In Vitro Wound Healing Properties of Novel Acidic Treatment Regimen in Enhancing Metabolic Activity and Migration of Skin Cells
Abstract
:1. Introduction
2. Results
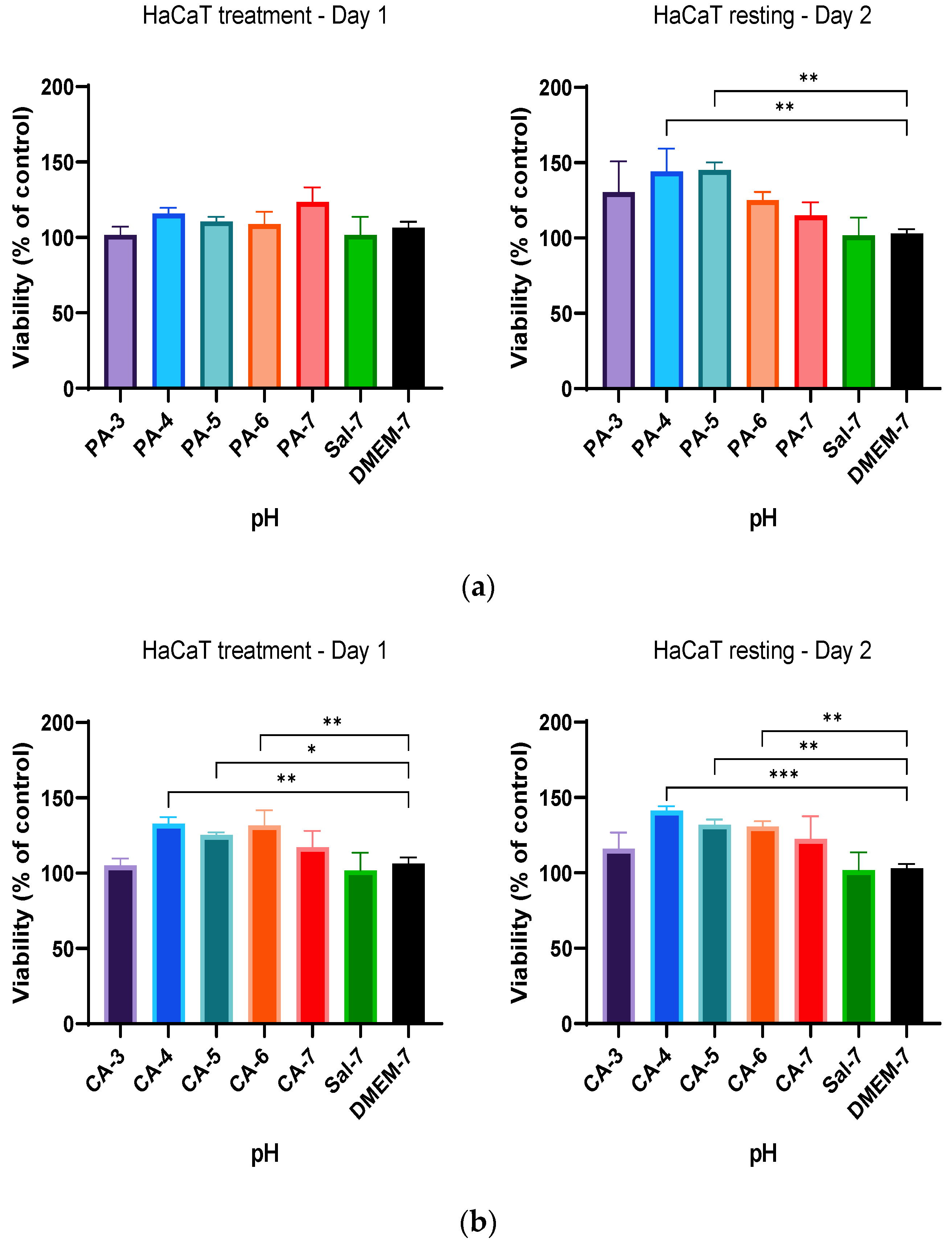
2.1. Low Ionic Strength Increases Metabolic Activity of Keratinocytes
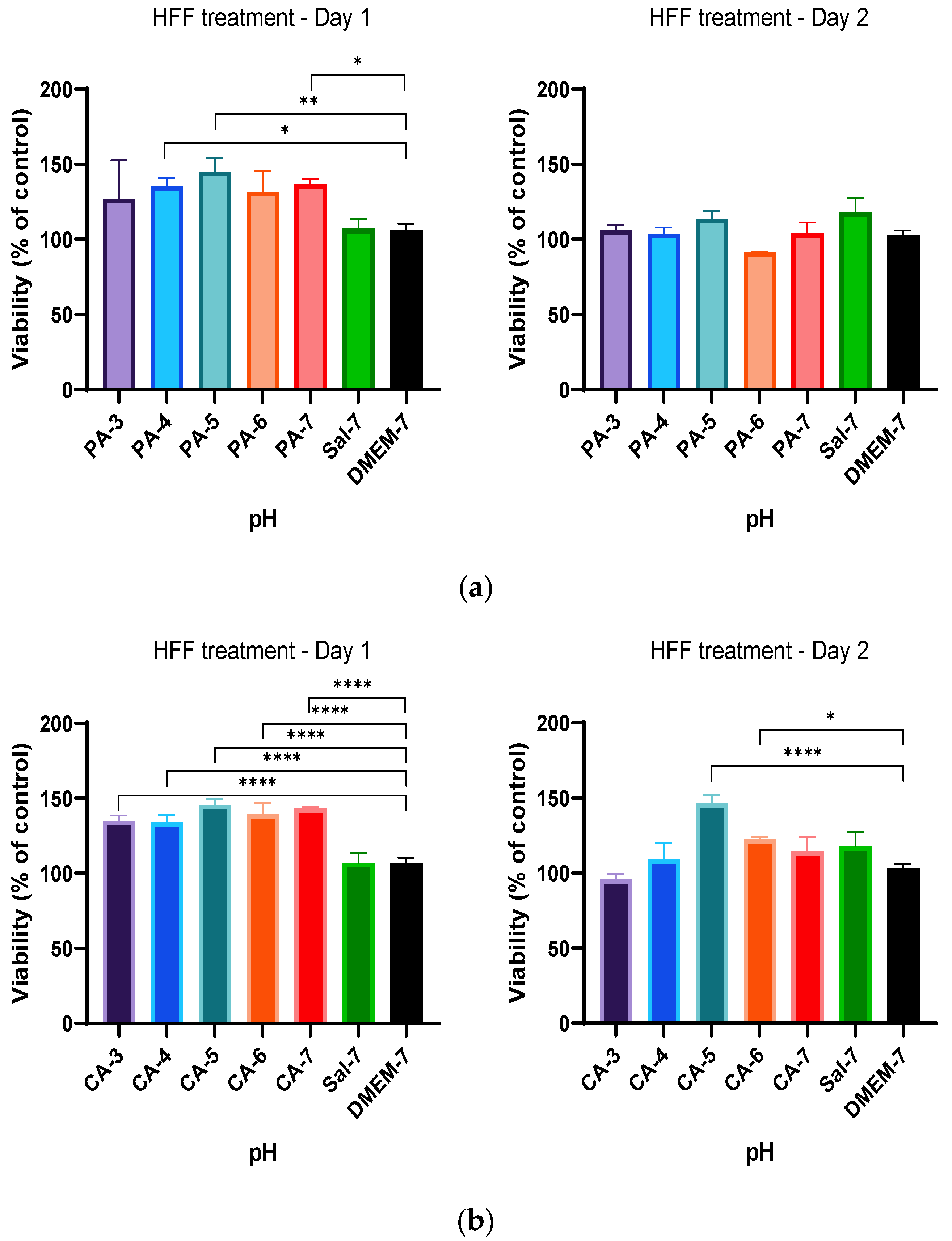
2.2. Acidification Enhances Metabolic Activity of Keratinocytes and Fibroblasts
2.3. Metabolic Activity Influenced by pH and Time-Dependent Acidification
2.4. Treatment Regimen C Improves the Proliferation Rate
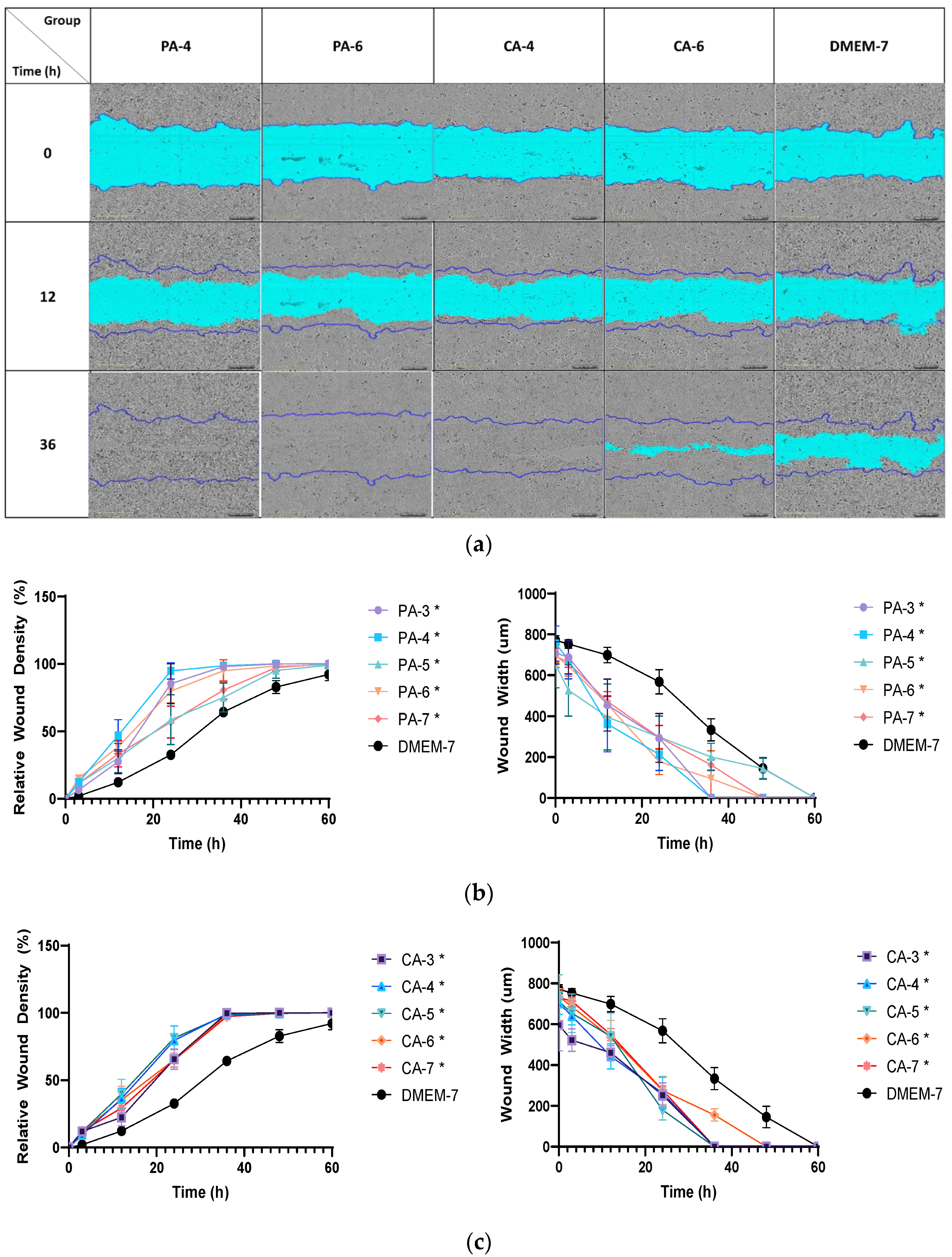
2.5. Acidification Increases Migration Rate of Keratinocytes and Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Buffer Preparation
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Cell Migration Assay
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaufman, T.; Eichenlaub, E.H.; Angel, M.F.; Levin, M.; Futrell, J.W. Topical acidification promotes healing of experimental deep partial thickness skin burns: A ran-domized double-blind preliminary study. Burns 1985, 12, 84–90. [Google Scholar] [CrossRef]
- Lengheden, A.; Jansson, L. PH effects on experimental wound healing of human fibroblasts in vitro. Eur. J. Oral Sci. 1995, 103, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ohgoda, O.; Sakai, A.; Koga, H.; Kanai, K.; Miyazaki, T.; Niwano, Y. Fibroblast-migration in a wound model of ascorbic acid-supplemented three-dimensional culture system: The effects of cytokines and malotilate, a new wound healing stimulant, on cell-migration. J. Dermatol. Sci. 1998, 17, 123–131. [Google Scholar] [CrossRef]
- Demetriou, A.A.; Levenson, S.M.; Rettura, G.; Seifter, E. Vitamin A and retinoic acid: Induced fibroblast differentiation in vitro. Surgery 1985, 98, 931–934. [Google Scholar]
- Duman, N.; Duman, R.; Tosun, M.; Akıcı, M.; Göksel, E.; Gökçe, B.; Alagöz, O. Topical folinic acid enhances wound healing in rat model. Adv. Med. Sci. 2018, 63, 347–352. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef]
- Hsu, Y.-Y.; Liu, K.-L.; Yeh, H.-H.; Lin, H.-R.; Wu, H.-L.; Tsai, J.-C. Sustained release of recombinant thrombomodulin from cross-linked gelatin/hyaluronic acid hydrogels potentiate wound healing in diabetic mice. Eur. J. Pharm. Biopharm. 2019, 135, 61–71. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Baier, R. Surface Chemistry in Epidermal Repair. In Epidermal Wound Healing; Year Book Medical Publishers, Inc.: Chicago, IL, USA, 1972; pp. 27–48. [Google Scholar]
- Bruno, M.; Trucchi, B.; Burlando, B.; Ranzato, E.; Martinotti, S.; Akkol, E.K.; Süntar, I.; Keleş, H.; Verotta, L. (+)-Usnic acid enamines with remarkable cicatrizing properties. Bioorg. Med. Chem. 2013, 21, 1834–1843. [Google Scholar] [CrossRef]
- Gao, Y.; Zhaoyu, L.; Xiangming, F.; Chunyi, L.; Jiayu, P.; Lu, S.; Jitao, C.; Liangcai, C.; Jifang, L. Abietic acid attenuates allergic airway inflammation in a mouse allergic asthma model. Int. Immunopharmacol. 2016, 38, 261–266. [Google Scholar] [CrossRef]
- González, M.A.; Pérez-Guaita, D.; Correa-Royero, J.; Zapata, B.; Agudelo, L.; Mesa-Arango, A.; Betancur-Galvis, L. Synthesis and biological evaluation of dehydroabietic acid derivatives. Eur. J. Med. Chem. 2010, 45, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, M.; Xu, F.; Chen, J.; Ma, S. Acceleration of wound healing activity with syringic acid in streptozotocin induced diabetic rats. Life Sci. 2019, 233, 116728. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Kumar, C.S. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Efficacies of fumaric acid and its mono and di-methyl esters in rodent models for analgesics and an-ti-inflammatory agents. EC Pharm. Sci. 2015, 1, 73–85. [Google Scholar]
- Basha, S.; Ghosh, S.; Vinothkumar, K.; Ramesh, B.; Kumari, P.H.P.; Mohan, K.M.; Sukumar, E. Fumaric acid incorporated Ag/agar-agar hybrid hydrogel: A multifunctional avenue to tackle wound healing. Mater. Sci. Eng. C 2020, 111, 110743. [Google Scholar] [CrossRef] [PubMed]
- Leveen, H.H.; Falk, G.E.R.A.L.D.; Borek, B.; Diaz, C.A.R.L.O.S.; Lynfield, Y.; Wynkoop, B.J.; Mabunda, G.A.; Rubricius, J.L.; Christoudias, G.C. Chemical acidification of wounds. An adjuvant to healing and the unfavorable action of alkalinity and ammonia. Ann. Surg. 1973, 178, 745. [Google Scholar] [CrossRef]
- Jeong, H.S.; Lee, B.H.; Lee, H.K.; Kim, H.S.; Moon, M.S.; Suh, I.S. Negative Pressure Wound Therapy of Chronically Infected Wounds Using 1% Acetic Acid Irrigation. Arch. Plast. Surg. 2015, 42, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Liang, H.; Maulu, S.; Ge, X.; Ren, M.; Xie, J.; Xi, B. Dietary phosphorus affects growth, glucolipid metabolism, antioxidant activity and immune status of juvenile blunt snout bream (Megalobrama amblycephala). Anim. Feed Sci. Technol. 2021, 274, 114896. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Liu, Y.J.; Mai, K.S.; Tian, L.X. Effect of dietary phosphorus levels on growth, body composition, muscle and bone mineral concen-trations for orange-spotted grouper Epinephelus coioides reared in floating cages. J. World Aquac. Soc. 2004, 35, 427–435. [Google Scholar] [CrossRef]
- Chen, X.; Lv, Q.; Liu, Y.; Deng, W. Effect of Food Additive Citric Acid on The Growth of Human Esophageal Carcinoma Cell Line EC109. Cell J. 2016, 18, 493–502. [Google Scholar] [CrossRef]
- Bodner, G.M. Metabolism Part II: The tricarboxylic acid (TCA), citric acid, or Krebs cycle. J. Chem. Educ. 1986, 63, 673. [Google Scholar] [CrossRef]
- Schreml, S.; Meier, R.J.; Kirschbaum, M.; Kong, S.C.; Gehmert, S.; Felthaus, O.; Küchler, S.; Sharpe, J.R.; Wöltje, K.; Weiß, K.T.; et al. Luminescent dual sensors reveal extracellular pH-gradients and hypoxia on chronic wounds that disrupt epidermal repair. Theranostics 2014, 4, 721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lönnqvist, S.; Emanuelsson, P.; Kratz, G. Influence of acidic pH on keratinocyte function and re-epithelialisation of human in vitro wounds. J. Plast. Surg. Hand Surg. 2015, 49, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reep-ithelialization: In vitro and in vivo study. Wound Repair Regen. 2017, 25, 260–269. [Google Scholar] [CrossRef]
- Li, N.-B.; Xu, W.-H.; Zhao, J.-H.; Xiao, G.-Y.; Lu, Y.-P. The significant influence of ionic concentrations and immersion temperatures on deposition behaviors of hydroxyapatite on alkali- and heat-treated titanium in simulated body fluid. Thin Solid Film. 2018, 646, 163–172. [Google Scholar] [CrossRef]
- Gethin, G.T.; Cowman, S.; Conroy, R.M. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int. Wound J. 2008, 5, 185–194. [Google Scholar] [CrossRef]
- Pipelzadeh, M.H.; Naylor, I.L. The in vitro enhancement of rat myofibroblast contractility by alterations to the pH of the physiological solution. Eur. J. Pharmacol. 1998, 357, 257–259. [Google Scholar] [CrossRef]
- Chang, P.C.; Sulik, G.I.; Soong, H.K.; Parkinson, W.C. Galvanotropic and galvanotaxic responses of corneal endothelial cells. J. Formos. Med. Assoc. 1996, 95, 623–627. [Google Scholar]
- Soong, H.K.; Parkinson, W.C.; Bafna, S.; Sulik, G.L.; Huang, S.C. Movements of cultured corneal epithelial cells and stromal fibroblasts in electric fields. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2278–2282. [Google Scholar]





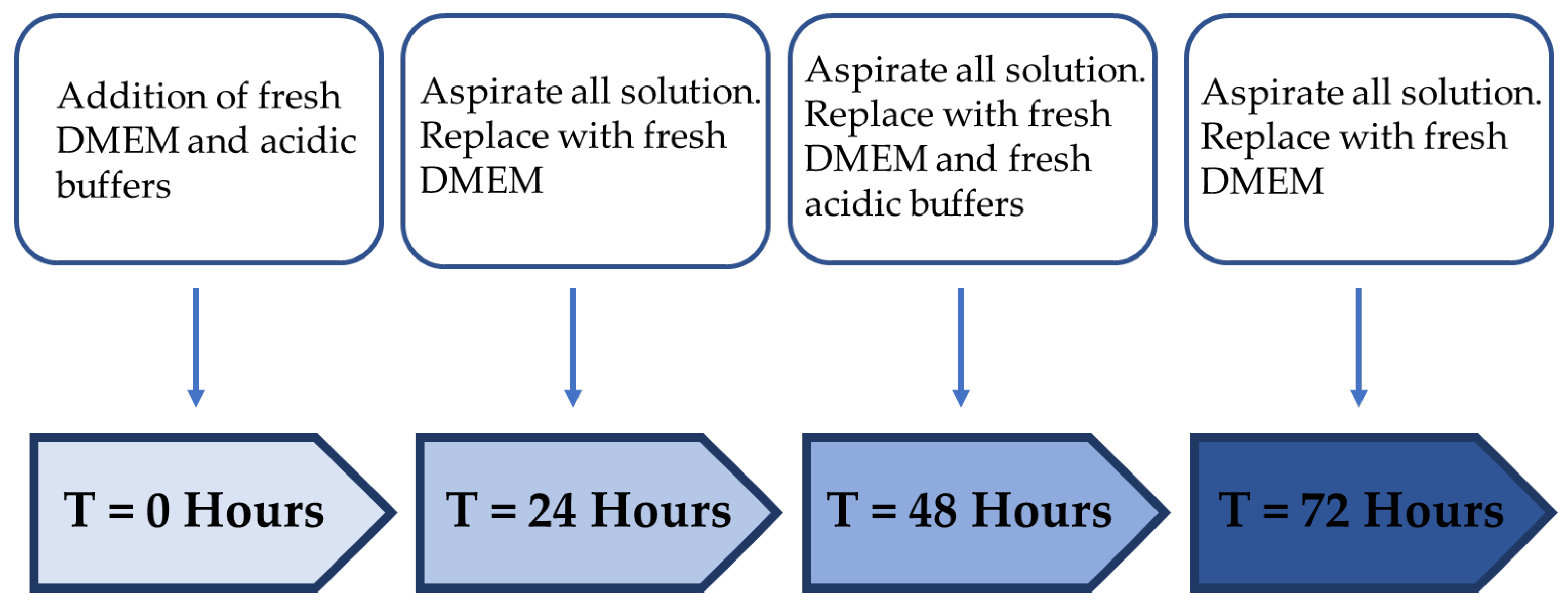
| Treatment Regimen | Protocol | |
|---|---|---|
| Treatment Period (Hour) | Resting Period (Hour) | |
| A | 24 | - |
| B | 12 | 24 |
| C | 24 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, P.; Song, Y.; Yang, G.N.; Cowin, A.J.; Garg, S. In Vitro Wound Healing Properties of Novel Acidic Treatment Regimen in Enhancing Metabolic Activity and Migration of Skin Cells. Int. J. Mol. Sci. 2022, 23, 7188. https://doi.org/10.3390/ijms23137188
Sim P, Song Y, Yang GN, Cowin AJ, Garg S. In Vitro Wound Healing Properties of Novel Acidic Treatment Regimen in Enhancing Metabolic Activity and Migration of Skin Cells. International Journal of Molecular Sciences. 2022; 23(13):7188. https://doi.org/10.3390/ijms23137188
Chicago/Turabian StyleSim, Pivian, Yunmei Song, Gink N. Yang, Allison J. Cowin, and Sanjay Garg. 2022. "In Vitro Wound Healing Properties of Novel Acidic Treatment Regimen in Enhancing Metabolic Activity and Migration of Skin Cells" International Journal of Molecular Sciences 23, no. 13: 7188. https://doi.org/10.3390/ijms23137188
APA StyleSim, P., Song, Y., Yang, G. N., Cowin, A. J., & Garg, S. (2022). In Vitro Wound Healing Properties of Novel Acidic Treatment Regimen in Enhancing Metabolic Activity and Migration of Skin Cells. International Journal of Molecular Sciences, 23(13), 7188. https://doi.org/10.3390/ijms23137188








