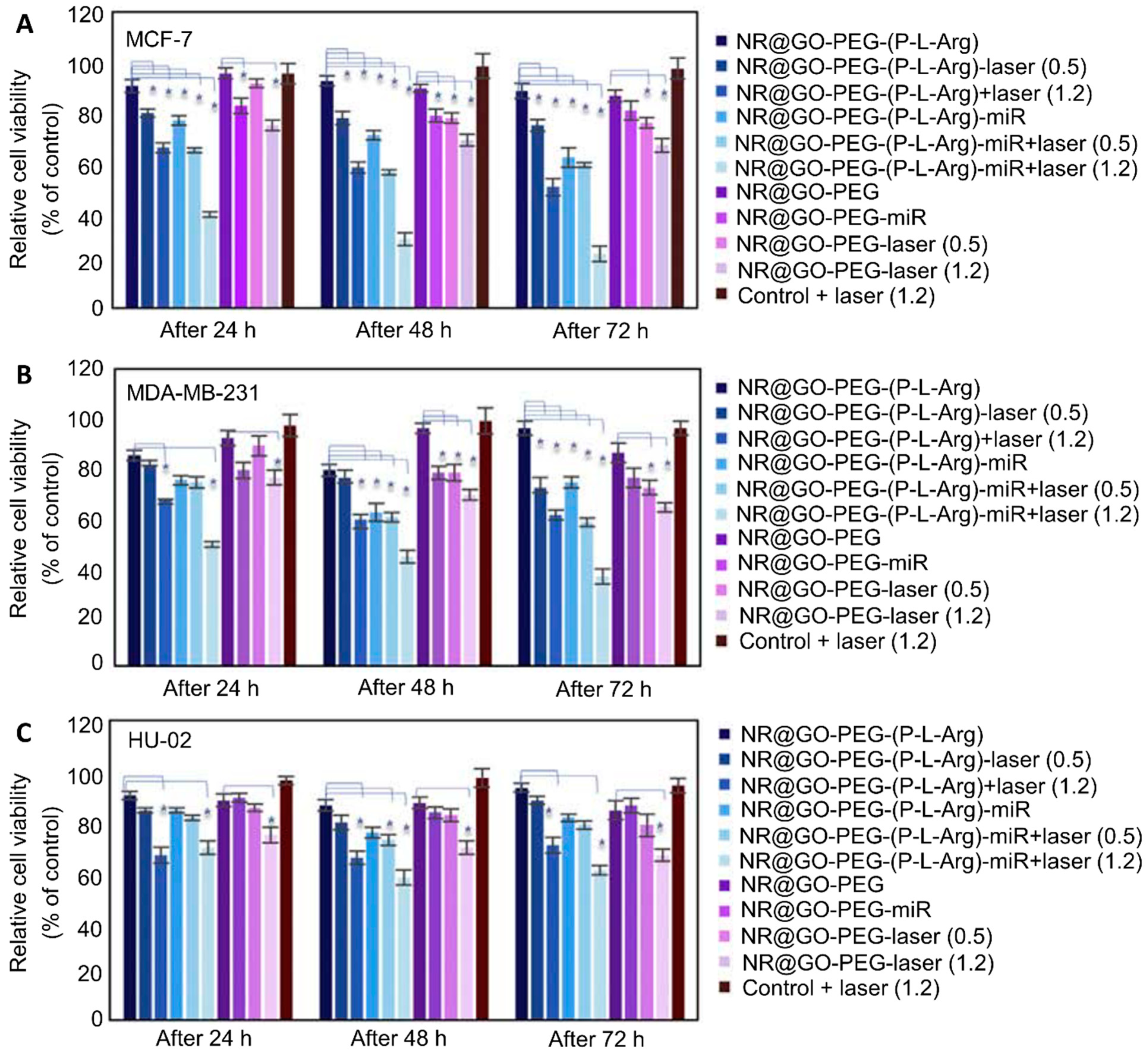
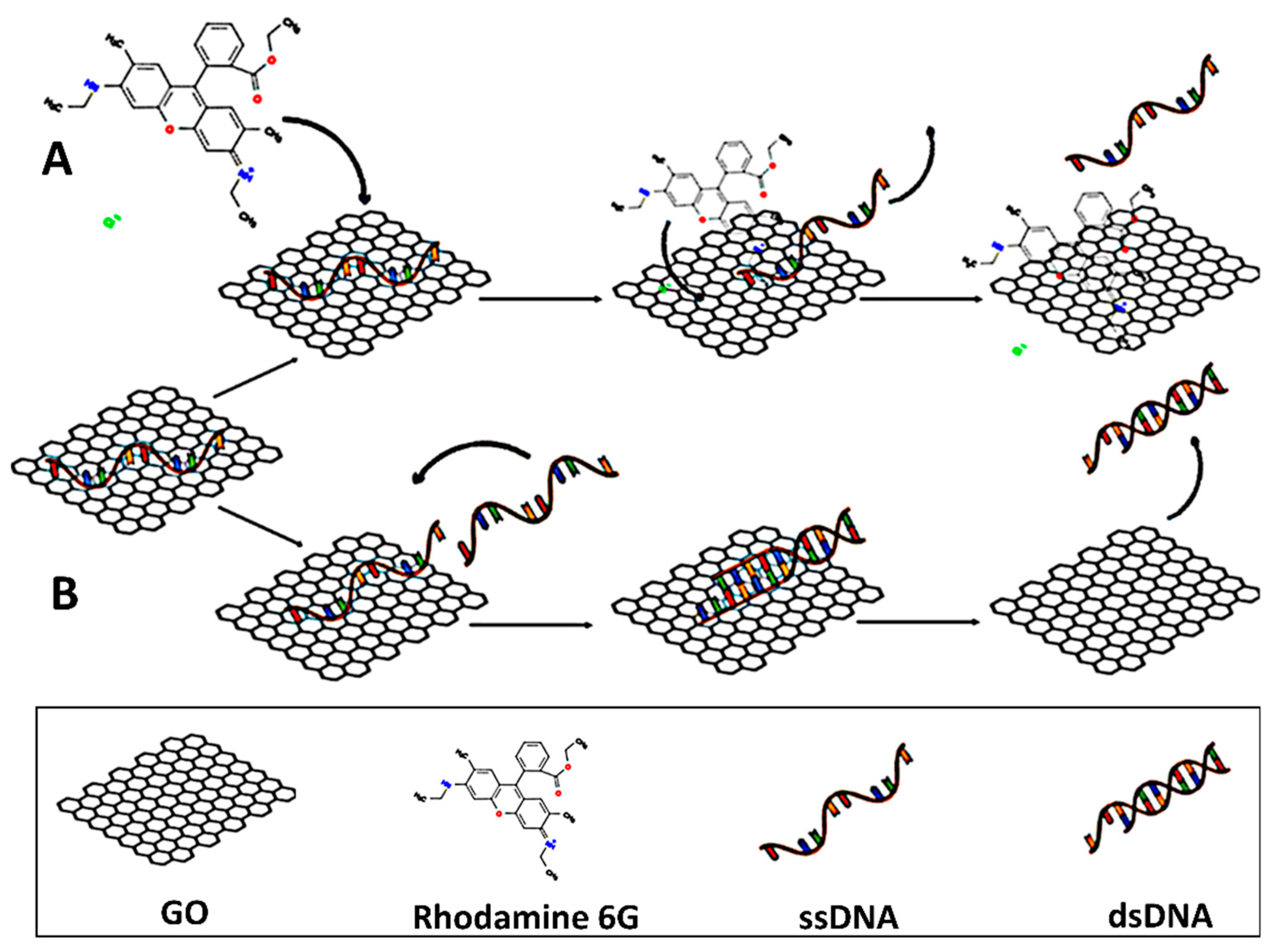
| GO Modification | Modification Mechanism | Agents |
|---|---|---|
| Covalent | Amidation | PEG [62,63,77,86] |
| PAMAM [16,17,87] | ||
| PEI [88,89] | ||
| Chitosan [64,65,66] | ||
| DEX [90] | ||
| Ring-opening reaction | PEG [17] | |
| APTES [53] | ||
| Esterification | PEG [91] | |
| Non-covalent | π-π stacking | MUC1 aptamer [72] |
| Electrostatic interactions | PEG [67,70] | |
| PEI [67] | ||
| Hydrophobic interactions | Phospholipids [52,70] |
| GO Modification | Payload Type (Targeting Agent) | N/P Ratio (w/w) | Gene Silencing | Cell Type |
|---|---|---|---|---|
| PEG | a miRNA 101 (PLA) [62] | 1−3.8 | - | MCF-7, MDA-MB-231 |
| a cd-siRNA (R8) [70] | 10 | - | ||
| a P-gp siRNA (FA) [88] | 4 | 70% | ||
| b Rictor siRNA (R8) [67] | 0.5 | 70% | ||
| a EPAC1-siRNA [17] | 2 | 62% | MDA-MB-231 | |
| PAMAM | a EPAC1-siRNA [17] | 2 | 62% | MDA-MB-231 |
| b miR-21i [16] | - | - | ||
| b MMP-9 shRNA [87] | 10 | 52% | MCF-7 | |
| PEI | a P-gp siRNA (FA) [88] | 4 | 70% | MCF-7, MDA-MB-231 |
| b Rictor siRNA (R8) [67] | 0.5 | 70% | ||
| Chitosan | b Survivin siRNA (anti-EpCAM) [51] | 30 | 44% | MCF-7 |
| a HIF-1α siRNA (HA) [65] | 6.5 | 62% | 4T1 | |
| a EGFR siRNA (FA) [66] | - | - | MCF-7, MDA-MB-231 | |
| Peptide | a cd-siRNA (R8) [70] | 10 | - | MCF-7, MDA-MB-231 |
| b Rictor siRNA (R8) [67] | 0.5 | 70% | ||
| b Survivin siRNA (anti-HER2) [64] | 40 | 50% | MCF-7 |
| Nanoparticle | Cell Type | Dose (µg/mL)/Dependency | Time (Day)/Dependency | ||
|---|---|---|---|---|---|
| GONR (PEG-DSPE) [122] | HeLa; MCF-7; SKBR; NIH3T3 | 10−400 | Y | 0.5−2 | Y |
| GO | PMO; J774A.1; LLC; MCF-7; HepG2; HUVEC [117] | 0−20 | Y | 1, 2, and 4 | - |
| HDF [129] | 5, 10, 20, 50, and 100 | Y | 1, 2, 3, 4, and 5 | Y | |
| A549 [130,131] | 10, 25, 50, 100, and 200 | N | 1, 2, and 3 | N | |
| HepG2 [132] | 4, 8, and 16 | Y | 1 and 3 | Y | |
| GO, GOD and GOP a [114] | MCF-7 | 2.4, 24, and 48 (μg/cm2) | Y | 1, 2, and 3 | N |
| GO-DEX-Apt-CUR b [90] | 4T1; MCF-7 | Up to 300 | Y | 1 and 2 | N |
| Lipid-rGO [119] | MDA-MB-231 | 10, 50, and 100 | Y | 1 and 2 | Y |
| MCF-7; MCF 10-A | N | N | |||
| rGO [118] | MDA-MB-231; ZR-75-1 | 25−300 | Y | 1 and 2 | Y |
| MCF-7; Hs 578T; T-47D | N | N | |||
| Graphene/SWCNT [128] | PC12 | 0.01, 0.1, 1, 10, and 100 | Y | 1−24 h | Y |
| Nanocarriee | Size (nm) | Zeta Potential (mV) | |
|---|---|---|---|
| GO | NP | ||
| GO-PEI [133] | 250−500 | −53 | +50 |
| GO-DOTAP [52] | 100−150 | −30 | +15 |
| rGO-MPAH-FA [136] | 100−230 | −45 | +40 |
| GO-anti HER2-R8 [51] | 120−260 | −49 | +26 |
| GO-anti EpCAM-Chitosan [64] | 70−350 | −42 | +38 |
| GO-PEG-PAMAM [17] | 54−220 | −30 | +10 |
| Release Trigger | Responsive Molecules | Responsive Functional Groups/Bonds | Mechanism |
|---|---|---|---|
| pH | GO + cationic molecules, pH-sensitive hydrogels | Amide [162], imine [189], hydrazone [190], ester [191], and oxime [192] | Protonation |
| Reducible intracellular environment | GO + molecules containing disulfide bonds | Disulfide bonds | Redox [123,185,193] |
| Enzymes | Phosphatase, collagenase, cathepsin | Peptide bonds | Degradation [186,194] |
| NIR irradiation | GO | Aromatic C-C bonds | PDT and PTT [168,195,196] |
| Temperature treatment | Thermo-sensitive hydrogels | Hydrogen bonds between the polymer molecules and/or water | Phase-volume transition [197,198] |
| Competitive molecules | GO + polyaromatic cationic molecules | Hydrophobic and electrostatic interactions between GO and the polyaromatic cationic molecules | Desorption of genes [187,188] |
| GO + cellular DNA | Hydrogen bonds between nitrogenous bases of the two DNA strands | Desorption of genes [187] |
| pH | Efficiency | GO Functionalization | Drug/Gene | Time (h) | Cell Type |
|---|---|---|---|---|---|
| <7 | 20% | GO-PAMAM [87] | DOX | 144 | MCF-7 |
| 7.4 | 17% | ||||
| <7 | 40% | PPG-FA a [88] | DOX | 48 | MCF-7/ADR |
| 7.4 | 10% | ||||
| <7 | 60% | CS-g-PMAA/GO b [202] | DOX | 220 | MCF-7 |
| 7.4 | 25% | ||||
| <7 | 71% | GO [200] | DXR | 30 | - |
| 7.4 | 11% | ||||
| <7 | 95% | FA-rGO/ZnS:Mn c [203] | DOX | 70 | MDA-MB 231 |
| 7.4 | 150% | ||||
| <7 | 28% | GO-Gel-BSA [186] | DOX | - | MCF-7 |
| 7.4 | 10% | ||||
| <7 | 58% | CGO-TMC-HA d [65] | Dinaciclib | 36 | 4T1 |
| 7.4 | 50% | ||||
| <7 | 90% | Chitosan [64] | Survivin_siRNA | 200 | MCF-7 |
| 7.4 | 30% | ||||
| <7 | 80% | Chitosan-HA [65] | HIF-1α_siRNA and Dinaciclib | 32 | 4T1, CT26, B16-F10, TM3 |
| 7.4 | 70% | ||||
| <7 | 12% | PAMAM [17] | siRNA | 72 | MDA-MB-231 |
| 7.4 | 61% |
| Trigger | NPs | Cell Type | Effect |
|---|---|---|---|
| 430 nm laser (100 J/cm2) | GO-Ag | MCF-7 | ROS production [214] |
| NIR | GO-FA | MDA-MB-231 | PTT [195] |
| nGO-CuSQDs | MCF-7 | Apoptosis [148] | |
| rGO-nZVI | MCF-7 | PTT [215] | |
| LED (660 nm) | GO-MB | MDA-MB-231 | PDT [168] |
| GQDs-MB | MCF-7 | ROS production and apoptosis [213] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grilli, F.; Hajimohammadi Gohari, P.; Zou, S. Characteristics of Graphene Oxide for Gene Transfection and Controlled Release in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 6802. https://doi.org/10.3390/ijms23126802
Grilli F, Hajimohammadi Gohari P, Zou S. Characteristics of Graphene Oxide for Gene Transfection and Controlled Release in Breast Cancer Cells. International Journal of Molecular Sciences. 2022; 23(12):6802. https://doi.org/10.3390/ijms23126802
Chicago/Turabian StyleGrilli, Francesca, Parisa Hajimohammadi Gohari, and Shan Zou. 2022. "Characteristics of Graphene Oxide for Gene Transfection and Controlled Release in Breast Cancer Cells" International Journal of Molecular Sciences 23, no. 12: 6802. https://doi.org/10.3390/ijms23126802






