The Contribution of Wnt Signaling to Vascular Complications in Type 2 Diabetes Mellitus
Abstract
:1. Wnt Signaling Pathway in the Vasculature
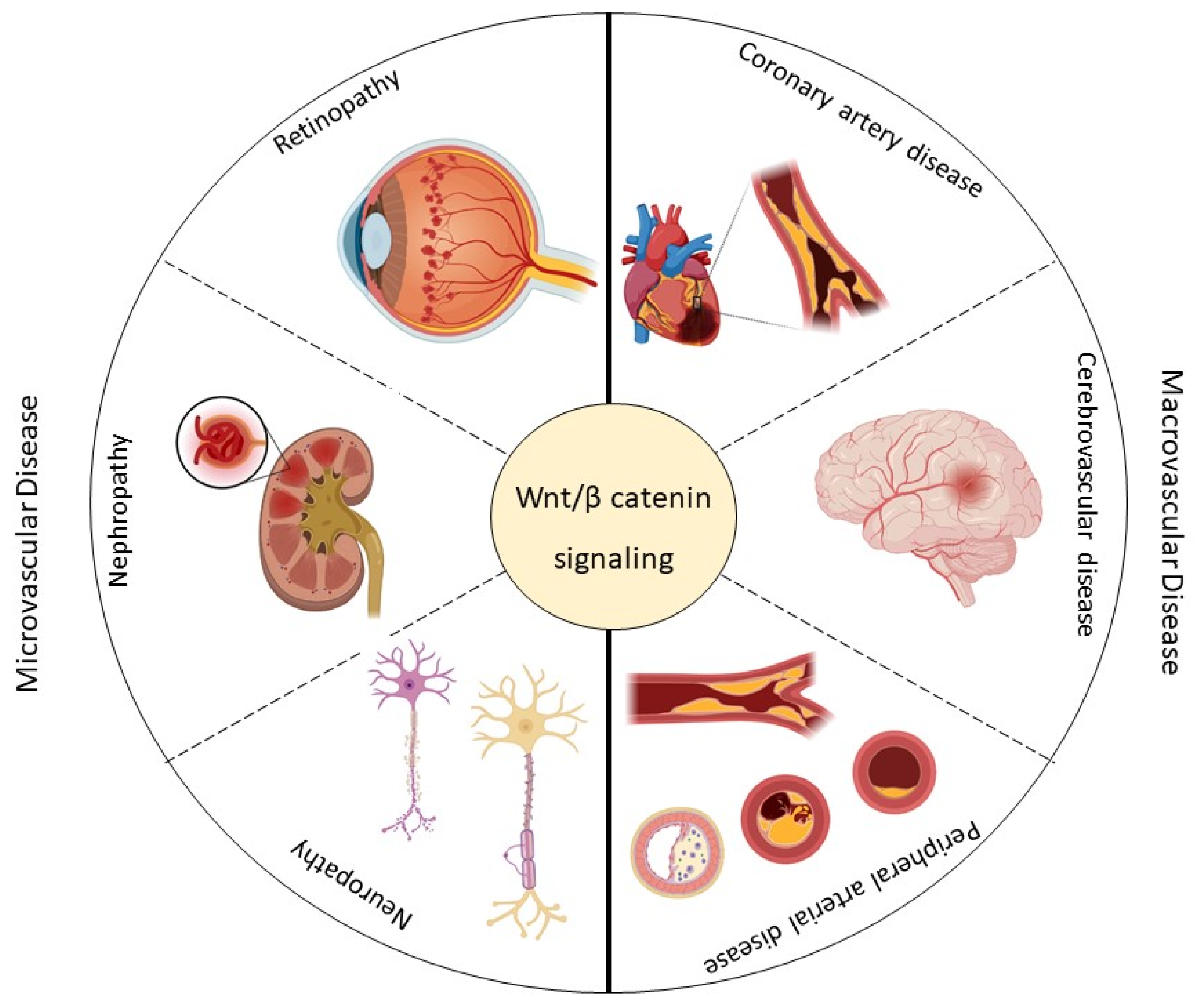
2. Vascular Complications of Type 2 Diabetes Mellitus
3. Wnt Pathway and Microvascular Disease in Type 2 Diabetes Mellitus
3.1. Retinopathy
3.2. Diabetic Kidney Disease
3.3. Neuropathy
4. Wnt Pathway and Macrovascular Disease in Type 2 Diabetes Mellitus
4.1. Coronary Artery Disease
4.2. Cerebrovascular Disease
4.3. Peripheral Arterial Disease
5. Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer disease |
| ADR | Adriamycin nephropathy |
| CAD | Coronary artery disease |
| CVD | Cardiovascular disease |
| CKD | Chronic kidney disease |
| Dkk | Dickkopf |
| CKD-MBD | CKD bone-mineral disorder syndrome |
| DKD | Diabetic kidney disease |
| DPN | Diabetic peripheral neuropathy |
| EC | Endothelial cell |
| ER | Endoplasmic reticulum |
| ESRD | End-stage renal disease |
| FRP | Follistatin-related protein |
| Fzd | Frizzled |
| FOXO | Forkhead box O |
| GLP-1 | Glucagon-like peptide-1 |
| IMT | Intima-media thickness |
| LDL | Low-density lipoprotein |
| LRP5 | LDL receptor-related protein |
| MCAO | Middle cerebral artery occlusion |
| mTOR | Mammalian target of rapamycin |
| PAD | Peripheral arterial disease |
| PAOD | Peripheral arterial occlusive disease |
| PPARs | peroxisome proliferator-activated receptors |
| PEDF | Pigment epithelium-derived factor |
| RAS | Renin–angiotensin–aldosterone system |
| ROS | Reactive oxygen species |
| SCAI | Spinal cord area index |
| SM | Salvia miltiorrhiza |
| SMC | Smooth muscle cell |
| STAT | Signal transducer and activator of transcription 3 |
| TCF/LEF | T-cell factor/lymphoid enhancer factor family |
| T2DM | Type 2 diabetes mellitus |
| TG | Triglycerides |
| VSMC | Vascular smooth muscle cell |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| VLN | Very LDL receptor extracellular domain |
| Wnt | Wingless-Int |
References
- Reis, M.; Liebner, S. Wnt signaling in the vasculature. Exp. Cell Res. 2013, 319, 1317–1323. [Google Scholar] [CrossRef]
- Marinou, K.; Christodoulides, C.; Antoniades, C.; Koutsilieris, M. Wnt signaling in cardiovascular physiology. Trends Endocrinol. Metab. 2012, 23, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Freese, J.L.; Pino, D.; Pleasure, S.J. Wnt signaling in development and disease. Neurobiol. Dis. 2010, 38, 148–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansson, L.; Kim, G.S.; Cheng, A.G. Making sense of Wnt signaling—Linking hair cell regeneration to development. Front. Cell. Neurosci. 2015, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, A.; Bellone, F.; Morabito, N.; Corica, F. Sclerostin and vascular pathophysiology. Int. J. Mol. Sci. 2020, 21, 4779. [Google Scholar] [CrossRef]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.N.; Komm, B.S.; Javed, A.; Van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef] [Green Version]
- Bundy, K.; Boone, J.; Simpson, C.L. Wnt signaling in vascular calcification. Front. Cardiovasc. Med. 2021, 8, 3–8. [Google Scholar] [CrossRef]
- Komori, T. Regulation of proliferation, differentiation and functions of osteoblasts by runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [Green Version]
- Duan, P.; Bonewald, L. The role of the Wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Matsumoto, T. Sclerostin: From bench to bedside. J. Bone Miner. Metab. 2021, 39, 332–340. [Google Scholar] [CrossRef]
- Gay, A.; Towler, D.A.; Wilson, M.E.; Opin, C.; Author, L. Wnt signaling in cardiovascular disease: Opportunities and challenges HHS public access author manuscript. Curr. Opin. Lipidol. 2017, 28, 387–396. [Google Scholar] [CrossRef] [PubMed]
- aFGF Alleviates Diabetic Endothelial Dysfunction by Decreasing Oxidative Stress via Wnt/β-Catenin-Mediated Upregulation of HXK2|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S2213231720310168?token=ACF7439396356846411802F2F5DA6A45B3962C9A3A11A6D4E8AAC1B6FB88D7C48A4219F0EC57BA9A38F5B755B2436306&originRegion=eu-west-1&originCreation=20220617104143 (accessed on 17 May 2022).
- Jia, Q.; Zhu, R.; Tian, Y.; Chen, B.; Li, R.; Li, L.; Wang, L.; Che, Y.; Zhao, D.; Mo, F.; et al. Salvia miltiorrhiza in diabetes: A review of its pharmacology, phytochemistry, and safety. Phytomedicine 2019, 58, 152871. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lin, T.; Shi, M.; Chen, X.; Wu, P. Liraglutide suppresses production of extracellular matrix proteins and ameliorates renal injury of diabetic nephropathy by enhancing Wnt/β-catenin signaling. Am. J. Physiol. Renal Physiol. 2020, 319, F458–F468. [Google Scholar] [CrossRef] [PubMed]
- Canto, E.D.; Ceriello, A.; Ryde, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W.J. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications diabetes cardiovascular risk. Eur. J. Prev. Cardiol. 2019, 26 (Suppl. S2), 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.; Kearney, P.; Reynolds, K.; Chen, J.; He, J. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Physiol. Behav. 2016, 134, 139–148. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Hsueh, W.A.; Anderson, P.W. Clinical conference hypertension, the endothelial cell, and the vascular complications of diabetes mellitus. Hypertension 1992, 20, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, R.; Emslie-smith, A.M.; Gardner, I.D.; Morris, A.D. Vascular complications of diabetes Microvascular complications Macrovascular complications. BMJ 2000, 320, 1062-6. [Google Scholar]
- Smits, M.M.; Tonneijck, L.; Muskiet, M.H.A.; Hoekstra, T.; Kramer, M.H.H.; Diamant, M.; Serné, E.H.; Van Raalte, D.H. GLP-1-based therapies have no microvascular effects in type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2125–2132. [Google Scholar] [CrossRef] [Green Version]
- Strain, W.D.; Paldánius, P.M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzoni, D.; Rosei, E.A. Small artery remodeling in hypertension and diabetes. Curr. Hypertens. Rep. 2006, 8, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Valero, K.; Marante, D.; Torres, M.R.; Ramírez, G.; Cortéz, R.; Carlini, R. Complicaciones microvasculares de la diabetes. Rev. Venez. Endocrinol. Metab. 2012, 10, 111–137. [Google Scholar]
- Summers, M.E.; Richmond, B.W.; Kropski, J.A.; Majka, S.A.; Bastarache, J.A.; Hatzopoulos, A.K.; Bylund, J.; Ghosh, M.; Petrache, I.; Foronjy, R.F.; et al. Balanced Wnt/Dickkopf-1 signaling by mesenchymal vascular progenitor cells in the microvascular niche maintains distal lung structure and function. Am. J. Physiol. Cell Physiol. 2021, 320, C119. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Zhou, T.; Zhou, K.K.; Mott, R.; Wu, M.; Boulton, M.; Lyons, T.J.; Gao, G.; Ma, J.X. Activation of the wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am. J. Pathol. 2009, 175, 2676–2685. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Dennison, R.J.; Sapieha, P.; Hua, J.; Hatton, C.J.; Juan, A.M.; Aderman, C.M.; et al. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation 2011, 124, 1871–1881. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Ma, J. xing Canonical Wnt signaling in diabetic retinopathy. Vis. Res. 2017, 139, 47–58. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, K.K.; Ma, J.X. Inhibition of connective tissue growth factor overexpression in diabetic retinopathy by SERPINA3K via blocking the WNT/β-catenin pathway. Diabetes 2010, 59, 1809–1816. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Cheng, R.; Lee, K.; Tyagi, P.; Ding, L.; Kompella, U.B.; Chen, J.; Xu, X.; Ma, J.X. Nanoparticle-mediated expression of a Wnt pathway inhibitor ameliorates ocular neovascularization. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Saishin, Y.; Saishin, Y.; Silva, R.L.; Oshima, Y.; Oshima, S.; Melia, M.; Paszkiet, B.; Zerby, D.; Kadan, M.J.; et al. Intraocular expression of endostatin reduces VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment. FASEB J. 2003, 17, 896–898. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; McBride, J.D.; Zhou, K.; Lee, K.; Zhou, Y.; Liu, Z.; Ma, J.X. Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical wnt pathway. Diabetes 2013, 62, 4228–4238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Lee, K.; Zhang, B.; Zhou, T.; He, X.; Gao, G.; Murray, A.R.; Ma, J.-X. Identification of a novel inhibitor of the canonical Wnt pathway. Mol. Cell. Biol. 2011, 31, 3038–3051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, Y.; Chen, Q.; Rajala, R.V.S.; Ma, J.X. MicroRNA-184 modulates canonical Wnt signaling through the regulation of frizzled-7 expression in the retina with ischemia-induced neovascularization. FEBS Lett. 2015, 589, 1143–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, M.; Almas, S.; Prabhakar, S. Wnt signaling and podocyte dysfunction in diabetic nephropathy. J. Investig. Med. 2017, 65, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shang, D. Transforming growth factor-β1 enhances proliferative and metastatic potential by up-regulating lymphoid enhancer-binding factor 1/integrin αMβ2 in human renal cell carcinoma. Mol. Cell. Biochem. 2020, 465, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Rooney, B.; O’Donovan, H.; Gaffney, A.; Browne, M.; Faherty, N.; Curran, S.P.; Sadlier, D.; Godson, C.; Brazil, D.P.; Crean, J. CTGF/CCN2 activates canonical Wnt signalling in mesangial cells through LRP6: Implications for the pathogenesis of diabetic nephropathy. FEBS Lett. 2011, 585, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Kiewisz, J.; Skowronska, A.; Winiarska, A.; Pawlowska, A.; Kiezun, J.; Rozicka, A.; Perkowska-Ptasinska, A.; Kmiec, Z.; Stompor, T. WNT4 expression in primary and secondary kidney diseases: Dependence on staging. Kidney Blood Press. Res. 2019, 44, 200–210. [Google Scholar] [CrossRef]
- Hruska, K.A.; Sugatani, T.; Agapova, O.; Fang, Y. The chronic kidney disease—Mineral bone disorder (CKD-MBD): Advances in pathophysiology. Bone 2017, 100, 80–86. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhou, L. The signaling of cellular senescence in diabetic nephropathy. Oxidative Med. Cell. Longev. 2019, 2019, 7495629. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, M.; Yang, S.; Liu, F.; Sun, L. A glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling in diabetic nephropathy. Biomed. Res. Int. 2013, 2013, 987064. [Google Scholar] [CrossRef] [Green Version]
- Beaton, H.; Andrews, D.; Parsons, M.; Murphy, M.; Gaffney, A.; Kavanagh, D.; McKay, G.J.; Maxwell, A.P.; Taylor, C.T.; Cummins, E.P.; et al. Wnt6 regulates epithelial cell differentiation and is dysregulated in renal fibrosis. Am. J. Physiol. Ren. Physiol. 2016, 311, F35–F45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resham, K.; Sharma, S.S. Pharmacologic inhibition of porcupine, disheveled, and β-catenin in Wnt signaling pathway ameliorates diabetic peripheral neuropathy in rats. J. Pain 2019, 20, 1338–1352. [Google Scholar] [CrossRef]
- Itokazu, T.; Hayano, Y.; Takahashi, R.; Yamashita, T. Involvement of Wnt/β-catenin signaling in the development of neuropathic pain. Neurosci. Res. 2014, 79, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Packham, D.K.; Alves, T.P.; Dwyer, J.P.; Atkins, R.; De Zeeuw, D.; Cooper, M.; Shahinfar, S.; Lewis, J.B.; Heerspink, H.J.L. Relative incidence of ESRD versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: Results from the DIAMETRIC (Diabetes mellitus treatment for renal insufficiency consortium) database. AJKD 2011, 59, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C.; Almeida, M. Gone with the Wnts:-Catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol. Endocrinol. 2007, 21, 2605–2614. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Sun, D.; Wang, L.; Wang, X.; Shi, M.; Jiang, X.; Gao, X. The role of STAT3/mTOR-regulated autophagy in angiotensin II-induced senescence of human glomerular mesangial cells. Cell. Signal. 2019, 53, 327–338. [Google Scholar] [CrossRef]
- Sangaralingham, S.J.; Wang, B.H.; Huang, L.; Kumfu, S.; Ichiki, T.; Krum, H.; Burnett, J.C. Cardiorenal fibrosis and dysfunction in aging: Imbalance in mediators and regulators of collagen. Peptides 2016, 76, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Zhou, S.; Zhou, Z.; Liu, Y.; Yang, L.; Liu, J.; Zhang, Y.; Li, H.; Liu, Y.; Hou, F.F.; et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J. Am. Soc. Nephrol. 2018, 29, 1238–1256. [Google Scholar] [CrossRef] [Green Version]
- Galer, B.S.; Gianas, A.; Jensen, M.P. Painful diabetic polyneuropathy: Epidemiology, pain description, and quality of life. Diabetes Res. Clin. Pract. 2000, 47, 123–128. [Google Scholar] [CrossRef]
- Tracy, J.A.; Dyck, P.J.B. The spectrum of diabetic neuropathies. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Dewanjee, S.; Das, S.; Das, A.K.; Bhattacharjee, N.; Dihingia, A.; Dua, T.K.; Kalita, J.; Manna, P. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur. J. Pharmacol. 2018, 833, 472–523. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Chen, Z.; Zhang, F.; Di, Z.; Zhang, J.; Cai, L. Gene expression profiling of the sciatic nerve in streptozotocin-induced diabetic rats with peripheral neuropathy. J. Diabetes Res. 2020, 2020, 5283284. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.; Flemmer, M. Diabetes and macrovascular disease. J. Diabetes Complicat. 2002, 16, 235–245. [Google Scholar] [CrossRef]
- Isea, J.; Viloria, J.L.; Ponte, C.I.; Gómez, J.R. Complicaciones macrovasculares de la diabetes mellitus: Cardíacas, vásculo cerebrales y enfermedad arterial periférica. Rev. Venez. Endocrinol. Metab. 2012, 10, 96–110. [Google Scholar]
- Couffinhal, T.; Dufourcq, P. Common pathway between wnt and growth factor signaling in vascular smooth muscle cell proliferation? Circ. Res. 2006, 99, 1287–1289. [Google Scholar] [CrossRef] [Green Version]
- Kun, D.; Grantham, R.N.; Trachte, A.L.; Mannion, J.D.; Wilson, C.L. Activation of the canonical Wnt/b -catenin pathway enhances monocyte adhesion to endothelial cells. Biochem. Biophys. Res. Commun. 2006, 347, 109–116. [Google Scholar] [CrossRef]
- Ku, M.; Dejana, E. The role of Wnt signaling in physiological and pathological angiogenesis. Circ. Res. 2010, 107, 1798–1806. [Google Scholar] [CrossRef] [Green Version]
- Novo-Rodríguez, C.; García-Fontana, B.; Luna-Del Castillo, J.D.D.; Andujar-Vera, F.; Avila-Rubio, V.; García-Fontana, C.; Morales-Santana, S.; Rozas-Moreno, P.; Munoz-Torres, M. Circulating levels of sclerostin are associated with cardiovascular mortality. PLoS ONE 2018, 13, e0199504. [Google Scholar] [CrossRef]
- Morales-Santana, S.; Rozas-Moreno, P.; Antonio, J.; Ia-Salcedo, G.; Reyes-Garc Ia, R.; Muñoz-Torres, M. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care 2013, 36, 1667–1674. [Google Scholar] [CrossRef] [Green Version]
- Ueland, T.; Åkerblom, A.; Ghukasyan, T.; Michelsen, A.E.; Becker, R.C.; Bertilsson, M.; Himmelmann, A.; James, S.K.; Siegbahn, A.; Storey, R.F.; et al. Admission levels of DKK1 (Dickkopf-1) are associated with future cardiovascular death in patients with acute coronary syndromes: Insights from the PLATO trial. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 294–302. [Google Scholar] [CrossRef]
- Souilhol, C.; Harmsen, M.C.; Evans, P.C.; Krenning, G. Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc. Res. 2018, 114, 565–577. [Google Scholar] [CrossRef]
- Go, G.W.; Srivastava, R.; Hernandez-Ono, A.; Gang, G.; Smith, S.B.; Booth, C.J.; Ginsberg, H.N.; Mani, A. The combined hyperlipidemia caused by impaired Wnt-LRP6 signaling is reversed by Wnt3a rescue. Cell Metab. 2014, 19, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relling, I.; Akcay, G.; Fangmann, D.; Knappe, C.; Schulte, D.M.; Hartmann, K.; Müller, N.; Türk, K.; Dempfle, A.; Franke, A.; et al. Role of Wnt5a in metabolic inflammation in humans. J. Clin. Endocrinol. Metab. 2018, 103, 4253–4264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohta, H.; Wada, H.; Niwa, T.; Kirii, H.; Iwamoto, N.; Fujii, H.; Saito, K.; Sekikawa, K.; Seishima, M. Disruption of tumor necrosis factor-α gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis 2005, 180, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Kiechl, S.; Qi, D.; Wang, X.; Song, Y.; Weger, S.; Mayr, A.; Le Bras, A.; Karamariti, E.; Zhang, Z.; et al. A cytokine-like protein dickkopf-related protein 3 is atheroprotective. Circulation 2017, 136, 1022–1036. [Google Scholar] [CrossRef] [Green Version]
- Uglow, E.B.; Slater, S.; Sala-Newby, G.B.; Aguilera-Garcia, C.M.; Angelini, G.D.; Newby, A.C.; George, S.J. Dismantling of cadherin-mediated cell-cell contacts modulates smooth muscle cell proliferation. Circ. Res. 2003, 92, 1314–1321. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.; Zhang, J.; Go, G.W.; Narayanan, A.; Nottoli, T.P.; Mani, A. Impaired LRP6-TCF7L2 activity enhances smooth muscle cell plasticity and causes coronary artery disease. Cell Rep. 2015, 13, 746–759. [Google Scholar] [CrossRef] [Green Version]
- Williams, H.; Mill, C.A.E.; Monk, B.A.; Hulin-Curtis, S.; Johnson, J.L.; George, S.J. Wnt2 and WISP-1/CCN4 induce intimal thickening via promotion of smooth muscle cell migration. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1417–1424. [Google Scholar] [CrossRef] [Green Version]
- Borrell-Pagès, M.; Romero, J.C.; Badimon, L. LRP5 deficiency down-regulates Wnt signalling and promotes aortic lipid infiltration in hypercholesterolaemic mice. J. Cell. Mol. Med. 2015, 19, 770–777. [Google Scholar] [CrossRef]
- He, X.W.; Wang, E.; Bao, Y.Y.; Wang, F.; Zhu, M.; Hu, X.F.; Jin, X.P. High serum levels of sclerostin and Dickkopf-1 are associated with acute ischaemic stroke. Atherosclerosis 2016, 253, 22–28. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, D.; Zhong, C.; Wang, A.; Xie, X.; Xu, T.; Chen, C.S.; Peng, Y.; Peng, H.; Li, Q.; et al. Serum Dkk-1 (Dickkopf-1) is a potential biomarker in the prediction of clinical outcomes among patients with acute ischemic stroke. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.D.; Li, X.M.; Liu, J.L.; Li, J.; Zhou, H. Effects of miR-150-5p on cerebral infarction rats by regulating the Wnt signaling pathway via p53. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3882–3891. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhu, W.; Jiang, J. miR-150-5p suppresses the stem cell-like characteristics of glioma cells by targeting the Wnt/β-catenin signaling pathway. Cell Biol. Int. 2020, 44, 1156–1167. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Shang, Y.C.; Hou, J.; Maiese, K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid. Med. Cell. Longev. 2010, 3, 153–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, K.A.; Zhang, X.; Predescu, D.; Huang, X.; MacHado, R.F.; Göthert, J.R.; Malik, A.B.; Valyi-Nagy, T.; Zhao, Y.Y. Endothelial β-catenin signaling is required for maintaining adult blood-brain barrier integrity and central nervous system homeostasis. Circulation 2016, 133, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Yi, R.; Xiao-Ping, G.; Hui, L. Atorvastatin prevents angiotensin II-induced high permeability of human arterial endothelial cell monolayers via ROCK signaling pathway. Biochem. Biophys. Res. Commun. 2015, 459, 94–99. [Google Scholar] [CrossRef]
- Ruan, W.; Hu, J.; Zhou, H.; Li, Y.; Xu, C.; Luo, Y.; Chen, T.; Xu, B.; Yan, F.; Chen, G. Intranasal Wnt-3a alleviates neuronal apoptosis in early brain injury post subarachnoid hemorrhage via the regulation of wnt target PPAN mediated by the moonlighting role of aldolase C. Neurochem. Int. 2020, 134, 104656. [Google Scholar] [CrossRef]
- Abe, T.; Zhou, P.; Jackman, K.; Capone, C.; Casolla, B.; Hochrainer, K.; Kahles, T.; Ross, M.E.; Anrather, J.; Iadecola, C. Lipoprotein receptor-related protein-6 protects the brain from ischemic injury. Stroke 2013, 44, 2284–2291. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, J.; Kim, D.W.; Ha, Y.; Ihm, M.H.; Kim, H.; Song, K.; Lee, I. Wnt5a induces endothelial inflammation via β-catenin–independent signaling. J. Immunol. 2010, 185, 1274–1282. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; Shan, G. MiR-148b regulates proliferation and differentiation of neural stem cells via Wnt/β-Catenin signaling in rat ischemic stroke model. Front. Cell. Neurosci. 2017, 11, 329. [Google Scholar] [CrossRef]
- Wang, B.; Pan, Y.; Yang, G.; Cui, Z.; Yu, W.; Liu, H.; Bai, B. Sfrp5/Wnt5a and leptin/adiponectin levels in the serum and the periarterial adipose tissue of patients with peripheral arterial occlusive disease. Clin. Biochem. 2021, 87, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Myngbay, A.; Manarbek, L.; Ludbrook, S.; Kunz, J. The role of collagen triple helix repeat-containing 1 protein (Cthrc1) in rheumatoid arthritis. Int. J. Mol. Sci. 2021, 22, 2426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, J.; Sun, X.; Yang, K.; Yang, L.; Kong, L.; Zhang, B.; Li, F.; Li, C.; Shi, B.; et al. Loss of m6A demethylase ALKBH5 promotes post-ischemic angiogenesis via post-transcriptional stabilization of WNT5A. Clin. Transl. Med. 2021, 11, e402. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; De Caterina, R. Cellular and molecular mechanisms of vascular injury in diabetes—Part II: Cellular mechanisms and therapeutic targets. Vascul. Pharmacol. 2011, 54, 75–79. [Google Scholar] [CrossRef]
- García-Martín, A.; Rozas-Moreno, P.; Reyes-García, R.; Morales-Santana, S.; García-Fontana, B.; García-Salcedo, J.A.; Muñ Oz-Torres, M. Circulating levels of sclerostin are increased inpatients with type 2 diabetes mellitus. J. Clin. Endocrinol Metab 2012, 97, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Goliasch, G.; Wiesbauer, F.; Kastl, S.P.; Katsaros, K.M.; Blessberger, H.; Maurer, G.; Schillinger, M.; Huber, K.; Wojta, J.; Speidl, W.S. Premature myocardial infarction is associated with low serum levels of Wnt-1. Atherosclerosis 2012, 222, 251–256. [Google Scholar] [CrossRef]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [Green Version]
- McCrimmon, R.J.; Ryan, C.M.; Frier, B.M. Diabetes and cognitive dysfunction. Lancet 2012, 379, 2291–2299. [Google Scholar] [CrossRef]
- Perciaccante, A.; Fiorentini, A.; Paris, A.; Serra, P.; Tubani, L. Circadian rhythm of the autonomic nervous system in insulin resistant subjects with normoglycemia, impaired fasting glycemia, impaired glucose tolerance, type 2 diabetes mellitus. BMC Cardiovasc. Disord. 2006, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Vigili de Kreutzenberg, S.; Tiengo, A.; Avogaro, A. Cerebrovascular disease in diabetes mellitus: The role of carotid intima-media thickness. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 667–673. [Google Scholar] [CrossRef]
- Mastroiacovo, F.; Busceti, C.L.; Biagioni, F.; Moyanova, S.G.; Meisler, M.H.; Battaglia, G.; Caricasole, A.; Bruno, V.; Nicoletti, F. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J. Cereb. Blood Flow Metab. 2009, 29, 264–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matei, X.N.; Camara, X.J.; Mcbride, X.D.; Camara, X.R.; Xu, X.N.; Tang, X.J.; Zhang, X.J.H.; Physiology, D.; Linda, L.; Vivian, L. Intranasal Wnt3a attenuates neuronal apoptosis through Frz1/PIWIL1a/FOXM1 pathway in MCAO rats. J. Neurosci. 2018, 38, 6787–6801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, R.; Nakamura, K.; Maclauchlan, S.; Ngo, D.T.; Shimizu, I.; Fuster, J.J.; Katanasaka, Y.; Yoshida, S.; Qiu, Y.; Yamaguchi, T.P.; et al. An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat. Med. 2014, 20, 1464–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.L.; Zhu, L.Y.; Han, R.; Sun, L.L.; Li, J.X.; Dou, J.T. Pathophysiology of peripheral arterial disease in diabetes mellitus. J. Diabetes 2017, 9, 133–140. [Google Scholar] [CrossRef]
- Lepäntalo, M.; Fiengo, L.; Biancari, F. Peripheral arterial disease in diabetic patients with renal insufficiency: A review. Diabetes. Metab. Res. Rev. 2012, 28 (Suppl. S1), 40–45. [Google Scholar] [CrossRef]
- Kirkpantur, A.; Balci, M.; Turkvatan, A.; Afsar, B. Independent association between serum sclerostin levels and carotid artery atherosclerosis in prevalent haemodialysis patients. Clin. Kidney J. 2015, 8, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Evrard, S.; Delanaye, P.; Kamel, S.; Cristol, J.; Cavalier, E.; Arnaud, J.; Zaoui, P.; Carlier, M.C.; Laville, M.; Fouque, D.; et al. Clinica chimica acta vascular calci fi cation: From pathophysiology to biomarkers. Clin. Chim. Acta 2015, 438, 401–414. [Google Scholar] [CrossRef]
- Teng, I.; Wang, J.; Lee, C.; Hou, J.; Hsu, B. Serum sclerostin as an independent marker of peripheral artery disease in elderly persons. Int. J. Clin. Exp. Pathol. 2018, 11, 2816–2821. [Google Scholar]
- Liu, L.; Chen, X.; Zhou, X.; Zhu, Q. The Wnt antagonist and secreted frizzled-related protein 5: Implications on lipid metabolism, inflammation, and type 2 diabetes mellitus. Biosci. Rep. 2018, 38, BSR20180011. [Google Scholar] [CrossRef]
- Gaudio, A.; Xourafa, A.; Rapisarda, R.; Castellino, P.; Signorelli, S.S. Peripheral artery disease and osteoporosis: Not only age-related (Review). Mol. Med. Rep. 2018, 18, 4787–4792. [Google Scholar] [CrossRef] [Green Version]
- Markham, A. Romosozumab: First global approval. Drugs 2020, 79, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT—β-catenin signalling—A versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, H.; Wang, H.; Luo, S.; Wang, L.; Chen, J.; Lu, H. GLP-1 promotes osteogenic differentiation of human ADSCs via the Wnt/GSK-3β/β-catenin pathway. Mol. Cell. Endocrinol. 2020, 515, 110921. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Priya, K.; Mehta, C.H.; Nayak, U.Y.; Kabekkodu, S.P.; Pai, K.S.R. Repositioning of antidiabetic drugs for Alzheimer’s disease: Possibility of Wnt signaling modulation by targeting LRP6 an in silico based study. J. Biomol. Struct. Dyn. 2021, 3, 1–15. [Google Scholar] [CrossRef]
- Liu, Y.; Neogi, A.; Mani, A. The role of Wnt signalling in development of coronary artery disease and its risk factors. Open Biol. 2020, 10, 200128. [Google Scholar] [CrossRef]
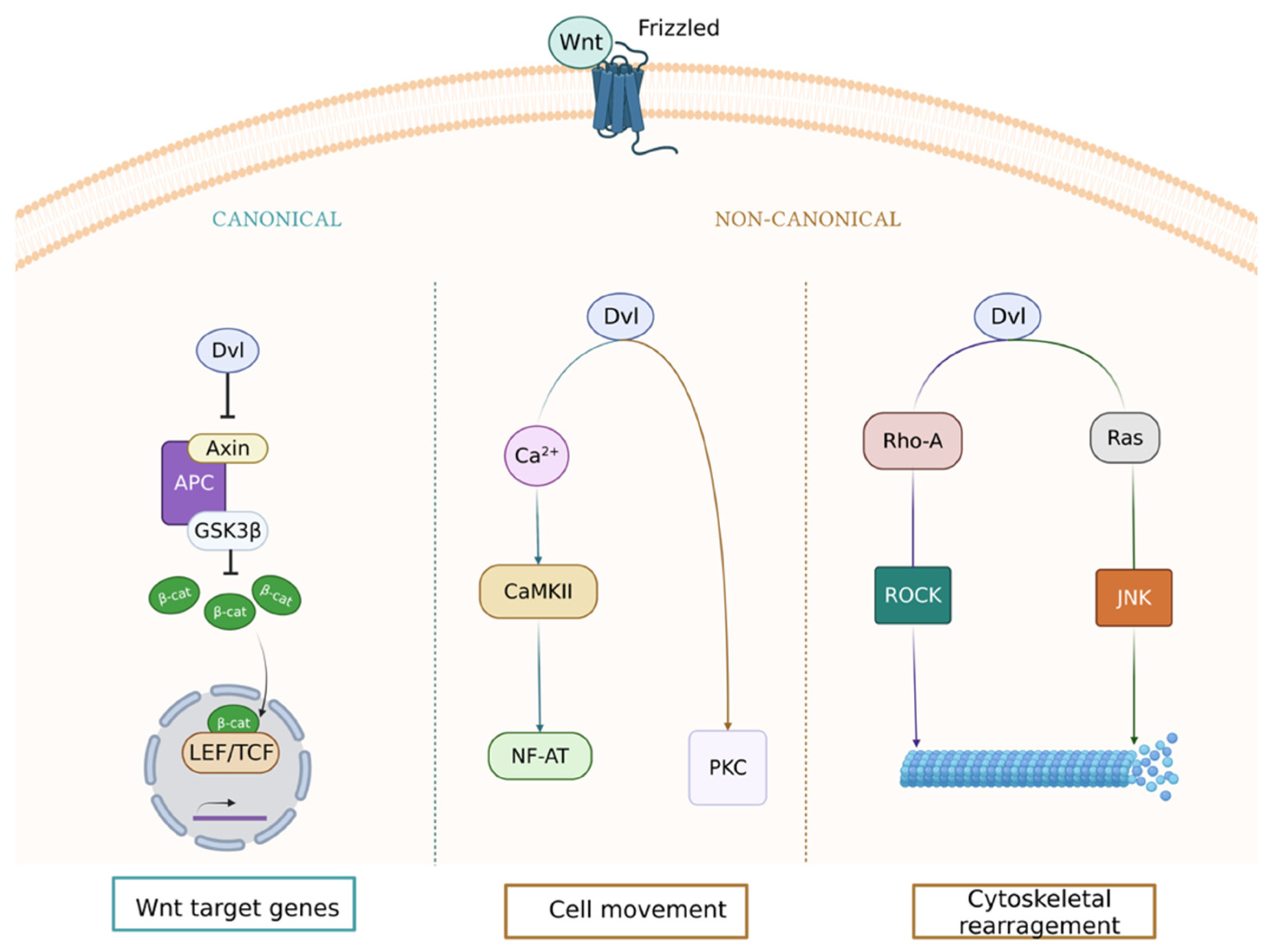
| Disease | Event | Component | Expression | In Vitro | In Vivo | Reference |
|---|---|---|---|---|---|---|
| Microvascular | Retinopathy | β-catenin | ↑ | Inflammation and angiogenesis | Retinal inflammation and vascular leakage | [26] |
| LRP5/6 | ↑ | Inflammation and angiogenesis | Retinal inflammation and vascular leakage | [26] | ||
| ↓ | Lack of deeper retinal vessels | Significant decrease in pathological retinal neovascularization Significant decrease in retinal vascularization during development Affects blood–retinal barrier formation | [27] | |||
| Dkk1 | ↑ | Inhibition of the generation of reactive oxygen species (ROS) | Mitigated retinal inflammation and blocked overexpression of proinflammatory factors such as ICAM-1 and COX-2 Reduction in retinal vascular leakage and improvement of ischemia-induced retinal neovascularization | [26] | ||
| Frizzled4 | ↑ | Angiogenesis | Pathological neovascularization | [27] | ||
| Dvl2 | ↓ | Impaired angiogenesis | Significant decrease in pathological retinal neovascularization | [27] | ||
| Claudin-5 | ↓ | Significant suppression of endothelial cell sprouting | Suppression of pathological vascular growth and development | [27] | ||
| Frizzled7 | ↑ | Inflammation, angiogenesis, and oxidative stress | Pathological neovascularization | [28] | ||
| SERPINA3K | ↑ | Inhibition of connective tissue growth factor overexpression | Antioxidation Anti-inflammatory Antifibrosis | [29] | ||
| VLDLR | ↑ | Anti-angiogenesis Inhibited endothelial cell proliferation, migration, and tube formation | Improvement of ocular neovascularization, | [30] | ||
| Endostatin | ↑ | Impaired angiogenesis | Reduced VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment | [31] | ||
| Kallistatin | ↑ | Anti-inflammation Anti-angiogenesis | Attenuation of ischemia-induced retinal neovascularization | [32] | ||
| PEDF | ↑ | Anti-inflammation Anti-angiogenesis | Ameliorated retinal inflammation, vascular leakage, and neovascularization | [33] | ||
| MiARN-184 | ↑ | Anti-angiogenesis | Improves inflammatory responses, vascular leakage, and neovascularization. | [34] | ||
| Nephropathy | β-catenin | ↑ | Reduced mesangial cell apoptosis Podocyte dysfunction | Glomerular albuminuria and subsequent glomerular injury | [35] | |
| ↓ | Mesangial cells apoptosis | Increased severity of streptozotocin-induced diabetes nephritis | [35] | |||
| LEF1 | ↑ | Enhanced proliferation and metastasis of renal cells | Renal cell carcinoma (RCC) | [36] | ||
| LRP6 | ↓ | Mesangial cell apoptosis | Attenuated renal inflammation, reduced proteinuria, and ameliorated fibrosis | [37] | ||
| Wnt4 | ↑ | Stimulation of mesenchymal-to-epithelial differentiation Podocyte dysfunction | Tubulo-interstitial fibrosis Glomerular albuminuria and subsequent glomerular injury | [35] | ||
| ↓ | Mesangial cell apoptosis | Kidney tissue disorganization, as well as disease development and progression | [38] | |||
| Dkk1 | ↑ | Amelioration of podocyte apoptosis and viability | Restored podocyte function and decreased albuminuriaBone-mineral disorder syndrome | [35,39] | ||
| TRPC6 | ↑ | Podocyte injury | Excessive calcium influx in podocytes leading to foot process effacement, podocyte apoptosis, and subsequent glomerular damage | [35] | ||
| Wnt9a | ↑ | Evoking of cell communication between senescent tubular cells and interstitial fibroblasts | Tubular senescence and renal fibrosis | [40] | ||
| Wnt5a | ↑ | Increased ROS production | Mesangial cell apoptosis | [41] | ||
| CTGF/CCN2 | ↑ | LRP6 phosphorylation and accumulation of β-catenin | Attenuated renal inflammation, reduced proteinuria, and ameliorated fibrosis Mesangial cell apoptosis | [37] | ||
| CTNNB1 | ↓ | Improved podocyte motility | Damage to the basement membrane, albuminuria, and increased susceptibility to glomerular injury | [41] | ||
| Wnt6 | ↓ | Damaged tubulo-interstitium | Renal fibrosis | [42] | ||
| Neuropathy | PORCN | ↓ | Slightly reduced expression of Wnt3a Significantly reduced expression of β-catenin, Dvl1, c-myc, GRP78, and MMP2 in the sciatic nerve | Decreased heat- and cold-induced hyperalgesia Increased motor nerve conduction speed Increased sensory nerve conduction speed Increased nerve blood flow Increased density of intraepidermal nerve fibers | [43] | |
| Dvl | ↓ | Significantly reduced expression of β-catenin, Dvl1, c-myc, GRP78, and MMP2 in the sciatic nerve | Decreased heat- and cold-induced hyperalgesia Increased motor nerve conduction speed Increased sensory nerve conduction speed Increased nerve blood flow Increased density of intraepidermal nerve fibers | [43] | ||
| β-catenin | ↓ | Significantly reduced expression of β-catenin, Dvl1, c-myc, GRP78, and MMP2 in the sciatic nerve | Decreased heat- and cold-induced hyperalgesia Increased motor nerve conduction speed Increased sensory nerve conduction speed Increased nerve blood flow Increased density of intraepidermal nerve fibers | [43] | ||
| Wnt3a | ↑ | Release of brain-derived neurotrophic factor in microglial cells | Allodynia | [44] | ||
| XAV939 | ↑ | - | Effective attenuation of neuropathic pain induction Drastic attenuation of the development of allodynia | [44] |
| Disease | Event | Component | Expression | In Vitro | In Vivo | Reference |
|---|---|---|---|---|---|---|
| Macrovascular | Coronary artery disease | Scl | ↑ | Endothelial dysfunction, alteration on proliferation, and migration of vascular smooth muscle cells | Atherosclerotic process, abnormal intima-media thickness, carotid plaques, aortic calcifications, and mortality | [59,60] |
| Dkk-1 | ↑ | Regulates platelet-mediated inflammation and contributes to plaque de-escalation | Ischemic stroke and cardiovascular death | [61] | ||
| ↑ | Endothelial activation and release of inflammatory cytokines Endothelial–mesenchymal transition in aortic endothelial cells | Onset and progression of atherosclerosis | [62] | |||
| LRP6 | ↓ | LDL uptake was significantly lower in lymphoblastoid cells | Elevated plasma cholesterol and elevated plasma LDL, triglyceride, and fatty liver levels | [63] | ||
| Wnt5a | ↑ | Induction of inflammatory gene expression GM-CSF, IL-1a, IL-3, IL-5, IL-6, IL-7, IL-8, CCL2, CCL8, and COX-2 in human aortic endothelial cells | Elevation of triglyceride levels, vascular insulin resistance, and endothelial dysfunction | [64] | ||
| ↑ | Macrophage activation | Increased recruitment of inflammatory cells and amplified inflammatory response | [65] | |||
| Dkk-3 | ↓ | Increased intima-media thickness of the carotid artery | Delayed reendothelialization and aggravated neointima formation | [66] | ||
| ↑ | Induces differentiation of vascular progenitors and fibroblasts into smooth muscle cells | Larger and more vulnerable atherosclerotic lesions with more macrophages, fewer smooth muscle cells, and less extracellular matrix deposition | [67] | |||
| TCF7L2 | ↓ | Loss of differentiation of vascular smooth muscle cells | Medial aortic hyperplasia | [68] | ||
| Wnt2 | ↑ | Regulates smooth muscle cell migration | Triggers intima-media thickening | [69] | ||
| LRP5 | ↓ | Activation of proinflammatory genes (interferon γ, IL15, IL18, and TNF ligand superfamily 13b). | Larger aortic atherosclerotic lesions | [70] | ||
| Cerebrovascular disease | Scl | ↑ | Arterial calcification | Ischemic stroke caused by atherosclerotic stroke of large arteries or occlusion of small arteries | [71] | |
| Dkk1 | ↑ | Biomarker for the presence of coronary atherosclerotic plaque | Carotid atherosclerosis, stable angina, and myocardial infarction Poor prognosis 1 year after ischemic stroke | [72] | ||
| miR-150-5p | ↑ | Regulates the Wnt signaling pathway and participates in cell proliferation and apoptosis by downregulating p53 | Inhibition of cell proliferation, colony formation, and tumor growth | [73] | ||
| ↓ | CD133− cells acquire a stem-cell-like phenotype | >Glioma | [74] | |||
| β-catenin | ↑ | Key regulators for cadherin-mediated cell–cell adhesion | Glioma Higher degree of malignancy of the tumor | [74] | ||
| Wnt1 | ↓ | Neuronal disappearance and increasing functional deficits | Oxidant stress and cerebral ischemia | [75] | ||
| claudin-1 | ↓ | Neuronal damage | Increased permeability of the blood–brain barrier, petechial hemorrhage in the brain, neuronal injury, and central nervous system inflammation | [76] | ||
| Claudin-3 | ↓ | Neuronal damage | Intracerebral petechial hemorrhages | [77] | ||
| Wnt3a | ↑ | Alleviates neuronal apoptosis at the cellular and subcellular levels | Neuroprotection in traumatic brain injury, and ischemic stroke | [78] | ||
| LRP6 | ↓ | Increased expression of inflammatory genes after middle artery occlusion | Risk of ischemic stroke, larger heart attack, and severe motor deficits | [79] | ||
| Wnt5 | ↑ | Enhanced endothelial activation type 1 inflammatory mediator to promote endothelial activation type 2 | Brain aging Inflamed atheroma plaques | [80] | ||
| miRNA-148b | ↓ | Attenuates neural stem-cell proliferation and differentiation | Reduces ischemic injury and improves neurological function | [81] | ||
| Peripheral arterial disease | Wnt5a | ↑ | Endothelial dysfunction | Increased risk of peripheral arterial occlusive disease, as well as metabolic and cardiovascular disorders | [82] | |
| Sfrp5 | ↓ | Inhibition of cardiac fibroblast proliferation and migration Inflammation and myocardial injury | ST-segment elevation myocardial infarction, metabolic syndrome, and increased risk of peripheral arterial occlusive disease | [82] | ||
| CTHRC1 | ↑ | Synovial hyperplasia, contributes to the inflammatory microenvironment, and promotes pannus invasion through increased motility and invasion of synoviocytes | Increased risk of systemic lupus erythematosus, development of rheumatoid arthritis, and severity of the disease | [83] | ||
| ALKBH5 | ↑ | Reduced proliferation and migration and decreased viability in hypoxic cardiac microvascular endothelial cells | Impaired hypoxic tube formation, but not the normoxic cardiac microvascular endothelial cells | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanabria-de la Torre, R.; García-Fontana, C.; González-Salvatierra, S.; Andújar-Vera, F.; Martínez-Heredia, L.; García-Fontana, B.; Muñoz-Torres, M. The Contribution of Wnt Signaling to Vascular Complications in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 6995. https://doi.org/10.3390/ijms23136995
Sanabria-de la Torre R, García-Fontana C, González-Salvatierra S, Andújar-Vera F, Martínez-Heredia L, García-Fontana B, Muñoz-Torres M. The Contribution of Wnt Signaling to Vascular Complications in Type 2 Diabetes Mellitus. International Journal of Molecular Sciences. 2022; 23(13):6995. https://doi.org/10.3390/ijms23136995
Chicago/Turabian StyleSanabria-de la Torre, Raquel, Cristina García-Fontana, Sheila González-Salvatierra, Francisco Andújar-Vera, Luis Martínez-Heredia, Beatriz García-Fontana, and Manuel Muñoz-Torres. 2022. "The Contribution of Wnt Signaling to Vascular Complications in Type 2 Diabetes Mellitus" International Journal of Molecular Sciences 23, no. 13: 6995. https://doi.org/10.3390/ijms23136995
APA StyleSanabria-de la Torre, R., García-Fontana, C., González-Salvatierra, S., Andújar-Vera, F., Martínez-Heredia, L., García-Fontana, B., & Muñoz-Torres, M. (2022). The Contribution of Wnt Signaling to Vascular Complications in Type 2 Diabetes Mellitus. International Journal of Molecular Sciences, 23(13), 6995. https://doi.org/10.3390/ijms23136995








