Combining Cortical Voltage Imaging and Hippocampal Electrophysiology for Investigating Global, Multi-Timescale Activity Interactions in the Brain
Abstract
:1. Introduction
2. Results
2.1. Simultaneous Cortical Voltage Imaging with Hippocampal Electrophysiology
2.2. Layer-Specific Hippocampal Signal during Integrative Visual Information Process
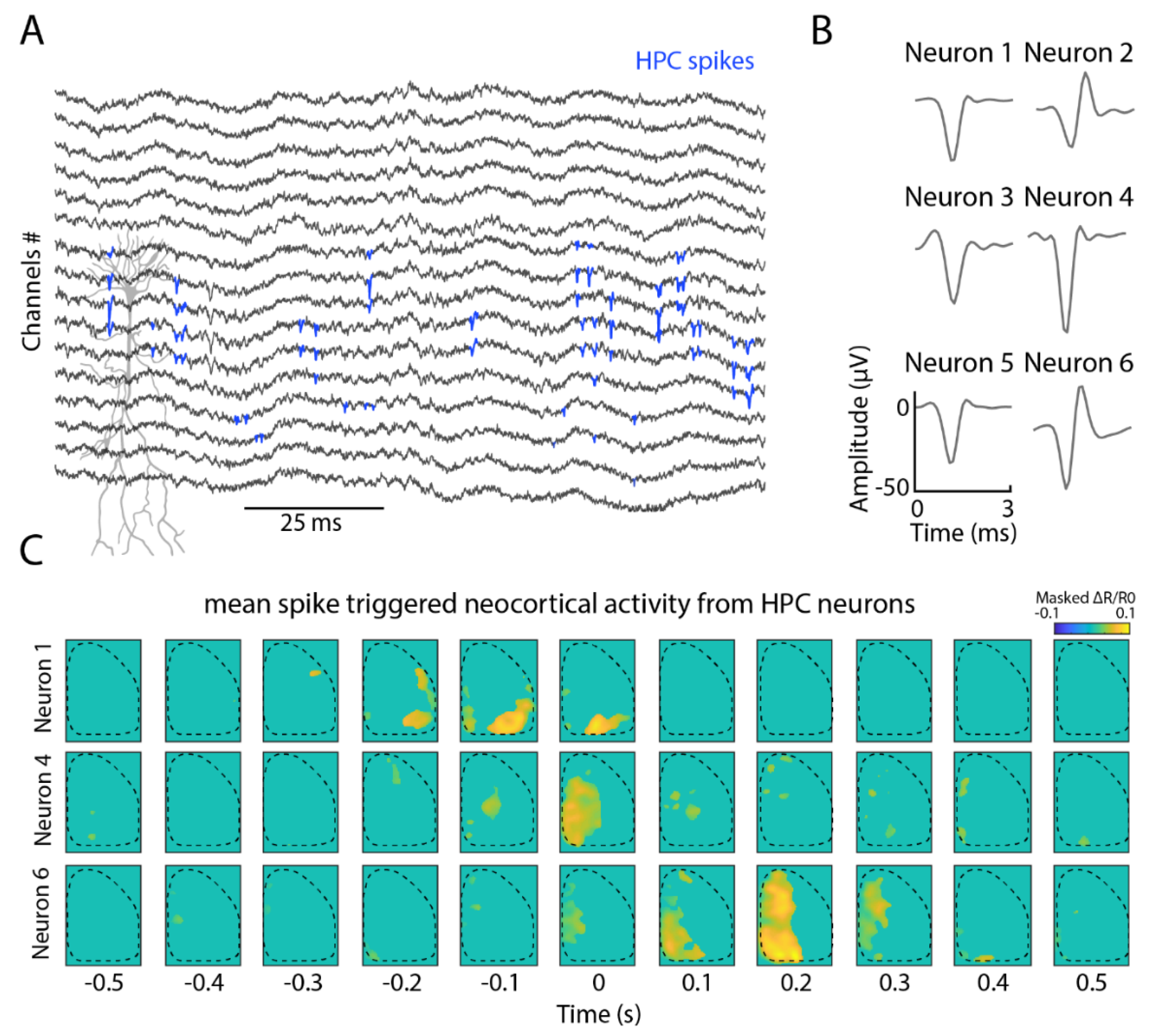
2.3. Hippocampal Neuronal Interrelation with Cortical Modules
2.4. Cortical Microstimulation and Hippocampal Electrophysiology
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedure for Combining Wide-Field Imaging with Hippocampal Electrophysiology
4.3. Combining Wide-Field Voltage Imaging with Hippocampal Electrophysiology
4.4. Visual Stimulus Presentation
4.5. Eye-Tracking and Pupil Detection
4.6. Cortical Microstimulation with Combined Cortical Voltage Imaging and Hippocampal Electrophysiology
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scoville, W.B.; Milner, B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 1957, 20, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Karimi Abadchi, J.; Nazari-Ahangarkolaee, M.; Gattas, S.; Bermudez-Contreras, E.; Luczak, A.; McNaughton, B.L.; Mohajerani, M.H. Spatiotemporal patterns of neocortical activity around hippocampal sharp-wave ripples. Elife 2020, 9. [Google Scholar] [CrossRef]
- Pedrosa, R.; Nazari, M.; Mohajerani, M.H.; Knöpfel, T.; Stella, F.; Battaglia, F. Hippocampal gamma and sharp wave/ripples mediate bidirectional interactions with cortical networks during sleep. bioRxiv 2022. [Google Scholar] [CrossRef]
- Peyrache, A.; Khamassi, M.; Benchenane, K.; Wiener, S.I.; Battaglia, F.P. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat. Neurosci. 2009, 12, 919–926. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.L.; Hay, Y.A.; Paulsen, O. Comparison of three gamma oscillations in the mouse entorhinal-hippocampal system. Eur. J. Neurosci. 2018, 48, 2795–2806. [Google Scholar] [CrossRef] [Green Version]
- Iwase, M.; Kitanishi, T.; Mizuseki, K. Cell type, sub-region, and layer-specific speed representation in the hippocampal-entorhinal circuit. Sci. Rep. 2020, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Klee, J.L.; Souza, B.C.; Battaglia, F.P. Learning differentially shapes prefrontal and hippocampal activity during classical conditioning. Elife 2021, 10. [Google Scholar] [CrossRef]
- Colgin, L.L.; Denninger, T.; Fyhn, M.; Hafting, T.; Bonnevie, T.; Jensen, O.; Moser, M.B.; Moser, E.I. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 2009, 462, 353–357. [Google Scholar] [CrossRef]
- Lopes-Dos-Santos, V.; van de Ven, G.M.; Morley, A.; Trouche, S.; Campo-Urriza, N.; Dupret, D. Parsing hippocampal theta oscillations by nested spectral components during spatial exploration and memory-guided behavior. Neuron 2018, 100, 940–952.e947. [Google Scholar] [CrossRef] [Green Version]
- Sirota, A.; Montgomery, S.; Fujisawa, S.; Isomura, Y.; Zugaro, M.; Buzsaki, G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 2008, 60, 683–697. [Google Scholar] [CrossRef] [Green Version]
- O’Keefe, J.; Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef]
- Battaglia, F.P.; Sutherland, G.R.; McNaughton, B.L. Hippocampal sharp wave bursts coincide with neocortical "up-state" transitions. Learn Mem. 2004, 11, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Driscoll, N.; Rosch, R.E.; Murphy, B.B.; Ashourvan, A.; Vishnubhotla, R.; Dickens, O.O.; Johnson, A.T.C.; Davis, K.A.; Litt, B.; Bassett, D.S.; et al. Multimodal in vivo recording using transparent graphene microelectrodes illuminates spatiotemporal seizure dynamics at the microscale. Commun. Biol. 2021, 4, 136. [Google Scholar] [CrossRef]
- Wei, Z.; Lin, B.J.; Chen, T.W.; Daie, K.; Svoboda, K.; Druckmann, S. A comparison of neuronal population dynamics measured with calcium imaging and electrophysiology. PLoS Comput. Biol. 2020, 16, e1008198. [Google Scholar] [CrossRef]
- Knopfel, T.; Diez-Garcia, J.; Akemann, W. Optical probing of neuronal circuit dynamics: Genetically encoded versus classical fluorescent sensors. Trends Neurosci. 2006, 29, 160–166. [Google Scholar] [CrossRef]
- Song, C.; Piscopo, D.M.; Niell, C.M.; Knopfel, T. Cortical signatures of wakeful somatosensory processing. Sci. Rep. 2018, 8, 11977. [Google Scholar] [CrossRef] [Green Version]
- Mishina, Y.; Mutoh, H.; Knopfel, T. Transfer of kv3.1 voltage sensor features to the isolated ci-vsp voltage-sensing domain. Biophys. J. 2012, 103, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Mishina, Y.; Mutoh, H.; Song, C.; Knopfel, T. Exploration of genetically encoded voltage indicators based on a chimeric voltage sensing domain. Front. Mol. Neurosci. 2014, 7, 78. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Barnes, S.; Knopfel, T. Mammalian cortical voltage imaging using genetically encoded voltage indicators: A review honoring professor amiram grinvald. Neurophotonics 2017, 4, 031214. [Google Scholar] [CrossRef] [Green Version]
- Clancy, K.B.; Orsolic, I.; Mrsic-Flogel, T.D. Locomotion-dependent remapping of distributed cortical networks. Nat. Neurosci. 2019, 22, 778–786. [Google Scholar] [CrossRef]
- MacDowell, C.J.; Buschman, T.J. Low-dimensional spatiotemporal dynamics underlie cortex-wide neural activity. Curr. Biol. 2020, 30, 2665–2680. [Google Scholar] [CrossRef]
- Musall, S.; Kaufman, M.T.; Juavinett, A.L.; Gluf, S.; Churchland, A.K. Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci. 2019, 22, 1677–1686. [Google Scholar] [CrossRef]
- Orsolic, I.; Rio, M.; Mrsic-Flogel, T.D.; Znamenskiy, P. Mesoscale cortical dynamics reflect the interaction of sensory evidence and temporal expectation during perceptual decision-making. Neuron 2021, 109, 1861–1875. [Google Scholar] [CrossRef]
- West, S.L.; Aronson, J.D.; Popa, L.S.; Feller, K.D.; Carter, R.E.; Chiesl, W.M.; Gerhart, M.L.; Shekhar, A.C.; Ghanbari, L.; Kodandaramaiah, S.B.; et al. Wide-field calcium imaging of dynamic cortical networks during locomotion. Cereb. Cortex 2022, 32, 2668–2687. [Google Scholar] [CrossRef]
- van Beest, E.H.; Mukherjee, S.; Kirchberger, L.; Schnabel, U.H.; van der Togt, C.; Teeuwen, R.R.M.; Barsegyan, A.; Meyer, A.F.; Poort, J.; Roelfsema, P.R.; et al. Mouse visual cortex contains a region of enhanced spatial resolution. Nat. Commun. 2021, 12, 4029. [Google Scholar] [CrossRef]
- Meyer, A.F.; Poort, J.; O’Keefe, J.; Sahani, M.; Linden, J.F. A head-mounted camera system integrates detailed behavioral monitoring with multichannel electrophysiology in freely moving mice. Neuron 2018, 100, 46–60. [Google Scholar] [CrossRef] [Green Version]
- Michaiel, A.M.; Abe, E.T.; Niell, C.M. Dynamics of gaze control during prey capture in freely moving mice. Elife 2020, 9. [Google Scholar] [CrossRef]
- Parker, P.R.L.; Brown, M.A.; Smear, M.C.; Niell, C.M. Movement-related signals in sensory areas: Roles in natural behavior. Trends Neurosci. 2020, 43, 581–595. [Google Scholar] [CrossRef]
- Eichenbaum, H. Prefrontal-hippocampal interactions in episodic memory. Nat. Rev. Neurosci. 2017, 18, 547–558. [Google Scholar] [CrossRef]
- Chen, G.; King, J.A.; Burgess, N.; O’Keefe, J. How vision and movement combine in the hippocampal place code. Proc. Natl. Acad. Sci. USA 2013, 110, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Save, E.; Cressant, A.; Thinus-Blanc, C.; Poucet, B. Spatial firing of hippocampal place cells in blind rats. J. Neurosci. 1998, 18, 1818–1826. [Google Scholar] [CrossRef] [Green Version]
- Mohajerani, M.H.; Chan, A.W.; Mohsenvand, M.; LeDue, J.; Liu, R.; McVea, D.A.; Boyd, J.D.; Wang, Y.T.; Reimers, M.; Murphy, T.H. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat. Neurosci. 2013, 16, 1426–1435. [Google Scholar] [CrossRef] [Green Version]
- Tambini, A.; Rimmele, U.; Phelps, E.A.; Davachi, L. Emotional brain states carry over and enhance future memory formation. Nat. Neurosci. 2017, 20, 271–278. [Google Scholar] [CrossRef]
- Kyweriga, M.; Sun, J.; Wang, S.; Kline, R.; Mohajerani, M.H. A large lateral craniotomy procedure for mesoscale wide-field optical imaging of brain activity. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Zavecz, Z.; Janacsek, K.; Simor, P.; Cohen, M.X.; Nemeth, D. Similarity of brain activity patterns during learning and subsequent resting state predicts memory consolidation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Akemann, W.; Mutoh, H.; Perron, A.; Park, Y.K.; Iwamoto, Y.; Knopfel, T. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J. Neurophysiol. 2012, 108, 2323–2337. [Google Scholar] [CrossRef] [Green Version]
- Carandini, M.; Shimaoka, D.; Rossi, L.F.; Sato, T.K.; Benucci, A.; Knopfel, T. Imaging the awake visual cortex with a genetically encoded voltage indicator. J. Neurosci. 2015, 35, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Scott, G.; Fagerholm, E.D.; Mutoh, H.; Leech, R.; Sharp, D.J.; Shew, W.L.; Knopfel, T. Voltage imaging of waking mouse cortex reveals emergence of critical neuronal dynamics. J. Neurosci. 2014, 34, 16611–16620. [Google Scholar] [CrossRef] [Green Version]
- Pachitariu, M.; Steinmetz, N.; Kadir, S.; Carandini, M.; Kenneth D., H. Kilosort: Realtime spike-sorting for extracellular electrophysiology with hundreds of channels. bioRxiv 2016, 061481. [Google Scholar] [CrossRef]
- Mathis, A.; Mamidanna, P.; Cury, K.M.; Abe, T.; Murthy, V.N.; Mathis, M.W.; Bethge, M. Deeplabcut: Markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 2018, 21, 1281–1289. [Google Scholar] [CrossRef]
- Meyer, A.F.; O’Keefe, J.; Poort, J. Two distinct types of eye-head coupling in freely moving mice. Curr. Biol. 2020, 30, 2116–2130. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa, R.; Song, C.; Knöpfel, T.; Battaglia, F. Combining Cortical Voltage Imaging and Hippocampal Electrophysiology for Investigating Global, Multi-Timescale Activity Interactions in the Brain. Int. J. Mol. Sci. 2022, 23, 6814. https://doi.org/10.3390/ijms23126814
Pedrosa R, Song C, Knöpfel T, Battaglia F. Combining Cortical Voltage Imaging and Hippocampal Electrophysiology for Investigating Global, Multi-Timescale Activity Interactions in the Brain. International Journal of Molecular Sciences. 2022; 23(12):6814. https://doi.org/10.3390/ijms23126814
Chicago/Turabian StylePedrosa, Rafael, Chenchen Song, Thomas Knöpfel, and Francesco Battaglia. 2022. "Combining Cortical Voltage Imaging and Hippocampal Electrophysiology for Investigating Global, Multi-Timescale Activity Interactions in the Brain" International Journal of Molecular Sciences 23, no. 12: 6814. https://doi.org/10.3390/ijms23126814
APA StylePedrosa, R., Song, C., Knöpfel, T., & Battaglia, F. (2022). Combining Cortical Voltage Imaging and Hippocampal Electrophysiology for Investigating Global, Multi-Timescale Activity Interactions in the Brain. International Journal of Molecular Sciences, 23(12), 6814. https://doi.org/10.3390/ijms23126814





