Abstract
Systemic sclerosis (SSc) is characterized by excessive collagen deposition in the skin and internal organs. Activated fibroblasts are the key effector cells for the overproduction of type I collagen, which comprises the α1(I) and α2(I) chains encoded by COL1A1 and COL1A2, respectively. In this study, we examined the expression patterns of α1(I) and α2(I) collagen in SSc fibroblasts, as well as their co-regulation with each other. The relative expression ratio of COL1A1 to COL1A2 in SSc fibroblasts was significantly higher than that in control fibroblasts. The same result was observed for type I collagen protein levels, indicating that α2(I) collagen is more elevated than α2(I) collagen. Inhibition or overexpression of α1(I) collagen in control fibroblasts affected the α2(I) collagen levels, suggesting that α1(I) collagen might act as an upstream regulator of α2(I) collagen. The local injection of COL1A1 small interfering RNA in a bleomycin-induced SSc mouse model was found to attenuate skin fibrosis. Overall, our data indicate that α2(I) collagen is a potent regulator of type I collagen in SSc; further investigations of the overall regulatory mechanisms of type I collagen may help understand the aberrant collagen metabolism in SSc.
1. Introduction
Systemic sclerosis (SSc) is a multisystem autoimmune disease characterized by excessive extracellular matrix (ECM) protein deposition in the skin and internal organs [1]. The pathogenesis of fibrosis in SSc includes inflammation, aberrant immune activation, and endothelial cell injury, resulting in fibroblast activation to increase ECM production, mainly type I collagen [2,3]. Transforming growth factor-β (TGF-β) is one of the major profibrotic cytokines and the most important factor involved in fibroblast activation [4,5]. TGF-β is also known to play an important role in excessive ECM production in SSc [6]. However, the mechanisms underlying excessive type I collagen production have not been fully elucidated.
Type I collagen, the main product of abnormal collagen metabolism in SSc [3], is typically a heterotrimeric protein comprising two α1(I) collagen (encoded by COL1A1 gene) polypeptides and one α2(I) collagen (encoded by COL1A2 gene) polypeptide [7,8]. Although COL1A1 and COL1A2 gene expression is thought to be regulated in concert, their regulatory factors are complex and remain unclear [9]. Electron microscopy studies have shown that immature collagen fibrils with uniform diameters are observed in the lower dermis of SSc [10]. The irregularity of collagen fibers in SSc suggests an abnormality in collagen amounts as well as in the properties of collagen [10,11,12].
The excessive accumulation of type I collagen in SSc is well known, but little is known regarding the ratio of α1(I) and α2(I) collagen and how they regulate each other in this disease. The blockade of type I collagen-related signaling, mainly TGF-β, has received attention as an anti-fibrosis treatment in SSc [13,14], but there has been little research focused on the direct inhibition of α1(I) and α2(I) collagen. Therefore, in this study, we evaluated the possibility that α1(I) and α2(I) collagen levels are altered in SSc fibroblasts and examined whether the direct inhibition of type I collagen expression using small interfering RNA (siRNA) has anti-fibrotic effects in a bleomycin-induced SSc mouse model.
2. Results
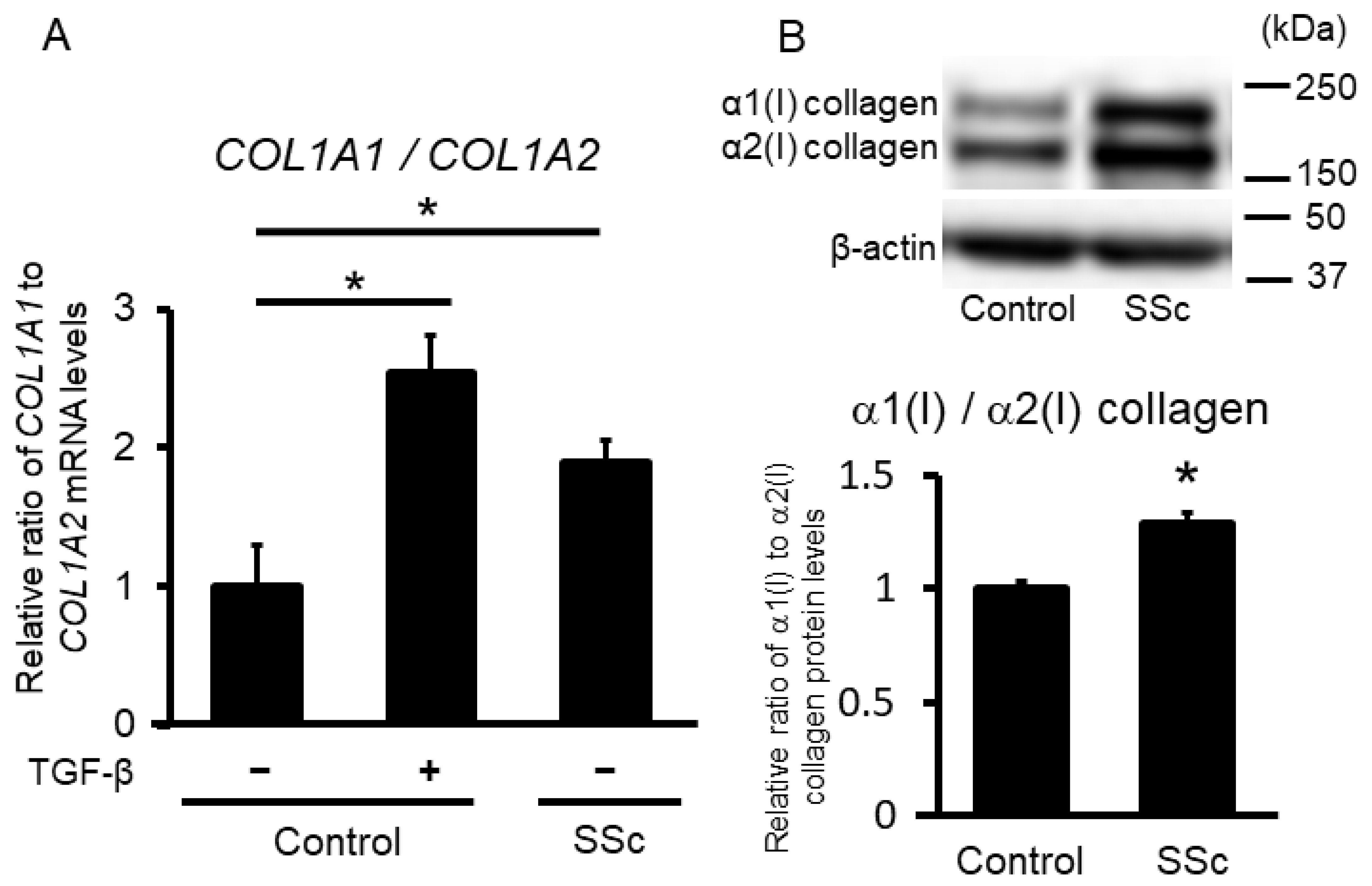
2.1. The Ratio of α1(I) and α2(I) Collagen Is Increased in SSc Dermal Fibroblasts
In an initial experiment to evaluate the expression patterns of type I procollagen, we extracted total RNA and protein from SSc and control dermal fibroblasts, with and without TGF-β stimulation. The relative ratio of COL1A1 to COL1A2 mRNA in SSc fibroblasts and control fibroblasts with TGF-β stimulation was significantly higher than that in control fibroblasts without TGF-β stimulation (Figure 1A). The relative ratio of α1(I) to α2(I) collagen protein expression determined by immunoblotting also showed the same tendency (Figure 1B). These results indicate that α1(1) collagen is predominantly expressed in SSc dermal fibroblasts, and this imbalance in type I procollagen expression may be attributed to stimulation of SSc fibroblasts with intrinsic TGF-β, as described in the introduction.

Figure 1.
Relative ratio of type 1 procollagen in control and systemic sclerosis (SSc) dermal fibroblasts. (A) Relative ratios of COL1A1 to COL1A2 mRNA were determined using real-time PCR in control, and SSc fibroblasts stimulated with or without TGF-β (n = 3 per group). (B) Control and SSc fibroblast lysates were subjected to immunoblotting. The graph shows the relative ratio of the type I collagen α1 chain to the α2 chain between control and SSc fibroblasts using densitometry (n = 4 per group). Each graph presents the mean ± standard deviation. The mean value in the control group was set as 1. * p < 0.05.
2.2. Forced Expression of α1(I) Collagen Affects α2(I) Collagen Expression in Control Dermal Fibroblasts
Little evidence is available regarding whether α1(I) and α2(I) collagen affect the expression of each other. Therefore, we performed experiments using control fibroblasts transfected with siRNAs targeting COL1A1 and COL1A2 expression. The depletion of COL1A1 in the control fibroblasts reduced the expression of COL1A2, whereas silencing COL1A2 did not affect COL1A1 gene expression (Figure 2A). A similar trend was observed at the protein level (Figure 2B). To determine whether the down-regulation of COL1A2 by COL1A1 silencing occurred at the transcriptional level, we performed a stability assay of COL1A2 mRNA using COL1A1-silenced control fibroblasts with actinomycin D stimulation. The relative decrease in COL1A2 levels upon incubation with actinomycin D over time was not altered in the presence or absence of COL1A1 siRNA (Figure 2C). This result indicated that COL1A2 expression was decreased by COL1A1 silencing without changing the stability of COL1A2 mRNA. Therefore, we hypothesized that the α1(I) collagen level might act as an upstream regulator of α2(I) collagen expression.

Figure 2.
COL1A1 silencing affects COL1A2 transcript levels in fibroblasts. (A) Relative mRNA levels of type 1 procollagen in control fibroblasts transfected with COL1A1 or COL1A2 siRNA were determined using real-time PCR. The mean values in the control siRNA treatment were set as 1 (n = 3 per group). * p < 0.05 versus the control. (B) Protein levels of type I collagen in control fibroblasts transfected with COL1A1 or COL1A2 siRNA are shown. The graph shows the mean relative type I collagen levels. The mean value in control siRNA was set as 1 (n = 3 per group). * p < 0.05 versus the control. (C) Control fibroblasts transfected with COL1A1 or control siRNA were incubated for 12 h after treatment with 2.5 μg/mL actinomycin D. COL1A2 mRNA expression was analyzed using real-time PCR (and normalized to GAPDH). The values in untreated fibroblasts were set as 100% (n = 3 per group). * p < 0.05.
To further test our hypothesis, we overexpressed COL1A1 by lentiviral transfection in the control fibroblasts. As expected, the overexpression of COL1A1 induced COL1A2 mRNA expression (Figure 3A). Figure 3B shows the same tendency in protein expression levels. As shown in Figure 2C, we compared the stability of COL1A2 mRNA between COL1A1-overexpressing and control fibroblasts. However, there was no significant difference in the stability of COL1A2 mRNA between the two groups (Figure 3C). Based on these results, we hypothesized that COL1A1 expression influences the transcriptional levels of COL1A2.

Figure 3.
COL1A1 overexpression affects COL1A2 transcript levels in fibroblasts. (A) Relative mRNA levels of type 1 procollagen in control fibroblasts treated with virus-containing medium with a control or COL1A1 expression vector were determined using real-time PCR. The mean value of the control vector was set as 1 (n = 3 per group). * p < 0.05 versus the control (B) Protein levels of type I collagen in control fibroblasts treated with virus-containing medium with a control or COL1A1 expression vector are shown. The graph shows the mean relative type I collagen levels. The mean value in the control vector was set as 1 (n = 3 per group). * p < 0.05 versus the control. (C) Control fibroblasts transfected with virus-containing medium with a control or COL1A1 expression vector were incubated for 12 h after treatment with 2.5 μg/mL actinomycin D. COL1A2 mRNA expression was analyzed using real-time PCR (and normalized to GAPDH). The values in untreated fibroblasts were set as 100% (n = 3 per group). * p < 0.05.
2.3. Local Administration of COL1A1 siRNA Attenuates Skin Fibrosis in a Bleomycin-Induced SSc Mouse Model
Considering the importance of α1(I) collagen, we examined whether the suppression of α1(I) collagen alone improves skin fibrosis in vivo. Prior to the in vivo experiments, we validated the effect of COL1A1 siRNA using mouse fibroblasts (NIH3T3 cells). The mRNA expression of both COL1A1 and COL1A2 was significantly knocked down in COL1A1-silenced NIH3T3 cells (Figure 4A), indicating a similar tendency as in human control fibroblasts transfected with COL1A1 siRNA. Furthermore, administration of COL1A1 siRNA decreased the mRNA expression of both COL1A1 and COL1A2 in mouse skin compared with that of the control siRNA administration (Figure 4B).

Figure 4.
COL1A1 silencing ameliorates skin fibrosis in a bleomycin-induced SSc mouse model. (A) Relative mRNA levels of type 1 procollagen in NIH3T3 cells transfected with COL1A1 or COL1A2 siRNA were determined using real-time PCR. The mean values of the control siRNA treatment were set as 1 (n = 3 per group). * p < 0.05 versus the control. (B) Relative mRNA levels of procollagen in mice skin injected with COL1A1 or control siRNA were determined using real-time PCR. The mean values in the control siRNA treatment were set as 1 (n = 5 per group). * p < 0.05 versus the control. (C) The protocol for (D) to (F) is shown. Bleomycin or PBS were injected intradermally into the back skin of C57BL/6 mice every other day for three weeks. COL1A1 or control siRNA mixed with atelocollagen were also injected into the back skin once weekly (for a total of three times). The back skin was obtained on the day after final bleomycin injection (D). Mouse skin sections were stained with hematoxylin and eosin. Representative results are shown. Scale bar = 200 μm. (E) Graph showing the results of dermal thickness (n = 5 per group) (F) Collagen content in mouse skin was measured using a hydroxyproline assay. Values are normalized relative to the PBS control group (n = 5 per group). * p < 0.05.
Finally, we evaluated the anti-fibrotic effects of COL1A1 siRNA in a bleomycin-induced SSc mouse model. Skin fibrosis induced using bleomycin injection in mice is a well-known model of SSc. Bleomycin was locally injected into the backs of C57BL/6 mice every other day for 3 weeks. Simultaneously, the control siRNA or COL1A1 siRNA mixed with atelocollagen was injected into the back skin once weekly (Figure 4C). Bleomycin-induced mouse skin showed dermal fibrosis, with an increased number of thickened collagen bundles (Figure 4D). COL1A1 siRNA significantly decreased dermal thickness and collagen content in the back skin of bleomycin-treated mice (Figure 4E,F). Collectively, these data indicate that COL1A1 inhibition attenuates skin fibrosis in an in vivo model of SSc.
3. Discussion
Fibrosis is a key feature of SSc, resulting in fibroblast activation and ECM accumulation, especially type I collagen, which comprises two α1(I) and one α2(I) collagen chains. Type I collagen is usually produced at a 2:1 ratio of α1(I) and α2(I) collagen. We first demonstrated that the relative ratio of α1(I) to α2(I) collagen was higher in SSc fibroblasts than that in control fibroblasts, suggesting that α1(I) collagen is predominantly expressed in SSc fibroblasts. Previous studies have also reported that the ratio of α1(I) to α2(I) collagen is high in SSc fibroblasts [15,16,17,18]. The same tendency was observed in control fibroblasts stimulated with TGF-β. These results suggest that the biased increase in α1(I) collagen compared with that in α2(I) collagen may result from activated endogenous TGF-β signaling. TGF-β also acts as a pro-fibrotic cytokine in chronic graft-versus-host disease (GVHD), an autoimmune disease characterized by inflammation and fibrosis of the dermis and subcutaneous tissue. The aberrant expression of α1 and α2 collagens has also been reported in other diseases. The imbalance of α1(I) and α2(I) collagen owing to the lack of COL1A2 has been reported in some orthopedic disorders and carcinomas [19,20,21]. Further, the loss of COL1A2 results in the homotrimers of three α1(I) collagen polypeptides and is reported to induce alteration of collagen structure [22], strength [23], and molecular stability owing to collagenase resistance [24,25]. The details of this mechanism have not been elucidated in this study, and future studies are needed to clarify this point.
Although the COL1A1 and COL1A2 genes are located on separate chromosomes, their expression is regulated coordinately [9]. In this study, COL1A2 expression was found to be altered with forced changes in COL1A1 expression, suggesting that COL1A1 expression acts as an upstream regulator of COL1A2. Additionally, an actinomycin D assay suggested that COL1A1 influences the transcriptional level of COL1A2 gene expression. We hypothesized that this could involve TGF-β because the biased expression of α1(I) collagen was found in the control fibroblasts upon TGF-β stimulation. Moreover, Dzobo et al. [26] reported that ECM components regulate the feedback pathway of COL1A2 gene expression. Alterations in α1(I) collagen expression may affect the regulation of collagen synthesis via a similar pathway. Further, the ratio of COL1A1 to COL1A2 has been reported to be altered by microRNA-29 [27]; thus, post-transcriptional mechanisms could also be involved in this regulation. Further studies are needed to clarify these points.
Finally, we tried to determine the effect of administering COL1A1 siRNA on skin fibrosis in a mouse model of SSc. siRNA technology has been widely studied for treating various diseases [28,29,30] and has attracted attention as a new approach for gene therapy in SSc [14,16,31]. Although previous reports have indicated the therapeutic effect of siRNA by knocking down the TGF-β signaling pathway, to the best of our knowledge, this is the first report describing the anti-fibrotic effect of COL1A1 siRNA by directly knocking down the expression of type I collagen in mouse skin. The anti-fibrotic effect of COL1A1 siRNA is assumed to have a direct as well as indirect inhibitory effect on type I collagen gene expression. For example, Vollmann et al. [32] indicated that COL1A1 siRNA significantly reduced PDGFRβ mRNA levels in a mouse model of liver fibrosis because of the feedback loop between ECM accumulation and PDGFRβ. Our data also suggest a possible therapeutic application of COL1A1 siRNA in patients with SSc; however, we could not elucidate its detailed anti-fibrotic mechanisms in this study.
In summary, this is the first report indicating the existence of a biased increase in α1(I) collagen compared with that in α2(I) collagen in SSc fibroblasts. There are only limited treatment options for patients with SSc. We demonstrated the potential of α1(I) collagen expression to affect α2(I) collagen levels. Therefore, inhibiting α1(I) collagen expression could be an efficient therapeutic strategy in SSc fibrosis. Although collagen metabolism in SSc remains unclear, further investigations regarding the regulation of type I collagen may facilitate a better understanding of SSc pathogenesis and provide new therapeutic approaches for this disease.
4. Materials and Methods
4.1. Reagents
Antibodies against type I collagen (1:1000, Cat.#1310-01) and β-actin (1:1000, Cat.#sc-47778) were purchased from Southern Biotechnologies (Birmingham, AL, USA) and Santa Cruz Biotechnology (Dallas, TX, USA), respectively. Recombinant human TGF-β1 (2 ng/mL, Cat.#240-B-002) was obtained from R&D Systems (Minneapolis, MN, USA). Actinomycin D (Cat.#018-21264) was purchased from Wako (Osaka, Japan).
4.2. Cell Cultures
SSc fibroblasts were obtained by skin biopsies from the affected areas (dorsal forearm) of three patients with diffuse cutaneous SSc and <2 years of skin thickening, as described previously [33]. Control fibroblasts obtained by skin biopsies from three healthy donors were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and Lonza (Walkersville, MD, USA). Mouse NIH3T3 cells were obtained from ATCC. Monolayer cultures of fibroblasts were maintained at 37 °C with 5% CO2. Cells were serum-starved for 12–24 h before all experiments [34]. All biopsies were performed according to the Declaration of Helsinki, with approval from the institutional review board, and written informed consent was obtained.
4.3. RNA Extraction, Reverse Transcription, and PCR Analysis of RNA Expression
The total RNA was extracted from cultured cells using ISOGEN (Nippon Gene, Tokyo, Japan). First-strand cDNA was synthesized using a PrimeScriptTM RT reagent kit (Takara Bio, Shiga, Japan). For quantitative real-time analysis, cDNA and primers were mixed with SYBR Premix Ex TaqTM II (Takara Bio, Shiga, Japan). Primer sets for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 18S ribosomal RNA (18SrRNA) were purchased from QIAGEN (Valencia, CA, USA) and Thermo Fisher Scientific (Waltham, MA, USA), respectively. Primer sets for human and mouse α1(I) and α2(I) collagen were obtained from Takara. The DNA was amplified over 40 cycles of denaturation for 5 s at 95 °C and annealing for 30 s at 60 °C, and relative expression was calculated using the ΔΔCt method [35].
4.4. Immunoblotting
The cultured human or mouse dermal fibroblasts were washed with PBS and lysed in RIPA buffer (Nacalai Tesque, Kyoto, Japan). Protein concentrations were quantified using a BCA Protein Assay kit (Thermo Fisher Scientific). Aliquots of cell lysates were separated by electrophoresis on 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes, which were blocked in blocking One P buffer (Nacalai Tesque) for 1 h and incubated overnight at 4 °C with primary antibody. The membranes were washed with TBS and 0.1% TBST, probed with HRP-conjugated secondary Ab for 1 h, and then washed with TBST again [36]. Immunoreactive bands were visualized using the ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA).
4.5. Transient Transfection
Human COL1A1 siRNA (ON-TARGETplus SMART pool), COL1A2 siRNA (ON-TARGETplus SMART pool), and control siRNA (ON-TARGETplus nontargeting control pool) were obtained from Dharmacon (Lafayette, CO, USA). Mouse COL1A1 siRNAs (5′-UGGCCUUGGAGGAAACUUU-3′ and 5′-AAAGUUUCCUCCAAGGCCA-3′) and control siRNA were purchased from Nippon Gene (Tokyo, Japan). For transfection, siRNAs (20 nM) mixed with Lipofectamine RNAiMAX (Thermo Fisher Scientific) were added to cells in 24-well culture dishes, followed by incubation for 24–72 h at 37 °C in 5% CO2 [36].
4.6. Lentiviral Gene Transfer
Lentiviral vector-mediated gene transfer was performed using CSII-EF-RfA, pCMV-VSV-G-RSV-Rev, and pHIVgp, which were kindly donated by Dr. Hiroyuki Miyoshi (RIKEN, Wako, Japan) [37,38]. cDNA fragments of the full-length human COL1A1 gene were generated and amplified by PCR using SuperScriptTM II Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) and PrimeSTAR® HS DNA Polymerase with GC Buffer (Takara), followed by cloning into CSII-EF-RfA [39]. Substitution mutations were generated using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) and were confirmed by sequencing [40].
4.7. Mice
To deliver mouse COL1A1 siRNA into mouse skin, mixtures of siRNA and AteloGene® Local Use (Koken, Tokyo, Japan) were prepared according to the manufacturer’s instructions. The COL1A1 siRNA oligo was obtained from Koken:5′-UGGCCUUGGAGGAAACUUU-3′ and 5′- AAAGUUUCCUCCAAGGCCA-3′. An irrelevant control siRNA was also obtained from Koken:5′-AUCCGCGCGAUAGUACGUA-3′ and 5′-UACGUACUAUCGCGCGGAU-3′. The samples were then annealed with one another. Then, 100 μL of the mixtures (siRNA concentration, 5 μM) were administered into the dermis of six-week-old male C57BL/6 mice (CLEA Japan, Tokyo, Japan) once weekly [41]. All of the mouse experiments were performed in accordance with the guidelines of the Institutional Animal Committee of Kumamoto University and approved by the Committee on Animal Research at Kumamoto University.
4.8. Bleomycin Treatment in Mice
Bleomycin (Nippon Kayaku, Tokyo, Japan) was dissolved in PBS at a concentration of 0.5 mg/mL and sterilized by filtration as described previously [42,43]. Bleomycin (100 μL) was then injected intradermally into the shaved backs of 6-week-old male C57BL/6 mice (CLEA Japan, Tokyo, Japan) every alternate day for 3 weeks. The back skin was removed on the day after the final bleomycin injection, and fibrosis was evaluated by histological analysis and a total collagen assay. The control mice were injected with equal volumes of PBS. The dermal thickness was evaluated by measuring the distance between the epidermal-dermal junction and dermal-fat junction in hematoxylin-eosin sections.
4.9. Measurement of Collagen Production in Mouse Skin
The collagen deposition in 8-mm mouse skin punch biopsy samples was measured using a Total Collagen Assay kit (QuickZyme Biosciences, Leiden, Netherlands) following the manufacturer’s protocol. Briefly, mouse skin samples were hydrolyzed using 12 mol/L of hydrochloric acid for 20 h at 95 °C. The samples were then added to the microplate wells, and dilution assay buffer was added to each well. After incubation for 20 min at room temperature, the detection reagent was added to each well. The samples were incubated for 60 min at 60 °C, and the absorbance of each sample was read at 570 nm using a spectrophotometer. The results are expressed as the relative hydroxyproline content [44].
4.10. Statistical Analysis
The values are presented as the mean ± standard deviation. Statistical analyses were performed using the Mann–Whitney U-test for a comparison of the two groups. One-way analysis of variance (ANOVA) with Tukey’s post-hoc test was used for multiple comparisons. All of the analyses were performed using Statcel4 software (OMS, Kurume, Japan). The statistical significance was defined as p < 0.05.
Author Contributions
S.S. (Soichiro Sawamura) and M.I. performed research and wrote the manuscript. M.J. contributed to experimental design. S.S. (Shuichi Shimada), I.K., T.M. and S.F. contributed data analysis. K.M. was the principal investigator and was involved in conception and design of the study, data analysis, and manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by a grant for scientific research from the Japanese Ministry of Education, Science, Sports and Culture (JSPS KAKENHI Grant Number JP19K17776) and by a research project on intractable diseases from the Japanese Ministry of Health, Labour and Welfare.
Institutional Review Board Statement
Upon informed written consent and in compliance with the institutional review board according to the Declaration of Helsinki, skin fibroblasts were obtained from SSc patients or healthy volunteers (Approval number: 1452, permission code: 31 March 2023, Kumamoto University). All mouse experiments were performed in accordance with the guidelines of the Institutional Animal Committee of Kumamoto University and were approved by the Committee on Animal Research at Kumamoto University (Approval number: A2021-007, permission code: 31 March 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets supporting the conclusions of this article are included in this published article.
Acknowledgments
We are grateful to Hironobu Ihn (Kumamoto, Japan) for his valuable suggestions and support in this study. We thank Chiemi Shiotsu for valuable technical assistance. Bleomycin was kindly provided by Nippon Kayaku (Tokyo, Japan).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ECM | extracellular matrix |
| siRNA | small interfering RNA |
| SSc | systemic sclerosis |
| TGF-β | transforming growth factor-β |
References
- Korn, J. Immunologic aspects of scleroderma. Curr. Opin. Rheumatol. 1989, 1, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Mauch, C.; Kreig, T. Fibroblast-matrix interactions and their role in the pathogenesis of fibrosis. Rheum. Dis. Clin. N. Am. 1990, 16, 93–107. [Google Scholar] [CrossRef]
- Trojanowska, M.; LeRoy, E.C.; Eckes, B.; Krieg, T. Pathogenesis of fibrosis: Type 1 collagen and the skin. J. Mol. Med. 1998, 76, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B.; Sporn, M.B.; Assoian, R.K.; Smith, J.M.; Roche, N.S.; Wakefield, L.M.; Heine, U.I.; Liotta, L.A.; Falanga, V.; Kehrl, J.H.; et al. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 4167–4171. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B.; Heine, U.I.; Flanders, K.C.; Sporn, M.B. Transforming growth factor-beta. Major role in regulation of extracellular matrix. Ann. N. Y. Acad. Sci. 1990, 580, 225–232. [Google Scholar] [CrossRef]
- Ihn, H. Autocrine TGF-beta signaling in the pathogenesis of systemic sclerosis. J. Dermatol. Sci. 2008, 49, 103–113. [Google Scholar] [CrossRef]
- Kivirikko, K.I. Collagen biosynthesis: A mini-review cluster. Matrix Biol. 1998, 16, 355–356. [Google Scholar] [CrossRef]
- Huerre, C.; Junien, C.; Weil, D.; Chu, M.L.; Morabito, M.; Van Cong, N.; Myers, J.C.; Foubert, C.; Gross, M.S.; Prockop, D.J.; et al. Human type I procollagen genes are located on different chromosomes. Proc. Natl. Acad. Sci. USA 1982, 79, 6627–6630. [Google Scholar] [CrossRef]
- Yamada, Y.; Mudryj, M.; de Crombrugghe, B. A uniquely conserved regulatory signal is found around the translation initiation site in three different collagen genes. J. Biol. Chem. 1983, 258, 14914–14919. [Google Scholar] [CrossRef]
- Fleischmajer, R.; Damiano, V.; Nedwich, A. Scleroderma and the subcutaneous tissue. Science 1971, 171, 1019–1021. [Google Scholar] [CrossRef]
- Moinzadeh, P.; Agarwal, P.; Bloch, W.; Orteu, C.; Hunzelmann, N.; Eckes, B.; Krieg, T. Systemic sclerosis with multiple nodules: Characterization of the extracellular matrix. Arch. Dermatol. Res. 2013, 305, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Fleischmajer, R.; Gay, S.; Perlish, J.S.; Cesarini, J.P. Immunoelectron microscopy of type III collagen in normal and scleroderma skin. J. Investig. Dermatol. 1980, 75, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.M.; Padilla, C.M.; McLaughlin, S.R.; Mathes, A.; Ziemek, J.; Goummih, S.; Nakerakanti, S.; York, M.; Farina, G.; Whitfield, M.L.; et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Investig. 2015, 125, 2795–2807. [Google Scholar] [CrossRef]
- Xiao, R.; Liu, F.Y.; Luo, J.Y.; Yang, X.J.; Wen, H.Q.; Su, Y.W.; Yan, K.L.; Li, Y.P.; Liang, Y.S. Effect of small interfering RNA on the expression of connective tissue growth factor and type I and III collagen in skin fibroblasts of patients with systemic sclerosis. Br. J. Dermatol. 2006, 155, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Pannu, J.; Gardner, H.; Shearstone, J.R.; Smith, E.; Trojanowska, M. Increased levels of transforming growth factor beta receptor type I and up-regulation of matrix gene program: A model of scleroderma. Arthritis Rheum. 2006, 54, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.; Chrobak, I.; Bujor, A.; Hant, F.; Mummery, C.; Ten Dijke, P.; Trojanowska, M. Endoglin promotes TGF-beta/Smad1 signaling in scleroderma fibroblasts. J. Cell Physiol. 2011, 226, 3340–3348. [Google Scholar] [CrossRef] [PubMed]
- Bujor, A.M.; Pannu, J.; Bu, S.; Smith, E.A.; Muise-Helmericks, R.C.; Trojanowska, M. Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J. Investig. Dermatol. 2008, 128, 1906–1914. [Google Scholar] [CrossRef]
- Honda, N.; Jinnin, M.; Kajihara, I.; Makino, T.; Makino, K.; Masuguchi, S.; Fukushima, S.; Okamoto, Y.; Hasegawa, M.; Fujimoto, M.; et al. TGF-beta-mediated downregulation of microRNA-196a contributes to the constitutive upregulated type I collagen expression in scleroderma dermal fibroblasts. J. Immunol. 2012, 188, 3323–3331. [Google Scholar] [CrossRef]
- Bailey, A.J.; Knott, L. Molecular changes in bone collagen in osteoporosis and osteoarthritis in the elderly. Exp. Gerontol. 1999, 34, 337–351. [Google Scholar] [CrossRef]
- Mann, V.; Hobson, E.E.; Li, B.; Stewart, T.L.; Grant, S.F.; Robins, S.P.; Aspden, R.M.; Ralston, S.H. A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J. Clin. Investig. 2001, 107, 899–907. [Google Scholar] [CrossRef]
- Pucci-Minafra, I.; Andriolo, M.; Basirico, L.; Alessandro, R.; Luparello, C.; Buccellato, C.; Garbelli, R.; Minafra, S. Absence of regular alpha2(I) collagen chains in colon carcinoma biopsy fragments. Carcinogenesis 1998, 19, 575–584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McBride, D.J., Jr.; Choe, V.; Shapiro, J.R.; Brodsky, B. Altered collagen structure in mouse tail tendon lacking the alpha 2(I) chain. J. Mol. Biol. 1997, 270, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Misof, K.; Landis, W.J.; Klaushofer, K.; Fratzl, P. Collagen from the osteogenesis imperfecta mouse model (oim) shows reduced resistance against tensile stress. J. Clin. Investig. 1997, 100, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.S.; Meyers, D.F.; Page, R.C.; Welgus, H.G. Action of mammalian collagenases on type I trimer collagen. Coll. Relat. Res. 1984, 4, 289–296. [Google Scholar] [CrossRef]
- Makareeva, E.; Han, S.; Vera, J.C.; Sackett, D.L.; Holmbeck, K.; Phillips, C.L.; Visse, R.; Nagase, H.; Leikin, S. Carcinomas contain a matrix metalloproteinase-resistant isoform of type I collagen exerting selective support to invasion. Cancer Res. 2010, 70, 4366–4374. [Google Scholar] [CrossRef]
- Dzobo, K.; Leaner, V.D.; Parker, M.I. Feedback regulation of the alpha2(1) collagen gene via the Mek-Erk signaling pathway. IUBMB Life 2012, 64, 87–98. [Google Scholar] [CrossRef]
- Van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef]
- Verma, U.N.; Surabhi, R.M.; Schmaltieg, A.; Becerra, C.; Gaynor, R.B. Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin. Cancer Res. 2003, 9, 1291–1300. [Google Scholar]
- Takeshita, F.; Minakuchi, Y.; Nagahara, S.; Honma, K.; Sasaki, H.; Hirai, K.; Teratani, T.; Namatame, N.; Yamamoto, Y.; Hanai, K.; et al. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 12177–12182. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Wang, J.C.; Lai, S.; Guo, X.; Zhang, X.; de Crombrugghe, B.; Sonnylal, S.; Arnett, F.C.; Zhou, X. Attenuation of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Res. Ther. 2010, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Vollmann, E.H.; Cao, L.; Amatucci, A.; Reynolds, T.; Hamann, S.; Dalkilic-Liddle, I.; Cameron, T.O.; Hossbach, M.; Kauffman, K.J.; Mir, F.F.; et al. Identification of Novel Fibrosis Modifiers by In Vivo siRNA Silencing. Mol. Ther. Nucleic Acids 2017, 7, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H.; LeRoy, E.C.; Trojanowska, M. Oncostatin M stimulates transcription of the human alpha2(I) collagen gene via the Sp1/Sp3-binding site. J. Biol. Chem. 1997, 272, 24666–24672. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Jinnin, M.; Yamane, K.; Honda, N.; Kajihara, I.; Makino, T.; Masuguchi, S.; Fukushima, S.; Okamoto, Y.; Hasegawa, M.; et al. Impaired IL-17 signaling pathway contributes to the increased collagen expression in scleroderma fibroblasts. J. Immunol. 2012, 188, 3573–3583. [Google Scholar] [CrossRef]
- Kudo, H.; Jinnin, M.; Asano, Y.; Trojanowska, M.; Nakayama, W.; Inoue, K.; Honda, N.; Kajihara, I.; Makino, K.; Fukushima, S.; et al. Decreased interleukin-20 expression in scleroderma skin contributes to cutaneous fibrosis. Arthritis Rheumatol. 2014, 66, 1636–1647. [Google Scholar] [CrossRef]
- Makino, K.; Jinnin, M.; Hirano, A.; Yamane, K.; Eto, M.; Kusano, T.; Honda, N.; Kajihara, I.; Makino, T.; Sakai, K.; et al. The downregulation of microRNA let-7a contributes to the excessive expression of type I collagen in systemic and localized scleroderma. J. Immunol. 2013, 190, 3905–3915. [Google Scholar] [CrossRef]
- Kitamura, T.; Koshino, Y.; Shibata, F.; Oki, T.; Nakajima, H.; Nosaka, T.; Kumagai, H. Retrovirus-mediated gene transfer and expression cloning: Powerful tools in functional genomics. Exp. Hematol. 2003, 31, 1007–1014. [Google Scholar] [CrossRef]
- Miyoshi, H.; Blomer, U.; Takahashi, M.; Gage, F.H.; Verma, I.M. Development of a self-inactivating lentivirus vector. J. Virol. 1998, 72, 8150–8157. [Google Scholar] [CrossRef]
- Tahara-Hanaoka, S.; Sudo, K.; Ema, H.; Miyoshi, H.; Nakauchi, H. Lentiviral vector-mediated transduction of murine CD34(−) hematopoietic stem cells. Exp. Hematol. 2002, 30, 11–17. [Google Scholar] [CrossRef]
- Egashira, S.; Jinnin, M.; Ajino, M.; Shimozono, N.; Okamoto, S.; Tasaki, Y.; Hirano, A.; Ide, M.; Kajihara, I.; Aoi, J.; et al. Chronic sun exposure-related fusion oncogenes EGFR-PPARGC1A in cutaneous squamous cell carcinoma. Sci. Rep. 2017, 7, 12654. [Google Scholar] [CrossRef]
- Takei, Y.; Kadomatsu, K.; Yuzawa, Y.; Matsuo, S.; Muramatsu, T. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res. 2004, 64, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Ruzehaji, N.; Avouac, J.; Elhai, M.; Frechet, M.; Frantz, C.; Ruiz, B.; Distler, J.H.; Allanore, Y. Combined effect of genetic background and gender in a mouse model of bleomycin-induced skin fibrosis. Arthritis Res. Ther. 2015, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Asano, Y.; Ichimura, Y.; Toyama, T.; Taniguchi, T.; Noda, S.; Akamata, K.; Tada, Y.; Sugaya, M.; Kadono, T.; et al. Amelioration of tissue fibrosis by toll-like receptor 4 knockout in murine models of systemic sclerosis. Arthritis Rheumatol. 2015, 67, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Shin, M.Y.; Suh, S.H.; Park, S. Lyso-globotriaosylceramide downregulates KCa3.1 channel expression to inhibit collagen synthesis in fibroblasts. Biochem. Biophys. Res. Commun. 2015, 468, 883–888. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).