Antimicrobial Combined Action of Graphene Oxide and Light Emitting Diodes for Chronic Wound Management
Abstract
:1. Introduction
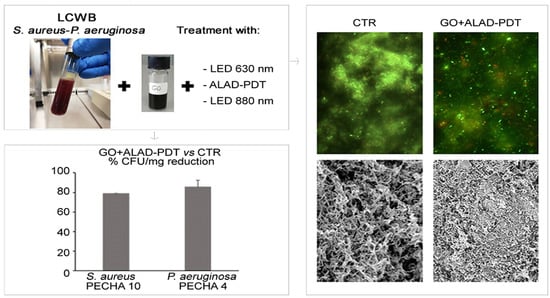
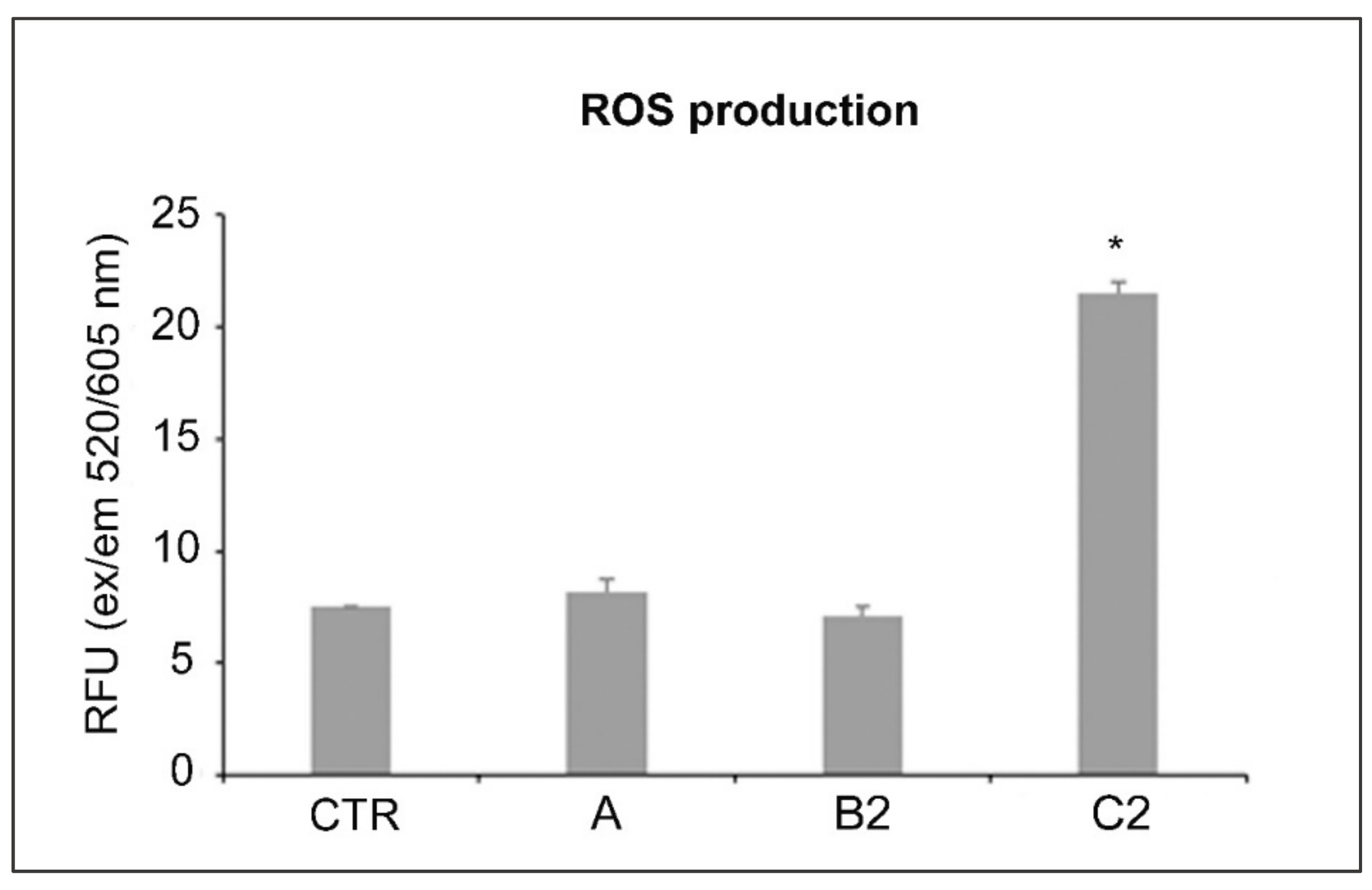
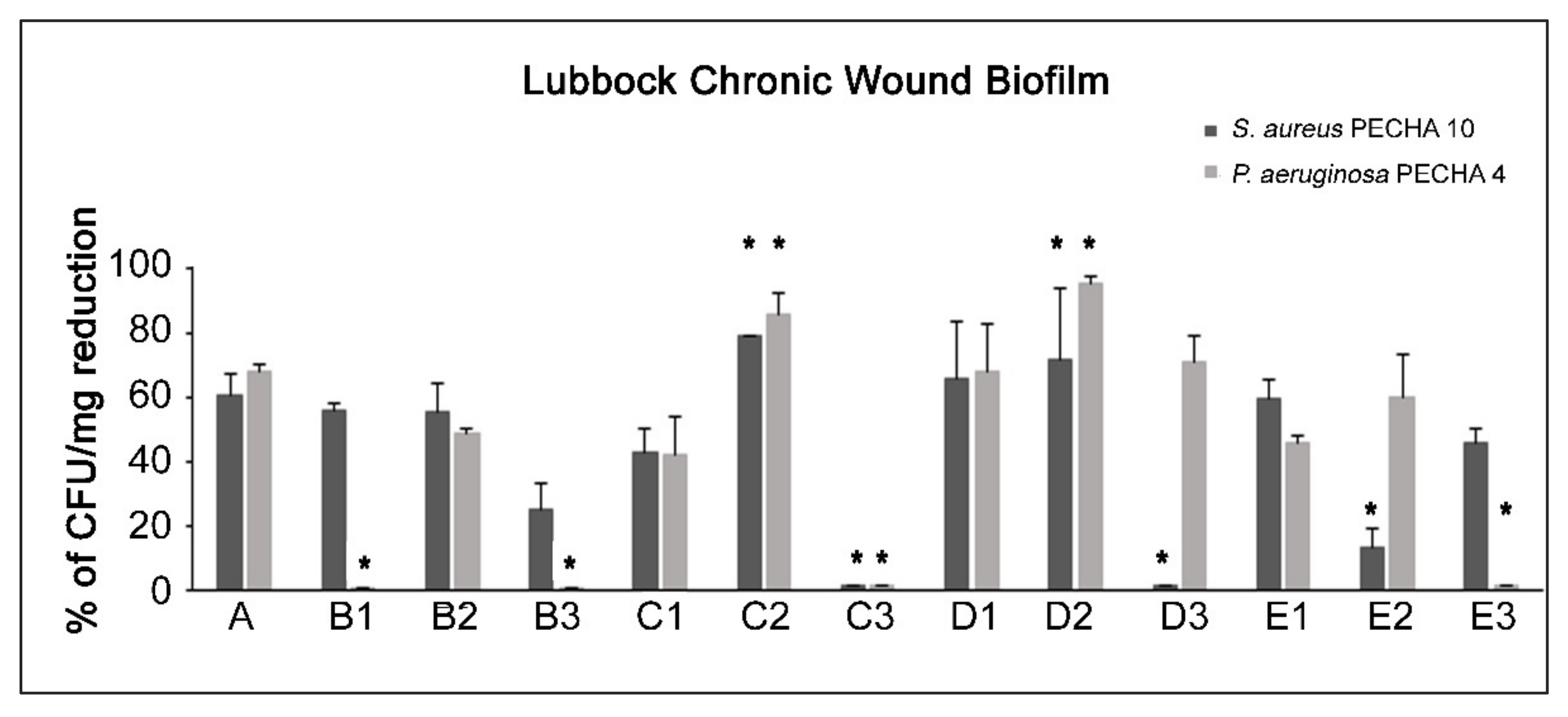
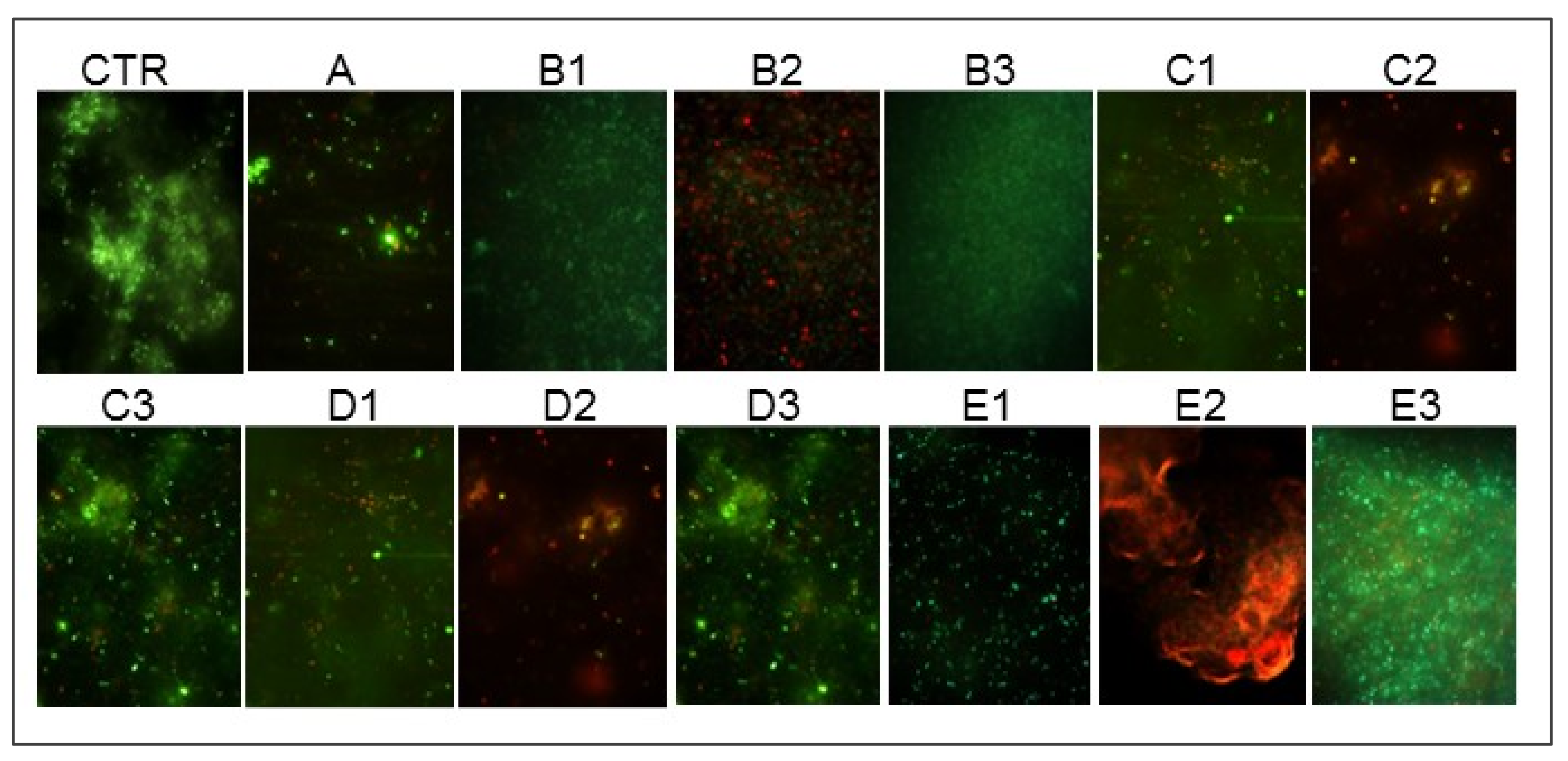
2. Results
3. Discussion
4. Materials and Methods
4.1. Bacterial Cultures
4.2. Substances
4.2.1. Preparation of Graphene Oxide Aqueous Dispersion
4.2.2. Aladent Gel
4.3. Light-Emitting Diode (LED) Devices
- An AlGaAs power LED device (TL-01), characterized by a 630 nm ± 10 nm FHWM nm wavelength, was used as light source (Alpha strumenti, Melzo, Milan, Italy). The handpiece constituted by one LED with a 6-mm diameter at the exit and a surface irradiance of 380 mW/cm2. During the experiments, the LED handpiece was mounted perpendicularly to the LCWB sample at a 0.5-mm distance. Irradiation was performed under a laminar flow hood in the dark, under aseptic conditions [20,28,42]. For the tests, 630 nm LED alone and plus ALAD (ALAD-PDT) were used.
4.4. Lubbock Chronic Wound Biofilm (LCWB) Model
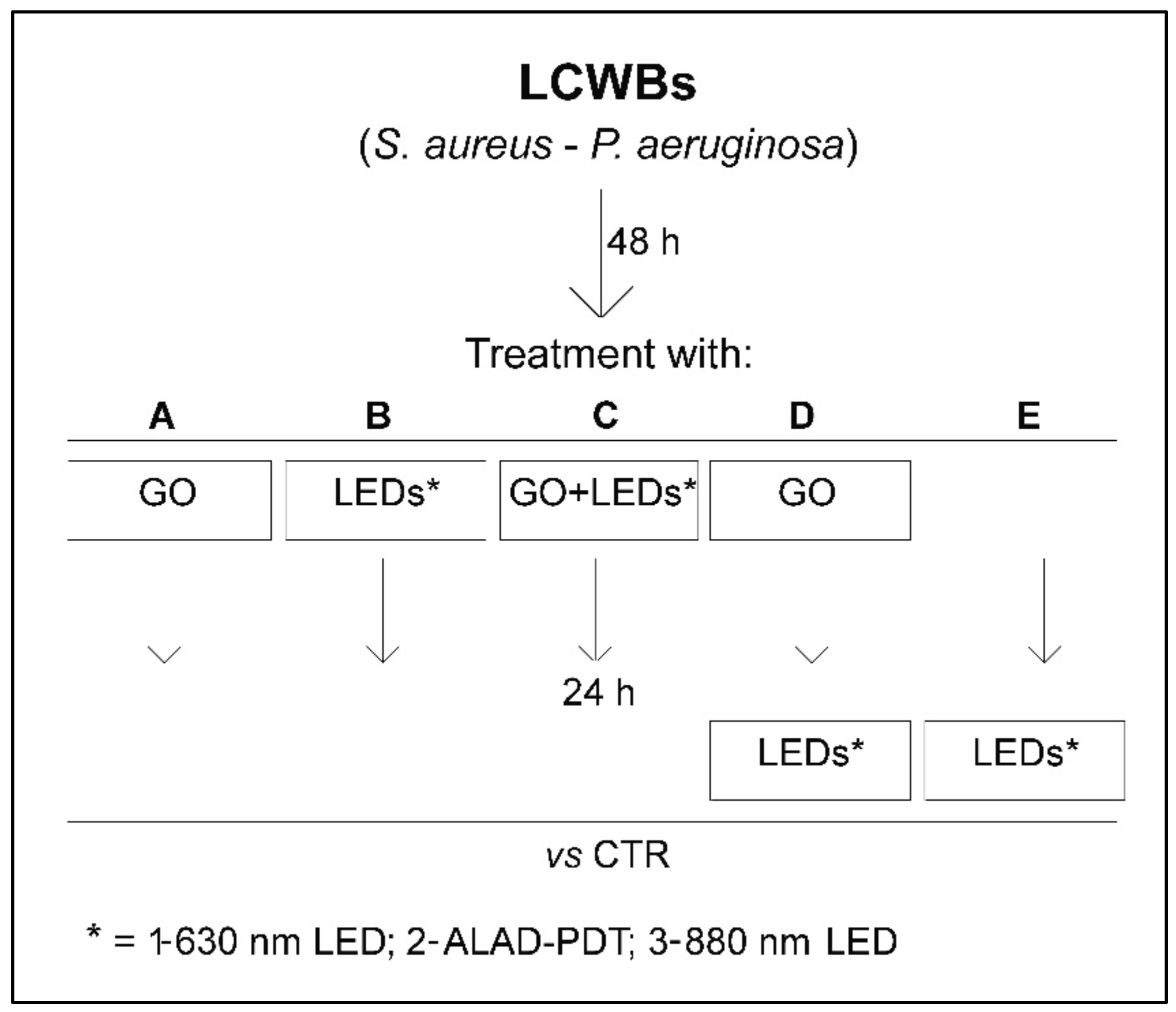
4.5. Effect of GO Alone and Combined with LEDs on LCWB Model
- B1: 630 nm LED
- B2: ALAD-PDT
- B3: 880 nm LED
- C1: 630 nm LED
- C2: ALAD-PDT
- C3: 880 nm LED
- D1: 630 nm LED
- D2: ALAD-PDT
- D3: 880 nm LED
- E1: 630 nm LED
- E2: ALAD-PDT
- E3: 880 nm LED
4.6. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.; Gómez, B.; McIntyre, M.; Dubick, M.; Christy, R.; Nicholson, S.; Burmeister, D. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giulio, M.; Di Lodovico, S.; Fontana, A.; Traini, T.; Di Campli, E.; Pilato, S.; D’Ercole, S.; Cellini, L. Graphene Oxide Affects Staphylococcus aureus and Pseudomonas aeruginosa Dual Species Biofilm in Lubbock Chronic Wound Biofilm Model. Sci. Rep. 2020, 10, 18525. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, S.; Clinton, A.; Fowler, H.; Everett, J.; Horswill, A.R.; Rumbaugh, K.P. Synergistic Interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro Wound Model. Infect. Immun. 2014, 82, 4718–4728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orazi, G.; O’Toole, G.A. Pseudomonas aeruginosa Alters Staphylococcus aureus Sensitivity to Vancomycin in a Biofilm Model of Cystic Fibrosis Infection. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batoni, G.; Maisetta, G.; Esin, S. Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections. Int. J. Mol. Sci. 2021, 22, 482. [Google Scholar] [CrossRef]
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Møller, K.; Jørgensen, B.; Andersen, A.S.; Krogfelt, K.A.; Givskov, M.; Tolker-Nielsen, T. Nonrandom Distribution of Pseudomonas aeruginosa and Staphylococcus aureus in Chronic Wounds. J. Clin. Microbiol. 2009, 47, 4084–4089. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Dowd, S.E.; Smith, E.; Rhoads, D.D.; Wolcott, R.D. In Vitro Multispecies Lubbock Chronic Wound Biofilm Model. Wound Rep. Regen. 2008, 16, 805–813. [Google Scholar] [CrossRef]
- Kucera, J.; Sojka, M.; Pavlik, V.; Szuszkiewicz, K.; Velebny, V.; Klein, P. Multispecies Biofilm in an Artificial Wound Bed—A Novel Model for in Vitro Assessment of Solid Antimicrobial Dressings. J. Microbiol. Methods 2014, 103, 18–24. [Google Scholar] [CrossRef]
- Thaarup, I.C.; Bjarnsholt, T. Current in Vitro Biofilm-Infected Chronic Wound Models for Developing New Treatment Possibilities. Adv. Wound Care 2020. [Google Scholar] [CrossRef]
- Ganesh, K.; Sinha, M.; Mathew-Steiner, S.S.; Das, A.; Roy, S.; Sen, C.K. Chronic Wound Biofilm Model. Adv. Wound Care 2015, 4, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.T. Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications. Molecules 2021, 26, 2797. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.P.; Marangoni, V.S.; de Faria, C.G.; Leite, I.S.; Silva, C.d.C.C.E.; Maroneze, C.M.; Pereira-da-Silva, M.A.; Bagnato, V.S.; Inada, N.M. Graphene Oxide Mediated Broad-Spectrum Antibacterial Based on Bimodal Action of Photodynamic and Photothermal Effects. Front. Microbiol. 2020, 10, 2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ercole, S.; Di Fermo, P.; Di Giulio, M.; Di Lodovico, S.; Di Campli, E.; Scarano, A.; Tripodi, D.; Cellini, L.; Petrini, M. Near-infrared NIR Irradiation and Sodium Hypochlorite: An Efficacious Association to Counteract the Enterococcus faecalis Biofilm in Endodontic Infections. J. Photochem. Photobiol. B 2020, 210, 111989. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Calderhead, R.G. Is Light-Emitting Diode Phototherapy (LED-LLLT) Really Effective? Laser Ther. 2011, 20, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.; Du, L.; Zubair, M.; Subedi, S.; Ullah, A.; Roopesh, M.S. Applications of Light-Emitting Diodes (LEDs) in Food Processing and Water Treatment. Food Eng. Rev. 2020, 12, 268–289. [Google Scholar] [CrossRef]
- Bekmukhametova, A.; Ruprai, H.; Hook, J.M.; Mawad, D.; Houang, J.; Lauto, A. Photodynamic Therapy With Nanoparticles to Combat Microbial Infection and Resistance. Nanoscale 2020, 12, 21034–21059. [Google Scholar] [CrossRef]
- Yang, D.; Lv, X.; Xue, L.; Yang, N.; Hu, Y.; Weng, L.; Fu, N.; Wang, L.; Dong, X. A Lipase-Responsive Antifungal Nanoplatform for Synergistic Photodynamic/Photothermal/Pharmaco-Therapy of Azole-Resistant Candida albicans Infections. Chem. Commun. 2019, 55, 15145–15148. [Google Scholar] [CrossRef]
- Ghate, V.; Leong, A.L.; Kumar, A.; Bang, W.S.; Zhou, W.; Yuk, H.-G. Enhancing the Antibacterial Effect of 461 and 521 Nm Light Emitting Diodes on Selected Foodborne Pathogens in Trypticase Soy Broth by Acidic and Alkaline PH Conditions. Food Microbiol. 2015, 48, 49–57. [Google Scholar] [CrossRef]
- Radunović, M.; Petrini, M.; Vlajic, T.; Iezzi, G.; Di Lodovico, S.; Piattelli, A.; D’Ercole, S. Effects of a Novel Gel Containing 5-Aminolevulinic Acid and Red LED against Bacteria Involved in Peri-Implantitis and Other Oral Infections. J. Photochem. Photobiol. B 2020, 205, 111826. [Google Scholar] [CrossRef]
- W.H.O.; Traditional Medicine Programme. Regulatory Situation of Herbal Medicines : A Worldwide Review. apps.who.int. 1998. [Google Scholar]
- Maslova, E.; Eisaiankhongi, L.; Sjöberg, F.; McCarthy, R.R. Burns and Biofilms: Priority Pathogens and In Vivo Models. NPJ Biofilms Microbiomes 2021, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Amos-Tautua, B.; Songca, S.; Oluwafemi, O. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef] [Green Version]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef]
- D’Ercole, S.; Spoto, G.; Trentini, P.; Tripodi, D.; Petrini, M. In Vitro Inactivation of Enterococcus faecalis with a Led Device. J. Photochem. Photobiol. B 2016, 160, 172–177. [Google Scholar] [CrossRef]
- Harris, F.; Pierpoint, L. Photodynamic Therapy Based on 5-Aminolevulinic Acid and Its Use as an Antimicrobial Agent. Med. Res. Rev. 2011, 32, 1292–1327. [Google Scholar] [CrossRef]
- Petrini, M.; Pierfelice, T.V.; D’Amico, E.; Carlesi, T.; Iezzi, G.; D’Arcangelo, C.; Di Lodovico, S.; Piattelli, A.; D’Ercole, S. Comparison Between Single and Multi-LED Emitters for Photodynamic Therapy: An in Vitro Study on Enterococcus Faecalis and Human Gingival Fibroblasts. Int. J. Environ. Res. 2022, 19, 3048. [Google Scholar] [CrossRef]
- Elias, L.; Taengua, R.; Frígols, B.; Salesa, B.; Serrano-Aroca, Á. Carbon Nanomaterials and LED Irradiation as Antibacterial Strategies against Gram-Positive Multidrug-Resistant Pathogens. Int. J. Mol. Sci. 2019, 20, 3603. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, H.; Pourhajibagher, M.; Alikhani, M.Y.; Bahador, A. The Effect of Antimicrobial Photodynamic Therapy on the Expression of Biofilm Associated Genes in Staphylococcus aureus Strains Isolated from Wound Infections in Burn Patients. Photodiagn. Photodyn. Ther. 2019, 25, 406–413. [Google Scholar] [CrossRef]
- Tan, Y.; Cheng, Q.; Yang, H.; Li, H.; Gong, N.; Liu, D.; Wu, J.; Lei, X. Effects of ALA-PDT on Biofilm Structure, Virulence Factor Secretion, and QS in Pseudomonas aeruginosa. Photodiagn. Photodyn. Ther. 2018, 24, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Trentini, P.; Tripodi, D.; Spoto, G.; D’Ercole, S. In Vitro Antimicrobial Activity of LED Irradiation on Pseudomonas aeruginosa. J. Photochem. Photobiol. B 2017, 168, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Probst-Rüd, S.; McNeill, K.; Ackermann, M. Thiouridine Residues in TRNAs Are Responsible for a Synergistic Effect of UVA and UVB Light in Photoinactivation of Escherichia coli. Environ. Microbiol. 2016, 19, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, H.; Tian, Q.; Zheng, G.; Hu, Y.; Fu, Y.; Tan, H. Effects of 5-Aminolevulinic Acid–Mediated Photodynamic Therapy on Antibiotic-Resistant Staphylococcal Biofilm: An in Vitro Study. J. Surg. Res. 2013, 184, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Tan, Y.; Zhang, W.; Yang, W.; Luo, J.; Chen, L.; Liu, H.; Yang, G.; Lei, X. Effects of ALA-PDT on the Healing of Mouse Skin Wounds Infected With Pseudomonas aeruginosa and Its Related Mechanisms. Front. Cell Dev. Biol. 2020, 8, 585132. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Bacchetti, T.; D’Ercole, S.; Covone, S.; Petrini, M.; Di Giulio, M.; Di Fermo, P.; Diban, F.; Ferretti, G.; Cellini, L. Complex Chronic Wound Biofilms Are Inhibited in Vitro by the Natural Extract of Capparis Spinose. Front. Microbiol. 2022, 13, 832919. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Di Stefano, A.; D’Aurizio, E.; Trubiani, O.; Grande, R.; Di Campli, E.; Di Giulio, M.; Di Bartolomeo, S.; Sozio, P.; Iannitelli, A.; Nostro, A.; et al. Viscoelastic Properties Of Staphylococcus aureus and Staphylococcus epidermidis mono-Microbial Biofilms. Microb. Biotechnol. 2009, 2, 634–641. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Chao, X.; Fei, M.; Dai, Y.; Liu, B. Analysis of S. epidermidis IcaA and IcaD Genes by Polymerase Chain Reaction and Slime Production: A Case Control Study. BMC Infect. Dis. 2013, 13, 242. [Google Scholar] [CrossRef] [Green Version]
- Lanotte, P. Genetic Features of Pseudomonas aeruginosa Isolates from Cystic Fibrosis Patients Compared with Those of Isolates from Other Origins. J. Med. Microbiol. 2004, 53, 73–81. [Google Scholar] [CrossRef]
- Fusco, L.; Garrido, M.; Martín, C.; Sosa, S.; Ponti, C.; Centeno, A.; Alonso, B.; Zurutuza, A.; Vázquez, E.; Tubaro, A.; et al. Skin Irritation Potential of Graphene-Based Materials Using a Non-Animal Test. Nanoscale 2020, 12, 610–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrini, M.; Di Lodovico, S.; Iezzi, G.; Cellini, L.; Tripodi, D.; Piattelli, A.; D’Ercole, S. Photodynamic Antibiofilm and Antibacterial Activity of a New Gel with 5-Aminolevulinic Acid on Infected Titanium Surfaces. Biomedicines 2022, 10, 572. [Google Scholar] [CrossRef] [PubMed]
- Di Fermo, P.; Ciociola, T.; Di Lodovico, S.; D’Ercole, S.; Petrini, M.; Giovati, L.; Conti, S.; Di Giulio, M.; Cellini, L. Antimicrobial Peptide L18R Displays a Modulating Action against Inter-Kingdom Biofilms in the Lubbock Chronic Wound Biofilm Model. Microorganisms 2021, 9, 1779. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lodovico, S.; Diban, F.; Di Fermo, P.; Petrini, M.; Fontana, A.; Di Giulio, M.; Piattelli, A.; D’Ercole, S.; Cellini, L. Antimicrobial Combined Action of Graphene Oxide and Light Emitting Diodes for Chronic Wound Management. Int. J. Mol. Sci. 2022, 23, 6942. https://doi.org/10.3390/ijms23136942
Di Lodovico S, Diban F, Di Fermo P, Petrini M, Fontana A, Di Giulio M, Piattelli A, D’Ercole S, Cellini L. Antimicrobial Combined Action of Graphene Oxide and Light Emitting Diodes for Chronic Wound Management. International Journal of Molecular Sciences. 2022; 23(13):6942. https://doi.org/10.3390/ijms23136942
Chicago/Turabian StyleDi Lodovico, Silvia, Firas Diban, Paola Di Fermo, Morena Petrini, Antonella Fontana, Mara Di Giulio, Adriano Piattelli, Simonetta D’Ercole, and Luigina Cellini. 2022. "Antimicrobial Combined Action of Graphene Oxide and Light Emitting Diodes for Chronic Wound Management" International Journal of Molecular Sciences 23, no. 13: 6942. https://doi.org/10.3390/ijms23136942